Abstract
The gut and the brain communicate bidirectionally through anatomic and humoral pathways, establishing what is known as the gut–brain axis. Therefore, interventions affecting one system will impact on the other, giving the opportunity to investigate and develop future therapeutic strategies that target both systems. Alterations in the gut–brain axis may arise as a consequence of changes in microbiota composition (dysbiosis), modifications in intestinal barrier function, impairment of enteric nervous system, unbalanced local immune response and exaggerated responses to stress, to mention a few. In this review we analyze and discuss several novel pharmacological targets within the gut–brain axis, with potential applications to improve intestinal and mental health.
Keywords: enteric nervous system, gut–brain axis, interleukin 22, intestinal microbiota, serotonin, vagus nerve
Introduction
Anatomic, endocrine and immune pathways of communication comprise the gut–brain (or brain–gut) axis. In a bidirectional connection, the central nervous system (CNS) sends information to the gut by the vagal, thoracolumbar and lumbosacral nerve pathways [Berthoud and Neuhuber, 2000; Brookes et al. 2013], or by soluble mediators, including various hormones, neurotransmitters and cytokines [Stasi et al. 2012; Dockray, 2014]. Conversely, changes at an intestinal level can modulate central function using similar communication strategies [Berthoud and Neuhuber, 2000; Stasi et al. 2012; Dockray, 2014].
The continuous communication between gut and brain regulates various physiological events, for example, the induction of satiety upon leptin release from the gut [Hussain and Bloom, 2013]. However, this axis may suffer alterations, and furthermore, there is comorbidity of gastrointestinal diseases and some CNS disorders. For example, clinical evidence indicates that mood disorders are commonly associated with gastrointestinal alterations, such as diarrhea or abdominal pain [Gros et al. 2009]. On the other hand, patients that present with digestive problems or gastrointestinal discomfort, display signs of anxiety and depression more frequently than the general population [Cheng et al. 2003; Mussell et al. 2008].
All the above has led us to consider the brain–gut axis as a functional unit and therefore, treatment of one of its components may have therapeutic relevance for another component. This review explores pharmacological targets in the brain–gut axis with the potential of modulating intestinal tissue and/or CNS function.
Gut microbiota
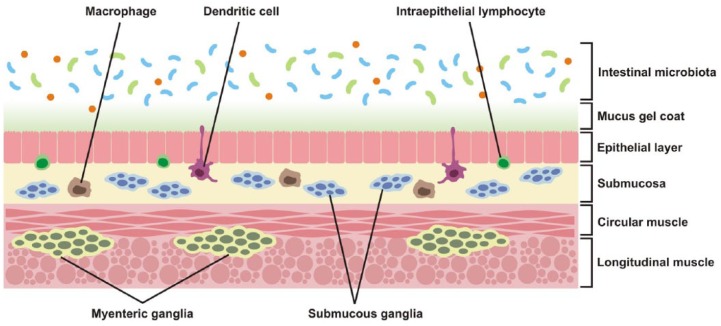
A diverse microbial community inhabits the gastrointestinal lumen of higher animals and is on a delicate equilibrium with the host (Figure 1). This group of bacteria, fungi and viruses is known as the intestinal microbiota and its composition depends on the host species, age and nutritional state, among others [Moeller and Ochman, 2013; David et al. 2014]. In the case of the human microbiota, the bacteria alone outnumber by tenfold the host’s body cell count, and they contribute by approximately 150 times the amount of genes of the human genome [Qin et al. 2010].
Figure 1.
Cellular components in the intestine that can be targeted to modulate gut–brain axis activity. Gut microbiota is a source of antigens and other bioactive mediators. Commensal bacteria and intestinal mucus restrict mucosal colonization by pathogenic entities [Johansson et al. 2013; Hansen et al. 2014]. Junctional complexes between intestinal epithelial cells also limit translocation of luminal contents [Berkes et al. 2003]. Enteroendocrine cells, with a major role in the production of neurotransmitters and hormones, are also located in the epithelial layer [Bertrand and Bertrand, 2010; Dockray, 2014]. Immune cells, some of which are in close association with the intestinal mucosa, are in a privileged location to sense and respond to changes in microbiota composition [Daneman and Rescigno, 2009]. Enteric neurons and glia are organized in ganglia and also release soluble mediators that affect epithelial and immune functions in the gut [Gulbransen and Sharkey, 2012].
Animals and their microbiota have evolved together in symbiotic association. For example, intestinal strains of Clostridium are able to modulate the host’s local immunity by affecting the number and function of mucosal CD4+ regulatory T cells [Atarashi et al. 2011]. Anomalies on the host–microbiota interaction or in the gut microbiota composition have been associated with pathologies such as obesity, type 2 diabetes, inflammatory bowel disease and allergies [Hansen et al. 2014; Thomas et al. 2014]. Interestingly, CNS pathologies such as autism, anxiety, depression and alcohol dependence have been related to dysbiosis, that is, perturbations in the amount or diversity of intestinal microbiota [Neufeld et al. 2011; De Angelis et al. 2013; Park et al. 2013; Leclercq et al. 2014], and therefore, the intestinal microbiota has been considered a potential therapeutic target for the above pathologies.
Changes on adult microbiota composition also affect the host’s behavior including activation of the hormonal axis of stress. Thus, it has been shown that treatment with probiotic bacteria such as Lactobacillus farciminis [Ait-Belgnaoui et al. 2012] and Lactobacillus rhamnosus JB-1 [Bravo et al. 2011b] has the effect of reducing corticosterone levels in experimental animals. Furthermore, they generate anxiolytic and antidepressive effects on healthy mice [Bravo et al. 2011b]. Also, the administration of Lactobacillus helveticus NS8 has been shown to prevent depression-like behaviors induced by chronic stress in rats, to improve cognition, to decrease corticosterone and acetylcholine (ACTH) plasma levels and to restore the content of serotonin (5-HT), norepinephrine (NE) and brain-derived neurotrophic factor (BDNF) in the hippocampus [Liang et al. 2015]. These data strongly suggest that probiotic supplementation has the potential to improve many behavioral and neurochemical perturbations associated with depression.
Animal data have shown that induction of depression-like behavior is accompanied by intestinal dysbiosis, but the changes in microbiota composition in patients with major depressive disorder (MDD) are still not completely described. Jiang and colleagues found increased amounts of bacteroidetes, proteobacteria and actinobacteria in stool samples from depressed patients, while firmicutes were significantly reduced compared with the healthy control group. Despite deep interindividual variability, levels of several predominant genera were significantly different between depressed patients and controls. In particular, the MDD group had increased levels of enterobacteria and Alistipes but Faecalibacterium levels were reduced. Also, a negative correlation between Faecalibacterium and severity of depressive symptoms was observed [Jiang et al. 2015]. These findings show either predominance of some potentially harmful bacteria groups, or a reduction of beneficial bacteria in depressed patients.
Intestinal microbiota can be transferred from mothers to their offspring at the moment of birth. Therefore, it has been suggested that patients under stress or undergoing depression that have an altered composition of the intestinal microbiota, could also transfer the pathological condition to their offspring. Zijlmans and colleagues prospectively investigated the development of the intestinal microbiota as a potential pathway for linking prenatal stress and maternal child health. The results showed that maternal prenatal stress, established either by patient reports or through elevated baseline concentrations of salivary cortisol in the mother or both, was strongly associated with the composition of the microbiota of infants as determined by a phylogenetic microarray. Babies of mothers with high cumulative stress during pregnancy had significantly higher relative abundances of proteobacterial groups that are known to contain pathogens and less lactic acid bacteria. Furthermore, this altered colonization pattern was accompanied by infant gastrointestinal symptoms and allergic reactions reported by their mothers. In conclusion, clear links were found between prenatal stress in the mother, and infant intestinal microbiota and health [Zijlmans et al. 2015], suggesting a potential for bacterial interventions to improve the health and development of pregnant women with stress and, consequently, an improvement in infant health.
