Abstract
Objective:
This study was designed to investigate the effects of Ocimum gratissimum (OG) on hematological parameters and oxidative stress in diabetic rats.
Materials and Methods:
Twenty-five male rats (150–200 g) were randomly grouped into five as control, normal + OG, diabetic untreated, diabetic + OG, and diabetic + glibenclamide groups. Diabetes was induced by 100 mg/kg of alloxan monohydrate in the diabetic untreated and diabetic + OG groups followed by treatment with distilled water and 400 mg/kg OG, respectively, whereas control, normal + OG, and diabetic + glibenclamide groups were treated with distilled water, 400 mg/kg OG, and 5 mg/kg glibenclamide, respectively. Body weight and fasting blood glucose level were monitored weekly. After 28 days of treatments, under anesthesia induced by 50 mg/kg sodium thiopental i.p., blood samples were obtained for hematological analysis, malondialdehyde (MDA) level determination, and superoxide dismutase (SOD) activity. Data were compared using analysis of variance and Student's t-test.
Results:
There was a significant decrease in the fasting blood glucose of the diabetic + OG animals compared to the diabetic untreated and the initial reduction in weight observed in this group was reversed at the end of the experiments. Packed cell volume, red blood cell count, and hemoglobin concentration were significantly increased (P < 0.05) in the diabetic + OG when compared with the untreated group. The MDA concentration was significantly lowered (P < 0.01) in the diabetic + OG group when compared with diabetic untreated while SOD activity was significantly reduced in the diabetic untreated group.
Conclusion:
It was concluded that OG reverses anemia secondary to alloxan-induced diabetes mellitus in rats probably via its antioxidant activity.
Keywords: Antioxidant, hematological parameters, lipid peroxidation, Ocimum gratissimum
INTRODUCTION
Diabetes is a metabolic disease characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both.[1] It is a major public health problem in both developed and developing countries. There are an estimated 246 million people with diabetes in the world, of whom about 80% reside in developing countries.[2] Globally, it is believed to be the fifth leading cause of death in the year 2000 after communicable diseases, cardiovascular disease, cancer, and injuries.[3] The prevalence of this disease continues to rise in developing countries, including Nigeria.
The chronic hyperglycemia causes a hypoxic environment in the renal interstitium which impairs the production of erythropoietin.[4] Erythropoietin is a glycoprotein hormone secreted by kidneys and stimulates erythropoiesis. Structural renal abnormalities may result in low concentrations of erythropoietin and also play a role in the etiology of anemia in diabetes;[5] erythropoietin depletion is thus closely associated with anemia in diabetes mellitus (DM).[6] Increased oxidative stress is widely accepted as a participant in the progression of diabetes and its complication, anemia in DM is also accompanied with increased free radicals or impaired antioxidant defenses.[7]
A prevalence of 30.4% anemia in DM was reported in an Iranian population[8] and several studies have also reported anemia in DM in animal models.[9,10] Regular screening and treatment of anemia in DM has been suggested to slow the progression of microvascular and macrovascular complications.[11]
Ocimum gratissimum (OG), Africa basil/sweet basil, is a plant belonging to Lamiaceae family known in Nigeria as efinrin, Nehonwu, and ai daya ta guda by the Yoruba, Igbo, and Hausa, respectively.[12] Its extract has gained popularity from its use in the curbing of various ailments. The crushed leaf juice is used in the treatment of convulsion, stomach pain, and catarrh while oil from the leaf has been shown to possess antiseptic, antibacterial, and antifungal activities.[13] Its leaves have antimicrobial and grain preservative potentials,[14] and used traditionally to treat DM.[15]
The hypoglycemic property of OG has been reported by several groups.[16,17] Furthermore, its antioxidant and hematological properties have been reported in normal rats;[18,19] this antioxidant activity was found comparable to that of gallic acid and ascorbic acid in an in vitro study.[20] However, it remains unknown, the effect of OG on diabetes-induced anemia and the probable role oxidative stress plays in its progression. Therefore, this study was designed to investigate the effect of OG on oxidative stress and hematological parameters in diabetic rats.
