Abstract
Nonalcoholic fatty liver disease (NAFLD) is emerging as a major public health problem because of its association with increased cardiovascular and liver-related morbidity and mortality. Both genetic factors and lifestyle contribute to the pathogenesis of NAFLD. Lifestyle, including dietary habits and physical activity, is a modifiable risk factor and thus represents the main target for the prevention and treatment of NAFLD. In this review, we summarize the evidence regarding nutritional aspects (i.e. total energy intake, saturated fat and carbohydrates intake, certain foods or drinks and dietary patterns as a whole) in the treatment of NAFLD. In addition, we analyze the evidence concerning the independent effect of physical activity, including aerobic and resistance training, in the treatment of NAFLD. A therapeutic algorithm according to results from intervention trials is also provided for clinicians and other healthcare professionals involved in the management of NAFLD.
Keywords: nonalcoholic fatty liver disease, diet, lifestyle, physical activity, coffee
Introduction
Nonalcoholic fatty liver disease (NAFLD) has been recognized as a major health burden. Estimates suggest that about 20–30% of adults in developed countries have excess fat accumulation in the liver [Propst et al. 1995; Bellentani et al. 2000; Falck-Ytter et al. 2001; Bedogni and Bellentani, 2004; Zelber-Sagi et al. 2006], 50% among people with diabetes and about 80% in obese and morbidly obese people [Bellentani et al. 2000; Del Gaudio et al. 2002; Gupte et al. 2004]. Data from the United States National Health and Nutrition Examination Surveys, collected between 1988 and 2008, show a twofold increased prevalence of NAFLD during this period, along with the increasing prevalence of metabolic alterations such as obesity and insulin resistance [Younossi et al. 2011]. Not only obesity but also weight gain is an important determinant in NAFLD incidence. A prospective study with 7 years of follow up emphasized that even a modest weight change of 3–5 kg is an independent predictor for the development and remission of NAFLD, regardless of baseline body mass index (BMI) [Zelber-Sagi et al. 2012]. The importance of modest weight gain, as low as 2 kg, in the development of NAFLD was also reported in two large Korean cohorts [Chang et al. 2009; Kim et al. 2009]. Indeed, it has been demonstrated that insulin resistance already develops during weight gain within the normal range of body weight [Erdmann et al. 2008] and that even modest weight gain results in increases in abdominal fat [Orr et al. 2008], which in turn cause free fatty acid (FFA) levels to increase in the portal and peripheral circulations [Ruderman et al. 1998]. In agreement with that, in a recent prospective cohort study, 13.5% of Hong Kong Chinese adults developed NAFLD within 3–5 years; this was associated with incident central obesity that developed in 31% of subjects with incident fatty liver and 6% of those without (p < 0.001) [Wong et al. 2015].
The major treatment offered for NAFLD remains lifestyle changes including weight reduction and prevention of weight gain, eating a healthy diet and performing regular physical activity. The literature testing these lifestyle components in animal studies, observational studies and clinical trials among NAFLD patients is reviewed here to provide a practical tool for clinicians treating NAFLD.
Role of energy restriction
Current management of NAFLD includes gradual weight reduction achieved by diet with or without increased physical activity, which leads to an improvement in serum liver enzymes, reduced hepatic fatty infiltration, reduced degree of hepatic inflammation and less consistently reduced fibrosis [Eriksson et al. 1986; Palmer and Schaffner, 1990; Andersen et al. 1991; Ueno et al. 1997; Luyckx et al. 1998; Dixon et al. 2004; Shah et al. 2009]. Uncontrolled or nonrandomized studies, which evaluated histological outcome, demonstrated a beneficial effect of a balanced diet with a gradual weight reduction that resulted in reduced hepatic steatosis, inflammation and nonalcoholic steatohepatitis (NASH) score [Ueno et al. 1997; Huang et al. 2005]. In a randomized controlled trial (RCT), 32 NASH patients were randomized to receive intensive 48-week lifestyle intervention or basic education about healthy lifestyle. The NAFLD activity score (NAS) improved significantly in the treatment arm. Participants who reduced weight by ⩾7% had significant improvements in steatosis, lobular inflammation, ballooning injury and NAS compared with those who reduced weight by <7% [Promrat et al. 2010]. In the Orlistat trial, a weight reduction of at least 9% was necessary to significantly improve NAS, although a 5% reduction was sufficient for improving steatosis [Harrison et al. 2009].
Another RCT tested the effect of a 12-month intensive lifestyle intervention on hepatic steatosis in patients with type 2 diabetes. The intervention included a moderate caloric restriction, increased physical activity and weekly meetings, whereas the control group received only general information on nutrition and physical activity. After 12 months, participants assigned to the intensive intervention lost more weight compared with the controls (–8.5% versus –0.05%; p < 0.01) and had a greater decline in steatosis (–50.8% versus –22.8%; p = 0.04), with a clear dose–response relationship between the level of weight loss and reduction of steatosis [Lazo et al. 2010].
A recent large RCT among 154 adults with NAFLD demonstrated that a 12-month lifestyle modification program (provided by a dietitian) led to 64% NAFLD remission rate in the intervention group versus 20% in the control group (p < 0.001) and a reduction in liver stiffness only in the intervention group [Wong et al. 2013]. Encouraging data on fibrosis regression following diet-induced weight reduction were recently published in a study from Cuba that included 261 NASH patients undergoing paired liver biopsies within 52 weeks. All patients who lost ⩾10% of their weight had reductions in NAS, 90% had resolution of NASH, and 45% had regression of fibrosis [Vilar-Gomez et al. 2015].
Three relatively large sample size studies that addressed the effect of diet on alanine aminotransferase (ALT) levels demonstrated improvement or normalization with weight loss as low as 5% from initial body weight [Suzuki et al. 2005; Oza et al. 2009; St George et al. 2009]. Importantly, a meta-analysis of 23 trials (6 randomized, 5 with repeated liver biopsy) concluded that lifestyle modifications including weight reduction and/or increased physical activity consistently reduced liver fat and improved liver histopathology [Thoma et al. 2012].
A low carbohydrate diet may seem more effective in reducing liver fat, but this is only in the short term. Obese insulin-resistant individuals randomized to 16-week hypocaloric diets containing either 60% carbohydrate/25% fat or 40% carbohydrate/45% fat had a greater decrease in ALT levels with the latter diet, despite equal weight loss [Ryan et al. 2007]. In a shorter term study, liver triglycerides decreased significantly more during 2 weeks of diet in those on a carbohydrate-restricted diet than in those on a calorie-restricted diet [Browning et al. 2011]. Moreover, at 48 hours, intrahepatic lipid content was shown to decrease more with a low carbohydrate diet versus a low fat diet, but reduction was similar in both diets after 7% weight loss [Kirk et al. 2009].
In a large long-term RCT, a total of 102 overweight and obese subjects were randomized to 6-month reduced carbohydrate (<90 g carbohydrates and a minimum of 30% fat of total energy intake) or reduced fat (<20% fat of total energy intake) – both energy restricted diets (70% of regular energy intake). Significant reductions were observed in both diets in intrahepatic lipid content and ALT without any difference between the two diet regimens [Haufe et al. 2011]. It should be mentioned that both diets were designed to be healthy, including reduced saturated fat intake. A meta-analysis summarizing the results of RCTs that compared the effect of low carbohydrate versus low fat caloric restriction demonstrated that the two regimens yield similar liver fat and ALT reduction [Musso et al. 2012].
In recent years, the rs738409 G allele in the patatin-like phospholipase domain-containing 3 (PNPLA3) gene was demonstrated to be associated with NAFLD [Romeo et al. 2008]. PNPLA3 gene variants may also influence the decrease of liver fat induced by lifestyle changes. Following a hypocaloric low carbohydrate diet for 6 days, 8 subjects homozygous for the rs738409 G allele (148MM) had a 2.5-fold greater liver fat reduction compared with 10 subjects who were homozygous for the rs738409 C allele (148II) despite similar weight reduction [Sevastianova et al. 2011].
Role of nutritional composition: fats, carbohydrates and other nutrients
In light of the difficulty in reducing weight and maintaining the weight reduction in the long term [Katan, 2009], changing dietary composition without necessarily reducing caloric intake may offer a more realistic and feasible alternative for the treatment of NAFLD patients. Interestingly, an increasing number of patients have been described with normal BMI [Lee et al. 1998; Banerji et al. 1999; Chitturi et al. 2002; Pagano et al. 2002]; this is called ‘lean NAFLD’ [Younossi et al. 2012]. Epidemiological studies [Musso et al. 2003; Assy et al. 2008; Yasutake et al. 2009] indicate that normal weight NAFLD patients may consume unhealthy dietary composition compared with controls, therefore emphasizing the importance of dietary composition.