Transfer of fecal content from a healthy individual to a patient is a (somewhat controversial) strategy to restore protective intestinal microbiota. This kind of treatment has been used to reverse mucosal-barrier disruption caused by acute dysbiosis such as that produced by recurrent Clostridium difficile infection [Rohlke and Stollman, 2012], Staphylococcus aureus infection [Wei et al. 2015a] or inflammatory bowel disease [Wei et al. 2015b]. In humans and rodents [Li et al. 2015], fecal transplants allow re-establishing not only the intestinal microbial communities, but also immune networks that are also affected in the aforementioned gastrointestinal diseases.
The investigation of antibiotic-exposed animal models has given insight to the importance of intestinal microbiota in particular developmental windows. In mice, gut bacterial depletion from weaning onwards by means of chronic antibiotic treatment has an impact on anxiety and cognitive behaviors during adulthood, and affects key neuromodulators of the gut–brain communication (tryptophan, monoamines and neuropeptides) in a way that resembles the results reported in germ-free mice [Desbonnet et al. 2015]. These data suggest that, despite the presence of a normal intestinal microbiota in early postnatal life, dysregulation of the microbiota–gut–brain axis in the postweaning period may contribute to the pathogenesis of disorders such as anxiety and alterations in cognition. These data suggest not only that acquisition of gut microbes from postnatal day one onwards is important for CNS development, but also suggest that at a juvenile age the microbiota is still shaping the way an individual copes with a stressful situation, making the juvenile microbiota an interesting pharmacological target for the treatment as well as prevention of stress-related pathologies.
Among the potential mechanisms involved in the beneficial effects observed after targeting gut microbiota, it has been suggested the modulation of blood–brain barrier (BBB) permeability. Braniste and colleagues found that germ-free mice displayed increased BBB permeability associated with reduced expression of the tight junction proteins occludin and claudin-5. Conversely, exposure of germ-free mice to gut microbiota obtained from pathogen-free mice decreased BBB permeability and upregulated the expression of the tight-junction proteins [Braniste et al. 2014]. Another potential therapeutic mechanism involves modulation of the intestinal serotonergic system. Over 90% of circulating serotonin (5-hydroxytriptamine or 5-HT) is produced by intestinal enterochromaffin cells (ECs), and as detailed further on, 5-HT modulates bowel motor and secretory activities [Julio-Pieper et al. 2012]. ECs are exposed to a variety of bacteria, although the interaction mechanism is not clear. Kashyap and colleagues reported that germ-free mice, when recolonized with healthy human or mice fecal microbiota, increased intestinal transit significantly [Kashyap et al. 2013]. This effect could be partially blocked by 5-HT3 and 5-HT4 receptor antagonists. The authors suggest that changes on 5-HT signaling in response to recolonization are due to alterations on the host’s serotonergic system.
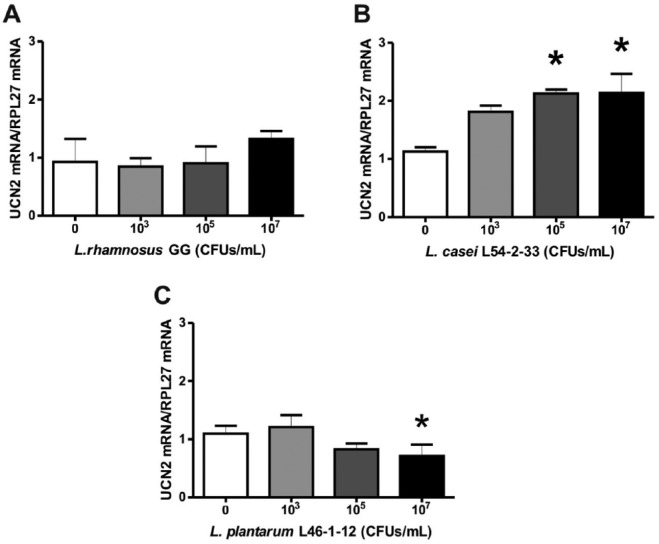
Moreover, our research group has found that lactobacilli are capable of modulating neuropeptide expression by human colonic epithelial cells in vitro. When Caco-2 cells were exposed to Lactobacillus casei L54-2-33 or Lactobacillus plantarum L46-1-12, the mRNA expression of Urocortin 2, a neuropeptide involved in gastrointestinal emptying and visceral pain perception [Martinez et al. 2004], was modified (Figure 2). This indicates that microbiota components may be able to regulate intestinal motility and gut pain responses, indirectly via epithelial production of neuroactive mediators.
Figure 2.
Relative expression of Urocortin 2 (UCN2) messenger ribonucleic acid (mRNA) in human Caco-2 cells stimulated by different lactobacilli. Cultured Caco-2 cells were exposed to different concentrations of lactobacilli [colony forming units per ml (CFUs/ml)] for three hours. The species used were: Lactobacillus rhamnosus GG (A), Lactobacillus casei L54-2-33 (B) and Lactobacillus plantarum L46-1-12 (C), where the latter two have been previously identified by Gotteland and collaborators [Gotteland et al. 2014]. The graphs show data from RT-PCR, were each bar is the ratio of UCN2 mRNA expression over RPL27 mRNA expression, which was used as a control housekeeping gene. Statistical analysis by one-way ANOVA reveals that there is an effect on UCN2 mRNA expression by L. casei L54-2-33 (F(3,2) = 9.943, p = 0.0096) and by L. plantarum L46-1-12 (F(3,2) = 13.36, p = 0.0046), but no effect on UCN2 mRNA was observed with L. rhamnosus GG (F(3,2) = 1093, p = 0.4214). Post hoc Tuckey tests show that there is a significant increase in relative UCN2 mRNA expression induced by L. casei L54-2-33 at 105 and 107 CFUs/ml in comparison with control (*p < 0.05), while L. plantarum L46-1-12 induces a decrease in relative UCN2 mRNA expression at 107 CFUs/ml in comparison with control (*p < 0.05). Data represent mean values ± SEM from triplicates.
Microbiota-modifying strategies not only involve the use of antibiotics or probiotic bacteria. Prebiotics are indigestible components of the diet that stimulate the growth and activity of health-promoting bacteria [Kashyap et al. 2013]. Tarr and colleagues tested whether oligosaccharides naturally found in high levels in human milk were able to prevent or attenuate stress-induced intestinal dysbiosis and anxiety-like behaviors in adult mice. Their study was the first to report that the prebiotics 3′sialyllactose and 6′ sialyllactose prevented social stress-induced alterations in microbiota composition, prevented anxiety-like behaviors and the stress-induced reduction of immature hippocampal neurons [Tarr et al. 2015]. Given the impact of oligosaccharides on bacterial metabolism, future studies should evaluate the synergic effects of probiotic–prebiotic combinations to prevent stress-induced disorders.
Intestinal microbiota composition and its alterations affect the host’s health, having impact on both gastrointestinal tract and CNS. For this reason the microbiota is considered a potential therapeutic target for the treatment of behavioral disorders.
Intestinal epithelial layer
Along with being a highly selective physical barrier, local epithelium maintains intestinal homeostasis due to the coordinate action of its different cell types (Figure 1). Among them, enteroendocrine cells (EECs), located at the base of intestinal crypts, transduce mechanic and chemical signals from the intestinal lumen to neighbor cells and the local neuronal network in a paracrine/endocrine fashion [Crowell, 2004; Symonds et al. 2015]. Specific nutrient receptors in EECs are associated with distinct signaling pathways and lead to the release of a variety of mediators. For example, the protein breakdown product receptor GPR93 and the short-chain fatty-acid receptor FFAR2 are highly expressed in EECs of human and mouse large intestine [Symonds et al. 2015]. Upon nutrient activation, the release of neuroactive molecules such as 5-HT and PYY is also differentially modulated [Symonds et al. 2015].