MATERIALS AND METHODS
Twenty-five male Wistar rats weighing between 150 and 200 g were used for this study. They were housed and acclimatized for 2 weeks in the Central Animal house of the College of Medicine, University of Ibadan, Ibadan, Nigeria. They were fed on standard rat pellet diet (Ladokun Feeds, Nigeria) and were allowed water ad libitum. The animals were maintained under standard laboratory conditions and were subjected to natural photoperiod of 12 h light: Dark cycle. All experimental protocols and handling were in compliance with the Guide for the Care and Use of Laboratory Animals.[21]
DM was induced by intraperitoneal injection of 100 mg/kg body weight of Alloxan monohydrate (Sigma®, St Louis, MO, USA). Diabetic status was confirmed after 72 h and animals with fasting blood glucose level ≥200 mg/dl were taken to be diabetic. Fresh leaves of OG were collected around Ibadan metropolis. Identification and authentication of the leaves were done at the Forestry Research Institute of Nigeria with voucher number: 110026. The fresh leaves were air-dried and pulverized; 1.3 kg of the powdery form was macerated in water for 24 h and filtered. The filtrate was concentrated in rotary evaporator and the total yield was 9.2%.
The animals were randomly divided into five groups of five animals each as - Group 1: Normal rats given distilled water (NC); Group 2: Normal rats treated with 400 mg/kg body weight of OG (NC + OG); Group 3: Diabetic untreated rats (DC); Group 4: Diabetic rats treated with 400 mg/kg body weight of OG (D + OG); Group 5: Diabetic rats given 5 mg/kg body weight of glibenclamide (D + GLB). All treatments were carried out for 28 days by oral gavage. Weight and blood glucose levels were monitored weekly throughout the experiment. Blood glucose level was determined using One Touch Ultra glucometer®. At the end of the experiments, the rats were anesthetized by intraperitoneal administration of 50 mg/kg b.wt. sodium thiopental (Rotex Medica, Trittau, Germany). A blood sample was collected through cardiac puncture into bottles containing lithium ethylenediaminetetraacetic acid for hematological analysis and plain samples bottles for determination of serum level of malondialdehyde (MDA) and superoxide dismutase (SOD).
Red blood cell count (RBC), packed cell volume (PCV), hemoglobin (Hb), white blood cell count (WBC), percentage lymphocyte, monocyte and granulocyte, and platelets (PLT) count were assayed using automated full blood count analyzer (Sysmex America Inc., Lincolnshire, IL60069, USA). Mean corpuscular volume (MCV), mean corpuscular Hb (MCH), and MCH concentration (MCHC) were calculated according to the methods described by Lewis et al.[22] For determining MDA concentrations, the assay method reported by Gutteridge and Wilkins[23] was adopted. The absorbance of the supernatant was read at 532 nm against a blank wherein serum was substituted with distilled water. The results were expressed as nanomoles MDA/ml. For determining SOD activity, the method described by Misra and Fridovich[24] was employed. For the determination of specific activity of SOD in 1 ml of blood serum, the rate of autoxidation of epinephrine was noted at 30 s intervals in all groups.
The data were expressed as mean and standard error of the mean. Statistical significance between the groups was assessed using one-way analysis of variance and Student's t-test. Differences in means were considered statistically significant at P < 0.05.
RESULTS
At the 2nd week of all treatments, all diabetic animals had significant weight loss when compared with the two normal animal groups. This initial weight loss were however reversed in the OG treated and glibenclamide treated diabetic rats in the 3rd week and at the end of the 4th week, there was a significant difference in the weight of the control and the diabetic untreated animals [Figure 1].
Figure 1.