Types of dietary fats
The diet of normal weight NASH patients compared with age, gender and BMI matched controls seems to be richer in saturated fat and cholesterol and poorer in polyunsaturated fatty acids (PUFA) [Musso et al. 2003]. These results are supported by a study in which the ratio of polyunsaturated to saturated fatty acid intake in both NASH and fatty liver patients was lower than the ratio in randomly selected controls [Toshimitsu et al. 2007]. Furthermore, epidemiological observational studies implicated a lower consumption of omega-3 PUFA and a higher n-6/n-3 ratio among NAFLD and NASH patients compared with controls [Cortez-Pinto et al. 2006; Zelber-Sagi et al. 2007].
Experimental studies have shown that diets enriched with n-3 PUFA increase insulin sensitivity in rats [Storlien et al. 1987], reduce intrahepatic triglyceride content and ameliorate steatohepatitis [Sekiya et al. 2003; Levy et al. 2004].
A meta-analysis of clinical trials pertaining to the effect of n-3 PUFA supplementation on NAFLD in humans included 9 eligible trials that were heterogeneous in study design (uncontrolled and controlled), duration (2–12 months) and dose (0.83–3.7 g). The data show that, despite the significant heterogeneity, marine omega-3 fatty acid supplementation in humans is associated with a positive effect on liver fat and this effect was also observed when only RCTs were included in the analysis [Parker et al. 2012]. More recent RCTs included liver histology and enabled us to learn the effect on NASH and fibrosis. An RCT, which included 15–18 months of treatment with docosahexaenoic acid (DHA) plus eicosapentaenoic acid (EPA) 4 g/day indicated that erythrocyte DHA enrichment occurring with DHA plus EPA treatment was independently associated with a decrease in liver fat percentage, but no improvement in fibrosis scores occurred [Scorletti et al. 2014]. In contrast, in a phase IIb multicenter, double-blind RCT, an EPA supplement provided for 12 months at high (2700 mg/day) or low (1800 mg/day) dose to 243 NASH patients led to no significant reduction of steatosis, inflammation, ballooning or fibrosis scores. Furthermore, there were no significant effects on levels of liver enzymes, insulin resistance, adiponectin, keratin 18, high-sensitivity C-reactive protein or hyaluronic acid. The only positive findings were that the high dosage group reduced serum triglyceride levels and there were no treatment-related serious adverse events [Sanyal et al. 2014]. In agreement with these findings, a smaller 1-year RCT among 34 NASH patients demonstrated that n-3 PUFA at 3000 mg/day did not lead to significant changes in the overall histological activity, although n-3 therapy was associated with reduced liver fat on biopsy and MRI, independent of weight loss [Argo et al. 2015].
Interestingly, in a population-based prospective cohort study of 90,296 Japanese subjects, consumption of n-3 PUFA-rich fish and individual types of n-3 PUFAs was inversely associated with hepatocellular carcinoma (HCC), irrespective of human hepatitis C virus (HCV) or hepatitis B virus (HBV) status [Sawada et al. 2012].
The conflicting results may stem in part from a different predisposing genotype that interacts with dietary intake of PUFA in determining liver fat retention [Romeo et al. 2008]. In a clinical trial, treatment efficacy of DHA on liver steatosis was affected by the PNPLA3 genotype; the rs738409 G allele was associated with lower response and the rs738409 C allele with greater response to DHA supplementation in children with NAFLD [Nobili et al. 2013]. In another pediatric cohort, the omega-6/omega-3 fatty acid intake ratio was positively correlated with liver fat content and ALT levels only in individuals homozygous for the rs738409 G allele (148II) of PNPLA3 [Santoro et al. 2012].
The Practice Guideline of the American Association for the Study of Liver Diseases (AASLD) summarizes that it is premature to recommend omega-3 fatty acids for the specific treatment of NAFLD or NASH, but they may be considered as first-line agents to treat hypertriglyceridemia in patients with NAFLD [Chalasani et al. 2012]. A monounsaturated fatty acid (MUFA)/oleic acid and the Mediterranean diet (MD) seem to play an important role in the metabolic profile of humans [Grosso et al. 2014]; n-9 oleic acid is the most prevalent MUFA in the diet and olive oil is one of its major sources (other sources are, nuts and avocado). MUFA has been demonstrated to have a favorable effect on lipid profile [Mensink et al. 2003] by reducing plasma triacylglycerol and very low density lipoprotein (VLDL) cholesterol concentrations and modestly increasing high density lipoprotein (HDL) cholesterol without adversely affecting low density lipoprotein (LDL) cholesterol concentrations [Garg, 1998].
In rats, olive oil was demonstrated to decrease the accumulation of triglycerides in the liver by 30% [Hussein et al. 2007], contributed to the recovery of the liver from hepatic steatosis [Hernandez et al. 2005] and protected against the development of fibrosis [Szende et al. 1994]. It has been suggested that adherence to the MD pattern (quantified by score) leads to a significant decrease in liver fat after 6 months of intervention among overweight patients with NAFLD [Trovato et al. 2015]. In a randomized parallel group design trial, 37 men and 8 women with type 2 diabetes were assigned to 1 of 2 isocaloric diets: either a high carbohydrate/high fiber diet or a high MUFA diet for an 8-week period. Liver fat content decreased more in the MUFA group (-29%) than in the high carbohydrate/high fiber group (-4%), despite stable weight in both groups. The different dietary composition was carbohydrate 52% versus 40%, fat 30% versus 42%, and MUFA (mostly olive oil) 16% versus 28% for the high carbohydrate/high fiber diet and MUFA diet, respectively [Bozzetto et al. 2012]. These results are in agreement with the benefits of a MD diet that were demonstrated among 12 nondiabetic NAFLD patients in a randomized, crossover 6-week dietary intervention. All subjects were treated with both the MD and a control diet, a low fat, high carbohydrate diet (LF/HCD). There was a significant relative reduction in hepatic steatosis after the MD compared with the LF/HCD: 39% versus 7%, despite a very modest weight loss that was not different between the two diets. The MD diet was based on the traditional Cretan MD; olives, dried fruits, nuts, Greek yoghurt, fish and olive oil. The LF/HCD was low in saturated and unsaturated fat and higher in carbohydrate than the MD [Ryan et al. 2013]. Despite these promising results, longer-term trials testing the MD diet are needed.
Experimental studies have demonstrated that, in mice, excess cholesterol intake leads to the development of NAFLD even in the absence of obesity [Matsuzawa et al. 2007, Wouters et al. 2008] and a diet containing 1% cholesterol induces steatohepatitis more than a simple high fat diet [Savard et al. 2013]. However, results from observational studies have been conflicting. Some studies did not demonstrate different dietary intakes of cholesterol between NAFLD patients and controls [Cortez-Pinto et al. 2006; Zelber-Sagi et al. 2007], but Musso and colleagues demonstrated a higher cholesterol consumption among normal weight NASH patients versus BMI matched controls [Musso et al. 2003]. A study that supports the role of dietary cholesterol in NAFLD compared 12 normal weight NAFLD patients to 44 obese NAFLD patients, showing that dietary cholesterol intake was significantly higher, while the intake of PUFAs was significantly lower in the non obese group. Therefore, this altered cholesterol and PUFA intake may be associated with the development of NAFLD in non obese patients [Yasutake et al. 2009]. In a large, nationally representative epidemiological study, dietary cholesterol consumption was independently associated with the development of cirrhosis [Ioannou et al. 2009]. Consistently, serum non-HDL cholesterol is an independent predictor of NAFLD [Zelber-Sagi et al. 2014b].
These findings may indicate that impairment of cholesterol regulation may have a causal relationship with liver steatogenesis. Indeed, excess intracellular cholesterol activates liver X receptors (LXRs) that regulate cholesterol homeostasis [Repa and Mangelsdorf, 2002], but induces hepatic steatosis [Fon Tacer and Rozman,. 2011] by activating sterol regulatory element-binding transcription factor 1c (SREBP-1c), a master transcriptional regulator of fatty acid synthesis in the liver [Schultz et al. 2000, Chen et al. 2004].
