Abstract
Irritable bowel syndrome is a functional bowel disorder with gastrointestinal symptoms (e.g. abdominal pain, straining, urgency, incomplete evacuation, nausea, and bloating) that occur alongside bowel function alterations (i.e. constipation, diarrhea, or both). Patients with irritable bowel syndrome may also experience comorbid anxiety and depression. Irritable bowel syndrome is common, with a prevalence estimated between 3% and 28%, affecting patient health and quality of life. Patients with moderate or severe irritable bowel syndrome generally seek medical care, whereas those with milder symptoms may choose self-management. Most patients with irritable bowel syndrome receive outpatient care, but irritable bowel syndrome-related hospitalizations do occur. The pathophysiology of irritable bowel syndrome is multifactorial (i.e. genetics, immune components, changes in the gut microbiota, disturbances in physiologic stress response systems, and psychosocial factors). Management of irritable bowel syndrome can include lifestyle changes, dietary interventions, counseling, psychologic medication, and agents that affect gastrointestinal motility. A number of therapies have emerged in recent years with clinical trial data demonstrating efficacy and safety for patients with irritable bowel syndrome, including agents that target gastrointestinal motility (i.e. linaclotide), gastrointestinal opioid receptors (i.e. asimadoline, eluxadoline), and gut microbiota (i.e. rifaximin). Linaclotide has been shown to significantly improve stool frequency and abdominal pain compared with placebo in constipation-predominant irritable bowel syndrome (number needed to treat, 5.1). Asimadoline shows efficacy in patients with moderate-to-severe irritable bowel syndrome-related pain. Rifaximin provided adequate relief of global irritable bowel syndrome symptoms versus placebo for a significantly greater percentage of patients with diarrhea-predominant irritable bowel syndrome (p < 0.001). Management that encompasses all aspects of irritable bowel syndrome (gastrointestinal symptoms) and comorbid psychologic symptoms (e.g. anxiety or depression) is important for improving overall patient health and well-being.
Keywords: asimadoline, functional bowel disorder, linaclotide, rifaximin
Introduction
Irritable bowel syndrome (IBS) is a functional bowel disorder characterized by symptoms of abdominal pain or discomfort that occur with changes in bowel function for at least 3 days/month for at least 3 months (Rome III criteria) [Drossman, 2006; Longstreth et al. 2006]. Patients with mild IBS have few symptoms, report good health-related quality of life (HRQOL), and generally seek medical care for symptoms approximately once per year, whereas patients with moderate or severe IBS have a greater number of symptoms (e.g. abdominal pain, bloating, dietary restrictions), report fair to poor HRQOL, and typically seek medical care approximately two to seven times per year [Drossman et al. 2009b, 2011]. IBS is further classified based on stool consistency, including constipation-predominant IBS (IBS-C), diarrhea-predominant IBS (IBS-D), IBS alternating between constipation and diarrhea (IBS-M), or unsubtyped IBS [Longstreth et al. 2006]. Patients with IBS often experience a number of additional gastrointestinal (GI)-related symptoms, including straining, urgency, incomplete evacuation, nausea, and bloating [Hungin et al. 2005, 2014; Longstreth et al. 2006; Ringel et al. 2009; Su et al. 2014]. Bloating has been considered to be one of the most bothersome IBS symptoms by patients; bloating led patients to seek medical care with significantly greater frequency than when bloating did not occur (p = 0.01) [Ringel et al. 2009]. Bloating adversely affected energy level, food intake, and the physical function subdomains of the IBS-quality of life (IBS-QOL) scale [Ringel et al. 2009]. Some symptoms tend to occur more often in a specific subtype of IBS. For example, a significantly greater percentage of patients with IBS-D and IBS-M reported experiencing urgency compared with patients with IBS-C (p < 0.001), while nausea was more commonly reported in patients with IBS-M than in patients with IBS-D or IBS-C (p = 0.01) [Su et al. 2014].
Epidemiology of IBS
The prevalence of IBS has been estimated between 3% and 28%, with IBS-M more prevalent than IBS-C and IBS-D [Brummond et al. 2015; Lin et al. 2014; Locke et al. 2000; Patel et al. 2015; Rasmussen et al. 2015; Rey de Castro et al. 2015; Ringel et al. 2009; Saito et al. 2002; Su et al. 2014]. IBS is also more prevalent in women compared with men [Lovell and Ford, 2012]. Diagnosis and management of patients with IBS occurs primarily in an outpatient setting; however, IBS accounted for 0.03% of US hospital discharges in 2010, with a mean duration of hospitalization of 3.7 days, accounting for a mean of US$21,153 in hospital costs [Mitchell and Drossman, 1987; Sethi et al. 2013]. A 2014 narrative review of studies that examined the cost of IBS reported an annual US cost of US$742–7547 per patient [depending on study year (1992 to 2004), relationship (direct versus indirect) to IBS care, and source of cost data] and a total projected annual cost of US$1.35 billion [Canavan et al. 2014]. Extraintestinal physical symptoms of IBS include fatigue, sleep problems, and back pain, reported by 69.3%, 47.5%, and 37.3% of patients, respectively [Choung et al. 2009; Patel et al. 2015]. In one study, patients reported the intensity of 12 different symptoms on a scale from 0 to 2 using the Patient Health Questionnaire (PHQ), with a score of 0 indicating ‘no bother at all,’ a score of 1 for ‘bothered a little,’ and a score of 2 for ‘bothered a lot,’ for a total score ranging between 0 and 24 [Patel et al. 2015]. Patients with IBS had greater odds of a medium or high total PHQ score (total score ⩾8) compared with patients of a GI clinic without IBS [odds ratio (OR) 1.7; 95% confidence interval (CI) 1.2–2.4]. A high total PHQ score (i.e. ⩾13), indicative of more severe physical symptoms, occurred in 31.7%, 22.5%, and 20.8% of patients with IBS-M, IBS-C, or IBS-D [Patel et al. 2015]. Low back pain is more common in patients with IBS-C than IBS-D (p < 0.01) [Schmulson et al. 1999]. Patients with IBS often experience improvement in pain symptoms after a bowel movement, particularly patients with pain-predominant IBS (i.e. abdominal pain >6 times/year), compared with patients with abdominal pain <6 times/year (50% versus 13%, respectively) [Talley et al. 1990]. Approximately one-quarter of patients in each of these groups had IBS-C. Patients with IBS who develop pelvic floor dyssynergy may experience worsening of IBS symptoms; interestingly, biofeedback therapy for dyssynergia has been shown to resolve IBS symptoms in some patients [Patcharatrakul and Gonlachanvit, 2011; Prott et al. 2010; Wong et al. 2010]. Thus, a number of physical symptoms appear to play a role in the overall well-being of patients with IBS and should not be overlooked or marginalized.
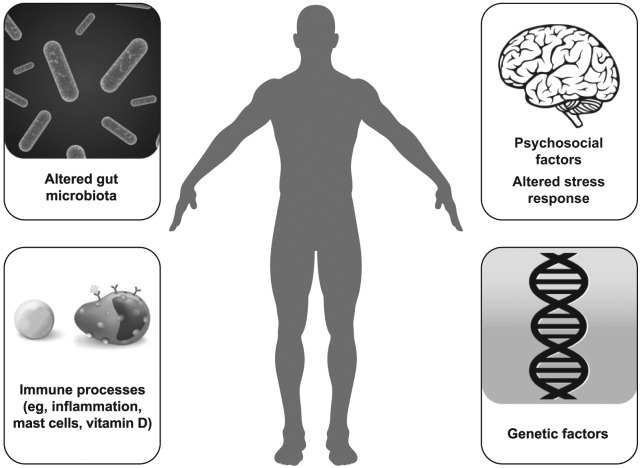
Pathophysiology of IBS
The pathophysiology of IBS has not been fully elucidated, but is multifactorial, and may include, but is not limited to, genetic factors, immune components, alterations in the gut microbiota, disturbances in physiologic stress response systems (e.g. hypothalamic–pituitary–adrenal), and psychosocial factors (Figure 1) [Camilleri, 2012]. A genetic component appears to play a role in IBS, as demonstrated by familial association studies and genome-wide association studies [Ek et al. 2015; Holliday et al. 2014; Levy et al. 2001; Locke et al. 2000] Genome-wide association studies of IBS have identified putative genetic risk loci for IBS [Ek et al. 2015; Holliday et al. 2014]. Further, additional genetic polymorphisms have been associated with IBS and specific disease characteristics (e.g. colonic transit) [Camilleri et al. 2014; Grasberger et al. 2013; Wouters et al. 2014].
Figure 1.
Pathophysiology of IBS.
(Credit: iStock.com/Stefan Alfonso (body silhouette), JDawnInk (DNA helix), and mstay (bacteria); and Shutterstock/maglyvi (brain), and Designua (immune processes artwork).)
Alterations in the immune system play a role in IBS, including cytokine imbalances, immune cell activation, inflammation, and increased GI membrane permeability. Results of a meta-analysis indicated that levels of the proinflammatory cytokine tumor necrosis factor (TNF)-α were significantly increased in patients with IBS-C, IBS-D, and IBS-M, compared with controls (p ⩽ 0.03 versus controls for all), while levels of the anti-inflammatory cytokine interleukin (IL)-10 did not differ between patients with either of these three subtypes and controls [Bashashati et al. 2014]. Further, serum levels of the proinflammatory cytokine IL-6 were significantly increased in patients with IBS-D compared with controls (32.2 versus 7.5 pg/ml, respectively; p < 0.001) [Rana et al. 2012]. Patients with IBS had increased levels of activated intestinal mucosa immune cells compared with patients of a gastroenterology clinic without IBS, or healthy individuals [Ahn et al. 2014; Chadwick et al. 2002; Coeffier et al. 2010; Guilarte et al. 2007; Martínez et al. 2012; Vivinus-Nébot et al. 2012, 2014]. In patients with IBS-D, the number of daily bowel movements and stool consistency were positively correlated with mucosal levels of activated B-cells and plasma cells [Vicario et al. 2014]. Mast cells, which play an important role in innate immunity, release granules containing histamine, serotonin, proteases, lipid mediators, and cytokines upon activation by stress [Barbara et al. 2006, 2011]. Indeed, patients with IBS-C and IBS-D had an increased number of duodenal mast cells compared with healthy individuals [Walker et al. 2009], and disease severity and abdominal pain were significantly correlated with mast cell counts [Vivinus-Nébot et al. 2012].
Vitamin D, which plays a role in inflammatory processes, may also be of relevance in the pathophysiology of IBS [Yin and Agrawal, 2014]. Vitamin D deficiency was significantly associated with depression, a condition not uncommon in patients with IBS [Fond et al. 2014; Hoang et al. 2011]. Although the role of vitamin D in the pathogenesis of IBS remains to be elucidated, vitamin D supplementation may improve symptoms of IBS, as well as anxiety and depression [Karaahmet et al. 2013; Sprake et al. 2012]. However, data to support this intriguing hypothesis are sparse and limited to case reports [Sprake et al. 2012].
Increased GI membrane permeability is thought to be an important factor in IBS pathogenesis [Martínez et al. 2012]. Patients with IBS appear to have increased GI membrane permeability compared with healthy individuals, with increased severity of disease and abdominal pain significantly associated with membrane permeability (p = 0.002 and p = 0.006, respectively) [Piche et al. 2009; Vivinus-Nébot et al. 2014]. Expression of genes related to membrane permeability and mast cell function was altered in patients with IBS-D compared with healthy individuals [Martínez et al. 2012]. Expression of the tight junction protein occludin was decreased in patients with IBS compared with healthy individuals [Coeffier et al. 2010; Martínez et al. 2012; Vivinus-Nébot et al. 2014]. Thus, impaired GI membrane permeability appears to play a role in IBS.