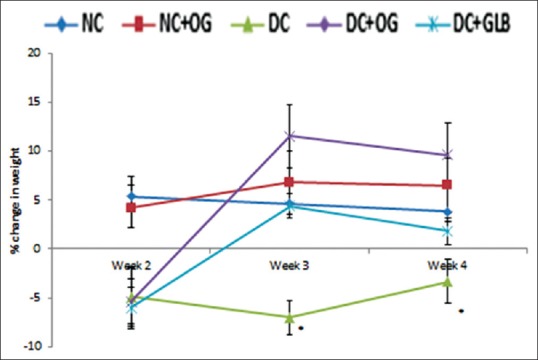
Effects of Ocimum gratissimum on weekly percentage change in weight in normal and diabetic rats. N = 5, *P < 0.05
Figure 2 shows a progressive reduction in the fasting blood glucose level of the diabetic rats treated with 400 mg/kg b.wt. OG. At the end of the 4th week, the reduction was significantly different from the diabetic untreated group (P < 0.05). The pattern of the observed reduction in the fasting blood glucose level of OG treated diabetic rats was similar to that of glibenclamide treated diabetic rats.
Figure 2.
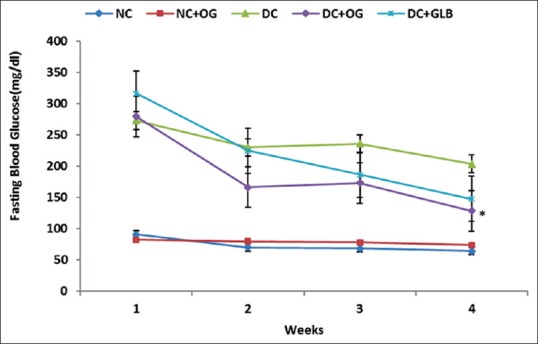
Effects of Ocimum gratissimum on fasting blood glucose level in normal and diabetic rats. N = 5, *P < 0.05
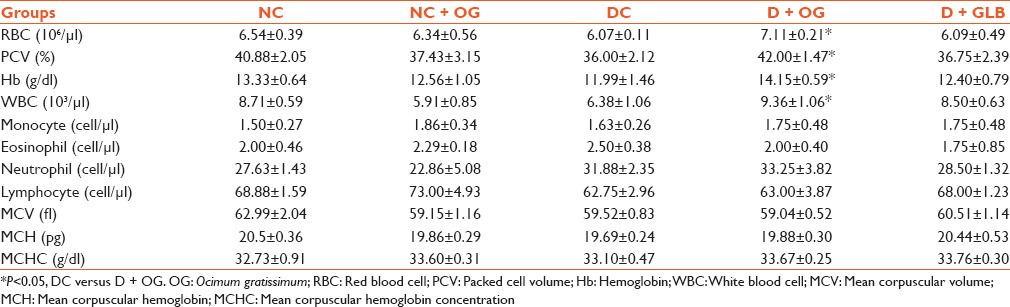
As shown in Table 1, there were significant increases in the RBC, PCV and Hb concentration of diabetic animals treated with OG when compared with the untreated group (P < 0.05). Although there was a significant increase in total WBC of the OG treated diabetic animals compared with the diabetic untreated (P < 0.05), there was no difference in the differential count of all treated groups. Furthermore, MCV, MCH and MCHC were not statistically different in all groups.
Table 1.
Effect of Ocimum gratissimum on hematological parameters in diabetic rats
There was a significant increase in the MDA level of diabetic untreated animals when compared with the control group (P < 0.05). This observed increase was significantly reduced in the diabetic animals treated with OG while there was no significant difference in the MDA levels of the diabetic and diabetic-glibenclamide treated group [Figure 3]. SOD activity was significantly decreased in the diabetic untreated group when compared with the control and all other treated groups [Figure 4].
Figure 3.