Types of dietary carbohydrates
‘Naturally occurring sugar’ refers to the sugar that is an integral constituent of whole fruit, vegetable and milk products, whereas ‘added sugar’ refers to sucrose or other refined sugars in soft drinks and incorporated into food, fruit drinks and other beverages [Howard and Wylie-Rosett, 2002]. Soft drinks are the leading source of added sugar in the world [Gaby, 2005]. Rats and humans that are fed either sucrose or fructose enriched diets develop fatty livers [Herman et al. 1970; Poulsom, 1986; Le et al. 2009; Sobrecases et al. 2010]. In addition, cola soft drinks contain caramel coloring, which is rich in advanced glycation end products (AGEs) that may increase insulin resistance and inflammation [Gaby, 2005]. Fructose also seems to be associated with alteration in intestinal microflora and a growing body of evidence supports a role for increased gut permeability and endotoxin in rodent and human NAFLD [Federico et al. 2016]. In animal studies, a high fructose diet induces changes similar to those seen in models of chronic alcohol intake and high fat diets, including increased gut permeability, endotoxemia, increased hepatic tumor necrosis factor alpha (TNF-α) production and hepatic steatosis [Federico et al. 2016]. Hepatic lipid accumulation, endotoxin levels in portal blood, lipid peroxidation and TNF-α expression were significantly higher in mice consuming fructose compared with glucose, sucrose or controls. Concomitant treatment of fructose fed mice with antibiotics markedly reduced hepatic lipid accumulation [Bergheim et al. 2008].
Several observational studies have been published on the association between soft drinks consumption and NAFLD, demonstrating a positive association [Zelber-Sagi et al. 2007; Assy et al. 2008; Ouyang et al. 2008; Abid et al. 2009]. Recently, the association between sugar-sweetened beverages (SSB), diet soda and fatty liver disease was tested in the Framingham Heart Study cohorts that included computed tomography (CT) in 2634 participants and ALT measurement in 5908 participants. A dose–response relationship was observed between SSB and fatty liver disease, with a 55% increased risk of fatty liver disease in daily consumers of SSB compared with non-SSB consumers. In addition, SSB consumption was positively associated with ALT levels. In contrast, there was no significant association between diet soda intake and either liver fat or ALT levels [Ma et al. 2015].
These findings are supported by an RCT in which overweight subjects (n = 47) were randomly assigned to 4 different test drinks (1 l/day for 6 months): regular cola, isocaloric semi-skimmed milk, aspartame-sweetened diet cola and water. The relative change in liver fat between baseline and the end of 6-month intervention was significantly higher in the regular cola group than in the 3 other groups [Maersk et al. 2012]. A large-scale study of 427 NAFLD patients expanded the understanding of the hepatic damage that may be related to overconsumption of fructose-containing beverages. After controlling for age, sex, BMI and total calorie intake, daily consumption of fructose-containing beverages was significantly associated with higher fibrosis stage [odds ratio (OR) = 3.2; 95% confidence interval (CI) 1.4–7.4 for ⩾7 versus <7 servings per week) [Abdelmalek et al. 2010].
One of the pathways by which SSBs can lead to fibrosis is by increasing serum uric acid (UA) levels in a dose–response manner. This increase stems from the large amounts of fructose in SSBs, which is the only carbohydrate known to increase uric acid levels [Choi et al. 2008]. A prospective observational study showed that elevation of serum UA levels independently predicted an increased risk for incident NAFLD [Xu et al. 2010]. In a cross-sectional analysis of real-world data of 82,608 people, obtained from a large health maintenance organization, a significant positive dose–response association between serum UA levels and the rate of elevated serum ALT was demonstrated in both men and women, and regardless of BMI [Zelber-Sagi et al. 2015a]. Elevated serum UA levels reflect and may also cause oxidative stress, insulin resistance and metabolic syndrome and, indeed, serum UA levels were demonstrated to be associated with the development of cirrhosis and the presence of elevated serum liver enzymes after adjustment for causes and risk factors of chronic liver disease (CLD) [Afzali et al. 2010].
Gene–diet interactions that contribute to fat accumulation in the liver have been identified with regard to carbohydrate and sugar consumption [Goran et al. 2012]. In a study of 153 Hispanic children, a nutrigenetic analysis revealed liver fat to be directly correlated with carbohydrate (r = 0.38, p = 0.02) and total sugar (r = 0.33, p = 0.04) intakes only in children homozygous for the rs738409 G allele (148MM) but not in the CC and CG genotypes, indicating a genetically determined metabolic response to dietary carbohydrates [Davis et al. 2010]. Trials assessing specific dietary interventions, based on genetic background, should be performed.
Other nutrients
Observational studies have demonstrated a favourable impact of coffee intake on health and in particular a protective effect from the metabolic syndrome [Grosso et al. 2015]. Several epidemiological studies, including prospective cohorts, have also indicated an inverse association between coffee consumption and liver cirrhosis and cancer development independently of etiology [Saab et al. 2013]. In recent years, cross-sectional studies have suggested an inverse association of coffee consumption with liver fibrosis in patients with NAFLD [Anty et al. 2012; Molloy et al. 2012; Bambha et al. 2014]. In the only study conducted so far, including both a prospective and cross-sectional cohorts from the Israeli general population, incident fatty liver diagnosed by abdominal ultrasound and quantified noninvasively by hepatorenal-ultrasound index and SteatoTest was not associated with baseline coffee consumption. However, in the cross- sectional cohort, high coffee consumption (⩾3 cups per day) was associated with lower odds for presumed clinically significant fibrosis measured by the FibroTest, also with adjustment for potential confounders [Zelber-Sagi et al. 2015b].
The specific components of coffee exerting beneficial effects have been partially elucidated [Godos et al. 2014]. Coffee contains hundreds of chemical ingredients including polyphenols, melanoidins and caffeine. Recently, caffeine was shown to inhibit hepatic stellate cell proliferation in vitro [Shim et al. 2013]. However, the hepatoprotective effects of coffee may be linked not only to caffeine but also to its polyphenolic fraction. In fact, in rats fed a high fat diet, consumption of decaffeinated coffee was demonstrated to be effective in preventing liver damage by inducing the expression of endogenous chaperones and antioxidant proteins [Vitaglione et al. 2010; Salomone et al. 2014]. The antioxidant activity of coffee appears relevant because progression of fibrosis in patients with NASH is associated with a lack of endogenous antioxidant defense [Salomone et al. 2013]. Other dietary polyphenols such as anthocyanins are promising candidates in the treatment of NAFLD and components of metabolic syndrome [Salamone et al. 2012a, 2012b], although RCTs are needed to establish their effects in patients.
A recent prospective cohort study supports a protective role for coffee also in HCC prevention. A US Multiethnic Cohort (MEC), which included 162,022 participants, demonstrated that compared with non coffee drinkers, those who drank 2–3 cups per day had a 38% reduction in risk for HCC and those who drank ⩾4 cups per day had a 41% reduction in HCC risk. Compared with non coffee drinkers, participants who consumed 2–3 cups coffee per day had a 46% reduction in risk of death from CLD and those who drank ⩾4 cups per day had a 71% reduction. The inverse associations were significant regardless of the participants’ ethnicity, sex, BMI, smoking status, alcohol intake or diabetes status [Setiawan et al. 2015].
Role of physical activity
Several observational studies indicated an inverse association between reported leisure time physical activity or cardiorespiratory fitness and the prevalence of NAFLD or hepatic fat content independently of body mass index [Magkos, 2010; Church et al. 2006; Perseghin et al. 2007]. Similar results were demonstrated in another study of the general population in which the NAFLD group engaged in less reported leisure time physical activity including total, aerobic and resistance training [Zelber-Sagi et al. 2008]. Few studies have tested the association with liver histology. In a small study on 37 NAFLD patients with liver biopsy, there was a lower cardiorespiratory fitness among patients with higher NAFLD activity score and NASH versus no NASH [Krasnoff et al. 2008]. Self-reported exercise intensity and histological severity of NAFLD was tested in a large cohort of adults with biopsy-proven NAFLD. Vigorous physical activity was associated with decreased BMI adjusted odds of having NASH in both minimum (⩾75 min/week) and extensive time (⩾150 min/week) of physical activity (35% and 44%, respectively). However, only extensive time spent in vigorous exercise was sufficient to reduce advanced fibrosis in almost 50% [Kistler et al. 2011]. In accordance with these findings, a study testing the beneficial effects of a varying volume of moderate to vigorous intensity physical activity among 169 obese men has shown that those enrolled for ⩾250 min/week of such activity (in comparison with <250 min/week) had a greater beneficial effect of reducing liver fat, inflammation and oxidative stress levels, and altering fatty acid metabolism. This was reflected by a greater reduction in levels of ferritin and lipid peroxidation, a significant increase in the adiponectin levels and favorable changes in the expression levels of genes involved in fatty acid synthesis and degradation following the 12-week intervention [Oh et al. 2015]. However, it has to be taken into consideration that increasing exercise frequency and dose to ⩾250 min/week may be difficult for most NAFLD patients.