The gut microbiota of patients with IBS differs qualitatively and quantitatively compared with that of healthy individuals [Carroll et al. 2011; Codling et al. 2010; Durbán et al. 2012, 2013]. Symptoms of IBS have been associated with specific gut microbiota profiles [Jeffery et al. 2012]. Bloating was associated with an increase in GI Cyanobacteria in patients with IBS, whereas increased colonic transit time and constipation were associated with 17 different taxa [Jeffery et al. 2012]. The GI tract of patients with IBS with depression has a lower concentration of bacteria belonging to the family Actinomycetaceae compared with patients with IBS without depression. However, healthy individuals have significantly less Actinomycetaceae than patients with IBS without depression (p = 0.002); the contribution of this bacterial family to the IBS pathology remains to be elucidated. Although specific symptoms have been correlated with expression of microbiota species in patients with IBS, symptom severity has been associated with instability in the gut microbiota, particularly for patients with IBS-D [Durbán et al. 2013]. However, instability of the microbiota was associated with recurrence and remission, with changes to the microbiota profile occurring rapidly (i.e. within days). Further, the microbiota profile differed by symptom severity for a patient with severe IBS-D, with differences between days with mild-to-moderate disease and severe disease. It is still unknown whether alterations in gut microbiota result in the development of IBS symptoms, or occur as a result of IBS.
The bidirectional brain–gut communication axis and brain processing of noxious stimuli appears to play an important role in the pathophysiology of IBS. Patients with IBS may have abnormal colonic transit [Tornblom et al. 2012] and visceral hypersensitivity [Camilleri et al. 2008; Larsson et al. 2012; Posserud et al. 2007] potentially related to altered processing of neuronal signals from the GI tract. A meta-analysis of 18 studies that examined brain region activation after rectal distension demonstrated that patients with IBS displayed differences in brain region activity compared with healthy individuals, particularly in brain regions engaged in emotional arousal [Tillisch et al. 2011]. Patients with IBS also display alterations in autonomic function [Salvioli et al. 2015] and basal levels of stress hormones [Chang et al. 2009], suggesting altered function of the hypothalamic–pituitary–adrenal axis.
Psychosocial factors (e.g. physical, sexual, or psychologic abuse; psychiatric conditions) may play a role in the pathophysiology of IBS; psychosocial problems tend to be reported more commonly in patients with more severe forms of IBS [Afari et al. 2014; Bradford et al. 2012; Chitkara et al. 2008; Drossman et al. 2011; Halland et al. 2014; Knight et al. 2015]. Although the exact role of anxiety and depression in the pathophysiology of IBS is currently unknown, both have been associated with increased risk for IBS. Indeed, anxiety and depression occurred in a significantly greater number of patients with IBS compared with individuals without IBS (p < 0.0001 for both anxiety and depression) [Koloski et al. 2012; Ladabaum et al. 2012]. Further, 38% of patients with IBS have reported thoughts of suicide and, although rare, some patients with IBS have also attempted suicide (5%) [Miller et al. 2004].
The severity of IBS and the intensity of abdominal pain have been positively correlated with anxiety and depression [Rey de Castro et al. 2015]. Patients with IBS-C and IBS-D, but not IBS-M, had significantly greater anxiety compared with healthy individuals (p = 0.04, p = 0.01, and p = 0.06, respectively); only patients with IBS-D had significantly greater incidence of depression than healthy individuals (p = 0.03) [Fond et al. 2014]. A greater percentage of patients with IBS with anxiety or depression had extraintestinal physical symptoms compared with patients without anxiety (44.8% versus 16.8%, respectively; p < 0.001) or depression (57.0% versus 21.5%, respectively; p < 0.001) [Patel et al. 2015]. A greater percentage of patients with IBS received anxiolytics and antidepressants compared with healthy controls (p < 0.0001 for both comparisons); notably, 62% of patients received these agents prior to receiving a diagnosis of IBS [Ladabaum et al. 2012]. Further, patients with IBS were more likely to have psychiatric conditions than individuals without IBS [Gulewitsch et al. 2011; Singh et al. 2012]. A significantly greater percentage of patients with severe IBS had at least one psychiatric disorder compared with patients with mild or moderate IBS (94.4% versus 35.7% and 76.1%, respectively; p = 0.003 and p = 0.02) [Singh et al. 2012]. Thus, the pathophysiology of IBS is multifactorial in nature.
Management of IBS: established approaches
Patients with IBS may benefit from lifestyle changes (e.g. exercise, dietary modification) [Fukudo et al. 2015]. In a study of patients with IBS undergoing military training, 62.9% of patients had improvement from baseline in bowel habits after 9 weeks of lifestyle modification imposed by the training (i.e. no smoking or alcohol consumption, regular meals, physical activity) [Kang et al. 2011]. After 9 weeks of training, mean stool frequency decreased from baseline, and the percentage of patients with normal stools (defined as Bristol stool scale score of 3, 4, or 5) increased from baseline (p = 0.05). In a ran-domized, controlled study of patients with IBS, patients in the group with increased physical activity experienced significant improvement from baseline in IBS severity compared with patients who had no change in physical activity after 12 weeks (p = 0.003) [Johannesson et al. 2011]. Further, patients who increased physical activity had significant improvement from baseline in the emotional (p = 0.002), sleep (p = 0.03), energy (p = 0.006), physical function (p = 0.001), social role (p = 0.001), and physical role (p = 0.008) dimensions of the IBS-QOL instrument after 12 weeks. Thus, increased exercise is a lifestyle change that appears to benefit patients with IBS.
Dietary modification is a therapeutic option preferred by patients with IBS, but may be limited by issues of long-term patient adherence and potential risk of nutritional deficiencies [Gibson et al. 2015]. A meta-analysis of four randomized, controlled clinical studies supported the efficacy of a low fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) diet in patients with IBS, with an estimated number needed to treat (NNT) of 2.2 (95% CI 1.9–2.5) [Khan et al. 2015]. However, long-term maintenance of a low-FODMAP diet may not be practical [Muir and Gibson, 2013] and its efficacy beyond that of traditionally recommended IBS diet (i.e. regular ingestion of meals and snacks, reduced intake of certain foods such as onions, avoidance of carbonated beverages and artificial sweeteners, and ingestion of fiber) is uncertain [Böhn et al. 2015]. Gluten-free diets may be recommended for patients with IBS, although it is unclear how gluten affects IBS symptoms [Vazquez-Roque et al. 2013]. In a randomized, controlled study of patients with IBS-D (based on Rome II criteria), those receiving a gluten-free diet for 4 weeks achieved a significant reduction in daily stool frequency compared with patients receiving diets containing gluten (p = 0.04). The National Institute for Health and Care Excellence recommends avoidance and exclusion diets (e.g. FODMAP) in patients who have persistent IBS symptoms; however, such patients should be under the care of a dietary management expert. Finally, the efficacy and safety of probiotics, which are one of the most common dietary supplements used for the treatment of GI conditions, are limited by the number of quality clinical studies (e.g. adequate sample size, variability in outcomes examined) supporting their use [Didari et al. 2015; Dossett et al. 2014; Whelan, 2014] It is currently unclear which probiotic strain might be appropriate for IBS symptoms, and at what daily therapeutic dose [Whelan, 2014]. Probiotics, if used, should be administered in combination with conventional treatments for IBS for best effect [Ringel and Ringel-Kulka, 2011].
Carbohydrate malabsorption is associated with symptoms of IBS (i.e. abdominal pain, constipation, diarrhea), although affected individuals may not have IBS [Goebel-Stengel et al. 2014]. In a retrospective study of patients with IBS-like abdominal symptoms, 36% and 64% of patients had symptomatic lactose and fructose malabsorption, respectively, as determined by breath testing. Of patients with diagnosed IBS (by Rome III criteria), 22% had fructose malabsorption as determined by breath testing [Melchior et al. 2014]. In these patients, carbohydrate malabsorption can be managed by an elimination diet [Goebel-Stengel et al. 2014].
Given the number of patients with IBS with anxiety or depression, patients with IBS may benefit from psychological (psychotropic) therapies [American College of Gastroenterology Task Force on IBS, 2009; Fukudo et al. 2015]. Indeed, findings of a systematic review and meta-analysis that evaluated 32 randomized, controlled trials of psychological therapies found that a greater percentage of patients receiving control therapy (i.e. monitoring of symptoms, physician’s ‘usual management’, supportive therapy, or placebo) reported that their symptoms of IBS did not improve (76.1%), compared with the percentage of patients that did not experience improvement with psychological therapy (i.e. cognitive behavioral therapy, multicomponent psychological therapy, stress management, and relaxation therapy; 51.9%) [Ford et al. 2014b]. Thus, results of this meta-analysis suggest that psychological therapies are an effective management option in patients with IBS.
Serotonin is a ubiquitous signaling molecule in the body, primarily produced and stored by enterochromaffin cells in the GI tract [Gershon, 2004; Gershon and Tack, 2007]. Serotonin is important for normal GI motility, secretion, and visceral sensitivity [Bennett and Whitney, 1966; Delvaux et al. 1998; Gershon, 1999]. Inactivation of serotonin is mediated by the serotonin reuptake transporter (SERT), which is expressed on GI enterocytes [Gershon, 2004; Wade et al. 1996]. Colonic serotonin and SERT levels were decreased in patients with IBS compared with healthy individuals [Coates et al. 2004]. Selective serotonin reuptake inhibitors (SSRIs) can increase serotonin levels in patients with IBS by inhibiting the reuptake of this neurotransmitter [Chang et al. 2014]. An analysis of pooled data from 7 or 11 randomized, controlled studies of SSRIs or tricyclic antidepressants, respectively, found active treatment was associated with decreased symptoms of IBS compared with placebo (NNT = 4 for both) [Ford et al. 2014a]. Although efficacious for patients with IBS, psychotropic medications can be associated with adverse events (AEs) including drowsiness, dry mouth, and lower libido, with a number needed to harm of 9 [Clayton and Montejo, 2006; Ford et al. 2014a]. The American College of Gastroenterology weakly recommends the use of SSRIs and tricyclic antidepressants for relief of symptoms and pain in patients with IBS, and the National Institute for Health and Care Excellence notes one could consider them as a second-line therapy [Ford et al. 2014a].
Whereas the majority of patients with IBS-C or IBS-D have normal colonic transit times, 12% of patients with IBS-C have slow, and 27% of patients with IBS-D have fast colonic transit times [Tornblom et al. 2012]. A number of therapeutic agents that influence colonic transit are available for the treatment of patients with IBS. Lubiprostone, a selective chloride channel activator indicated for the treatment of women with IBS-C, had exhibited a greater degree of efficacy than placebo for relief of global IBS-C symptoms [Takeda Pharmaceuticals America, Inc., 2013; Drossman et al. 2009a; Ford et al. 2014a]. Results of two phase III clinical studies demonstrated that a significantly larger percentage of patients with IBS-C receiving lubiprostone for 12 weeks achieved overall response (i.e. moderate or significant relief of global IBS symptoms for ⩾2 of 3 months by patients self-report) compared with placebo (17.9% versus 10.1%, respectively; p = 0.001) [Drossman et al. 2009a]. However, lubiprostone has been associated with mild-to-moderate nausea, which may negatively impact use for some patients [Drossman et al. 2009a]. Loperamide, an effective antidiarrheal agent, is not currently recommended for the treatment of patients with IBS, based on insufficient evidence of global IBS symptom relief [Ford et al. 2014a]. Alosetron is a selective 5-hydroxytryptamine (5-HT3) receptor antagonist indicated for the treatment of women with severe, chronic IBS-D refractory to other therapies [Prometheus Laboratories, Inc., 2014]. Early clinical studies of alosetron in patients with IBS included mostly women and failed to demonstrate improved efficacy compared with placebo in men [Bardhan et al. 2000; Camilleri et al. 1999]. Alosetron provided adequate relief of IBS pain and discomfort, and improvement of stool consistency, in men with IBS-D, but did not improve stool frequency, urgency, bloating, or number of pain-free days compared with placebo [Chang et al. 2005]. Because of the risk of severe constipation and ischemic colitis in some patients, prescribing of alosetron is limited to healthcare providers enrolled in a special prescribing program, and patients must sign an acknowledgment form before initiating treatment [Prometheus Laboratories, Inc., 2014; Chang et al. 2010; Schiller and Johnson, 2008]. Analysis of postmarketing data acquired after implementation of the risk management program demonstrated that the incidence of ischemic colitis and constipation remained rare and decreased between 2002 and 2011 [Tong et al. 2013]. Another 5-HT3 receptor antagonist, ondansetron, has also been shown to improve symptoms in patients with IBS-D. In a randomized, placebo-controlled crossover trial, patients with IBS-D who received ondansetron 12–24 mg/day for 5 weeks experienced significantly greater improvement from baseline in mean stool form than placebo (p < 0.001) [Garsed et al. 2014]. In addition, patients who received ondansetron had reduced mean numbers of days per week with urgency (p < 0.001) and mean numbers of days per week with bloating (p = 0.002) compared with placebo [Garsed et al. 2014]. The most frequent AE with ondansetron was constipation, which occurred in a greater percentage of patients who received ondansetron (9%) than placebo (2%); however, most patients responded to dose reduction and continued with the trial [Garsed et al. 2014].