ECs, a subset of EECs, are the main source of body 5-HT. The substrate for 5-HT synthesis is tryptophan, an essential amino acid which can be metabolized on the kynurenic acid pathway and the 5-HT pathway [Crowell, 2004; O’Mahony et al. 2015]. In healthy volunteers subjected to acute stress, the 5-HT pathway competes with the kynurenic acid pathway for the precursor tryptophan, resulting in decreased plasma 5-HT levels and an unbalance in serotonin production related to peripheral and CNS disorders [Keszthelyi et al. 2012]. This was one important piece of evidence leading to the suggestion that changes in the 5-HT metabolism may contribute to the pathophysiology of irritable bowel syndrome (IBS) and other disorders where gastrointestinal (GI) and CNS alterations are present. Furthermore, Keszthelyi and colleagues demonstrated that IBS patients had reduced mucosal levels and higher plasma concentration of both kynurenic acid and 5-HT, compared to healthy patients. They also found a positive correlation between depression signs and 5-HT concentrations in mucosa but not in plasma [Keszthelyi et al. 2013].
Once synthetized and released, 5-HT is able to bind different receptors in the GI tract where it leads to well described responses. For example, activation of 5-HT3 and 5-HT4 receptors increases GI motility, and these receptors have been targeted to reduce intestinal transit on IBS patients with diarrhea predominance [Manocha and Khan, 2012; Stasi et al. 2014]. In addition, 5-HT4 agonists have been shown to relieve visceral pain and promote intestinal motility in an animal model of chronic constipation [Hoffman et al. 2012]. Research has also focused on the study of antidepressants classified as selective serotonin reuptake inhibitors (SSRIs). These act on 5-HT transporters increasing 5-HT levels in the synaptic space. As discussed below, these drugs have been used to ease pain in chronic GI disorders [Vanuytsel et al. 2014]; however, the pharmacological mechanism at the intestinal level has not been elucidated.
The intestinal epithelium is characterized by a high metabolic rate and cell turnover [Darwich et al. 2014]. There is a close association between metabolite fate and homeostatic coordination of GI function, which is modulated mainly by vagal sensomotor reflexes. For instance, intestinal vagal afferents respond to an increase in glucose levels, which leads to higher 5-HT3 receptor availability on the vagal afferent membrane, in addition to an elevated 5-HT conductance towards the cell’s interior [Babic et al. 2012]. Interestingly, this serotonergic sensitization is coherent with the release of this neurotransmitter by ECs, since this mechanism is also glucose-dependent, although in an indirect manner. An investigation using human ECs determined that adenosine triphosphate (ATP) induces 5-HT release, possibly via ionotropic purinergic receptor activation. P2X, for instance, allows calcium entrance, thereby activating synaptotagmins capable of promoting cell and 5-HT vesicle membrane fusion, required for 5-HT release [Linan-Rico et al. 2013]. Interestingly, using intestine explants Patel demonstrated that ileum 5-HT release depends on ATP whereas colon does not release 5-HT upon ATP stimulation [Patel, 2014]. These data show that metabolic activity differentially modulates 5-HT bioavailability in different gut regions, which needs to be taken into account when investigating the pharmacology of the intestinal 5-HT system.
Mucosal immune machinery
The GI epithelium is in close proximity to potentially pathogenic bacteria, viruses, fungi and helminths. Therefore, the presence of a local immune system [composed of macrophages, lymphocytes and dendritic cells residing in the mucosa (see Figure 1)] is essential for maintaining homeostasis between the host and luminal microbiota. Such homeostasis can be altered to an aberrant secretion of pro-inflammatory cytokines that have the potential to induce epithelial damage and has been associated with gut disorders such as inflammatory bowel disease (IBD) and GI tissue neoplasia [Li et al. 2014]. As discussed later, elevation of pro-inflammatory cytokines, both locally in the mucosa as well as systemically, can cause activation of the endocrine stress axis [El Aidy et al. 2014]. Therefore, the mucosal immune component may not only be a target to prevent intestinal tissue damage but to potentially alleviate stress-related conditions.
In the search for pharmacological targets to specifically treat inflammatory GI disorders, the interleukin 22 (IL-22) pathway appears to be a potential candidate. IL-22 belongs to the family of interleukin-10 cytokines, which are produced by cells of the innate and adaptive immune system. It plays a systemic pro-inflammatory role, inducing secretion of antimicrobial peptides, cell proliferation and activation of antiapoptotic signaling cascades in the gut via the signal transducer and activator of transcription protein 3 (STAT3), having a role both in tissue defense and repair [Li et al. 2014]. For example, actively inflamed tissues obtained from ulcerative colitis patients contain significantly fewer T helper cells that are IL-22 positive when compared with uninflamed tissue from the same patient [Leung et al. 2014]. However, in colon cancer there is increased IL-22 expression [Ji et al. 2014; Sabat et al. 2014].
Interesting data have been obtained in obese mice that display chronic inflammation and poor mucosal immunity: upon challenge of mice with flagellin, a bacterial component, it was found that innate lymphoid cells obtained from obese mice produced less IL-22 than those isolated from lean controls. This was verified in ob/ob, db/db and diet-induced models of obesity [Wang et al. 2014]. Also, obese mice and mice deficient in IL-22 receptor had metabolic disorders such as insulin resistance and hyperglycemia, and were more susceptible to infection by the intestinal pathogen Citrobacter rodentium. Administration of exogenous IL-22 normalized metabolic, endocrine and immune alterations and reduced intestinal inflammation [Wang et al. 2014].
Regarding potential effects of IL-22 on the neuronal component of the gut–brain axis, because no receptors have been found in the central, peripheral or enteric nervous system (ENS), it is likely that this cytokine acts indirectly via modulation of neuroactive molecule release. For example, food intake was decreased in obese mice that received exogenous IL-22, and this effect was accompanied by increased serum levels of PYY, a gut hormone with an anorexigenic effect [Wang et al. 2014]. On the other hand, whole blood cultures treated with antidepressant compounds such as citalopram, escitalopram and mirtazapine displayed increased expression of pro-inflammatory cytokines including IL-22 [Munzer et al. 2013] that may be relevant due to the high comorbidity of depression, anxiety and inflammatory gut disorders.
Short-chain fatty acids (SCFAs) are generated in the gut lumen when intestinal bacteria metabolize indigestible fiber and are proposed as modulators in diet-induced therapeutic effect in models of immune pathologies [Haghikia et al. 2015]. On the other hand, long-chain fatty acids (LCFAs) that are abundant in Western diets have been linked to an increased risk of disease. Using autoimmune encephalomyelitis (AE) to model T-cell-mediated autoimmunity in the mouse, Haghikia and collaborators showed that LCFA-rich diet decreased SCFAs in the gut and also increased Th1-cell- and Th17-cell-mediated CNS autoimmunity in vivo, therefore exacerbating the disease. Ex vivo flow cytometry analyses showed that Th17 cell abundance in the small intestine displayed a maximum that coincided with the onset of AE symptoms. Interestingly, treatment with SCFAs imprinted a protective phenotype on Treg cells derived from intestinal lamina propria, which was associated with ameliorated AE and a higher degree of axonal preservation [Haghikia et al. 2015]. It would be interesting to evaluate whether metabolites from a healthy intestinal microbiota prevent the adaptive immune system from recognizing the host’s antigens. Moreover, the data suggests that dietary alterations in critical periods of development (i.e. western diet during childhood) could affect the way the immune system gains tolerance towards the host, which in turn could promote the appearance of autoimmune diseases later in life. It cannot be ruled out that alterations in immune tolerance, as a result of early-life dysbiosis, could also give rise to the appearance of CNS alterations.
The hypothalamus–pituitary–adrenal axis
Stress hormones, organized in the hypothalamus–pituitary–adrenal (HPA) axis (see Figure 3), are part of the humoral communication between the intestinal tract and the CNS. Much of the evidence showing the involvement of the HPA axis in this communication has been obtained from observations in IBS patients. They have higher basal cortisol levels than healthy controls [Heitkemper et al. 1996; Dinan et al. 2006; McKernan et al. 2011], together with an exaggerated release of ACTH and cortisol in response to intravenous administration of corticotropin-releasing factor (CRF) [Dinan et al. 2006]. Both normal and exaggerated HPA axis responses have been reported for subgroups of patients with IBS [Fukudo et al. 1998; Elsenbruch et al. 2001; Heim et al. 2001; Dickhaus et al. 2003; Bohmelt et al. 2005; Dinan et al. 2006]; it has been suggested that these enhanced responses could be related to traumatic events experienced early in life [Heim et al. 2001; Chang et al. 2009]. Also, HPA axis hypersuppression has been associated with functional gastrointestinal symptoms in patients suffering from major depression [Karling et al. 2015]. The above evidence suggests that alterations in the HPA axis are present together with gastrointestinal pathology.