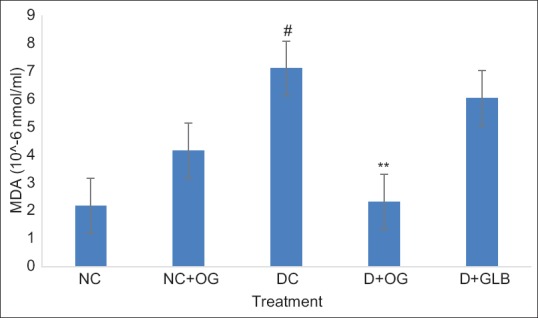
Effects of Ocimum gratissimum on malondialdehyde level in normal and diabetic rats. N = 5, #P < 0.05 (NC vs. DC), **P < 0.01 (DC vs. D + OG)
Figure 4.
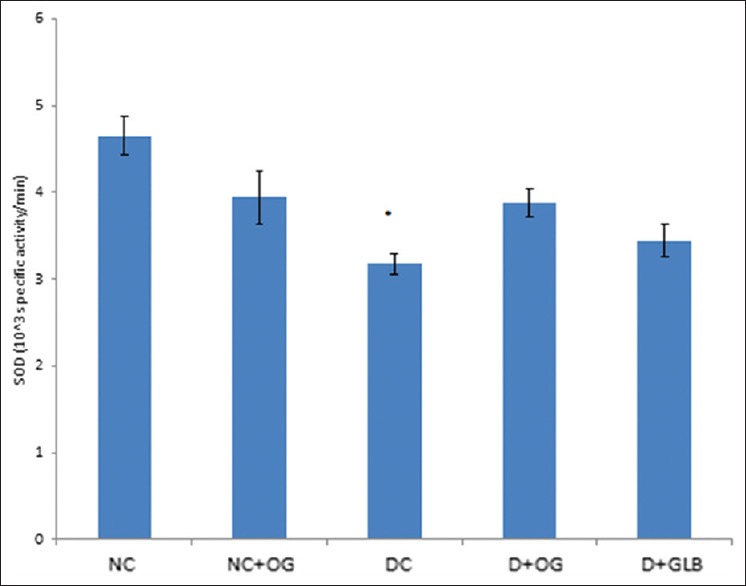
Effects of Ocimum gratissimum on superoxide dismutase activity in normal and diabetic rats. N = 5, *P < 0.05 (DC vs. D + OG)
DISCUSSION
The observed reversal of the initial weight loss in the diabetic animals treated with OG is in accordance with a previous report[25] that OG could ameliorate diabetes-induced weight loss in rats and the observed decreased in blood glucose level of diabetic animals following OG administration is in line with its well-documented hypoglycemic effect.
DM is associated with increased risk of anemia in rats;[9] this anemia could be attributed to the increased nonenzymatic glycosylation of RBC membrane protein which accompanies hyperglycemia.[26] The oxidation of these glycosylated membrane proteins and hyperglycemia in DM cause an increase in the production of lipid peroxides causing hemolysis of RBCs. Lipid peroxidation, an index of oxidative stress in vivo, is often characterized by high concentration of MDA, a biomarker of oxidative stress.[27] A high level of MDA had been reported in diabetic rats.[28] This is consistent with the observed increased in MDA level and decreased RBC count of the diabetic rats in this study.
Contrary to the report[19] that OG increased PCV and RBC in normal rats, there was no significant difference in the PCV, RBC, and Hb in the normal rats treated with OG in this study. This observation is also at variance with the decreased hematological variables reported by Jimoh et al.[29] in normal rats.