Abnormal contraction of smooth muscle within the GI tract may underlie some IBS symptoms, especially pain; therefore, agents that relax smooth muscles such as antispasmodics and peppermint oil have been evaluated in patients with IBS. A 2014 systematic review of available randomized, placebo- or ‘no treatment’-controlled trials for various antispasmodics (23 trials; 2154 patients) demonstrated that antispasmodics significantly improved IBS symptoms to a greater extent than placebo [NNT for antispasmodics 5 (95% CI 4–9)]. However, the effectiveness of individual antispasmodic agents varied, with only otilonium, hyoscine bromide, cimetropium bromide, pinaverium bromide, and dicyclomine hydrochloride showing benefits above placebo. AEs with antispasmodics were more common than with placebo [relative risk (RR) 1.6; 95% CI 1.1–2.4; number needed to harm 20 (95% CI 9.5–333)]; nonserious AEs of dry mouth, dizziness, and blurred vision were commonly reported [Ford et al. 2014a]. Two systematic reviews of ran-domized, placebo-controlled trials of enteric-coated peppermint oil in patients with IBS (5 trials, 482 patients; 9 trials, 726 patients) showed that peppermint oil was more efficacious than placebo for improving IBS symptoms (RR 2.2; 95% CI 1.8-2.8; NNT 3) [Ford et al. 2014a; Khanna et al. 2014]. Data are somewhat conflicting regarding the incidence of AEs. A pooled analysis study that included 7 randomized, placebo-controlled trials (474 patients) demonstrated a significantly greater risk of experiencing an AE than placebo (RR 1.7; 95% CI 1.3–2.4), whereas a separate study that pooled data from 5 randomized, placebo-controlled trials (482 patients) showed no significant difference from placebo (RR 1.3; 95% CI 0.8–2.1). This dissimilarity may reflect differences in the inclusion of individual studies for the pooled analysis rather than an actual clinical difference in AE occurrence.
The therapies that have been discussed within have been available for patients with IBS for a number of years and, in many cases, much longer. The purpose of this review is to provide gastroenterologists with an update on more recent therapies that have become available (i.e. since 2012), or are currently in development, for the treatment of patients with IBS.
Methods
A PubMed search of English-language articles available through 8 May 2015 was conducted using the following keywords to identify ran-domized, controlled studies performed in humans: ‘irritable bowel syndrome guidelines’, ‘rifaximin and irritable bowel syndrome’, ‘linaclotide and irritable bowel syndrome’, ‘alosetron and irritable bowel syndrome’, ‘ramosetron and irritable bowel syndrome’, ‘asimadoline and irritable bowel syndrome’, and ‘eluxadoline and irritable bowel syndrome’. Reference lists from review articles were used to identify additional studies for inclusion. Further, a ClinicalTrials.gov search of phase III or IV randomized, controlled studies of IBS (primary condition) was conducted on 15 February 2015 to identify additional agents for inclusion in this review.
Newer agents for the treatment of IBS
Targeting GI motility
The prosecretory agent linaclotide is a guanylate cyclase-C agonist indicated for the treatment of patients with moderate-to-severe IBS-C [Forest Laboratories, Inc., 2014; Yu and Rao, 2014]. Linaclotide binding activates guanylate cyclase-C, which leads to phosphorylation of the cystic fibrosis transmembrane conductance regulator (CFTR). Chloride and bicarbonate ions are secreted through the CFTR, while sodium absorption is decreased, leading to an increase of intestinal fluid into the GI lumen, and a subsequent increase in GI transit time [Layer and Stanghellini, 2014].
Linaclotide was shown to be well tolerated and efficacious for the treatment of patients with IBS-C in 2 randomized, double-blind, placebo-controlled studies, as well as additional post-hoc analyses (Table 1) [Castro et al. 2013; Chey et al. 2012; Macdougall et al. 2013; Quigley et al. 2013; Rao et al. 2012, 2014]. In one randomized, controlled study, a significantly greater percentage of patients receiving linaclotide had improvement in IBS symptoms as evaluated using the US Food and Drug Administration (FDA) efficacy endpoint [i.e. change from baseline ⩾30% in mean daily worst abdominal pain scores and an increase from baseline ⩾1 complete spontaneous bowel movement (CSBM) for ⩾6 of the first 12 weeks of treatment] compared with placebo (6 weeks, 33.7% versus 13.9%, respectively; p < 0.0001) [Chey et al. 2012]. A greater percentage of patients who received linaclotide versus placebo met criteria for the individual components of the combined endpoint [i.e. ⩾30% improvement in abdominal pain (48.9% versus 34.5%, respectively) and an increase of ⩾1 in the number of weekly CSBMs from baseline for ⩾6 of 12 weeks (47.6% versus 22.6%, respectively)]. Improvement in the FDA efficacy endpoint was sustained for 13 weeks, with 32.4% and 13.2% of patients receiving linaclotide or placebo, respectively, achieving the FDA efficacy endpoint (p < 0.0001) [Chey et al. 2012]. The percentages of patients who had ⩾30% reduction in average daily worst abdominal pain (36.9 versus 17.4% with linaclotide and placebo, respectively; p < 0.0001) and ⩾3 CSBMs with an increase of ⩾1 CSBM from baseline (15.7 versus 3.5%, respectively; p < 0.0001) also remained significantly greater with linaclotide than placebo during an additional 13 weeks of treatment [Chey et al. 2012]. These findings were supported by a second randomized, controlled study, which found that 33.6% and 21.0% of patients receiving linaclotide or placebo, respectively, achieved response as defined by the FDA efficacy endpoint (p < 0.0001) [Rao et al. 2012]. However, in this study, when patients who received linaclotide for 12 weeks were reassigned to receive placebo for 4 weeks, they experienced an increase in mean daily worst abdominal pain and a decrease in CSBMs comparable with that of patients who had received placebo for 12 weeks.
Table 1.
Summary of randomized, controlled clinical studies of linaclotide in patients with IBSa.
| Study design and patients | Treatment and duration evaluated | Efficacy outcomes | Quality of life | Safety |
|---|---|---|---|---|
| Primary Analyses | ||||
| R, DB, PBO-C [Chey et al. 2012] IBS-C (Rome II criteria) |
Linaclotide 290 µg qd (n = 402) versus PBO (n = 403) for 26 weeks | Linaclotide versus PBO: FDA endpointb: 6 weeks: 33.7% versus 13.9%, p < 0.0001; 13 weeks: 32.4% versus 13.2%, p < 0.0001 Decrease from baseline ⩾30% in abdominal pain: for ⩾6 weeks: 48.9% versus 34.5%, p < 0.0001; for ⩾9 weeks: 38.9% versus 19.6%, p < 0.0001; for ⩾20 weeks: 36.9% versus 17.4%, p < 0.0001 Increase from baseline ⩾1 CSBM: for ⩾6 weeks: 47.6% versus 22.6%, p < 0.0001; for ⩾9 weeks: 18.0% versus 5.0%, p < 0.0001; for ⩾20 weeks: 15.7% versus 3.5%, p < 0.0001 Decrease from baseline ⩾30% in abdominal pain and ⩾3 CSBMs and increase from baseline ⩾1 CSBM: for ⩾9 weeks: 12.7% versus 3.0%, p < 0.0001; for ⩾20 weeks: 12.0 versus 2.5%, p < 0.0001 |
Not evaluated | Linaclotide versus PBO: AEs: 65.4% versus 56.6% Diarrhea: 19.7% versus 2.5% |
| R, DB, PBO-C [Rao et al. 2012] IBS-C (Rome II criteria) |
Linaclotide 290 µg qd (n = 405) versus PBO (n = 395) for 12 weeks Withdrawal phase: patients receiving linaclotide for 12 weeks randomized to linaclotide (n = 158) or PBO (n = 154) for 4 weeks; patients receiving PBO for 12 weeks assigned to linaclotide (n = 335) for 4 weeks |
Linaclotide versus PBO: 12-week tx phase: FDA endpointb: 33.6% versus 21.0%, p < 0.0001 Decrease from baseline ⩾30% in abdominal pain for ⩾6 weeks: 50.1% versus 37.5%, p = 0.0003 Increase from baseline ⩾1 CSBM for ⩾6 weeks: 48.6% versus 29.6%, p < 0.0001 Decrease from baseline ⩾30% in abdominal pain for ⩾9 weeks: 34.3% versus 27.1%, p = 0.03 ⩾3 CSBMs and increase from baseline ⩾1 CSBM for ⩾9 weeks: 19.5% versus 6.3%, p < 0.0001 Decrease from baseline ⩾30% in abdominal pain and ⩾3 CSBMs and increase from baseline ⩾1 CSBM for ⩾9 weeks: 12.1% versus 5.1%, p = 0.0004 4-week withdrawal phase: Re-randomized from linaclotide to PBO: increase in worst abdominal pain and decrease in CSBMs comparable with PBO group in 12-week tx phase Continuation of linaclotide: continued improvement in worst abdominal pain and CSBMs Switch from PBO to linaclotide: improvement in worst abdominal pain and CSBMs comparable with linaclotide group in 12-week tx phase |
Not evaluated | Linaclotide versus PBO: 12-week tx phase: AEs: 56.2% versus 53.0% Diarrhea: 19.5% versus 3.5% Abdominal pain: 5.4% versus 2.5% Headache: 4.9% versus 3.5% Flatulence: 4.9% versus 1.5% Abdominal distension: 2.2% versus 0.8% 4-week withdrawal phase: Linaclotide-linaclotide: 22.2% Linaclotide-PBO: 22.1% PBO-linaclotide: 30.6% |
| Post hoc analyses | ||||
| R, DB, PBO-C [Castro et al. 2013] Post hoc longitudinal responder analysis of patients with IBS-C |
Linaclotide 290 µg qd versus PBO for 26 weeks (pooled,N = 805) | Decrease ⩾30% from baseline in abdominal pain in patients receiving linaclotide Week 3: >50% of patients Week 7: >60% of patients Week 26: ~70% of patients (p < 0.005 versus PBO for each of 26 weeks) |
Not evaluated | Not evaluated |
| R, DB, PBO-C [Quigley et al. 2013] Post hoc analysis [Cheyet al. 2012; Rao et al. 2012] of patients with IBS-C (Rome II criteria) and mean daily abdominal pain severity score ⩾3.0 |
Linaclotide 290 µg qd versus PBO for 12 weeks | Linaclotide versus PBO: EMA endpoint for abdominal pain or discomfortc: Trial 31: 54.8% versus 41.8%, p < 0.001 Trial 302: 54.1% versus 38.5%, p < 0.0001 EMA endpoint for degree of relief of IBS symptomsd: Trial 31: 37.0% versus 18.5%, p < 0.0001 Trial 302: 39.4% versus 16.6%, p < 0.0001 |
Linaclotide versus PBO (LS mean change): Improvement in IBS-QOL from baseline to Week 12: Trial 31: 18.4 versus 15.2,p = 0.004; Trial 302: 16.6 versus 11.1,p < 0.0001 Improvement in EQ-5D from baseline to Week 12: Trial 31: 0.08 versus 0.05,p = 0.001; Trial 302: 0.08 versus 0.04,p = 0.0005 Improvement in EQ-5D VAS from baseline to Week 12: Trial 31: 5.6 versus 3.7,p = 0.06; Trial 302: 7.1 versus 4.4,p = 0.006 |
Not further evaluated |
| R, DB, PBO-C [Macdougallet al. 2013] Post hoc analysis [Cheyet al. 2012; Rao et al. 2012] of patients with IBS-C (Rome II criteria) |
Linaclotide 290 µg qd versus PBO for 12 weeks (pooled, N = 1602) | Linaclotide versus PBO: FDA endpointb: 33.7% versus 17.4%, p < 0.0001 |
Not evaluated | Not evaluated |
| R, DB, PBO-C [Rao et al. 2014] Post hoc analysis [Cheyet al. 2012; Rao et al. 2012] of patients with IBS-C (Rome II criteria) and baseline abdominal symptom severity score ⩾7.0) Patients with severe abdominal symptoms: fullness (44%), bloating (44%), discomfort (32%), pain (23%), cramping (22%) |
Linaclotide 290 µg qd (n = 805) versus PBO (n = 797) for 12 weeks (pooled, N = 1602) | Significant change from baseline in severe abdominal symptoms (pain, discomfort, bloating, fullness, cramping) with linaclotide versus PBO at Week 12 (p < 0.0001 for all comparisons) Adequate relief of IBS symptoms significantly greater with linaclotide versus PBO at Week 12 (59–61% versus 28–32%, p < 0.0001) |
Response by IBS-QOL in 62–68% of patients receiving linaclotide versus 45–47% of patients receiving PBO (p < 0.01) |
Linaclotide versus PBO: Diarrhea: 18.3–19.8% versus 1.6–2.1% Flatulence: 4.2–5.7% versus 1.8–2.5% Abdominal pain: 2.1–4% versus 2.2–2.5% |
Studies presented are limited to the previous 4 years, due to the large number of clinical studies of linaclotide.