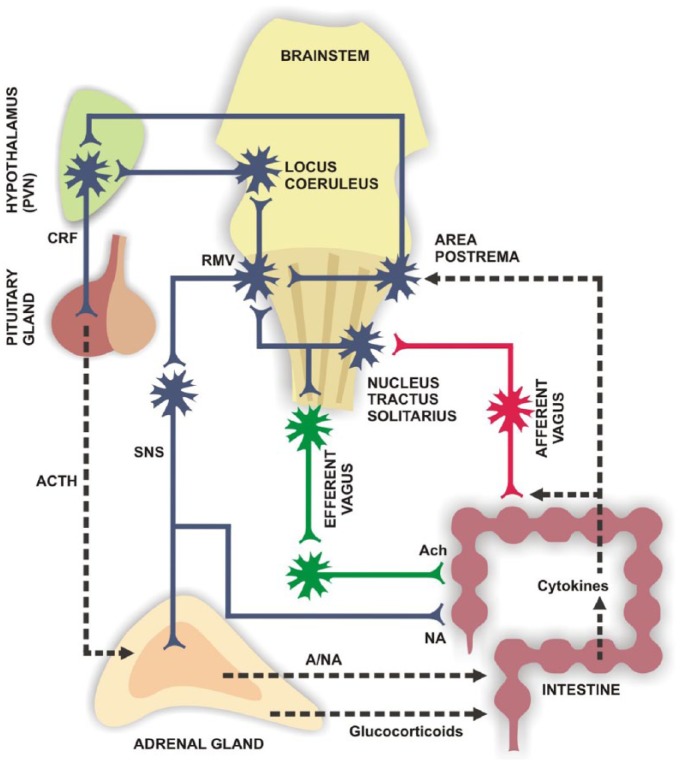
Figure 3.
Peripheral and CNS targets relevant to gut–brain axis modulation. Intestinal motility function responds to cholinergic signals from vagal efferent terminals [Jones et al. 2006]. On the other hand, sensory vagal afferences are responsive to cytokines released by immune, epithelial and nervous cells in the gut [Tracey, 2007] and relay on the dorsal vagal complex, which connects in turn to the hypothalamic paraventricular nucleus [Pavlov et al. 2003], triggering an early anti-inflammatory response. Acetylcholine released by vagal efferent terminals also has anti-inflammatory effects on gut immune cells, which depend on nicotinic receptors [Tracey, 2007]. Muscarinic receptor activation can also modulate local lymphocyte function [Kawashima and Fujii, 2000; Fujii et al. 2007]. (Adapted from Díaz-Zepeda et al. [2015].)
A, adrenalin; Ach, acetylcholine; ACTH, adrenocorticotrophic hormone; CRF, corticotrophin releasing factor; NA, noradrenalin; PVN, paraventricular nucleus; RMV, rostral ventromedial medulla; SNS, sympathetic nervous system.
Studies in animal models have examined the involvement of the endocrine axis of stress on the gut–brain functional unit. For example, maternal separation as a model of early-life stress in rodents induces an increase in basal corticosterone levels in adults, while reducing visceral pain threshold and inducing intestinal dysbiosis [O’Mahony et al. 2009]. In Rhesus monkeys, maternal separation decreases the content of Lactobacillus fecalis and increases cortisol plasma levels 3 days post-separation [Bailey and Coe, 1999]. Also, a week into maternal separation, the levels of Lactobacillus species are similar to those of unstressed controls and cortisol levels are somewhat reduced [Bailey and Coe, 1999]. These findings suggest that early-life stress not only affects the expression of genes relevant to the appropriate stress response [Bravo et al. 2011a; O’Malley et al. 2011], but also alters proprioception, microbiota composition and glucocorticoid plasma levels, with consequences extending into adulthood.
Moreover, rodents subjected to chronic-restraint stress display enhanced colonization by pathogenic bacteria such as Citrobacter rodentium [Bailey et al. 2010], while social-defeat stress alters the relative abundance of cecal bacteria [Bailey et al. 2011]. This type of stress also increased levels of interleukin 6 (IL-6) and monocyte chemo-attractant protein 1 (MCP-1). Interestingly, IL-6 and MCP-1 are reduced by antibiotic treatment directed against pathogenic bacteria [Bailey et al. 2011], suggesting that bacteria could modulate physiology by altering the host’s immune functions. Thus, an increase in pro-inflammatory cytokines in the intestine would activate a rapid vagus nerve-mediated anti-inflammatory response [Tracey, 2010] and also allow HPA axis activation [Hosoi et al. 2000] (see Figure 3). In addition, Zimomra and colleagues demonstrated that mice infected with Escherichia coli had increased glucocorticoid release; however, this effect was independent of ACTH release, and it involved the synthesis of prostaglandin E2 [Zimomra et al. 2011]. This shows that intestinal dysbiosis induced by pathogenic bacteria also has an effect on glucocorticoid release, whether it is through HPA axis activation via cytokines, including vagal sensory-fiber activations, or independently of pituitary ACTH release.
The HPA axis responds to microbiota changes; however, this axis is also sensitive to the environment, stressors, and CNS activity. Considering the significant immunomodulatory effect of the HPA axis (for example, the effect on anti-inflammatory glucocorticoids) and its role in the modulation of intestinal permeability [Moussaoui et al. 2014], glucocorticoids can significantly affect intestinal physiology, which could in turn have an impact on the intestinal microbiota composition, therefore establishing a bidirectional communication between these two components.
Dexamethasone-suppression test and CRF challenge have been used to evaluate antidepressant treatment efficacy [Lozano-Ortiz et al. 2012]. On the other hand, tricyclic antidepressants and SSRIs have been used to alleviate abdominal pain in IBS patients, but in lower doses than those used to treat mood disorders [Vanuytsel et al. 2014]. Although it is not known whether such doses affect HPA axis function in IBS patients, current evidence suggests the use of antidepressants in cases of comorbidity between mood disorders and IBS. This strategy might be of help in reducing symptomatology at both ends of the gut–brain axis, although the mechanism of action on both systems is still subject of debate.
Ghrelin is an orexigenic-peptide hormone produced centrally and in the periphery, and it has the ability to stimulate food intake by acting in the arcuate nucleus of the hypothalamus [Schellekens et al. 2013]. Fasting stimulates a rise in plasma ghrelin levels, and these are decreased after a meal [Cummings et al. 2001]. Also, administration of exogenous ghrelin promotes food-seeking behavior [Tong et al. 2011]. Brain imaging analyses show that ghrelin administration in healthy volunteers induced acute hedonic responses similar to those evoked by fasting, involving activation of corticolimbic reward–cognitive systems [Goldstone et al. 2014]. Circulating levels of ghrelin are increased by stress in both humans [Rouach et al. 2007] and rodents [Kristenssson et al. 2006]. Conversely, mice show less anxiety and depressive-like behaviors following caloric restriction or subcutaneous ghrelin injection [Lutter et al. 2008], suggesting that elevated plasma ghrelin may have anxiolytic and antidepressant-like effects. Moreover, ghrelin-lacking mice (ghr -/-) show more anxiety-like behaviors in the elevated plus maze and open field tests than wild-type control animals [Spencer et al. 2012], and furthermore, these ghr -/- mice secrete less ACTH than wild-type mice, suggesting a mechanism by which ghrelin modulates HPA axis activity (i.e. regulating anterior pituitary release of ACTH), an effect which also impacts on anxiety-like behaviors [Spencer et al. 2012].
The enteric nervous system
The intestinal tissue is highly innervated by intrinsic neurons mainly grouped in the myenteric and submucous plexus (Figure 1). Recent evidence also shows an important role of intestinal glia, that are even more ubiquitous than neurons, in gut physiology [Gulbransen and Sharkey, 2012]. The ENS releases the same mediators and neurotransmitters that are found in the CNS, modulating epithelial cell proliferation and differentiation, paracelullar permeability, water and electrolyte transport, nutrient absorption and muscle contractile motility, to name a few functions [Li et al. 2011; Neunlist et al. 2013].