The beneficial effect of exercise is supported by clinical trials and by a recent meta-analysis concluding that there is a clear evidence for a benefit of exercise therapy on liver fat with minimal or no weight loss and at exercise levels below current exercise recommendations for obesity management [Keating et al. 2012]. Nevertheless, the ability of exercise alone to improve other aspects of liver histology remains unknown [Chalasani et al. 2012].
An RCT assessed the effect of short-term (4 weeks) aerobic exercise training (3 cycle sessions per week of 30–45 min each versus stretching) in 19 sedentary obese men and women resulting in a reduction of 21% in hepatic triglyceride concentration with no change in weight or dietary intake [Johnson et al. 2009]. Furthermore, two other clinical trials – one in obese adolescents [van der Heijden et al. 2010] and the other in healthy elderly [Finucane et al. 2010] – support the beneficial effect of aerobic exercise. In both trials, 12-week aerobic exercise led to about 35% reduction in hepatic fat without diet or weight loss. However, the exact intensity and volume which would be optimal to reduce liver fat is unknown. A randomized trial compared different 8-week exercise regiments among inactive obese adults: 50% VO2 peak, 60 min, 4 days/week; 70% VO2 peak, 45 min, 3 days/week; 50% VO2 peak, 45 min, 3 days per week; and placebo. There was no difference in the efficacy of liver fat reduction by either aerobic exercise dose or intensity [Keating et al. 2015]. It appears that any reasonable amount of physical activity is better than nothing, since prolonged sitting time by itself was demonstrated to be positively associated with the prevalence of NAFLD in a large sample of Korean adults [Ryu et al. 2015].
Resistance training, without a concomitant weight loss diet, improves insulin sensitivity and fasting glycaemia and decreases abdominal fat [Ibanez et al. 2005]. In a small RCT including 19 sedentary adult NAFLD patients, 8 weeks of resistance training consisting of 45-minute sessions trice weekly, led to a reduction in liver fat without weight loss [Hallsworth et al. 2011]. In another RCT, patients were randomized to either resistance training (n = 33) or stretching arm (n = 31); 3-month resistance training improved hepatic fat content as tested by the hepatorenal-ultrasound index accompanied by favorable changes in body composition and ferritin [Zelber-Sagi et al. 2014a].
In a larger RCT that compared the effect of 8-month aerobic training versus resistance training and versus the combination of both, only aerobic training led to significant reductions in liver fat and serum ALT levels; moreover the effect of the combined training was indistinguishable from the effect of aerobic training alone [Slentz et al. 2011]. However, the aerobic training and combined training groups lost a small but significant body weight (2 kg) whereas the resistance training group did not, a difference that may partially explain the difference in outcomes. In contrast, in an RCT among 31 sedentary adults with type 2 diabetes and NAFLD, 4-month aerobic versus resistance training 3 times per week led to reduction in hepatic fat content to a similar extent in both training groups (mean relative reduction from baseline −32.8% versus −25.9%, respectively). Additionally, hepatic steatosis (defined as hepatic fat content >5.56%) regressed in almost one-quarter of the patients in each intervention group [Bacchi et al. 2013]. The combination of both types of activities may be best as demonstrated in a recent large RCT among 304 adolescents, in which 22-week combined aerobic and resistance training was superior to aerobic training alone in decreasing percentage body fat and waist circumference [Sigal et al. 2014].
Until more studies in large samples of NAFLD patients are performed, we can adopt the 2013 American College of Cardiology/American Heart Association guideline on lifestyle management to reduce cardiovascular risk, advising healthy adults to engage in aerobic physical activity 3–4 sessions a week, lasting on average 40 minutes per session, and involving moderate-to-vigorous intensity physical activity [Eckel et al. 2014]. The European guidelines on cardiovascular disease prevention recommend 2.5–5 hours a week of aerobic exercise training of at least moderate intensity, or 1–2.5 hours a week on vigorous intense exercise [Perk et al. 2012]. However, no specific recommendations were provided for resistance training or the combination of both aerobic and resistance training due to limited data [Eckel et al. 2014].
Conclusion
Lifestyle changes are crucial for the treatment of NAFLD. The position statement by the European Association for the Study of the Liver (EASL) on NAFLD/NASH [Ratziu et al. 2010] recommends a weight loss of 7% on the basis of an extensive body of literature. The American Association for the Study of Liver Diseases (AASLD) practice guideline indicates that loss of at least 3–5% of body weight appears necessary to improve steatosis, but a greater weight loss (up to 10%) may be needed to improve necroinflammation [Chalasani et al. 2012]. There is presently no convincing evidence that long-term low carbohydrate diets are better than low fat diets, and the diet of choice should be the one which individuals are able to adhere for years rather than weeks. Studies performed so far identified soft drinks as an important modifiable risk factor. Physicians and dietitians should routinely include questions regarding soft drink consumption as part of the patient’s history and advise patients to minimize its consumption. Reducing the consumption of fast food would be wise since it is served in large energy dense portions and combines several potential hepatotoxic nutrients, i.e. saturated fat, refined carbohydrates, fructose, caramel coloring, industrially produced trans fatty acids, and is low in fiber [Kechagias et al. 2008; Marchesini et al. 2008; Sobrecases et al. 2010]. Physical activity should be integrated into behavioral therapy in NAFLD, as even small gains in physical activity and fitness may have significant health benefits.
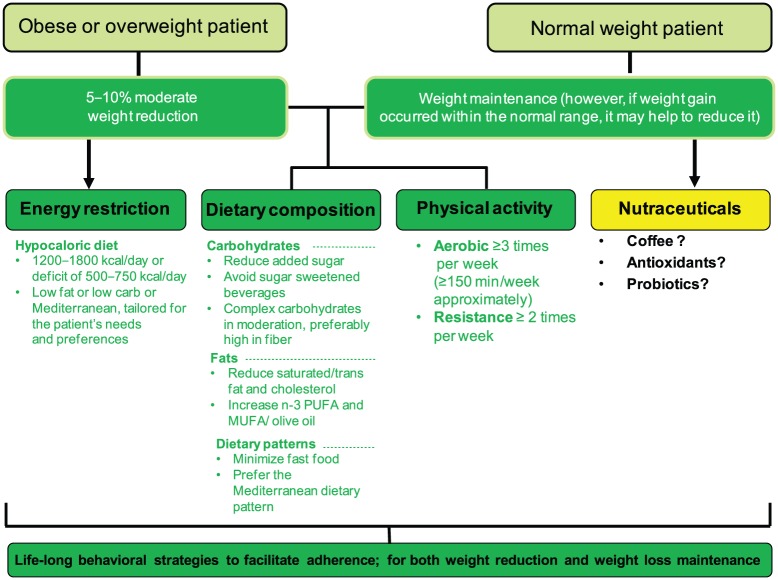
The suggested NAFLD’s lifestyle therapeutic algorithm is depicted in Figure 1 and represents the importance of all components in the treatment of NAFLD. Table 1 summarizes the main features and results of lifestyle intervention trials conducted so far.
Figure 1.
Algorithm for lifestyle changes in nonalcoholic fatty liver disease (NAFLD).
MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
Table 1.