Defined as meeting both an improvement from baseline ⩾30% in mean of daily worst abdominal pain scores and an increase from baseline ⩾1 CSBM for ⩾6 weeks of first 12 weeks of treatment.
Abdominal pain responder defined as patient with improvement from baseline ⩾30% in mean weekly worst abdominal pain score or mean weekly abdominal discomfort score for ⩾6 weeks of first 12 weeks of treatment, with neither score worsening from baseline. Scoring of abdominal pain or discomfort ranged from 0 (none) to 10 (very severe).
Degree-of-relief responder defined as patient with ‘considerable’ or ‘complete’ relief of IBS symptoms (i.e. score ⩽2) for ⩾6 of first 12 weeks of treatment. Symptoms were rated weekly on a scale of 1 to 7, with rating of 1 for ‘completely relieved’, 4 for ‘unchanged’, and 7 for ‘as bad as I can imagine’.
AE, adverse event; CSBM, complete spontaneous bowel movement; DB, double-blind; EMA, European Medicines Agency; EQ-5D, European Quality of Life - 5 Dimensions; EQ-5D VAS, European Quality of Life - 5 Dimensions Visual Analogue Scale; IBS-C, constipation-predominant irritable bowel syndrome; IBS-QOL, Irritable Bowel Syndrome - Quality Of Life questionnaire; LS, least squares; PBO, placebo; PBO-C, placebo-controlled; qd, once daily; R, randomized; tx, treatment.
Post hoc analysis of the two randomized, controlled studies [Chey et al. 2012; Rao et al. 2012] showed that linaclotide significantly improved IBS-QOL from baseline to 12 weeks compared with placebo (p = 0.004 and p < 0.0001 for the two studies) [Quigley et al. 2013]. Indeed, all subscales of the IBS-QOL were significantly improved with linaclotide compared with placebo (p < 0.05 for all comparisons), with the exception of the activity interference subscale for patients in the Rao and colleagues study (p = 0.6). Although linaclotide improved symptoms of IBS and patient quality of life, this agent was associated with diarrhea [Chang et al. 2014; Chey et al. 2012; Quigley et al. 2013; Rao et al. 2012].
Targeting opioid receptors
The µ-, δ-, and κ-opioid receptors expressed in the GI tract are important for the regulation of gut motility and secretion [Bagnol et al. 1997; Holzer, 2009; Lamki and Sullivan, 1983]. Expression of the κ-opioid receptor is increased during inflammation and chronic visceral hypersensitivity [Hughes et al. 2014a]. In mice, expression of δ-opioid receptors increased under conditions of stress compared with no stress [Wade et al. 2012]. Patients with IBS had decreased blood and colonic levels of the endogenous opioid β-endorphin compared with healthy individuals [Hughes et al. 2014b].
The mixed µ-opioid receptor agonist and δ-opioid receptor antagonist eluxadoline was approved in May 2015 for the treatment of IBS-D. Patients with IBS-D receiving eluxadoline in a phase II dose-ranging study had greater efficacy compared with patients receiving placebo after 12 weeks [Dove et al. 2013]. A significantly greater percentage of patients receiving eluxadoline 25 mg or 200 mg twice daily achieved clinical response (i.e. decrease from baseline in mean worst abdominal pain ⩾30% and ⩾2 points, with a daily Bristol Stool Scale score of 3 or 4 on ⩾66% of daily diary entries within a week) after 4 weeks of treatment compared with placebo (12% or 13.8% versus 5.7%, respectively; p = 0.04 for 25 mg and p = 0.02 for 200 mg versus placebo). Clinical response was also significantly greater in patients receiving eluxadoline 100 mg twice daily after 12 weeks of treatment compared with placebo (20.2% versus 11.3%, respectively; p < 0.05). Eluxadoline improved the number of daily bowel movements and decreased the episodes of urgency and incontinence experienced by patients during the 3-month treatment period. Eluxadoline had an overall favorable safety profile, with nausea, abdominal pain, vomiting, and constipation the most commonly reported AEs. Constipation was most common in the eluxadoline 100 mg group (6%); however, no patients in this group discontinued from the study or rated the intensity of constipation as severe. Three serious incidences of pancreatitis were observed (two within the first two doses of eluxadoline 200 mg twice daily and one within 18 days of 25 mg twice-daily dosing), but all incidences quickly resolved without sequelae. Furthermore, US prescribing information warns of an increased risk of sphincter of Oddi spasm, resulting in pancreatitis, as well as an increased risk of pancreatitis not associated with sphincter of Oddi spasm [Patheon Pharmaceuticals, Inc., 2015].
Asimadoline is a κ-opioid receptor agonist currently in development for the management of patients with IBS-D with moderate-to-severe pain (Table 2) [Delvaux et al. 2004; Mangel et al. 2008; Mangel and Hicks, 2012; Szarka et al. 2007]. In a phase II, dose-ranging study of patients with IBS with a mean abdominal pain score of 1.5 (on a scale of 0 to 3, with 0 = no pain, 1 = mild pain, 2 = moderate pain, and 3 = severe pain), treatment with asimadoline 0.15, 0.5, or 1 mg twice daily for 12 weeks did not improve response (i.e. number of months with adequate relief of IBS symptoms) compared with placebo [Mangel et al. 2008]. However, response was significantly improved in patients with moderate or severe pain (pain score ⩾2) receiving asimadoline 0.5 and 1 mg, compared with placebo (p = 0.006 and p = 0.005, respectively). Further, subgroup analysis by IBS type showed that 46.7% and 20% of patients with IBS-D receiving asimadoline 0.5 mg or placebo, respectively, had adequate relief of pain symptoms for ⩾3 of 4 weeks (p = 0.01). A significantly greater percentage of patients with IBS-D receiving asimadoline 0.5 mg achieved a 25% increase in the number of pain-free days during the 12 weeks of treatment compared with placebo (42.9% versus 18%, respectively; p = 0.001). Bowel function improved in patients with IBS-D after treatment with asimadoline 0.5 mg: the number of daily bowel movements decreased significantly from baseline during month 3 with asimadoline compared with placebo (2.3 versus 0.3, respectively; p < 0.05) and the number of days with urgency was decreased during the 3 months of treatment at 0.5 or 1.0 mg. Thus, asimadoline may be a potential future therapy for the treatment of patients with IBS-D.
Table 2.
Summary of randomized, controlled studies of asimadoline in patients with IBS.
| Study design and patient population | Treatment | Primary efficacy outcomes | Secondary efficacy outcomes | Safety |
|---|---|---|---|---|
| R, DB, PBO-C, CO [Delvaux et al. 2004] IBS (Rome II criteria) with pain threshold ⩽32 mmHg during first colonic distension attempt |
Asimadoline 0.5 mg 1 h prior to colonic distension attempt on Day 1 or 2, alternating with PBO (n = 20) | Pain intensity significantly decreased with asimadoline versus PBO (p = 0.04) Pain thresholds and compliance following colonic distension did not differ between groups (p = 0.2) |
Not evaluated | |
| R, DB, PBO-C [Szarka et al. 2007] Women with IBS (Rome II criteria) |
Asimadoline 0.5 mg prn up to 1.0 mg qid (n = 60) or PBO (n = 40) for 4 weeks | Mean decrease in pain severity from first daily dose to 2 h post-dose when patient had pain level ⩾30 mm on VAS did not differ between groups | Percentage of daily adequate relief of IBS pain and discomforta did not differ between groups and percentage of patients with adequate relief >50% of days with pain was comparable | AEs comparable between groups, except greater percentage of GI-related AEs with PBO |
| R, DB, PBO-C [Mangel et al. 2008] IBS-D, IBS-C, IBS-M (Rome II criteria) with a mean abdominal pain/discomfort severity score ⩾1.5 |
Asimadoline 0.15 mg (n = 149), 0.5 mg (n = 152), 1.0 mg (n = 144) bid, or PBO (n = 151) for 12 weeks |
Asimadoline 0.15, 0.5, 1.0 mg, versus PBO Total months patients had adequate relief of IBS pain or discomfortb during 3 months of tx: 33%, 37%, 37%, 33%, respectively Moderate pain or greater (score ⩾2.0): Asimadoline 0.5 mg and 1.0 mg versus PBO, 40% and 40% versus 23%, respectively (p = 0.006 and p = 0.005 versus PBO, respectively) Subgroups: IBS-D: 47% and 37% versus 20%, respectively (p = 0.01 and p = 0.05 versus PBO, respectively) IBS-M: 50% versus 28%, respectively (p = 0.02) IBS-C: no benefit with asimadoline |
Abdominal pain or discomfort scoresc: Increase from baseline in pain/discomfort-free days, asimadoline 0.5 mg versus PBO, 42.9% versus 18% (p = 0.001) in patients with IBS-D Stool consistency (Bristol Stool Scale) not improved from baseline in patients with IBS-D or IBS-M Daily stool frequency decreased from baseline to 3 months: asimadoline 0.5 mg versus PBO: 2.3 versus 0.3, p < 0.05 Sense of urgencyd decreased in patients with IBS-D during 3 months of tx Straininge not affected by asimadoline in patients with IBS-D or IBS-M Bloatingf significantly improved from baseline in patients with IBS-D with asimadoline 0.5 mg and 1.0 mg only during Month 2 |
Asimadoline 0.15, 0.5, 1.0 mg, versus PBO: Diarrhea: 13.4%, 5.9%, 11.1%, versus 7.9% Constipation: 12.8%, 10.5%, 7.6%, versus 4.6% Headache: 9.4%, 4.6%, 5.6%, versus 7.3% Nausea: 5.4%, 8.6%, 2.8%, versus 3.3% Sinusitis: 4.7%, 2.6%, 6.3%, versus 1.3% Abdominal pain: 5.4%, 4.6%, 4.2%, versus 4.0% |
Seven-point scale.
Adequate relief of IBS pain or discomfort evaluated by a weekly answer to the question, ‘In the past 7 days have you had adequate relief of your IBS pain or discomfort?’. Patients were considered to have adequate relief on a monthly basis if they answered ‘yes’ ⩾3 of 4 weeks per month. The total number of months that patients had adequate relief was calculated as a percentage of 3 months.
Scoring determined by answer to question, ‘Have you experienced abdominal pain or discomfort in the past 24 hours? If yes, how would you rate the maximum severity of abdominal pain or discomfort you have experienced in the past 24 hours? Mild, 1; Moderate, 2; Severe, 3.’.
Evaluated by the answer to the question, ‘Have you felt or experienced a sense of urgency in the past 24 hours?’.
Rating determined by the answer to the question, ‘Please rate the amount of straining you experienced with your stool in the past 24 hours. 1, no straining; 2, acceptable straining; 3, too much straining.’.
Evaluated by the answer to the question ‘Have you experienced bloating or abdominal distension in the past 24 hours?’.
AE, adverse event; bid, twice daily; CO, crossover; DB, double-blind; GI, gastrointestinal; IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhea-predominant IBS; IBS-M, IBS alternating between constipation and diarrhea; IBS-QOL, Irritable Bowel Syndrome - Quality Of Life questionnaire; PBO, placebo; PBO-C, placebo-controlled; prn, as needed; qid, 4 times a day; R, randomized; tx, treatment; VAS, visual analogue scale.