The ENS has been syndicated as an important signal transductor between the CNS and the gut. In rats under acute restraint stress, an increase in ACTH release in the intestinal tissue has been associated with augmented electrolyte secretion and permeability of the intestinal epithelium, responses that were prevented when atropine was used [Saunders et al. 1997]. Interestingly, using an in vitro coculture of human submucous neurons and human colon epithelial cells, it was shown that when submucosal neurons were activated by electrical-field stimulation there was a reduction in epithelial permeability together with an increase in the expression of tight-junction protein zona occludens 1 (ZO-1), important for maintaining the paracelullar epithelial barrier. These effects were blocked by tetrodotoxin treatment and also when a vasoactive intestinal peptide (VIP) receptor antagonist was used [Neunlist et al. 2003]. These data suggest that brain-to-gut communication involving ACTH or VIP signaling may be important for maintaining the intestinal epithelial barrier.
Considering that the gut is the major source of 5-HT, this has been proposed as one relevant signaling molecule between the gut and the CNS, and the ENS is considered a possible intermediary. Studies in knockout mice for different tryptophan hydroxylase (TPH) isoforms (the limiting enzyme in 5-HT synthesis) establish that constitutive intestinal motility depends mostly on the activity of the intestinal serotonergic neurons rather than ECs. Moreover, the early differentiation of serotonergic neurons in the immature ENS is key to promoting the development and survival of other neuron types, included the dopaminergic neurons [Li et al. 2011]. Therefore, these are potential targets for drugs destined to modify intestinal epithelium permeability and motility.
Studies focused in the opposite communication pathway (epithelium–ENS or epithelium–ENS–CNS) are more limited. Neunlist and Schemann explain that the presence or absence of specific nutrients in the intestinal lumen may induce changes mediated by enteroendocrine cells that affect the expression of neurotransmitters in the ENS as well as the survival of these neurons [Neunlist and Schemann, 2014]. The authors propose that this may have an impact on the intestinal motility, secretion and epithelial permeability. It will be interesting to establish if those changes at the ENS level could also affect CNS function and behavior.
The vagus nerve
The vagus nerve is one of the twelve nerves that innervate the thoracic and abdominal tissues [Berthoud and Neuhuber, 2000]. It possesses a left and a right cervical pathway; for each of them there are afferent and efferent projections that converge in the cranium where either information coming from the organs is processed or chemical signals are sent to the periphery [Berthoud and Neuhuber, 2000]. The vagus nerve plays an important role in gut–brain communication; for example, vagal activation has anti-inflammatory effects that are prevented by subdiaphragmatic vagotomy [Tracey, 2007]. On the other hand, electrical stimulation, specific pharmacological and nutritional interventions, behavioral modification, meditation, cognitive and relaxation therapies have been reported to stimulate vagus nerve activity [Tracey, 2007; Bonaz and Bernstein, 2013], potentially having the opposite effect .
In the rat, subdiaphragmatic vagal deafferentation (SDA) consists of a total disconnection of the vagal afferent abdominal projections, and has been shown to induce changes in anxiety-like behaviors, fear behaviors and in the levels of neurochemical mediators in the limbic system [Klarer et al. 2014]. Using the open field, elevated plus maze and fear conditioning tests it was demonstrated that SDA rats present significantly lower levels of anxiety compared with a sham group. SDA rats also showed attenuation in the response to fear conditioning test and region-specific changes in GABA and noradrenalin (NA) levels in the limbic system, without alterations in the basal or stress-induced corticosterone levels [Klarer et al. 2014]. These data demonstrated that there is a basal tone of information originating from abdominal regions that travel through vagal afferents and it is relevant for some behavioral and neurochemical aspects of the rat limbic system. However, it is impossible to discriminate which tissue (or tissues) innervated by the vagus nerve regulates the observed events. In another approximation, Bravo and collaborators demonstrated that in healthy mice, subdiaphragmatic vagotomy prevents the antidepressive and anxiolytic effects observed after a sustained oral administration of L. rhamnosus JB-1 [Bravo et al. 2011b]. In this model, vagotomy also prevented changes observed in mRNA levels of gamma-aminobutyric acid (GABA) receptors GABAB1b y GABAAα2 in different regions of the brain. Although the evidence suggests a direct gut–brain communication mediated by the vagus nerve, some reports describe that this may occur independently.
Van der Kleij and collaborators studied the effect of Lactobacillus reuteri and Bifidobacterium infantis, both with anti-inflammatory effect, in vagotomized mice with acute colitis, induced by dextran sulfate sodium (DSS), and also in a chronic colitis model induced by transference of lymphocytes from a healthy mouse to inmunodeficient mice. In the DSS-treated animals there was a decrease in pro-inflammatory cytokines when mice received a probiotic species that was not enhanced by vagotomy, suggesting that not all of the effects generated by manipulations on the enteric microbiota go through stimulation of vagal afferents. In addition, in the lymphocyte T model of colitis animals displayed a potentiated anti-inflammatory effect of the probiotics when they were vagotomized [Van Der Kleij et al. 2008], again suggesting a vagus-independent effect on the immune system.
Another effect mediated through vagal activation involves hormones. For instance, food ingestion and satiety are mediated by an array of gut hormones such as cholecystokinin (CCK), which inhibits food intake and gastric emptying [Smith et al. 1985]. When this hormone acts at CCK1 receptors on vagal afferent neurons it stimulates the expression of Y2 receptors and also that of the neuropeptide CART, a process mediated by activation of the transcriptional factor CREB [De Lartigue et al. 2010]. Both events are associated with the induction of satiety. On the other hand, the peptide ghrelin appears as a signal to the absence of food in the gut and is associated with the stimulation of food intake, also acting on vagal afferent neurons [Date et al. 2005]. Ghrelin inhibited the effects of CCK by promoting the nuclear exclusion of CREB [Date et al. 2002, 2005].
Vagal alterations have also been associated with the pathophysiology of Crohn’s disease (CD) and IBS. Vagal tone and markers of stress and inflammation were studied in IBS and CD patients and compared to those of healthy controls [Pellissier et al. 2014]. CD and IBS patients displayed higher scores in questionnaires for anxiety and depressive symptoms. Among control subjects, those with high vagal tone had significantly lower evening salivary cortisol levels than subjects with low vagal tone. However, this association was not present in CD and IBS patients, which suggests an uncoupling between vagal tone and cortisol level in those patients. Vagal tone and TNF-alpha levels were inversely correlated only in CD patients, indicating that the cholinergic anti-inflammatory pathway response was blunted in CD patients with low vagal tone. In IBS patients, vagal tone was inversely correlated with plasma epinephrine which may be sign of nonresponsive high sympathetic activity. It is important to state that no association was found between vagal tone and anxiety or depressive symptomatology in any group. The authors conclude that there is an imbalance between the HPA axis and the vagal tone in CD and IBS patients, and highlight that only a small proportion of these patients would be benefited by vagal reinforcement [Pellissier et al. 2014].
The use of nonpharmacological interventions in the management of visceral pain syndromes have also shed light on the antihyperalgesic and analgesic properties of the peripheral nervous system, including the vagus nerve. The technique of deep breathing allowed an increase in cardiac vagal tone and increased pain threshold in healthy individuals subjected to an acid challenge to the esophagus [Botha et al. 2015]. Atropine prevented both effects, again reinforcing the relevance of the vagal cholinergic anti-inflammatory pathway in visceral pain perception.
Concluding remarks
The gut–brain axis is a functional unit presenting a variety of potential pharmacological targets. Modifying a target within this unit may have consequences on the entire axis. For example, interventions on the gut serotonergic system by using 5-HT3/5-HT4 receptor agonists or 5-HT reuptake modulators may influence visceral pain perception and intestinal motility but also modify HPA axis function. Microbiota-modifying strategies involving the use of antibiotics, probiotic bacteria, as well as prebiotic compounds may have the potential to improve not only gastrointestinal alterations but also behavioral and neurochemical perturbations associated with stress.