Lifestyle intervention trials for nonalcoholic fatty liver disease (NAFLD) treatment.
| Reference | Country | Study design | Patients, (% men) | Liver fat content evaluation | Intervention (type, time) | Effects on liver fat content | Effects on liver histological endpoints |
|---|---|---|---|---|---|---|---|
| DIET INTERVENTIONS | |||||||
| Huang et al. [2005] | USA | Intervention without control arm | 16 obese, (50) | Liver biopsy | NC, 12 months | No effect | Decreased ballooning/ inflammation |
| Kirk et al. [2009] | USA | RCT | 22 obese, (18) | MRS | Low calorie HCD versus LCD, 11 weeks | Decreased in HCD and LCD | NP |
| Haufe et al. [2011] | Germany | RCT | 102 obese, (18) | MRS | Low calorie LCD versus LFD, 6 months | Decreased in LCD and LFD | NP |
| Bozzetto et al. [2012] | Italy | RCT | 36 diabetic, (81) | MRS | CHO/fiber versus MUFA, CHO/fiber+ exercise, versus MUFA + exercise, 8 weeks | Decreased in MUFA and MUFA + exercise | NP |
| Ryan et al. [2013] | Australia | RCT | 12 obese, (50) | MRS | MD versus LF/HCD | Decreased | NP |
| Trovato et al. [2015] | Italy | Intervention without control arm | 90 obese, (49) | US | MD, 6 months | Decreased | NP |
| EXERCISE INTERVENTIONS | |||||||
| Johnson et al. [2009] | Australia | RCT | 19 obese, (NA) | MRS | Aerobic training versus regular stretching, 4 weeks | Decreased | NP |
| Van der Heijden et al. [2010] | USA | Intervention without control arm | 15 obese and 14 lean (58) | MRS | Aerobic training obese versus lean, 12 weeks | Decreased | NP |
| Slentz et al. [2011] | USA | RCT | 144 obese, (44) | CT | Resistance training versus aerobic training versus combined, 8 months | Decreased in aerobic arm and aerobic + resistance training | NP |
| Hallsworthet al. [2011] | UK | RCT | 19 obese, (NA) | MRS | Resistance training versus SC, 8 weeks | Decreased | NP |
| Bacchi et al. [2013] | Italy | RCT | 30 obese, (73.3) | MRS | Aerobic training versus resistance training, 4 months | Decreased | NP |
| Zelber-Sagiet al. [2014a] | Israel | RCT | 64 obese, (53.1) | US | Resistance training versus home stretching, 3 months | Decreased | NP |
| DIET AND EXERCISE INTERVENTIONS | |||||||
| Ueno et al. [1997] | Japan | Intervention without control arm | 25 obese, (52) | Liver biopsy | Restricted diet and exercise versus control, 3 months | Decreased | Decreased steatosis |
| Shah et al. [2009] | USA | RCT | 18 obese, (28) | MRS | Diet versus diet with exercises, 6 months | Decreased in both arms | NP |
| Oza et al. [2009] | Japan | Intervention without control arm | 22 overweight (54.5) | CT | Weight loss (caloric restriction), 3 or 6 months | Decreased | NP |
| Lazo et al. [2010] | USA | RCT | 96 obese, (51) | MRS | Weight loss (caloric restriction, increased physical activity) versus DSE, 12 months | Decreased | NP |
| Promrat et al. [2010] | USA | RCT | 31 obese, (71) | Liver biopsy | Weight loss (caloric restriction, increased physical activity) versus control, 48 weeks | Decreased | Decreased steatosis and ballooning, no change in fibrosis score |
| Browninget al. [2011] | USA | Intervention without control arm | 18 obese, (68) | MRS | Low calorie versus low carbohydrate, 2 weeks | Decreased in both arms | NP |
| Wong et al. [2013] | Hong-Kong | RCT | 154 normal weight, (25) | MRS | Weight loss (caloric restriction, increased physical activity) versus SC, 12 months | Decreased | NP |
| Vilar-Gomez et al. [2015] | Cuba | Intervention without control arm | 261 obese, (25) | Liver biopsy | Weight loss (caloric restriction, increased physical activity) versus SC, 52 weeks | Decreased | Decreased steatosis, ballooning and fibrosis |
CHO/fiber, high carbohydrate/high fiber/low glycemic index; CT, computed tomography; DSE, diabetes support and education; HCD, high carbohydrate diet; LCD, low carbohydrate diet; LFD, low fat diet; LF/HCD, low fat/high carbohydrate diet; MD, Mediterranean diet; MRS, magnetic resonance spectroscopy; MUFA, monounsaturated fatty acids; NA, not available; NC, nutritional counseling,; NP, not pertinent; RCT, randomized trial; SC, standard counseling; US, ultrasonography.
In the future, one of the treatment goals would be to establish a tailored treatment approach for NAFLD according to diet and genotype interactions.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Shira Zelber-Sagi, School of Public Health, Faculty of Social Welfare and Health Sciences, University of Haifa; Department of Gastroenterology, Tel-Aviv Sourasky Medical Center, Israel.
Justyna Godos, Department of Biomedical and Biotechnological Sciences, University of Catania, Catania, Italy.
Federico Salomone, Division of Gastroenterology, Ospedale di Acireale, Azienda Sanitaria Provinciale di Catania, Catania, Italy.
References
- Abdelmalek M., Suzuki A., Guy C., Unalp-Arida A., Colvin R., Johnson R., et al. (2010) Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 51: 1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abid A., Taha O., Nseir W., Farah R., Grosovski M., Assy N. (2009) Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome. J Hepatol 51: 918–924. [DOI] [PubMed] [Google Scholar]
- Afzali A., Weiss N., Boyko E., Ioannou G. (2010) Association between serum uric acid level and chronic liver disease in the United States. Hepatology 52: 578–589. [DOI] [PubMed] [Google Scholar]
- Andersen T., Gluud C., Franzmann M., Christoffersen P. (1991) Hepatic effects of dietary weight loss in morbidly obese subjects. J Hepatol 12: 224–229. [DOI] [PubMed] [Google Scholar]
- Anty R., Marjoux S., Iannelli A., Patouraux S., Schneck A., Bonnafous S., et al. (2012) Regular coffee but not espresso drinking is protective against fibrosis in a cohort mainly composed of morbidly obese European women with NAFLD undergoing bariatric surgery. J Hepatol 57: 1090–1096. [DOI] [PubMed] [Google Scholar]
- Argo C., Patrie J., Lackner C., Henry T., De Lange E., Weltman A., et al. (2015) Effects of n-3 fish oil on metabolic and histological parameters in nash: a double-blind, randomized, placebo-controlled trial. J Hepatol 62: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assy N., Nasser G., Kamayse I., Nseir W., Beniashvili Z., Djibre A., et al. (2008) soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol 2: 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi E., Negri C., Targher G., Faccioli N., Lanza M., Zoppini G., et al. (2013) both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 randomized trial). Hepatology 58: 1287–1295. [DOI] [PubMed] [Google Scholar]
- Bambha K., Wilson L., Unalp A., Loomba R., Neuschwander-Tetri B., Brunt E., et al. (2014) Coffee consumption in NAFLD patients with lower insulin resistance is associated with lower risk of severe fibrosis. Liver Int 34: 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji M., Faridi N., Atluri R., Chaiken R., Lebovitz H. (1999) Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab 84: 137–144. [DOI] [PubMed] [Google Scholar]
- Bedogni G., Bellentani S. (2004) Fatty liver: how frequent is it and why? Ann Hepatol 3: 63–65. [PubMed] [Google Scholar]
- Bellentani S., Saccoccio G., Masutti F., Croce L., Brandi G., Sasso F., et al. (2000) Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med 132: 112–117. [DOI] [PubMed] [Google Scholar]
- Bergheim I., Weber S., Vos M., Kramer S., Volynets V., Kaserouni S., et al. (2008) Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol 48: 983–992. [DOI] [PubMed] [Google Scholar]
- Bozzetto L., Prinster A., Annuzzi G., Costagliola L., Mangione A., Vitelli A., et al. (2012) Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care 35: 1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning J., Baker J., Rogers T., Davis J., Satapati S., Burgess S. (2011) short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr 93: 1048–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani N., Younossi Z., Lavine J., Diehl A., Brunt E., Cusi K., et al. (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55: 2005–2023. [DOI] [PubMed] [Google Scholar]
- Chang Y., Ryu S., Sung E., Woo H., Cho S., Yoo S., et al. (2009) Weight gain within the normal weight range predicts ultrasonographically detected fatty liver in healthy Korean men. Gut 58: 1419–1425. [DOI] [PubMed] [Google Scholar]