Targeting gut microbiota
The nonsystemic antibiotic rifaximin appears to have anti-inflammatory, host-response, and gut microbiota modulatory activities [Bajaj et al. 2013; Brown et al. 2010; Cheng et al. 2010; Debbia et al. 2008; DuPont and Jiang, 2004; Hopkins et al. 2014; Jiang et al. 2010a, 2010b; Maccaferri et al. 2010; Mencarelli et al. 2010, 2011; Schrodt et al. 2013; Terc et al. 2014; Xu et al. 2014]. Rifaximin received regulatory approval for the treatment of IBS-D in May 2015; several studies indicated a favorable efficacy and safety profile for rifaximin in IBS-D (Table 3) [Di Stefano et al. 2011; Pimentel et al. 2006, 2011, 2014]. The identically designed, randomized, placebo-controlled, phase III TARGET 1 and 2 studies examined the safety and efficacy of rifaximin 550 mg 3 times daily for 2 weeks in patients with IBS-D [Pimentel et al. 2011]. The primary efficacy endpoint (i.e. percentage of patients with adequate relief of global IBS symptoms for ⩾2 of the first 4 weeks after the 2-week treatment ended) was achieved by 40.7% and 31.7% of patients receiving rifaximin or placebo, respectively (p < 0.001). Further, a significantly greater percentage of patients receiving rifaximin had adequate relief of IBS-related bloating during the first 4 weeks after the 2-week treatment phase compared with placebo (40.2% versus 30.3%, respectively; p < 0.001). The results of these studies support that rifaximin has a sustained effect in patients with IBS-D, with a 2-week treatment course providing patients with IBS-D benefit for at least 3 months. In addition, three meta-analyses of five randomized, controlled trials of rifaximin for IBS (any subtype) demonstrated significant improvement in overall IBS symptoms with rifaximin compared with placebo (NNT 9–10.6) [Ford et al. 2014a; Menees et al. 2012; Shah et al. 2012]. The safety of rifaximin was favorable and generally comparable with that of placebo; no Clostridium difficile–associated diarrhea was reported in the TARGET 1 and 2 studies. Regarding its safety profile, meta-analyses of rifaximin trials showed no difference in the overall incidence of AEs compared with placebo [Ford et al. 2014a; Menees et al. 2012; Shah et al. 2012]. Furthermore, data indicated that 846 patients would benefit from rifaximin treatment for every 1 patient harmed (number needed to harm 8971; NNT 10.6) [Shah et al. 2012].
Table 3.
Randomized, controlled studies of rifaximin in patients with IBS.
| Study and design | Patients | Treatment | Primary efficacy outcome(s) | Secondary efficacy outcome(s) | Safety |
|---|---|---|---|---|---|
| Rifaximin 550 mg | |||||
| R, DB, PBO-C [Pimentel et al. 2011] | IBS (Rome II criteria) with abdominal pain and discomfort | Rifaximin 550 mg tid (n = 624) versus PBO (n = 634)a for 2 weeks | Adequate reliefb of global IBS symptoms: rifaximin versus PBO: 40.7% versus 31.7% (p < 0.001) | Adequate reliefb of IBS-related bloating: rifaximin versus PBO, 40.2% versus 30.3%, p < 0.001 | AEs comparable between groups Rifaximin versus PBO: Headache: 6.1% versus 6.6% Upper respiratory tract infection: 5.6% versus 6.2% Abdominal pain: 4.6% versus 5.5% |
| R, DB, PBO-C [Pimentel et al. 2014] | IBS-C (Rome II criteria) <3 CSBMs/week and breath methane >3 ppm |
Rifaximin 550 mg tid (n = 16) or PBO (n = 16), both in combination with neomycin 500 mg bid for 2 weeks | Constipation severity 1 week post-tx: rifaximin versus PBO: 28.6 versus 61.2 (p = 0.002) | Baseline to 4 weeks post-tx (rifaximin versus PBO): Constipation severity(p = 0.0007); bloating (p = 0.02); straining (p = 0.02); abdominal pain(p = 0.5) 4 weeks post-tx: Methane ⩽3 ppm: Rifaximin (67%) PBO (69%) |
Rifaximin versus PBO: Nausea: 47% versus 63% Bloating and distension: 47% versus 56% Abdominal pain: 20% versus 38% Constipation: 13% versus 13% Diarrhea: 1% versus 13% Urgency: 0% versus 13% |
| Rifaximin 400 mg | |||||
| R, DB, PBO-C [Pimentel et al. 2006] | IBS (Rome I criteria) | Rifaximin 400 mg tid (n = 43) versus PBO (n = 44) for 10 days | Global improvement in symptoms favored tx with rifaximin versus PBO after 10 weeks (36.4% versus 21%, p = 0.02) | Bloating significantly improved with rifaximin versus PBO after 10 weeks (p = 0.01) No significant improvement in VAS scores for abdominal pain (p = 0.3), diarrhea (p = 0.7), and constipation (p = 0.07) |
Incidence of AEs comparable between groups Most common AEs: abdominal pain, diarrhea, and bad taste in mouth |
| R, DB, PBO-C, CO [Di Stefano et al. 2011] | IBS-C (Rome III criteria) with moderate-to-severe bloating | Rifaximin 400 mg bid versus PBO (n = 24) for 7 days; washout of 4 weeks |
Symptom severity significantly decreased from baseline with rifaximin, but not PBO: Bloating (p = 0.002); abdominal distention (p = 0.03); abdominal pain (p = 0.002); flatulence (p = 0.004); borborygmi (p = 0.008); nausea (NS) Cumulative breath hydrogen excretion significantly decreased from baseline with rifaximin (p < 0.005), but not PBO (NS) |
Not reported | |
Patients included in modified intention-to-treat analysis.
Defined as relief of symptoms for ⩾2 of first 4 weeks of treatment by self-report.
AE, adverse event; bid, twice daily; CO, crossover; DB, double-blind; CSBM, complete and spontaneous bowel movement; IBS, irritable bowel syndrome; IBS-C, constipation-predominant irritable bowel syndrome; NS, not significant; PBO, placebo; PBO-C, placebo-controlled; ppm, parts per million; R, randomized; tid, 3 times daily; tx, treatment; VAS, visual analogue scale.
An additional phase III study (TARGET 3) indicated that repeat treatment (up to three 2-week cycles of rifaximin 550 mg three times daily) with rifaximin in patients with IBS-D was significantly more efficacious than placebo in improving IBS symptoms (both abdominal pain and stool consistency) and treatment was well tolerated in patients with IBS-D [Lembo et al. 2014]. Rifaximin was not associated with clinically meaningful adverse effects on pathogen emergence or bacterial susceptibility to common antibiotic classes and no sustained disturbance of the overall gut (stool) microbiota was observed. Although not indicated for IBS-C, rifaximin has also been examined in patients with IBS-C, specifically in combination with neomycin [Pimentel et al. 2014]. Rifaximin plus neomycin significantly improved severity of constipation, and symptoms of bloating, straining, and abdominal pain for up to 4 weeks following 2-week treatment, compared with placebo [Pimentel et al. 2014].
Bloating and flatulence in patients with IBS may, in part, be due to bacterial overgrowth, may be diagnosed by breath testing, and is a distinct medical condition from IBS [Saadi and McCallum, 2013; Shah et al. 2013]. Antibiotics (e.g. ciprofloxacin, metronidazole) are generally used to treat small intestinal bacterial overgrowth (SIBO), which is thought to affect up to half of patients with IBS [Saadi and McCallum, 2013; Shah et al. 2013]. In the rifaximin TARGET 1, 2, and 3 studies, SIBO was not tested as part of the inclusion criteria, and also not included in the diagnosis of IBS. Rifaximin, with its favorable safety profile and demonstrated efficacy, appears to be a viable therapeutic option for patients with IBS with or without comorbid SIBO [Scarpellini et al. 2007].
Additional agents for treatment of IBS
The neurokinin-2 receptor antagonist ibodutant is currently in clinical development; however, data from randomized, controlled studies in patients with IBS have not yet been published [Trinkley and Nahata, 2014]. Plecanatide, an analog of the natural peptide uroguanylin, which regulates digestive activity, is currently in phase III trials in patients with IBS-C. Data from a phase II, randomized, controlled trial in patients with IBS-C (n = 424) indicated that various doses of plecanatide (including 1.0, 3.0, and 9.0 mg) provided significant improvement in the number of weekly CSBMs versus placebo (p ⩽ 0.05 for all comparisons) [Miner et al. 2014]. The most common AE with plecanatide was diarrhea [Miner et al. 2014]. Ramosetron, a selective serotonin receptor antagonist, is currently indicated for the treatment of men with IBS-D in Japan, although efficacy (i.e. improvement in global relief of IBS symptoms by patient report) was also demonstrated in women in one study [Matsueda et al. 2008a]. Efficacy was demonstrated in both men and women with IBS-D in a second, phase III study [Matsueda et al. 2008b]. In this study, overall response (i.e. global relief of IBS symptoms by patient report) was significantly greater with ramosetron compared with placebo after 1, 2, 3, and 4 months of treatment (p < 0.001) [Matsueda et al. 2008b], However, when stratified by sex, women reported significant relief of IBS symptoms at month 2 only compared with placebo, whereas men reported significant relief of IBS symptoms versus placebo at all time points. Further, the incidence of AEs, including abdominal distension, constipation, hard stool, and decreased white blood cell count, was ⩾3% higher for women than men. Thus, there are apparent differences in response in men versus women, but the reasons for these differences are currently unknown. Another treatment being examined for patients with IBS-C is chenodeoxycholic acid (CDC), a bile acid traditionally used for gallstone dissolution [Rao et al. 2010]. In a double-blind RCT, patients with IBS-C received placebo, CDC 500 mg, or CDC 1000 mg for 4 days [Rao et al. 2010]. CDC significantly accelerated overall colonic transit within 24 hours compared with placebo (p = 0.005) [Rao et al. 2010]. In addition, among females, CDC significantly improved stool consistency (p = 0.003), increased stool frequency (p = 0.18), and improved ease of passage (p = 0.24) versus placebo [Rao et al. 2010]. Lower abdominal cramping/pain was the most common AE with CDC and was significantly more prevalent in the CDC groups (42% to 45%) than in the placebo group (0%; p = 0.01). Diarrhea (17%-18% with CDC versus 0% with placebo) and nausea (9% to 25% versus 0%) were also common with CDC and were more prevalent with CDC compared with placebo [Rao et al. 2010]. Herbal preparations, such as STW 5 (Iberogast®; Bayer Corporation, Morrisville, NC) may improve IBS symptoms [Madisch et al. 2004]; however, clinical data are limited. There is also preliminary evidence that ketotifen, a mast cell stabilizer used in the treatment of asthma, may improve IBS symptoms [Klooker et al. 2010], but additional adequately powered studies are needed. Agents that have shown efficacy in other GI conditions [e.g. chronic constipation (elobixibat, or A3309 [Chey et al. 2011]), inflammatory bowel disease (AZD9056 [Eser et al. 2015] and 5-aminosalicylic acid [Feagan and Macdonald, 2012; Ford et al. 2011])] may also be beneficial for patients with IBS, but further research is needed.
Conclusions
IBS is a common condition managed primarily in an outpatient setting. Although patients with moderate-to-severe IBS seek medical care with greater frequency compared with patients with mild IBS, it is important to improve the health and overall well-being of all patients with IBS. Treatment should therefore include identification and management of psychologic comorbidities, such as anxiety and depression, as appropriate. The pathophysiology of IBS is unclear, but is thought to include genetic, immunologic, microbial, physiologic stress response, and psychosocial components. Management of patients with IBS includes lifestyle changes, dietary modification, use of psychotropic medications, psychological therapies, and over-the-counter agents targeting GI motility. Newer therapies targeting GI motility (i.e. linaclotide for IBS-C), GI opioid receptors (i.e. eluxadoline for IBS-D), and gut microbiota (i.e. rifaximin for IBS-D) have demonstrated efficacy and safety in patients with IBS. Further, additional therapies currently in phase II and III of development appear to show promise for the treatment of patients with IBS.