Targeting the immune system, another key component in this axis, may affect the anti-inflammatory response initiated by activation of the vagus nerve. Vagal-mediated anti-inflammatory response can also activate the HPA axis, modifying the release of glucocorticoids which are important immunomodulators. Therefore, the gut–brain axis presents an opportunity for the development of novel pharmacological (and nutritional) strategies. This can be especially favorable in disorders where behavioral alterations and gut functional diseases coexist.
Footnotes
Funding: Marcela Julio-Pieper’s work was funded by Fondecyt (grant number 1130213) and PUCV-DI (grant number 037.470/2015). Javier A. Bravo’s work was funded by Fondecyt (grant number 1140776) and PUCV-DI (grant number 037.386/2014).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Camila González-Arancibia, Grupo de NeuroGastroBioquímica, Instituto de Química, Facultad de Ciencias, Pontificia Universidad Católica de Valparaíso, Valparaíso, Chile.
Jorge Escobar-Luna, Grupo de NeuroGastroBioquímica, Instituto de Química, Facultad de Ciencias, Pontificia Universidad Católica de Valparaíso, Valparaíso, Chile.
Camila Barrera-Bugueño, Grupo de NeuroGastroBioquímica, Instituto de Química, Facultad de Ciencias, Pontificia Universidad Católica de Valparaíso, Valparaíso, Chile.
Camilo Díaz-Zepeda, Grupo de NeuroGastroBioquímica, Instituto de Química, Facultad de Ciencias, Pontificia Universidad Católica de Valparaíso, Valparaíso, Chile.
María P. González-Toro, Grupo de NeuroGastroBioquímica, Instituto de Química, Facultad de Ciencias, Pontificia Universidad Católica de Valparaíso, Valparaíso, Chile
Loreto Olavarría-Ramírez, Grupo de NeuroGastroBioquímica, Instituto de Química, Facultad de Ciencias, Pontificia Universidad Católica de Valparaíso, Valparaíso, Chile.
Francesca Zanelli-Massai, Grupo de NeuroGastroBioquímica, Instituto de Química, Facultad de Ciencias, Pontificia Universidad Católica de Valparaíso, Valparaíso, Chile.
Martin Gotteland, Departamento de Nutrición, Facultad de Medicina, Universidad de Chile, Santiago, Chile.
Javier A. Bravo, Grupo de NeuroGastroBioquímica, Instituto de Química, Facultad de Ciencias, Pontificia Universidad Católica de Valparaíso, Valparaíso, Chile
Marcela Julio-Pieper, Grupo de NeuroGastroBioquímica, Instituto de Química, Facultad de Ciencias, Pontificia Universidad Católica de Valparaíso, Avenida Universidad 330, Curauma, Valparaíso, Chile.
References
- Ait-Belgnaoui A., Durand H., Cartier C., Chaumaz G., Eutamene H., Ferrier L., et al. (2012) Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 37: 1885–1895. [DOI] [PubMed] [Google Scholar]
- Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., et al. (2011) Induction of colonic regulatory T cells by indigenous clostridium species. Science 331: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic T., Troy A., Fortna S., Browning K. (2012) Glucose-dependent trafficking of 5-HT3 receptors in rat gastrointestinal vagal afferent neurons. Neurogastroenterol Motil 24: e476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M., Coe C. (1999) Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol 35: 146–155. [PubMed] [Google Scholar]
- Bailey M., Dowd S., Galley J., Hufnagle A., Allen R., Lyte M. (2011) Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 25: 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M., Dowd S., Parry N., Galley J., Schauer D., Lyte M. (2010) Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by citrobacter rodentium. Infect Immun 78: 1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkes J., Viswanathan V., Savkovic S., Hecht G. (2003) Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 52: 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H., Neuhuber W. (2000) Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85: 1–17. [DOI] [PubMed] [Google Scholar]
- Bertrand P., Bertrand R. (2010) Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci 153: 47–57. [DOI] [PubMed] [Google Scholar]
- Bohmelt A., Nater U., Franke S., Hellhammer D., Ehlert U. (2005) Basal and stimulated hypothalamic–pituitary–adrenal axis activity in patients with functional gastrointestinal disorders and healthy controls. Psychosom Med 67: 288–294. [DOI] [PubMed] [Google Scholar]
- Bonaz B., Bernstein C. (2013) Brain–gut interactions in inflammatory bowel disease. Gastroenterology 144: 36–49. [DOI] [PubMed] [Google Scholar]
- Botha C., Farmer A., Nilsson M., Brock C., Gavrila A., Drewes A., et al. (2015) Preliminary report: modulation of parasympathetic nervous system tone influences oesophageal pain hypersensitivity. Gut 64: 611–617. [DOI] [PubMed] [Google Scholar]
- Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Toth M., et al. (2014) The gut microbiota influences blood–brain barrier permeability in mice. Sci Transl Med 6: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo J., Dinan T., Cryan J. (2011a) Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disorders. Int J Neuropsychopharmacol 14: 666–683. [DOI] [PubMed] [Google Scholar]
- Bravo J., Forsythe P., Chew M., Escaravage E., Savignac H., Dinan T., et al. (2011b) Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 108: 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes S., Spencer N., Costa M., Zagorodnyuk V. (2013) Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol 10: 286–296. [DOI] [PubMed] [Google Scholar]
- Chang L., Sundaresh S., Elliott J., Anton P., Baldi P., Licudine A., et al. (2009) Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil 21: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Chan A., Hui W., Lam S. (2003) Coping strategies, illness perception, anxiety and depression of patients with idiopathic constipation: a population-based study. Aliment Pharmacol Ther 18: 319–326. [DOI] [PubMed] [Google Scholar]
- Crowell M. (2004) Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol 141: 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D., Purnell J., Frayo R., Schmidova K., Wisse B., Weigle D. (2001) A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719. [DOI] [PubMed] [Google Scholar]
- Daneman R., Rescigno M. (2009) The gut immune barrier and the blood–brain barrier: are they so different? Immunity 31: 722–735. [DOI] [PubMed] [Google Scholar]
- Darwich A., Aslam U., Ashcroft D., Rostami-Hodjegan A. (2014) Meta-analysis of the turnover of intestinal epithelia in preclinical animal species and humans. Drug Metab Dispos 42: 2016–2022. [DOI] [PubMed] [Google Scholar]
- Date Y., Murakami N., Toshinai K., Matsukura S., Niijima A., Matsuo H., et al. (2002) The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123: 1120–1128. [DOI] [PubMed] [Google Scholar]
- Date Y., Toshinai K., Koda S., Miyazato M., Shimbara T., Tsuruta T., et al. (2005) Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology 146: 3518–3525. [DOI] [PubMed] [Google Scholar]
- David L., Materna A., Friedman J., Campos-Baptista M., Blackburn M., Perrotta A., et al. (2014) Host lifestyle affects human microbiota on daily timescales. Genome Biol 15: R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis M., Piccolo M., Vannini L., Siragusa S., De Giacomo A., Serrazzanetti D., et al. (2013) Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One 8: e76993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lartigue G., Dimaline R., Varro A., Raybould H., De La Serre C., Dockray G. (2010) Cocaine- and amphetamine-regulated transcript mediates the actions of cholecystokinin on rat vagal afferent neurons. Gastroenterology 138: 1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L., Clarke G., Traplin A., O’Sullivan O., Crispie F., Moloney R., et al. (2015) Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun 48: 165–173. [DOI] [PubMed] [Google Scholar]
- Díaz-Zepeda C., Escobar-Luna J., González-Arancibia C., González-Toro M., Olavarría-Ramírez L., Zanelli-Massai F., et al. (2015) Blancos farmacológicos en el eje intestino–cerebro. Rev Farmacol Chile 8: 12. [Google Scholar]
- Dickhaus B., Mayer E., Firooz N., Stains J., Conde F., Olivas T., et al. (2003) Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. Am J Gastroenterol 98: 135–143. [DOI] [PubMed] [Google Scholar]