- Chen G., Liang G., Ou J., Goldstein J., Brown M. (2004) Central role for Liver X receptor in insulin-mediated activation of SREBP-1C transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci U S A 101: 11245–11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitturi S., Abeygunasekera S., Farrell G., Holmes-Walker J., Hui J., Fung C., et al. (2002) NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology 35: 373–379. [DOI] [PubMed] [Google Scholar]
- Choi J., Ford E., Gao X., Choi H. (2008) Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the third National Health and Nutrition Examination Survey. Arthritis Rheum 59: 109–116. [DOI] [PubMed] [Google Scholar]
- Church T., Kuk J., Ross R., Priest E., Biltoft E., Blair S. (2006) Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology 130: 2023–2030. [DOI] [PubMed] [Google Scholar]
- Cortez-Pinto H., Jesus L., Barros H., Lopes C., Moura M., Camilo M. (2006) how different is the dietary pattern in non-alcoholic steatohepatitis patients? Clin Nutr 25: 816–823. [DOI] [PubMed] [Google Scholar]
- Davis J., Le K., Walker R., Vikman S., Spruijt-Metz D., Weigensberg M., et al. (2010) Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am J Clin Nutr 92: 1522–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Gaudio A., Boschi L., Del Gaudio G., Mastrangelo L., Munari D. (2002) Liver damage in obese patients. Obesity Surgery 12: 802–804. [DOI] [PubMed] [Google Scholar]
- Dixon J., Bhathal P., Hughes N., O’Brien P. (2004) Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology 39: 1647–1654. [DOI] [PubMed] [Google Scholar]
- Eckel R., Jakicic J., Ard J., De Jesus J., Houston Miller N., Hubbard V., et al. (2014) 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129: S76–S99. [DOI] [PubMed] [Google Scholar]
- Erdmann J., Kallabis B., Oppel U., Sypchenko O., Wagenpfeil S., Schusdziarra V. (2008) Development of hyperinsulinemia and insulin resistance during the early stage of weight gain. Am J Physiol Endocrinol Metab 294: E568–E575. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Eriksson K., Bondesson L. (1986) Nonalcoholic steatohepatitis in obesity: a reversible condition. Acta Medica Scandinavica 220: 83–88. [DOI] [PubMed] [Google Scholar]
- Falck-Ytter Y., Younossi Z., Marchesini G., Mccullough A. (2001) clinical features and natural history of nonalcoholic steatosis syndromes. Semin Liver Dis 21: 17–26. [DOI] [PubMed] [Google Scholar]
- Federico A., Dallio M., Godos J., Loguercio C., Salomone F. (2016) Targeting gut-liver axis for the treatment of nonalcoholic steatohepatitis: translational and clinical evidence. Transl Res 167: 116–124. [DOI] [PubMed] [Google Scholar]
- Finucane F., Sharp S., Purslow L., Horton K., Horton J., Savage D., et al. (2010) The effects of aerobic exercise on metabolic risk, insulin sensitivity and intrahepatic lipid in healthy older people from the Hertfordshire cohort study: a randomised controlled trial. Diabetologia 53: 624–631. [DOI] [PubMed] [Google Scholar]
- Fon Tacer K., Rozman D. (2011) Nonalcoholic fatty liver disease: focus on lipoprotein and lipid deregulation. J Lipids 2011: 783976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaby A. (2005) Adverse effects of dietary fructose. Altern Med Rev 10: 294–306. [PubMed] [Google Scholar]
- Garg A. (1998) High-monounsaturated-fat diets for patients with diabetes mellitus: a meta-analysis. Am J Clin Nutr 67: 577S–582S. [DOI] [PubMed] [Google Scholar]
- Godos J., Pluchinotta F., Marventano S., Buscemi S., Li Volti G., Galvano F., et al. (2014) Coffee components and cardiovascular risk: beneficial and detrimental effects. Int J Food Sci Nutr 65: 925–936. [DOI] [PubMed] [Google Scholar]
- Goran M., Walker R., Allayee H. (2012) Genetic-related and carbohydrate-related factors affecting liver fat accumulation. Curr Opin Clin Nutr Metab Care 15: 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso G., Mistretta A., Frigiola A., Gruttadauria S., Biondi A., Basile F., et al. (2014) Mediterranean diet and cardiovascular risk factors: a systematic review. Crit Rev Food Sci Nutr 54: 593–610. [DOI] [PubMed] [Google Scholar]
- Grosso G., Stepaniak U., Micek A., Topor-Madry R., Pikhart H., Szafraniec K., et al. (2015) Association of daily coffee and tea consumption and metabolic syndrome: results from the Polish arm of the HAPIEE study. Eur J Nutr 54: 1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte P., Amarapurkar D., Agal S., Baijal R., Kulshrestha P., Pramanik S., et al. (2004) Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol 19: 854–858. [DOI] [PubMed] [Google Scholar]
- Hallsworth K., Fattakhova G., Hollingsworth K., Thoma C., Moore S., Taylor R., et al. (2011) Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 60: 1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S., Fecht W., Brunt E., Neuschwander-Tetri B. (2009) Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology 49: 80–86. [DOI] [PubMed] [Google Scholar]
- Haufe S., Engeli S., Kast P., Bohnke J., Utz W., Haas V., et al. (2011) randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology 53: 1504–1514. [DOI] [PubMed] [Google Scholar]
- Herman R., Zakim D., Stifel F. (1970) Effect of diet on lipid metabolism in experimental animals and man. Fed Proc 29: 1302–1307. [PubMed] [Google Scholar]
- Hernandez R., Martinez-Lara E., Canuelo A., Del Moral M., Blanco S., Siles E., et al. (2005) Steatosis recovery after treatment with a balanced sunflower or olive oil-based diet: involvement of perisinusoidal stellate cells. World J Gastroenterol 11: 7480–7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B., Wylie-Rosett J. (2002) Sugar and cardiovascular disease: a statement for healthcare professionals from the Committee on Nutrition of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation 106: 523–527. [DOI] [PubMed] [Google Scholar]
- Huang M., Greenson J., Chao C., Anderson L., Peterman D., Jacobson J., et al. (2005) one-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol 100: 1072–1081. [DOI] [PubMed] [Google Scholar]
- Hussein O., Grosovski M., Lasri E., Svalb S., Ravid U., Assy N. (2007) monounsaturated fat decreases hepatic lipid content in non-alcoholic fatty liver disease in rats. World J Gastroenterol 13: 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez J., Izquierdo M., Arguelles I., Forga L., Larrion J., Garcia-Unciti M., et al. (2005) Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care 28: 662–667. [DOI] [PubMed] [Google Scholar]
- Ioannou G., Morrow O., Connole M., Lee S. (2009) Association between dietary nutrient composition and the incidence of cirrhosis or liver cancer in the United States population. Hepatology 50: 175–184. [DOI] [PubMed] [Google Scholar]
- Johnson N., Sachinwalla T., Walton D., Smith K., Armstrong A., Thompson M., et al. (2009) Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 50: 1105–1112. [DOI] [PubMed] [Google Scholar]
- Katan M. (2009) Weight-loss diets for the prevention and treatment of obesity. N Engl J Med 360: 923–925. [DOI] [PubMed] [Google Scholar]
- Keating S., Hackett D., George J., Johnson N. (2012) Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 57: 157–166. [DOI] [PubMed] [Google Scholar]
- Keating S., Hackett D., Parker H., O’Connor H., Gerofi J., Sainsbury A., et al. (2015) Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol 63: 174–182. [DOI] [PubMed] [Google Scholar]
- Kechagias S., Ernersson A., Dahlqvist O., Lundberg P., Lindstrom T., Nystrom F. (2008) Fast-food-based hyper-alimentation can induce rapid and profound elevation of serum alanine aminotransferase in healthy subjects. Gut 57: 649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Park J., Lee K., Lee G., Jeon S., Kim J., et al. (2009) Effect of body weight and lifestyle changes on long-term course of nonalcoholic fatty liver disease in Koreans. Am J Med Sci 337: 98–102. [DOI] [PubMed] [Google Scholar]
- Kirk E., Reeds D., Finck B., Mayurranjan S., Patterson B., Klein S. (2009) Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 136: 1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler K., Brunt E., Clark J., Diehl A., Sallis J., Schwimmer J. (2011) Physical Activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol 106: 460–468; quiz 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnoff J., Painter P., Wallace J., Bass N., Merriman R. (2008) Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology 47: 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo M., Solga S., Horska A., Bonekamp S., Diehl A., Brancati F., et al. (2010) Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care 33: 2156–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le K., Ith M., Kreis R., Faeh D., Bortolotti M., Tran C., et al. (2009) fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr 89: 1760–1765. [DOI] [PubMed] [Google Scholar]