Interestingly, RBC, PCV, and Hb were all significantly increased in the diabetic rats following OG administration which agrees with recent findings in diabetic rats[30] and phenylhydrazine-induced anemic rats;[31] this indicates that OG might probably have a boosting effect on hematological variables in this group of animals as a concurrent significant reduction was observed in their MDA level. These findings are also supported by the observed significantly increased SOD level of these diabetic animals following OG treatment when compared with the untreated animals. SOD is a first-line antioxidant enzyme which catalyzes the dismutation of superoxide anion (O−). This anion could react with nitric oxide to produce a potent oxidant and nitrosating agent, peroxynitrite (ONOO−), which can damage proteins, lipids, and DNA directly.[32] Flavonoids and phenolic compounds such as cirsilineol, cirsimaritin, isothymusin, isothymonin, apigenin, and rosmarinic acid which are present in the leaves of OG are known to exhibit antioxidant activities as well as scavenge superoxide radicals[33] accordingly a preliminary phytochemical screening of the extract used in this study showed presence of flavonoid and phenol.
Low Hb concentration is strongly associated with diabetic profiles[34] as it contributes to the development of cardiovascular disease in patients with diabetes.[35] Although this study did not assess the glycosylated Hb as an indicator of progression of diabetic treatment, it is known that oxygen carrying capacity is proportional to the circulating Hb concentration[36] and this might suggests an unhindered oxidative metabolism particularly when the level is within the physiological limit, 11–18 g/dl in rat.[37] In spite of the significant increase in WBC, there was no effect of OG on lymphocyte, neutrophil, eosinophil, basophil, and PLT count in all treated groups.
CONCLUSION
It is concluded from this study that the aqueous leaf extract of OG may have hemopoetic roles in diabetic rats through its antioxidant properties.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:581–90. [Google Scholar]
- 2.Sicree R, Shaw J, Zimmet P. Diabetes and impaired glucose tolerance. In: Gan D, editor. Diabetes Atlas. 3rd ed. Belgium: International Diabetes Federation; 2006. pp. 15–103. [Google Scholar]
- 3.Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, et al. The burden of mortality attributable to diabetes: Realistic estimates for the year 2000. Diabetes Care. 2005;28:2130–5. doi: 10.2337/diacare.28.9.2130. [DOI] [PubMed] [Google Scholar]
- 4.Singh DK, Winocour P, Farrington K. Erythropoietic stress and anemia in diabetes mellitus. Nat Rev Endocrinol. 2009;5:204–10. doi: 10.1038/nrendo.2009.17. [DOI] [PubMed] [Google Scholar]
- 5.Inomata S, Itoh M, Imai H, Sato T. Serum levels of erythropoietin as a novel marker reflecting the severity of diabetic nephropathy. Nephron. 1997;75:426–30. doi: 10.1159/000189580. [DOI] [PubMed] [Google Scholar]
- 6.Winkler AS, Marsden J, Chaudhuri KR, Hanbley H, Watkins PJ. Erythropoietin depletion and anemia in diabetes mellitus. Diabet Med. 1999;16:813–9. doi: 10.1046/j.1464-5491.1999.00172.x. [DOI] [PubMed] [Google Scholar]
- 7.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 8.Hosseini MS, Rostami Z, Saadat A, Saadatmand SM, Naeimi E. Anemia and microvascular complications in patients with type 2 diabetes mellitus. Nephrourol Mon. 2014;6:e19976. doi: 10.5812/numonthly.19976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adeyi AO, Idowu BA, Mafiana CF, Oluwalana SA, Ajayi OL, Akinloye OA. Rat model of food-induced non-obese-type 2 diabetes mellitus: Comparative pathophysiology and histopathology. Int J Physiol Pathophysiol Pharmacol. 2012;4:51–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Akomas SC, Okafor A, Ikechukwu I, Solomon N. Glucose level, hematological parameters and lipid profile in ficus surtreated diabetic rats. Compr J Agric Biol Sci. 2014;2:5–11. [Google Scholar]
