Abstract
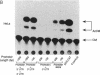
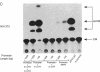
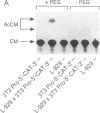
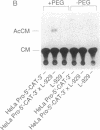
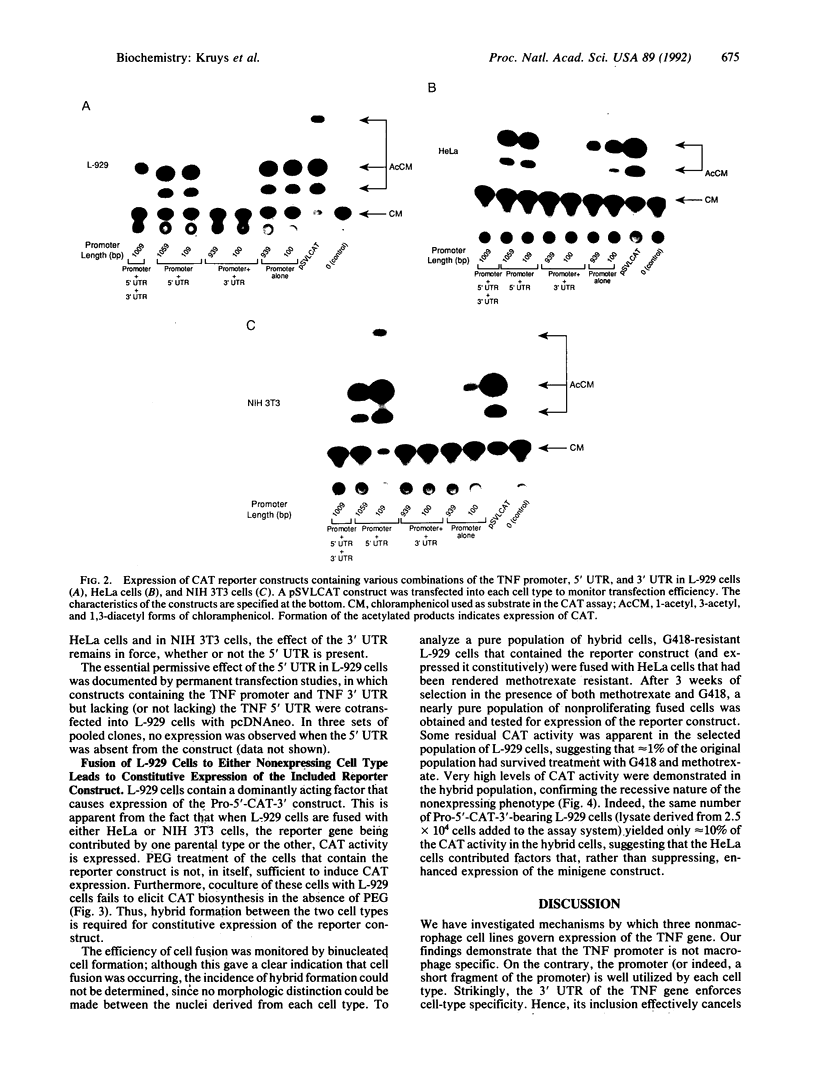
The role of the mouse tumor necrosis factor (TNF) promoter, 5' untranslated region (UTR), and 3' UTR in TNF gene expression has been examined in three nonmacrophage cell lines (HeLa, NIH 3T3, and L-929). The TNF promoter is not macrophage-specific. On the contrary, it constitutively drives reporter gene expression in all three cell lines. Not only the full-length promoter but also truncated versions of the promoter, lacking NF-kappa B binding motifs, are active in each type of cell. The TNF 3' UTR effectively cancels reporter gene expression in HeLa cells and in NIH 3T3 cells but fails to block expression in L-929 cells. L-929 cells contain a factor that overcomes the inhibitory influence of the TNF 3' UTR. Its action depends upon the presence of sequences found in the TNF 5' UTR. Cell-fusion experiments reveal that this activator is trans-dominant. These studies highlight the essential role played by the TNF 3' UTR, which silences the TNF gene in cells that might otherwise express TNF. They also reveal the existence of an escape mechanism whereby inappropriate synthesis of TNF might occur.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B., Brown T. A CAT reporter construct allows ultrasensitive estimation of TNF synthesis, and suggests that the TNF gene has been silenced in non-macrophage cell lines. J Clin Invest. 1991 Apr;87(4):1336–1344. doi: 10.1172/JCI115137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuturi M. C., Murphy M., Costa-Giomi M. P., Weinmann R., Perussia B., Trinchieri G. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. J Exp Med. 1987 Jun 1;165(6):1581–1594. doi: 10.1084/jem.165.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degliantoni G., Murphy M., Kobayashi M., Francis M. K., Perussia B., Trinchieri G. Natural killer (NK) cell-derived hematopoietic colony-inhibiting activity and NK cytotoxic factor. Relationship with tumor necrosis factor and synergism with immune interferon. J Exp Med. 1985 Nov 1;162(5):1512–1530. doi: 10.1084/jem.162.5.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Brown T., Beutler B. Endotoxin-responsive sequences control cachectin/tumor necrosis factor biosynthesis at the translational level. J Exp Med. 1990 Feb 1;171(2):465–475. doi: 10.1084/jem.171.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneel C. V., Shakhov A. N., Nedospasov S. A., Cerottini J. C. Molecular control of tissue-specific expression at the mouse TNF locus. Eur J Immunol. 1989 Mar;19(3):549–552. doi: 10.1002/eji.1830190321. [DOI] [PubMed] [Google Scholar]
- Kruys V., Marinx O., Shaw G., Deschamps J., Huez G. Translational blockade imposed by cytokine-derived UA-rich sequences. Science. 1989 Aug 25;245(4920):852–855. doi: 10.1126/science.2672333. [DOI] [PubMed] [Google Scholar]
- Laskov R., Lancz G., Ruddle N. H., McGrath K. M., Specter S., Klein T., Djeu J. Y., Friedman H. Production of tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) by murine pre-B and B cell lymphomas. J Immunol. 1990 May 1;144(9):3424–3430. [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Sullivan S. A., Williamson B. D., Carswell E. A., Old L. J. Nonhematopoietic cells selected for resistance to tumor necrosis factor produce tumor necrosis factor. J Exp Med. 1986 Oct 1;164(4):1350–1355. doi: 10.1084/jem.164.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhov A. N., Collart M. A., Vassalli P., Nedospasov S. A., Jongeneel C. V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990 Jan 1;171(1):35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Spriggs D. R., Imamura K., Rodriguez C., Sariban E., Kufe D. W. Tumor necrosis factor expression in human epithelial tumor cell lines. J Clin Invest. 1988 Feb;81(2):455–460. doi: 10.1172/JCI113341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs D., Imamura K., Rodriguez C., Horiguchi J., Kufe D. W. Induction of tumor necrosis factor expression and resistance in a human breast tumor cell line. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6563–6566. doi: 10.1073/pnas.84.18.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Jung L. K., Walters J. A., Chen W., Wang C. Y., Fu S. M. Production of tumor necrosis factor/cachectin by human B cell lines and tonsillar B cells. J Exp Med. 1988 Nov 1;168(5):1539–1551. doi: 10.1084/jem.168.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]