- Lee J., Rhee P., Lee J., Lee K., Kim J., Koh K., et al. (1998) Role of hyperinsulinemia and glucose intolerance in the pathogenesis of nonalcoholic fatty liver in patients with normal body weight. Korean J Intern Med 13: 12–14. [PubMed] [Google Scholar]
- Levy J., Clore J., Stevens W. (2004) Dietary n-3 polyunsaturated fatty acids decrease hepatic triglycerides in Fischer 344 Rats. Hepatology 39: 608–616. [DOI] [PubMed] [Google Scholar]
- Luyckx F., Desaive C., Thiry A., Dewe W., Scheen A., Gielen J., et al. (1998) Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. International Journal of Obesity and Related Metabolic Disorders 22: 222–226. [DOI] [PubMed] [Google Scholar]
- Ma J., Fox C., Jacques P., Speliotes E., Hoffmann U., Smith C., et al. (2015) sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J Hepatol 63: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maersk M., Belza A., Stodkilde-Jorgensen H., Ringgaard S., Chabanova E., Thomsen H., et al. (2012) Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 95: 283–289. [DOI] [PubMed] [Google Scholar]
- Magkos F. (2010) Exercise and fat accumulation in the human liver. Curr Opin Lipidol 21: 507–517. [DOI] [PubMed] [Google Scholar]
- Marchesini G., Ridolfi V., Nepoti V. (2008) Hepatotoxicity of fast food? Gut 57: 568–570. [DOI] [PubMed] [Google Scholar]
- Matsuzawa N., Takamura T., Kurita S., Misu H., Ota T., Ando H., et al. (2007) Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology 46: 1392–1403. [DOI] [PubMed] [Google Scholar]
- Mensink R., Zock P., Kester A., Katan M. (2003) Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 77: 1146–1155. [DOI] [PubMed] [Google Scholar]
- Molloy J., Calcagno C., Williams C., Jones F., Torres D., Harrison S. (2012) Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology 55: 429–436. [DOI] [PubMed] [Google Scholar]
- Musso G., Cassader M., Rosina F., Gambino R. (2012) Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia 55: 885–904. [DOI] [PubMed] [Google Scholar]
- Musso G., Gambino R., De Michieli F., Cassader M., Rizzetto M., Durazzo M., et al. (2003) Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 37: 909–916. [DOI] [PubMed] [Google Scholar]
- Nobili V., Bedogni G., Donati B., Alisi A., Valenti L. (2013) The I148M variant of PNPLA3 reduces the response to docosahexaenoic acid in children with non-alcoholic fatty liver disease. J Med Food 16: 957–960. [DOI] [PubMed] [Google Scholar]
- Oh S., Shida T., Yamagishi K., Tanaka K., So R., Tsujimoto T., et al. (2015) Moderate to vigorous physical activity volume is an important factor for managing non-alcoholic fatty liver disease: a retrospective Study. Hepatology 61: 1205–1215. [DOI] [PubMed] [Google Scholar]
- Orr J., Gentile C., Davy B., Davy K. (2008) Large artery stiffening with weight gain in humans: role of visceral fat accumulation. Hypertension 51: 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X., Cirillo P., Sautin Y., Mccall S., Bruchette J., Diehl A., et al. (2008) Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 48: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza N., Eguchi Y., Mizuta T., Ishibashi E., Kitajima Y., Horie H., et al. (2009) A pilot trial of body weight reduction for nonalcoholic fatty liver disease with a home-based lifestyle modification intervention delivered in collaboration with interdisciplinary medical staff. J Gastroenterol 44: 1203–1208. [DOI] [PubMed] [Google Scholar]
- Pagano G., Pacini G., Musso G., Gambino R., Mecca F., Depetris N., et al. (2002) Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology 35: 367–372. [DOI] [PubMed] [Google Scholar]
- Palmer M., Schaffner F. (1990) Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology 99: 1408–1413. [DOI] [PubMed] [Google Scholar]
- Parker H., Johnson N., Burdon C., Cohn J., O’Connor H., George J. (2012) Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 56: 944–951. [DOI] [PubMed] [Google Scholar]
- Perk J., De Backer G., Gohlke H., Graham I., Reiner Z., Verschuren M., et al. (2012) European Practice guidelines on cardiovascular disease prevention in clinical (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 33: 1635–1701. [DOI] [PubMed] [Google Scholar]
- Perseghin G., Lattuada G., De Cobelli F., Ragogna F., Ntali G., Esposito A., et al. (2007) Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care 30: 683–688. [DOI] [PubMed] [Google Scholar]
- Poulsom R. (1986) Morphological changes of organs after sucrose or fructose feeding. Prog Biochem Pharmacol 21: 104–134. [PubMed] [Google Scholar]
- Promrat K., Kleiner D., Niemeier H., Jackvony E., Kearns M., Wands J., et al. (2010) Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 51: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propst A., Propst T., Judmaier G., Vogel W. (1995) Prognosis in nonalcoholic steatohepatitis. Gastroenterology 108: 1607. [DOI] [PubMed] [Google Scholar]
- Ratziu V., Bellentani S., Cortez-Pinto H., Day C., Marchesini G. (2010) A position statement on NAFLD/NASH based on the EASL 2009 Special Conference. J Hepatol 53: 372–384. [DOI] [PubMed] [Google Scholar]
- Repa J., Mangelsdorf D. (2002) The liver X receptor gene team: potential new players in atherosclerosis. Nat Med 8: 1243–1248. [DOI] [PubMed] [Google Scholar]
- Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L., et al. (2008) Genetic variation in PNPLa3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 40: 1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N., Chisholm D., Pi-Sunyer X., Schneider S. (1998) The metabolically obese, normal-weight individual revisited. Diabetes 47: 699–713. [DOI] [PubMed] [Google Scholar]
- Ryan M., Abbasi F., Lamendola C., Carter S., McLaughlin T. (2007) serum alanine aminotransferase levels decrease further with carbohydrate than fat restriction in insulin-resistant adults. Diabetes Care 30: 1075–1080. [DOI] [PubMed] [Google Scholar]
- Ryan M., Itsiopoulos C., Thodis T., Ward G., Trost N., Hofferberth S., et al. (2013) The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with nonalcoholic fatty liver disease. J Hepatol 59: 138–143. [DOI] [PubMed] [Google Scholar]
- Ryu S., Chang Y., Jung H., Yun K., Kwon M., Choi Y., et al. (2015) Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J Hepatol 63: 1229–1237. [DOI] [PubMed] [Google Scholar]
- Saab S., Mallam D., Cox Ii G., Tong M. (2013) Impact of coffee on liver diseases: a systematic review. Liver Int 34: 495–504. [DOI] [PubMed] [Google Scholar]
- Salamone F., Galvano F., Marino Gammazza A., Paternostro C., Tibullo D., Bucchieri F., et al. (2012a) Silibinin improves hepatic and myocardial injury in mice with nonalcoholic steatohepatitis. Dig Liver Dis 44: 334–342. [DOI] [PubMed] [Google Scholar]
- Salamone F., Li Volti G., Titta L., Puzzo L., Barbagallo I., La Delia F., et al. (2012b) Moro orange juice prevents fatty liver in mice. World J Gastroenterol 18: 3862–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomone F., Li Volti G., Rosso C., Grosso G., Bugianesi E. (2013) Unconjugated bilirubin, a potent endogenous antioxidant, is decreased in patients with non-alcoholic steatohepatitis and advanced fibrosis. J Gastroenterol Hepatol 28: 1202–1208. [DOI] [PubMed] [Google Scholar]
- Salomone F., Li Volti G., Vitaglione P., Morisco F., Fogliano V., Zappala A., et al. (2014) Coffee enhances the expression of chaperones and antioxidant proteins in rats with nonalcoholic fatty liver disease. Transl Res 163: 593–602. [DOI] [PubMed] [Google Scholar]
- Santoro N., Savoye M., Kim G., Marotto K., Shaw M., Pierpont B., et al. (2012) hepatic fat accumulation is modulated by the interaction between the rs738409 variant in the PNPLA3 gene and the dietary omega6/omega3 PUFA intake. PLoS One 7: e37827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A., Abdelmalek M., Suzuki A., Cummings O., Chojkier M. (2014) No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology 147: 377–384. [DOI] [PubMed] [Google Scholar]