- Dinan T., Quigley E., Ahmed S., Scully P., O’Brien S., O’Mahony L., et al. (2006) Hypothalamic–pituitary–gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology 130: 304–311. [DOI] [PubMed] [Google Scholar]
- Dockray G. (2014) Gastrointestinal hormones and the dialogue between gut and brain. J Physiol 592: 2927–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Aidy S., Dinan T., Cryan J. (2014) Immune modulation of the brain–gut–microbe axis. Front Microbiol 5: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsenbruch S., Lovallo W., Orr W. (2001) Psychological and physiological responses to postprandial mental stress in women with the irritable bowel syndrome. Psychosom Med 63: 805–813. [DOI] [PubMed] [Google Scholar]
- Fujii T., Takada-Takatori Y., Kawashima K. (2007) Roles played by lymphocyte function-associated antigen-1 in the regulation of lymphocytic cholinergic activity. Life Sci 80: 2320–2324. [DOI] [PubMed] [Google Scholar]
- Fukudo S., Nomura T., Hongo M. (1998) Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut 42: 845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone A., Prechtl C., Scholtz S., Miras A., Chhina N., Durighel G., et al. (2014) Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. Am J Clin Nutr 99: 1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotteland M., Cires M., Carvallo C., Vega N., Ramirez M., Morales P., et al. (2014) Probiotic screening and safety evaluation of lactobacillus strains from plants, artisanal goat cheese, human stools, and breast milk. J Med Food 17: 487–495. [DOI] [PubMed] [Google Scholar]
- Gros D., Antony M., McCabe R., Swinson R. (2009) Frequency and Severity of the Symptoms of irritable bowel syndrome across the anxiety disorders and depression. J Anxiety Disord 23: 290–296. [DOI] [PubMed] [Google Scholar]
- Gulbransen B., Sharkey K. (2012) Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 9: 625–632. [DOI] [PubMed] [Google Scholar]
- Haghikia A., Jorg S., Duscha A., Berg J., Manzel A., Waschbisch A., et al. (2015) Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43: 817–829. [DOI] [PubMed] [Google Scholar]
- Hansen A., Friis Hansen C., Krych L., Nielsen D. (2014) Impact of the gut microbiota on rodent models of human disease. World J Gastroenterol 20: 17727–17736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Newport D., Bonsall R., Miller A., Nemeroff C. (2001) Altered pituitary–adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry 158: 575–581. [DOI] [PubMed] [Google Scholar]
- Heitkemper M., Jarrett M., Cain K., Shaver J., Bond E., Woods N., et al. (1996) Increased urine catecholamines and cortisol in women with irritable bowel syndrome. Am J Gastroenterol 91: 906–913. [PubMed] [Google Scholar]
- Hoffman J., Tyler K., Maceachern S., Balemba O., Johnson A., Brooks E., et al. (2012) Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 142: 844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi T., Okuma Y., Nomura Y. (2000) Electrical stimulation of afferent vagus nerve induces IL-1beta expression in the brain and activates HPA axis. Am J Physiol Regul Integr Comp Physiol 279: R141–147. [DOI] [PubMed] [Google Scholar]
- Hussain S., Bloom S. (2013) The regulation of food intake by the gut–brain axis: implications for obesity. Int J Obes (Lond) 37: 625–633. [DOI] [PubMed] [Google Scholar]
- Ji Y., Yang X., Li J., Lu Z., Li X., Yu J., et al. (2014) IL-22 promotes the migration and invasion of gastric cancer cells via IL-22r1/Akt/MMP-9 Signaling. Int J Clin Exp Pathol 7: 3694–3703. [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y., et al. (2015) Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 48: 186–194. [DOI] [PubMed] [Google Scholar]
- Johansson M., Sjovall H., Hansson G. (2013) The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol 10: 352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M., Dilley J., Drossman D., Crowell M. (2006) Brain–gut connections in functional GI disorders: anatomic and physiologic relationships. Neurogastroenterol Motil 18: 91–103. [DOI] [PubMed] [Google Scholar]
- Julio-Pieper M., O’Mahony C., Clarke G., Bravo J., Dinan T., Cryan J. (2012) Chronic stress-induced alterations in mouse colonic 5-HT and defecation responses are strain dependent. Stress 15: 218–226. [DOI] [PubMed] [Google Scholar]
- Karling P., Wikgren M., Adolfsson R., Norrback K. (2015) HPA-axis hypersuppression is associated with gastrointestinal symptoms in major depression. J Neurogastroenterol Motil. 26 October 2015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap P., Marcobal A., Ursell L., Larauche M., Duboc H., Earle K., et al. (2013) Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 144: 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima K., Fujii T. (2000) Extraneuronal cholinergic system in lymphocytes. Pharmacol Ther 86: 29–48. [DOI] [PubMed] [Google Scholar]
- Keszthelyi D., Troost F., Jonkers D., Kruimel J., Leue C., Masclee A. (2013) Decreased levels of kynurenic acid in the intestinal mucosa of IBS patients: relation to serotonin and psychological state. J Psychosom Res 74: 501–504. [DOI] [PubMed] [Google Scholar]
- Keszthelyi D., Troost F., Jonkers D., Van Donkelaar E., Dekker J., Buurman W., et al. (2012) Does acute tryptophan depletion affect peripheral serotonin metabolism in the intestine? Am J Clin Nutr 95: 603–608. [DOI] [PubMed] [Google Scholar]
- Klarer M., Arnold M., Gunther L., Winter C., Langhans W., Meyer U. (2014) Gut vagal afferents differentially modulate innate anxiety and learned fear. J Neurosci 34: 7067–7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristenssson E., Sundqvist M., Astin M., Kjerling M., Mattsson H., Dornonville De La Cour C., et al. (2006) Acute psychological stress raises plasma ghrelin in the rat. Regul Pept 134: 114–117. [DOI] [PubMed] [Google Scholar]
- Leclercq S., Matamoros S., Cani P., Neyrinck A., Jamar F., Starkel P., et al. (2014) Intestinal permeability, gut–bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A 111: E4485–4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J., Davenport M., Wolff M., Wiens K., Abidi W., Poles M., et al. (2014) Il-22-producing CD4+ cells are depleted in actively inflamed colitis tissue. Mucosal Immunol 7: 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Gong C., Zhao M., Feng B. (2014) Role of interleukin-22 in inflammatory bowel disease. World J Gastroenterol 20: 18177–18188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Liang P., Li Z., Wang Y., Zhang G., Gao H., et al. (2015) Fecal microbiota transplantation and bacterial consortium transplantation have comparable effects on the re-establishment of mucosal barrier function in mice with intestinal dysbiosis. Front Microbiol 6: 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Chalazonitis A., Huang Y., Mann J., Margolis K., Yang Q., et al. (2011) Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci 31: 8998–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S., Wang T., Hu X., Luo J., Li W., Wu X., et al. (2015) Administration of lactobacillus helveticus ns8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310: 561–577. [DOI] [PubMed] [Google Scholar]
- Linan-Rico A., Wunderlich J., Grants I., Frankel W., Xue J., Williams K., et al. (2013) Purinergic autocrine regulation of mechanosensitivity and serotonin release in a human EC model: ATP-gated P2X3 channels in EC are downregulated in ulcerative colitis. Inflamm Bowel Dis 19: 2366–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Ortiz R., Marin-Lacasa R., Pascual-Garcia A., Santacruz-Abion M., Sebastian-Perez F., Orea-Ramon B. (2012) Therapeutic monitoring of escitalopram by dexamethasone suppression test. Actas Esp Psiquiatr 40: 275–280. [PubMed] [Google Scholar]
- Lutter M., Sakata I., Osborne-Lawrence S., Rovinsky S., Anderson J., Jung S., et al. (2008) The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci 11: 752–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manocha M., Khan W. (2012) Serotonin and GI disorders: an update on clinical and experimental studies. Clin Transl Gastroenterol 3: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V., Wang L., Million M., Rivier J., Tache Y. (2004) Urocortins and the regulation of gastrointestinal motor function and visceral pain. Peptides 25: 1733–1744. [DOI] [PubMed] [Google Scholar]