Acknowledgments
Technical editorial and medical writing assistance was provided under the direction of A.E.F.-O. by Sophie Bolick, PhD, Synchrony Medical Communications, LLC, West Chester, PA. Funding for this support was provided by Salix, a Division of Valeant Pharmaceuticals North America LLC, Bridgewater, NJ, USA.
Footnotes
Funding: This work was supported by Salix.
Conflict of interest statement: A.E.F.-O. has served on the speakers’ bureau for Salix, and has received payments for lectures.
References
- Afari N., Ahumada S., Wright L., Mostoufi S., Golnari G., Reis V., et al. (2014) Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med 76: 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J., Lee K., Choi C., Kim J., Lee H., Kim J., et al. (2014) Colonic mucosal immune activity in irritable bowel syndrome: comparison with healthy controls and patients with ulcerative colitis. Dig Dis Sci 59: 1001–1011. [DOI] [PubMed] [Google Scholar]
- American College of Gastroenterology Task Force on IBS (2009) An evidence-based systematic review on the management of irritable bowel syndrome. Am J Gastroenterol 104: S1–S35. [DOI] [PubMed] [Google Scholar]
- Bagnol D., Mansour A., Akil H., Watson S.J. (1997) Cellular localization and distribution of the cloned mu and kappa opioid receptors in rat gastrointestinal tract. Neuroscience 81: 579–591. [DOI] [PubMed] [Google Scholar]
- Bajaj J., Heuman D., Sanyal A., Hylemon P., Sterling R., Stravitz R., et al. (2013) Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One 8: e60042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara G., Cremon C., Carini G., Bellacosa L., Zecchi L., De Giorgio R., et al. (2011) The immune system in irritable bowel syndrome.J Neurogastroenterol Motil 17: 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara G., Stanghellini V., De Giorgio R., Corinaldesi R. (2006) Functional gastrointestinal disorders and mast cells: implications for therapy. Neurogastroenterol Motil 18: 6–17. [DOI] [PubMed] [Google Scholar]
- Bardhan K., Bodemar G., Geldof H., Schütz E., Heath A., Mills J., et al. (2000) A double-blind, randomized, placebo-controlled dose-ranging study to evaluate the efficacy of alosetron in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 14: 23–34. [DOI] [PubMed] [Google Scholar]
- Bashashati M., Rezaei N., Shafieyoun A., McKernan D., Chang L., Ohman L., et al. (2014) Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil 26: 1036–1048. [DOI] [PubMed] [Google Scholar]
- Bennett A., Whitney B. (1966) A pharmacological study of the motility of the human gastrointestinal tract. Gut 7: 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhn L., Störsrud S., Liljebo T., Collin L., Lindfors P., Tornblom H., et al. (2015) Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology 149: 1399–1407. [DOI] [PubMed] [Google Scholar]
- Bradford K., Shih W., Videlock E., Presson A., Naliboff B., Mayer E., et al. (2012) Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol 10: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E., Xue Q., Jiang Z., Xu Y., DuPont H. (2010) Pretreatment of epithelial cells with rifaximin alters bacterial attachment and internalization profiles. Antimicrob Agents Chemother 54: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummond N., Locke G., III, Choung R., Chang J., Schleck C., Zinsmeister A., et al. (2015) Effects of birth cohorts on the irritable bowel syndrome support early-life risk factors. Dig Dis Sci 60: 2112–2118. [DOI] [PubMed] [Google Scholar]
- Camilleri M. (2012) Peripheral mechanisms in irritable bowel syndrome. N Engl J Med 367:1626–1635. [DOI] [PubMed] [Google Scholar]
- Camilleri M., Klee E., Shin A., Carlson P., Li Y., Grover M., et al. (2014) Irritable bowel syndrome-diarrhea: characterization of genotype by exome sequencing, and phenotypes of bile acid synthesis and colonic transit. Am J Physiol Gastrointest Liver Physiol 306: G13–G26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M., Mayer E., Drossman D., Heath A., Dukes G., McSorley D., et al. (1999) Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther 13: 1149–1159. [DOI] [PubMed] [Google Scholar]
- Camilleri M., McKinzie S., Busciglio I., Low P., Sweetser S., Burton D., et al. (2008) Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 6: 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavan C., West J., Card T. (2014) Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther 40: 1023–1034. [DOI] [PubMed] [Google Scholar]
- Carroll I., Ringel-Kulka T., Keku T., Chang Y., Packey C., Sartor R., et al. (2011) Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 301: G799–G807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J., Harrington A., Hughes P., Martin C., Ge P., Shea C., et al. (2013) Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3’,5’-monophosphate. Gastroenterology 145:1334–1346. [DOI] [PubMed] [Google Scholar]
- Chadwick V., Chen W., Shu D., Paulus B., Bethwaite P., Tie A., et al. (2002) Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 122: 1778–1783. [DOI] [PubMed] [Google Scholar]
- Chang L., Ameen V., Dukes G., McSorley D., Carter E., Mayer E. (2005) A dose-ranging, phase II study of the efficacy and safety of alosetron in men with diarrhea-predominant IBS. Am J Gastroenterol 100: 115–123. [DOI] [PubMed] [Google Scholar]
- Chang L., Lembo A., Sultan S. (2014) American Gastroenterological Association technical review on the pharmacological management of irritable bowel syndrome. Gastroenterology 147: 1149–1172. [DOI] [PubMed] [Google Scholar]
- Chang L., Sundaresh S., Elliott J., Anton P., Baldi P., Licudine A., et al. (2009) Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil 21: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Tong K., Ameen V. (2010) Ischemic colitis and complications of constipation associated with the use of alosetron under a risk management plan: clinical characteristics, outcomes, and incidences. Am J Gastroenterol 105: 866–875. [DOI] [PubMed] [Google Scholar]
- Cheng J., Shah Y., Ma X., Pang X., Tanaka T., Kodama T., et al. (2010) Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation. J Pharmacol Exp Ther 335: 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey W., Camilleri M., Chang L., Rikner L., Graffner H. (2011) A randomized placebo-controlled phase IIb trial of a3309, a bile acid transporter inhibitor, for chronic idiopathic constipation. Am J Gastroenterol 106: 1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey W., Lembo A., Lavins B., Shiff S., Kurtz C., Currie M., et al. (2012) Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol 107: 1702–1712. [DOI] [PubMed] [Google Scholar]
- Chitkara D., van Tilburg M., Blois-Martin N., Whitehead W. (2008) Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol 103: 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choung R., Locke G., III, Zinsmeister A., Schleck C., Talley N. (2009) Psychosocial distress and somatic symptoms in community subjects with irritable bowel syndrome: a psychological component is the rule. Am J Gastroenterol 104: 1772–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A., Montejo A. (2006) Major depressive disorder, antidepressants, and sexual dysfunction.J Clin Psychiatry 67(Suppl. 6): 33–37. [PubMed] [Google Scholar]
- Coates M., Mahoney C., Linden D., Sampson J., Chen J., Blaszyk H., et al. (2004) Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 126: 1657–1664. [DOI] [PubMed] [Google Scholar]
- Codling C., O’Mahony L., Shanahan F., Quigley E., Marchesi J. (2010) A molecular analysis of fecal and mucosal bacterial communities in irritable bowel syndrome. Dig Dis Sci 55: 392–397. [DOI] [PubMed] [Google Scholar]
- Coeffier M., Gloro R., Boukhettala N., Aziz M., Lecleire S., Vandaele N., et al. (2010) Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am J Gastroenterol 105: 1181–1188. [DOI] [PubMed] [Google Scholar]
- Debbia E., Maioli E., Roveta S., Marchese A. (2008) Effects of rifaximin on bacterial virulence mechanisms at supra- and sub-inhibitory concentrations. J Chemother 20: 186–194. [DOI] [PubMed] [Google Scholar]
- Delvaux M., Beck A., Jacob J., Bouzamondo H., Weber F., Frexinos J. (2004) Effect of asimadoline, a kappa opioid agonist, on pain induced by colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther 20: 237–246. [DOI] [PubMed] [Google Scholar]
- Delvaux M., Louvel D., Mamet J., Campos-Oriola R., Frexinos J. (1998) Effect of alosetron on responses to colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther 12: 849–855. [DOI] [PubMed] [Google Scholar]
- Di Stefano M., Tana P., Mengoli C., Miceli E., Pagani E., Corazza G. (2011) Colonic hypersensitivity is a major determinant of the efficacy of bloating treatment in constipation-predominant irritable bowel syndrome. Intern Emerg Med 6: 403–411. [DOI] [PubMed] [Google Scholar]
- Didari T., Mozaffari S., Nikfar S., Abdollahi M. (2015) Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol 21: 3072–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossett M., Davis R., Lembo A., Yeh G. (2014) Complementary and alternative medicine use by US adults with gastrointestinal conditions: results from the 2012 National Health Interview Survey. Am J Gastroenterol 109: 1705–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove L., Lembo A., Randall C., Fogel R., Andrae D., Davenport J., et al. (2013) Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology 145: 329–338. [DOI] [PubMed] [Google Scholar]
- Drossman D., (2006) Rome III diagnostic criteria for functional gastrointestinal disorders. In: Rome III: The functional GI disorders. MacLean, VA: Degnon Associates, Inc., pp 885–897. [Google Scholar]
- Drossman D., Chang L., Bellamy N., Gallo-Torres H., Lembo A., Mearin F., et al. (2011) Severity in irritable bowel syndrome: a Rome Foundation Working Team report. Am J Gastroenterol 106: 1749–1759. [DOI] [PubMed] [Google Scholar]
- Drossman D., Chey W., Johanson J., Fass R., Scott C., Panas R., et al. (2009a) Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome–results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther 29: 329–341. [DOI] [PubMed] [Google Scholar]
- Drossman D., Morris C., Schneck S., Hu Y., Norton N., Norton W., et al. (2009b) International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J Clin Gastroenterol 43: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont H., Jiang Z. (2004) Influence of rifaximin treatment on the susceptibility of intestinal Gram-negative flora and Enterococci. Clin Microbiol Infect 10: 1009–1011. [DOI] [PubMed] [Google Scholar]
- Durbán A., Abellán J., Jiménez-Hernández N., Artacho A., Garrigues V., Ortiz V., et al. (2013) Instability of the faecal microbiota in diarrhoea-predominant irritable bowel syndrome. FEMS Microbiol Ecol 86: 581–589. [DOI] [PubMed] [Google Scholar]
- Durbán A., Abellán J., Jimenéz-Hernández N., Salgado P., Ponce M., Ponce J., et al. (2012) Structural alterations of faecal and mucosa-associated bacterial communities in irritable bowel syndrome. Environ Microbiol Rep 4: 242–247. [DOI] [PubMed] [Google Scholar]
- Ek W., Reznichenko A., Ripke S., Niesler B., Zucchelli M., Rivera N., et al. (2015) Exploring the genetics of irritable bowel syndrome: a GWA study in the general population and replication in multinational case-control cohorts [published online ahead of print September 23, 2014]. Gut 64: 1774–1782. [DOI] [PubMed] [Google Scholar]
- Eser A., Colombel J., Rutgeerts P., Vermeire S., Vogelsang H., Braddock M., et al. (2015) Safety and efficacy of an oral inhibitor of the purinergic receptor P2X7 in adult patients with moderately to severely active Crohn’s disease: a randomized placebo-controlled, double-blind, phase IIa study. Inflamm Bowel Dis 21: 2247–2253. [DOI] [PubMed] [Google Scholar]
- Feagan B., Macdonald J. (2012) Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 10: CD000543. [DOI] [PubMed] [Google Scholar]
- Fond G., Loundou A., Hamdani N., Boukouaci W., Dargel A., Oliveira J., et al. (2014) Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 264: 651–660. [DOI] [PubMed] [Google Scholar]
- Ford A., Achkar J., Khan K., Kane S., Talley N., Marshall J., et al. (2011) Efficacy of 5-aminosalicylates in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol 106: 601–616. [DOI] [PubMed] [Google Scholar]