- Savard C., Tartaglione E., Kuver R., Haigh W., Farrell G., Subramanian S., et al. (2013) Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology 57: 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada N., Inoue M., Iwasaki M., Sasazuki S., Shimazu T., Yamaji T., et al. (2012) Consumption of n-3 fatty acids and fish reduces risk of hepatocellular carcinoma. Gastroenterology 142: 1468–1475. [DOI] [PubMed] [Google Scholar]
- Schultz J., Tu H., Luk A., Repa J., Medina J., Li L., et al. (2000) Role of LXRS in control of lipogenesis. Genes Dev 14: 2831–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorletti E., Bhatia L., Mccormick K., Clough G., Nash K., Hodson L., et al. (2014) Effects of purified eicosapentaenoic and docosahexaenoic acids in non-alcoholic fatty liver disease: results from the Welcome* study. Hepatology 60: 1211–1221. [DOI] [PubMed] [Google Scholar]
- Sekiya M., Yahagi N., Matsuzaka T., Najima Y., Nakakuki M., Nagai R., et al. (2003) Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology 38: 1529–1539. [DOI] [PubMed] [Google Scholar]
- Setiawan V., Wilkens L., Lu S., Hernandez B., Le Marchand L., Henderson B. (2015) Association of coffee intake with reduced incidence of liver cancer and death from chronic liver disease in the US multiethnic cohort. Gastroenterology 148: 118–125; quiz e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevastianova K., Kotronen A., Gastaldelli A., Perttila J., Hakkarainen A., Lundbom J., et al. (2011) Genetic variation in PNPLA3 (adiponutrin) confers sensitivity to weight loss-induced decrease in liver fat in humans. Am J Clin Nutr 94: 104–111. [DOI] [PubMed] [Google Scholar]
- Shah K., Stufflebam A., Hilton T., Sinacore D., Klein S., Villareal D. (2009) Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity 17: 2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim S., Jun D., Kim E., Saeed W., Lee K., Lee H., et al. (2013) Caffeine attenuates liver fibrosis via defective adhesion of hepatic stellate cells in cirrhotic model. J Gastroenterol Hepatol 28: 1877–1884. [DOI] [PubMed] [Google Scholar]
- Sigal R., Alberga A., Goldfield G., Prud’homme D., Hadjiyannakis S., Gougeon R., et al. (2014) Effects of aerobic training, resistance training, or both on percentage body fat and cardiometabolic risk markers in obese adolescents: the healthy eating aerobic and resistance training in youth randomized clinical trial. JAMA Pediatr 168: 1006–1014. [DOI] [PubMed] [Google Scholar]
- Slentz C., Bateman L., Willis L., Shields A., Tanner C., Piner L., et al. (2011) Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab 301: E1033–E1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrecases H., Le K., Bortolotti M., Schneiter P., Ith M., Kreis R., et al. (2010) Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab 36: 244–246. [DOI] [PubMed] [Google Scholar]
- St George A., Bauman A., Johnston A., Farrell G., Chey T., George J. (2009) Effect of a lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J Gastroenterol Hepatol 24: 399–407. [DOI] [PubMed] [Google Scholar]
- Storlien L., Kraegen E., Chisholm D., Ford G., Bruce D., Pascoe W. (1987) Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 237: 885–888. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Lindor K., St Saver J., Lymp J., Mendes F., Muto A., et al. (2005) Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol 43: 1060–1066. [DOI] [PubMed] [Google Scholar]
- Szende B., Timar F., Hargitai B. (1994) Olive oil decreases liver damage in rats caused by carbon tetrachloride (CCl4). Exp Toxicol Pathol 46: 355–359. [DOI] [PubMed] [Google Scholar]
- Thoma C., Day C., Trenell M. (2012) Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol 56: 255–266. [DOI] [PubMed] [Google Scholar]
- Toshimitsu K., Matsuura B., Ohkubo I., Niiya T., Furukawa S., Hiasa Y., et al. (2007) Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition 23: 46–52. [DOI] [PubMed] [Google Scholar]
- Trovato F., Catalano D., Martines G., Pace P., Trovato G. (2015) Mediterranean diet and non-alcoholic fatty liver disease: the need of extended and comprehensive interventions. Clin Nutr 34: 86–88. [DOI] [PubMed] [Google Scholar]
- Ueno T., Sugawara H., Sujaku K., Hashimoto O., Tsuji R., Tamaki S., et al. (1997) Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol 27: 103–107. [DOI] [PubMed] [Google Scholar]
- Van der Heijden G., Wang Z., Chu Z., Sauer P., Haymond M., Rodriguez L., et al. (2010) A 12-week aerobic exercise program reduces hepatic fat accumulation and insulin resistance in obese, hispanic adolescents. Obesity 18: 384–390. [DOI] [PubMed] [Google Scholar]
- Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L., et al. (2015) Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149: 367–378 e365; quiz e314-365. [DOI] [PubMed] [Google Scholar]
- Vitaglione P., Morisco F., Mazzone G., Amoruso D., Ribecco M., Romano A., et al. (2010) Coffee reduces liver damage in a rat model of steatohepatitis: the underlying mechanisms and the role of polyphenols and melanoidins. Hepatology 52: 1652–1661. [DOI] [PubMed] [Google Scholar]
- Wong V., Chan R., Wong G., Cheung B., Chu W., Yeung D., et al. (2013) Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol 59: 536–542. [DOI] [PubMed] [Google Scholar]
- Wong V., Wong G., Yeung D., Lau T., Chan C., Chim A., et al. (2015) Incidence of non-alcoholic fatty liver disease in Hong Kong: a population study with paired proton-magnetic resonance spectroscopy.J Hepatol 62: 182–189. [DOI] [PubMed] [Google Scholar]
- Wouters K., van Gorp P., Bieghs V., Gijbels M., Duimel H., Lutjohann D., et al. (2008) Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 48: 486–474. [DOI] [PubMed] [Google Scholar]
- Xu C., Yu C., Xu L., Miao M., Li Y. (2010) high serum uric acid increases the risk for nonalcoholic fatty liver disease: a prospective observational study. PLoS One 5: e11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasutake K., Nakamuta M., Shima Y., Ohyama A., Masuda K., Haruta N., et al. (2009) Nutritional investigation of non-obese patients with non-alcoholic fatty liver disease: the significance of dietary cholesterol. Scand J Gastroenterol 44: 471–477. [DOI] [PubMed] [Google Scholar]
- Younossi Z., Stepanova M., Afendy M., Fang Y., Younossi Y., Mir H., et al. (2011) Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 9: 524–530 [DOI] [PubMed] [Google Scholar]
- Younossi Z., Stepanova M., Negro F., Hallaji S., Younossi Y., Lam B., et al. (2012) Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine 91: 319–327. [DOI] [PubMed] [Google Scholar]
- Zelber-Sagi S., Ben-Assuli O., Rabinowich L., Goldstein A., Magid A., Shalev V., et al. (2015a) The association between the serum levels of uric acid and alanine aminotransferase in a population-based cohort. Liver Int 35: 2408–2415. [DOI] [PubMed] [Google Scholar]
- Zelber-Sagi S., Buch A., Yeshua H., Vaisman N., Webb M., Harari G., et al. (2014a) Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol 20: 4382–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelber-Sagi S., Lotan R., Shlomai A., Webb M., Harrari G., Buch A., et al. (2012) Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol 56: 1145–1151. [DOI] [PubMed] [Google Scholar]
- Zelber-Sagi S., Nitzan-Kaluski D., Goldsmith R., Webb M., Blendis L., Halpern Z., et al. (2007) Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol 47: 711–717. [DOI] [PubMed] [Google Scholar]
- Zelber-Sagi S., Nitzan-Kaluski D., Goldsmith R., Webb M., Zvibel I., Goldiner I., et al. (2008) Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology 48: 1791–1798. [DOI] [PubMed] [Google Scholar]
- Zelber-Sagi S., Nitzan-Kaluski D., Halpern Z., Oren R. (2006) Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int 26: 856–863. [DOI] [PubMed] [Google Scholar]
- Zelber-Sagi S., Salomone F., Webb M., Lotan R., Yeshua H., Halpern Z., et al. (2015b) Coffee consumption and nonalcoholic fatty liver onset: a prospective study in the general population. Transl Res 165: 428–436. [DOI] [PubMed] [Google Scholar]
- Zelber-Sagi S., Salomone F., Yeshua H., Lotan R., Webb M., Halpern Z., et al. (2014b) Non-high-density lipoprotein cholesterol independently predicts new onset of non-alcoholic fatty liver disease. Liver Int 34: e128–e135. [DOI] [PubMed] [Google Scholar]