- McKernan D., Gaszner G., Quigley E., Cryan J., Dinan T. (2011) Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment Pharmacol Ther 33: 1045–1052. [DOI] [PubMed] [Google Scholar]
- Moeller A., Ochman H. (2013) Factors that drive variation among gut microbial communities. Gut Microbes 4: 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaoui N., Braniste V., Ait-Belgnaoui A., Gabanou M., Sekkal S., Olier M., et al. (2014) Changes in intestinal glucocorticoid sensitivity in early life shape the risk of epithelial barrier defect in maternal-deprived rats. PLoS One 9: e88382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzer A., Sack U., Mergl R., Schonherr J., Petersein C., Bartsch S., et al. (2013) Impact of antidepressants on cytokine production of depressed patients in vitro. Toxins (Basel) 5: 2227–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussell M., Kroenke K., Spitzer R., Williams J., Herzog W., Lowe B. (2008) Gastrointestinal symptoms in primary care: prevalence and association with depression and anxiety. J Psychosom Res 64: 605–612. [DOI] [PubMed] [Google Scholar]
- Neufeld K., Kang N., Bienenstock J., Foster J. (2011) Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 23: 255–264. [DOI] [PubMed] [Google Scholar]
- Neunlist M., Schemann M. (2014) Nutrient-induced changes in the phenotype and function of the enteric nervous system. J Physiol 592: 2959–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M., Toumi F., Oreschkova T., Denis M., Leborgne J., Laboisse C., et al. (2003) Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via vipergic pathways. Am J Physiol Gastrointest Liver Physiol 285: G1028–1036. [DOI] [PubMed] [Google Scholar]
- Neunlist M., Van Landeghem L., Mahe M., Derkinderen P., Des Varannes S., Rolli-Derkinderen M. (2013) The digestive neuronal–glial–epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol 10: 90–100. [DOI] [PubMed] [Google Scholar]
- O’Mahony S., Clarke G., Borre Y., Dinan T., Cryan J. (2015) Serotonin, tryptophan metabolism and the brain–gut–microbiome axis. Behav Brain Res 277: 32–48. [DOI] [PubMed] [Google Scholar]
- O’Mahony S., Marchesi J., Scully P., Codling C., Ceolho A., Quigley E., et al. (2009) Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 65: 263–267. [DOI] [PubMed] [Google Scholar]
- O’Malley D., Dinan T., Cryan J. (2011) Neonatal maternal separation in the rat impacts on the stress responsivity of central corticotropin-releasing factor receptors in adulthood. Psychopharmacology 214: 221–229. [DOI] [PubMed] [Google Scholar]
- Park A., Collins J., Blennerhassett P., Ghia J., Verdu E., Bercik P., et al. (2013) Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil 25: 733–740, e574–e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B. (2014) Mucosal adenosine triphosphate mediates serotonin release from ileal but not colonic guinea pig enterochromaffin cells. Neurogastroenterol Motil 26: 237–246. [DOI] [PubMed] [Google Scholar]
- Pavlov V., Wang H., Czura C., Friedman S., Tracey K. (2003) The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med 9: 125–134. [PMC free article] [PubMed] [Google Scholar]
- Pellissier S., Dantzer C., Mondillon L., Trocme C., Gauchez A., Ducros V., et al. (2014) Relationship between vagal tone, cortisol, TNF-alpha, epinephrine and negative affects in Crohn’s disease and irritable bowel syndrome. PLoS One 9: e105328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Li R., Raes J., Arumugam M., Burgdorf K., Manichanh C., et al. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlke F., Stollman N. (2012) Fecal microbiota transplantation in relapsing clostridium difficile infection. Therap Adv Gastroenterol 5: 403–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach V., Bloch M., Rosenberg N., Gilad S., Limor R., Stern N., et al. (2007) The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology 32: 693–702. [DOI] [PubMed] [Google Scholar]
- Sabat R., Ouyang W., Wolk K. (2014) Therapeutic opportunities of the IL-22-IL-22r1 system. Nat Rev Drug Discov 13: 21–38. [DOI] [PubMed] [Google Scholar]
- Saunders P., Hanssen N., Perdue M. (1997) Cholinergic nerves mediate stress-induced intestinal transport abnormalities in wistar-kyoto rats. Am J Physiol 273: G486–490. [DOI] [PubMed] [Google Scholar]
- Schellekens H., Dinan T., Cryan J. (2013) Ghrelin at the interface of obesity and reward. Vitam Horm 91: 285–323. [DOI] [PubMed] [Google Scholar]
- Smith G., Jerome C., Norgren R. (1985) Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol 249: R638–641. [DOI] [PubMed] [Google Scholar]
- Spencer S., Xu L., Clarke M., Lemus M., Reichenbach A., Geenen B., et al. (2012) Ghrelin regulates the hypothalamic–pituitary–adrenal axis and restricts anxiety after acute stress. Biol Psychiatry 72: 457–465. [DOI] [PubMed] [Google Scholar]
- Stasi C., Bellini M., Bassotti G., Blandizzi C., Milani S. (2014) Serotonin receptors and their role in the pathophysiology and therapy of irritable bowel syndrome. Tech Coloproctol 18: 613–621. [DOI] [PubMed] [Google Scholar]
- Stasi C., Rosselli M., Bellini M., Laffi G., Milani S. (2012) Altered neuro–endocrine–immune pathways in the irritable bowel syndrome: the top-down and the bottom-up model. J Gastroenterol 47: 1177–1185. [DOI] [PubMed] [Google Scholar]
- Symonds E., Peiris M., Page A., Chia B., Dogra H., Masding A., et al. (2015) Mechanisms of activation of mouse and human enteroendocrine cells by nutrients. Gut 64: 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr A., Galley J., Fisher S., Chichlowski M., Berg B., Bailey M. (2015) The prebiotics 3’sialyllactose and 6’sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: evidence for effects on the gut–brain axis. Brain Behav Immun 50: 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L., Ockhuizen T., Suzuki K. (2014) Exploring the influence of the gut microbiota and probiotics on health: a symposium report. Br J Nutr 112: S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J., Mannea E., Aime P., Pfluger P., Yi C., Castaneda T., et al. (2011) Ghrelin enhances olfactory sensitivity and exploratory sniffing in rodents and humans. J Neurosci 31: 5841–5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. (2007) Physiology and immunology of the cholinergic anti-inflammatory pathway. J Clin Invest 117: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. (2010) Understanding immunity requires more than immunology. Nat Immunol 11: 561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Kleij H., O’Mahony C., Shanahan F., O’Mahony L., Bienenstock J. (2008) Protective effects of lactobacillus rhamnosus [corrected] and bifidobacterium infantis in murine models for colitis do not involve the vagus nerve. Am J Physiol Regul Integr Comp Physiol 295: R1131–1137. [DOI] [PubMed] [Google Scholar]
- Vanuytsel T., Tack J., Boeckxstaens G. (2014) Treatment of abdominal pain in irritable bowel syndrome. J Gastroenterol 49: 1193–1205. [DOI] [PubMed] [Google Scholar]
- Wang X., Ota N., Manzanillo P., Kates L., Zavala-Solorio J., Eidenschenk C., et al. (2014) Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 514: 237–241. [DOI] [PubMed] [Google Scholar]
- Wei Y., Gong J., Zhu W., Guo D., Gu L., Li N., et al. (2015a) Fecal microbiota transplantation restores dysbiosis in patients with methicillin resistant staphylococcus aureus enterocolitis. BMC Infect Dis 15: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Zhu W., Gong J., Guo D., Gu L., Li N., et al. (2015b) Fecal microbiota transplantation improves the quality of life in patients with inflammatory bowel disease. Article id: 517597 Gastroenterol Res Pract 2015: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M., Korpela K., Riksen-Walraven J., De Vos W., De Weerth C. (2015) Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 53: 233–245. [DOI] [PubMed] [Google Scholar]
- Zimomra Z., Porterfield V., Camp R., Johnson J. (2011) Time-dependent mediators of HPA axis activation following live escherichia coli. Am J Physiol Regul Integr Comp Physiol 301: R1648–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]