- Ford A., Moayyedi P., Lacy B., Lembo A., Saito Y., Schiller L., et al. ; Task Force on the Management of Functional Bowel Disorders. (2014a) American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol 109: S2–S26. [DOI] [PubMed] [Google Scholar]
- Ford A., Quigley E., Lacy B., Lembo A., Saito Y., Schiller L., et al. (2014b) Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol 109: 1350–1365. [DOI] [PubMed] [Google Scholar]
- Forest Laboratories, Inc. (2014) Linzess (linaclotide) capsules, for oral use [package insert]. St. Louis, MO: Forest Laboratories, Inc. [Google Scholar]
- Fukudo S., Kaneko H., Akiho H., Inamori M., Endo Y., Okumura T., et al. (2015) Evidence-based clinical practice guidelines for irritable bowel syndrome. J Gastroenterol 50: 11–30. [DOI] [PubMed] [Google Scholar]
- Garsed K., Chernova J., Hastings M., Lam C., Marciani L., Singh G., et al. (2014) A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut 63: 1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon M. (1999) Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther 13(Suppl. 2): 15–30. [PubMed] [Google Scholar]
- Gershon M. (2004) Review article: serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther 20(Suppl. 7): 3–14. [DOI] [PubMed] [Google Scholar]
- Gershon M., Tack J. (2007) The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132: 397–414. [DOI] [PubMed] [Google Scholar]
- Gibson P., Varney J., Malakar S., Muir J. (2015) Food components and irritable bowel syndrome. Gastroenterology 148: 1158–1174. [DOI] [PubMed] [Google Scholar]
- Goebel-Stengel M., Stengel A., Schmidtmann M., Voort I., Kobelt P., Monnikes H. (2014) Unclear abdominal discomfort: pivotal role of carbohydrate malabsorption. J Neurogastroenterol Motil 20: 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasberger H., Chang L., Shih W., Presson A., Sayuk G., Newberry R., et al. (2013) Identification of a functional TPH1 polymorphism associated with irritable bowel syndrome bowel habit subtypes. Am J Gastroenterol 108: 1766–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte M., Santos J., de Torres I, Alonso C., Vicario M., Ramos L., et al. (2007) Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut 56: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulewitsch M., Enck P., Hautzinger M., Schlarb A. (2011) Irritable bowel syndrome symptoms among German students: prevalence, characteristics, and associations to somatic complaints, sleep, quality of life, and childhood abdominal pain. Eur J Gastroenterol Hepatol 23: 311–316. [DOI] [PubMed] [Google Scholar]
- Halland M., Almazar A., Lee R., Atkinson E., Larson J., Talley N., et al. (2014) A case-control study of childhood trauma in the development of irritable bowel syndrome. Neurogastroenterol Motil 26: 990–998. [DOI] [PubMed] [Google Scholar]
- Hoang M., Defina L., Willis B., Leonard D., Weiner M., Brown E. (2011) Association between low serum 25-hydroxyvitamin D and depression in a large sample of healthy adults: the Cooper Center longitudinal study. Mayo Clin Proc 86: 1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday E., Attia J., Hancock S., Koloski N., McEvoy M., Peel R., et al. (2014) Genome-wide association study identifies two novel genomic regions in irritable bowel syndrome. Am J Gastroenterol 109: 770–772. [DOI] [PubMed] [Google Scholar]
- Holzer P. (2009) Opioid receptors in the gastrointestinal tract. Regul Pept 155: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins K., Mushtaq S., Richardson J., Doumith M., de Pinna E., Cheasty T., et al. (2014) In vitro activity of rifaximin against clinical isolates of Escherichia coli and other enteropathogenic bacteria isolated from travellers returning to the UK. Int J Antimicrob Agents 45: 431–437. [DOI] [PubMed] [Google Scholar]
- Hughes P., Castro J., Harrington A., Isaacs N., Moretta M., Hicks G., et al. (2014a) Increased kappa-opioid receptor expression and function during chronic visceral hypersensitivity. Gut 63: 1199–1200. [DOI] [PubMed] [Google Scholar]
- Hughes P., Moretta M., Lim A., Grasby D., Bird D., Brierley S., et al. (2014b) Immune derived opioidergic inhibition of viscerosensory afferents is decreased in irritable bowel syndrome patients. Brain Behav Immun 42: 191–203. [DOI] [PubMed] [Google Scholar]
- Hungin A., Molloy-Bland M., Claes R., Heidelbaugh J., Cayley W., Jr, Muris J., et al. (2014) Systematic review: the perceptions, diagnosis and management of irritable bowel syndrome in primary care - a Rome Foundation working team report. Aliment Pharmacol Ther 40: 1133–1145. [DOI] [PubMed] [Google Scholar]
- Hungin A., Chang L., Locke G., Dennis E., Barghout V. (2005) Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther 21: 1365–1375. [DOI] [PubMed] [Google Scholar]
- Jeffery I., O’Toole P., Ohman L., Claesson M., Deane J., Quigley E., et al. (2012) An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 61: 997–1006. [DOI] [PubMed] [Google Scholar]
- Jiang Z., DuPont H., La Rocco M., Garey K. (2010a) In vitro susceptibility of Clostridium difficile to rifaximin and rifampin in 359 consecutive isolates at a university hospital in Houston, Texas. J Clin Pathol 63: 355–358. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Ke S., DuPont H. (2010b) Rifaximin-induced alteration of virulence of diarrhoea-producing Escherichia coli and Shigella sonnei. Int J Antimicrob Agents 35: 278–281. [DOI] [PubMed] [Google Scholar]
- Johannesson E., Simren M., Strid H., Bajor A., Sadik R. (2011) Physical activity improves symptoms in irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol 106: 915–922. [DOI] [PubMed] [Google Scholar]
- Kang S., Choi S., Lee S., Chung W., Lee H., Chung K., et al. (2011) The effects of lifestyle modification on symptoms and quality of life in patients with irritable bowel syndrome: a prospective observational study. Gut Liver 5: 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaahmet F., Basar O., Yuksel I., Coban S., Yuksel O. (2013) Letter: vitamin D supplementation and the irritable bowel syndrome. Aliment Pharmacol Ther 37: 499. [DOI] [PubMed] [Google Scholar]
- Khan M., Nusrat S., Khan M., Nawras A., Bielefeldt K. (2015) Low-FODMAP diet for irritable bowel syndrome: is it ready for prime time? Dig Dis Sci 60: 1169–1177. [DOI] [PubMed] [Google Scholar]
- Khanna R., Macdonald J., Levesque B. (2014) Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis.J Clin Gastroenterol 48: 505–512. [DOI] [PubMed] [Google Scholar]
- Klooker T., Braak B., Koopman K., Welting O., Wouters M., van der Heide S., et al. (2010) The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 59: 1213–1221. [DOI] [PubMed] [Google Scholar]
- Knight J., Locke G., III, Zinsmeister A., Schleck C., Talley N. (2015) Family history of mental illness or alcohol abuse and the irritable bowel syndrome.J Psychosom Res 78: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koloski N., Jones M., Kalantar J., Weltman M., Zaguirre J., Talley N. (2012) The brain–gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut 61: 1284–1290. [DOI] [PubMed] [Google Scholar]
- Ladabaum U., Boyd E., Zhao W., Mannalithara A., Sharabidze A., Singh G., et al. (2012) Diagnosis, comorbidities, and management of irritable bowel syndrome in patients in a large health maintenance organization. Clin Gastroenterol Hepatol 10: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamki L., Sullivan S. (1983) A study of gastrointestinal opiate receptors: the role of the Mu receptor on gastric emptying: concise communication. J Nucl Med 24: 689–692. [PubMed] [Google Scholar]
- Larsson M., Tillisch K., Craig A., Engstrom M., Labus J., Naliboff B., et al. (2012) Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology 142: 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layer P., Stanghellini V. (2014) Review article: linaclotide for the management of irritable bowel syndrome with constipation. Aliment Pharmacol Ther 39: 371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo A., Pimentel M., Rao S., Schoenfeld P., Cash B., Weinstock L.et al. (2014) Efficacy and safety of repeat treatment with rifaximin for diarrhea-predominant irritable bowel syndrome (IBS-D): results of the TARGET 3 study. Presented at: American College of Gastroenterology (ACG) 2014 Annual Scientific Meeting, Philadelphia, PA, 17–22 October 2014. [Google Scholar]
- Levy R., Jones K., Whitehead W., Feld S., Talley N., Corey L. (2001) Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology 121: 799–804. [DOI] [PubMed] [Google Scholar]
- Lin S., Mooney P., Kurien M., Aziz I., Leeds J., Sanders D. (2014) Prevalence, investigational pathways and diagnostic outcomes in differing irritable bowel syndrome subtypes. Eur J Gastroenterol Hepatol 26: 1176–1180. [DOI] [PubMed] [Google Scholar]
- Locke G., III, Zinsmeister A., Talley N., Fett S., Melton L., III. (2000) Familial association in adults with functional gastrointestinal disorders. Mayo Clin Proc 75: 907–912. [DOI] [PubMed] [Google Scholar]
- Longstreth G., Thompson W., Chey W., Houghton L., Mearin F., Spiller R. (2006) Functional bowel disorders. Gastroenterology 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- Lovell R., Ford A. (2012) Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol 107: 991–1000. [DOI] [PubMed] [Google Scholar]
- Maccaferri S., Vitali B., Klinder A., Kolida S., Ndagijimana M., Laghi L., et al. (2010) Rifaximin modulates the colonic microbiota of patients with Crohn’s disease: an in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother 65: 2556–2565. [DOI] [PubMed] [Google Scholar]
- Macdougall J., Johnston J., Lavins B., Nelson L., Williams V., Carson R., et al. (2013) An evaluation of the FDA responder endpoint for IBS-C clinical trials: analysis of data from linaclotide Phase 3 clinical trials. Neurogastroenterol Motil 25: 481–486. [DOI] [PubMed] [Google Scholar]
- Madisch A., Holtmann G., Plein K., Hotz J. (2004) Treatment of irritable bowel syndrome with herbal preparations: results of a double-blind, randomized, placebo-controlled, multi-centre trial. Aliment Pharmacol Ther 19: 271–279. [DOI] [PubMed] [Google Scholar]
- Mangel A., Bornstein J., Hamm L., Buda J., Wang J., Irish W., et al. (2008) Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther 28: 239–249. [DOI] [PubMed] [Google Scholar]
- Mangel A., Hicks G. (2012) Asimadoline and its potential for the treatment of diarrhea-predominant irritable bowel syndrome: a review. Clin Exp Gastroenterol 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez C., Vicario M., Ramos L., Lobo B., Mosquera J., Alonso C., et al. (2012) The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol 107: 736–746. [DOI] [PubMed] [Google Scholar]
- Matsueda K., Harasawa S., Hongo M., Hiwatashi N., Sasaki D. (2008a) A phase II trial of the novel serotonin type 3 receptor antagonist ramosetron in Japanese male and female patients with diarrhea-predominant irritable bowel syndrome. Digestion 77: 225–235. [DOI] [PubMed] [Google Scholar]
- Matsueda K., Harasawa S., Hongo M., Hiwatashi N., Sasaki D. (2008b) A randomized, double-blind, placebo-controlled clinical trial of the effectiveness of the novel serotonin type 3 receptor antagonist ramosetron in both male and female Japanese patients with diarrhea-predominant irritable bowel syndrome. Scand J Gastroenterol 43: 1202–1211. [DOI] [PubMed] [Google Scholar]
- Melchior C., Gourcerol G., Déchelotte P., Leroi A., Ducrotté P. (2014) Symptomatic fructose malabsorption in irritable bowel syndrome: A prospective study. United Eur Gastroenterol J 2: 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencarelli A., Migliorati M., Barbanti M., Cipriani S., Palladino G., Distrutti E., et al. (2010) Pregnane-X-receptor mediates the anti-inflammatory activities of rifaximin on detoxification pathways in intestinal epithelial cells. Biochem Pharmacol 80: 1700–1707. [DOI] [PubMed] [Google Scholar]
- Mencarelli A., Renga B., Palladino G., Claudio D., Ricci P., Distrutti E., et al. (2011) Inhibition of NF-kB by a PXR-dependent pathway mediates counter-regulatory activities of rifaximin on innate immunity in intestinal epithelial cells. Eur J Pharmacol 668: 317–324. [DOI] [PubMed] [Google Scholar]
- Menees S., Maneerattannaporn M., Kim H., Chey W. (2012) The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol 107: 28–35. [DOI] [PubMed] [Google Scholar]
- Miller V., Hopkins L., Whorwell P. (2004) Suicidal ideation in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2: 1064–1068. [DOI] [PubMed] [Google Scholar]
- Miner P., DeLuca R., La Portilla M., Padila E., Koltun W., Wiltz O., et al. (2014) 1831. Plecanatide, a novel uroguanylin analog: a 12-week, randomized, double-blind, placebo-controlled, dose-ranging trail to evaluate efficacy and safety in patients with irritable bowel syndrome with constipation (IBS-C). Am J Gastroenterol: S541–S541. [Google Scholar]
- Mitchell C., Drossman D. (1987) Survey of the AGA membership relating to patients with functional gastrointestinal disorders. Gastroenterology 92: 1282–1284. [DOI] [PubMed] [Google Scholar]
- Muir J., Gibson P. (2013) The low FODMAP diet for treatment of irritable bowel syndrome and other gastrointestinal disorders. Gastroenterol Hepatol (N Y ) 9: 450–452. [PMC free article] [PubMed] [Google Scholar]
- Patheon Pharmaceuticals, Inc. (2015) Viberzi (eluxadoline) tablets, for oral use, CIV [package insert]. Cincinnati, OH: Patheon Pharmaceuticals, Inc. [Google Scholar]
- Patcharatrakul T., Gonlachanvit S. (2011) Outcome of biofeedback therapy in dyssynergic defecation patients with and without irritable bowel syndrome. J Clin Gastroenterol 45: 593–598. [DOI] [PubMed] [Google Scholar]
- Patel P., Bercik P., Morgan D., Bolino C., Pintos-Sanchez M., Moayyedi P., et al. (2015) Irritable bowel syndrome is significantly associated with somatisation in 840 patients, which May drive bloating. Aliment Pharmacol Ther 41: 449–458. [DOI] [PubMed] [Google Scholar]
- Piche T., Barbara G., Aubert P., Bruley des Varannes S., Dainese R., Nano J., et al. (2009) Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 58: 196–201. [DOI] [PubMed] [Google Scholar]
- Pimentel M., Chang C., Chua K., Mirocha J., DiBaise J., Rao S., et al. (2014) Antibiotic treatment of constipation-predominant irritable bowel syndrome. Dig Dis Sci 59: 1278–1285. [DOI] [PubMed] [Google Scholar]
- Pimentel M., Lembo A., Chey W., Zakko S., Ringel Y., Yu J., et al. ; TARGET Study Group. (2011) Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 364: 22–32. [DOI] [PubMed] [Google Scholar]
- Pimentel M., Park S., Mirocha J., Kane S., Kong Y. (2006) The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med 145: 557–563. [DOI] [PubMed] [Google Scholar]
- Posserud I., Syrous A., Lindstrom L., Tack J., Abrahamsson H., Simren M. (2007) Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology 133: 1113–1123. [DOI] [PubMed] [Google Scholar]
- Prometheus Laboratories, Inc. (2014) Lotronex® (alosetron hydrochloride) tablets [package insert]. San Diego, CA: Prometheus Laboratories, Inc. [Google Scholar]
- Prott G., Shim L., Hansen R., Kellow J., Malcolm A. (2010) Relationships between pelvic floor symptoms and function in irritable bowel syndrome. Neurogastroenterol Motil 22: 764–769. [DOI] [PubMed] [Google Scholar]
- Quigley E., Tack J., Chey W., Rao S., Fortea J., Falques M., et al. (2013) Randomised clinical trials: linaclotide phase 3 studies in IBS-C - a prespecified further analysis based on European Medicines Agency-specified endpoints. Aliment Pharmacol Ther 37: 49–61. [DOI] [PubMed] [Google Scholar]
- Rana S., Sharma S., Sinha S., Parsad K., Malik A., Singh K. (2012) Pro-inflammatory and anti-inflammatory cytokine response in diarrhoea-predominant irritable bowel syndrome patients. Trop Gastroenterol 33: 251–256. [DOI] [PubMed] [Google Scholar]
- Rao A., Wong B., Camilleri M., Odunsi-Shiyanbade S., McKinzie S., Ryks M.et al. (2010) Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology 139: 1549–58, 1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Lembo A., Shiff S., Lavins B., Currie M., Jia X., et al. (2012) A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol 107: 1714–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Quigley E., Shiff S., Lavins B., Kurtz C., Macdougall J., et al. (2014) Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol 12: 616–623. [DOI] [PubMed] [Google Scholar]
- Rasmussen S., Jensen T., Henriksen S., Haastrup P., Larsen P., Sondergaard J., et al. (2015) Overlap of symptoms of gastroesophageal reflux disease, dyspepsia and irritable bowel syndrome in the general population. Scand J Gastroenterol 50: 162–169. [DOI] [PubMed] [Google Scholar]
- Rey de, Castro N., Miller V., Carruthers H., Whorwell P. (2015) Irritable bowel syndrome: a comparison of subtypes. J Gastroenterol Hepatol 30: 279–285. [DOI] [PubMed] [Google Scholar]
- Ringel Y., Ringel-Kulka T. (2011) The rationale and clinical effectiveness of probiotics in irritable bowel syndrome. J Clin Gastroenterol 45: S145–S148. [DOI] [PubMed] [Google Scholar]
- Ringel Y., Williams R., Kalilani L., Cook S. (2009) Prevalence, characteristics, and impact of bloating symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 7: 68–72. [DOI] [PubMed] [Google Scholar]
- Saadi M., McCallum R. (2013) Rifaximin in irritable bowel syndrome: rationale, evidence and clinical use. Ther Adv Chronic Dis 4: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Schoenfeld P., Locke G., III (2002) The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol 97: 1910–1915. [DOI] [PubMed] [Google Scholar]
- Salvioli B., Pellegatta G., Malacarne M., Pace F., Malesci A., Pagani M., et al. (2015) Autonomic nervous system dysregulation in irritable bowel syndrome. Neurogastroenterol Motil. [DOI] [PubMed] [Google Scholar]
- Scarpellini E., Gabrielli M., Lauritano C., Lupascu A., Merra G., Cammarota G., et al. (2007) High dosage rifaximin for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther 25: 781–786. [DOI] [PubMed] [Google Scholar]
- Schiller L., Johnson D. (2008) Balancing drug risk and benefit: toward refining the process of FDA decisions affecting patient care. Am J Gastroenterol 103: 815–819. [DOI] [PubMed] [Google Scholar]
- Schmulson M., Lee O., Chang L., Naliboff B., Mayer E. (1999) Symptom differences in moderate to severe IBS patients based on predominant bowel habit. Am J Gastroenterol 94: 2929–2935. [DOI] [PubMed] [Google Scholar]
- Schrodt C., McHugh E., Gawinowicz M., DuPont H., Brown E. (2013) Rifaximin-mediated changes to the epithelial cell proteome: 2-D gel analysis. PLoS One 8: e68550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S., Wadhwa V., LeClair J., Mikami S., Park R., Jones M., et al. (2013) In-patient discharge rates for the irritable bowel syndrome - an analysis of national trends in the United States from 1997 to 2010. Aliment Pharmacol Ther 38: 1338–1346. [DOI] [PubMed] [Google Scholar]
- Shah E., Kim S., Chong K., Lembo A., Pimentel M. (2012) Evaluation of harm in the pharmacotherapy of irritable bowel syndrome. Am J Med 125: 381–393. [DOI] [PubMed] [Google Scholar]
- Shah S., Day L., Somsouk M., Sewell J. (2013) Meta-analysis: antibiotic therapy for small intestinal bacterial overgrowth. Aliment Pharmacol Ther 38: 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Agnihotri A., Pathak M., Shirazi A., Tiwari R., Sreenivas V., et al. (2012) Psychiatric, somatic and other functional gastrointestinal disorders in patients with irritable bowel syndrome at a tertiary care center. J Neurogastroenterol Motil 18: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprake E., Grant V., Corfe B. (2012) Vitamin D3 as a novel treatment for irritable bowel syndrome: single case leads to critical analysis of patient-centered data. BMJ Case Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su A., Shih W., Presson A., Chang L. (2014) Characterization of symptoms in irritable bowel syndrome with mixed bowel habit pattern. Neurogastroenterol Motil 26: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarka L., Camilleri M., Burton D., Fox J., McKinzie S., Stanislav T., et al. (2007) Efficacy of on-demand asimadoline, a peripheral kappa-opioid agonist, in females with irritable bowel syndrome. Clin Gastroenterol Hepatol 5: 1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Pharmaceuticals America, Inc. (2013) Amitiza (lubiprostone) capsules [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc. [Google Scholar]
- Talley N., Phillips S., Melton L., Mulvihill C., Wiltgen C., Zinsmeister A. (1990) Diagnostic value of the Manning criteria in irritable bowel syndrome. Gut 31: 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terc J., Hansen A., Alston L., Hirota S. (2014) Pregnane X receptor agonists enhance intestinal epithelial wound healing and repair of the intestinal barrier following the induction of experimental colitis. Eur J Pharm Sci 55: 12–19. [DOI] [PubMed] [Google Scholar]
- Tillisch K., Mayer E., Labus J. (2011) Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 140: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong K., Nicandro J., Shringarpure R., Chuang E., Chang L. (2013) A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Therap Adv Gastroenterol 6: 344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornblom H., Van O., Sadik R., Abrahamsson H., Tack J., Simren M. (2012) Colonic transit time and IBS symptoms: what’s the link? Am J Gastroenterol 107: 754–760. [DOI] [PubMed] [Google Scholar]
- Trinkley K., Nahata M. (2014) Medication management of irritable bowel syndrome. Digestion 89: 253–267. [DOI] [PubMed] [Google Scholar]
- Vazquez-Roque M., Camilleri M., Smyrk T., Murray J., Marietta E., O’Neill J., et al. (2013) A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 144: 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario M., Gonzalez-Castro A., Martinez C., Lobo B., Pigrau M., Guilarte M., et al. (2014) Increased humoral immunity in the jejunum of diarrhoea-predominant irritable bowel syndrome associated with clinical manifestations. Gut 64: 1379–1388. [DOI] [PubMed] [Google Scholar]
- Vivinus-Nébot M., Dainese R., Anty R., Saint-Paul M., Nano J., Gonthier N., et al. (2012) Combination of allergic factors can worsen diarrheic irritable bowel syndrome: role of barrier defects and mast cells. Am J Gastroenterol 107: 75–81. [DOI] [PubMed] [Google Scholar]
- Vivinus-Nébot M., Frin-Mathy G., Bzioueche H., Dainese R., Bernard G., Anty R., et al. (2014) Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut 63: 744–752. [DOI] [PubMed] [Google Scholar]
- Wade P., Chen J., Jaffe B., Kassem I., Blakely R., Gershon M. (1996) Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci 16: 2352–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade P., Palmer J., McKenney S., Kenigs V., Chevalier K., Moore B., et al. (2012) Modulation of gastrointestinal function by MuDelta, a mixed micro opioid receptor agonist/ micro opioid receptor antagonist. Br J Pharmacol 167: 1111–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M., Talley N., Prabhakar M., Pennaneac’h C., Aro P., Ronkainen J., et al. (2009) Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther 29: 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan K. (2014) Editorial: The importance of systematic reviews and meta-analyses of probiotics and prebiotics. Am J Gastroenterol 109: 1563–1565. [DOI] [PubMed] [Google Scholar]
- Wong R., Palsson O., Turner M., Levy R., Feld A., Von K., et al. (2010) Inability of the Rome III criteria to distinguish functional constipation from constipation-subtype irritable bowel syndrome. Am J Gastroenterol 105: 2228–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters M., Lambrechts D., Knapp M., Cleynen I., Whorwell P., Agreus L., et al. (2014) Genetic variants in CDC42 and NXPH1 as susceptibility factors for constipation and diarrhoea predominant irritable bowel syndrome. Gut 63: 1103–1111. [DOI] [PubMed] [Google Scholar]
- Xu D., Gao J., Gillilland M., III, Wu X., Song I., Kao J., et al. (2014) Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 146: 484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin K., Agrawal D. (2014) Vitamin D and inflammatory diseases. J Inflamm Res 7: 69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Rao S. (2014) Advances in the management of constipation-predominant irritable bowel syndrome: the role of linaclotide. Therap Adv Gastroenterol 7: 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]