- 11.McGill JB, Bell DS. Anemia and the role of erythropoietin in diabetes. J Diabetes Complications. 2006;20:262–72. doi: 10.1016/j.jdiacomp.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Ayinla MT, Dada SO, Shittu ST, Olayaki LA, Akiode AO, Ojulari SL. Anti-hyperlipidemic effect of aqueous leaf extract of Ocimum gratissimum in alloxan induced diabetic rats. Int J Med Med Sci. 2011;3:360–3. [Google Scholar]
- 13.Ezekwesili CN, Obiorah KA, Ugwu OP. Evaluation of anti-diarrheal effect of Ocimum gratissimum crude extract on albino rats. Biokemistri. 2004;16:122–31. [Google Scholar]
- 14.Mann A. Phytochemical constituents and antimicrobial and grain protectant activities of clove basil (Ocimum gratissimum L.) grown in Nigeria. Int J Plant Res. 2012;2:51–8. [Google Scholar]
- 15.Bailey CJ, Day C. Traditional plant medicine as treatment for diabetes. Diabetes Care. 1989;12:553–64. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 16.Aguiyi JC, Obi CI, Gang SS, Igweh AC. Hypoglycaemic activity of Ocimum gratissimum in rats. Fitoterapia. 2000;71:444–6. doi: 10.1016/s0367-326x(00)00143-x. [DOI] [PubMed] [Google Scholar]
- 17.Egesie UG, Adelaiye AB, Ibu JO, Egesie OJ. Safety and hypoglycaemic properties of aqueous leaf extract of Ocimum gratissimum in streptozotocin induced diabetic rats. Niger J Physiol Sci. 2006;1:31–5. doi: 10.4314/njps.v21i1-2.53971. [DOI] [PubMed] [Google Scholar]
- 18.Aprioku JS, Obianime AW. Antioxidant activity of the aqueous crude extract of Ocimum gratissimum Linn. leafon basal and cadmium-induced serum levels of phosphatases in male guinea-pigs. J Appl Sci Environ Manage. 2008;12:33–9. [Google Scholar]
- 19.Ofem OE, Ani EJ, Eno AE. Effect of aqueous leaves extract of Ocimum gratissimum on hematological parameters in rats. Int J Appl Basic Med Res. 2012;2:38–42. doi: 10.4103/2229-516X.96807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akinmoladun AC, Ibukun EO, Afor E, Obutor EM, Farombi EO. Phytochemical constituents and antioxidant activity of extract from the leaves of Ocimum gratissimum. Sci Res Essay. 2007;2:163–6. [Google Scholar]
- 21.8th ed. Washington (DC): National Academies Press (US); 2011. National Research Council. Guide for the Care and Use of Laboratory Animals; p. 209. [PubMed] [Google Scholar]
- 22.Lewis SM, Bain BJ, Bates I. 10th ed. Philadelphia: Churchill Livingstone; 2006. Dacie and Lewis Practical Hematology; pp. 25–54. [Google Scholar]
- 23.Gutteridge JM, Wilkins S. Copper-dependent hydroxyl radical damage to ascorbic acid: Formation of a thiobarbituric acid-reactive product. FEBS Lett. 1982;25; 137:327–30. doi: 10.1016/0014-5793(82)80377-3. [DOI] [PubMed] [Google Scholar]
- 24.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;25; 247:3170–5. [PubMed] [Google Scholar]
- 25.Okon UA, Ita SO, Ekpenyong CE, Davies KG, Inyang OI. Reduction of platelet and lymphocyte counts and elevation of neutrophil counts in rats treated with aqueous leaf extract of Ocimum gratissimum. Afr J Biochem Res. 2011;5:303–6. [Google Scholar]
- 26.Kennedy L, Baynes JW. Non-enzymatic glycosylation and the chronic complications of diabetes: An overview. Diabetologia. 1984;26:93–8. doi: 10.1007/BF00281113. [DOI] [PubMed] [Google Scholar]
- 27.Aruoma IO. Free radicals, oxidative stress, and antioxidants in human health and disease. JAOCS. 1998;75:199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moussa SA. Oxidative stress in diabetes mellitus. Rom J Biophys. 2008;18:225–36. [Google Scholar]
- 29.Jimoh OR, Olaore J, Olayaki LA, Olawepo AO, Biliaminu SA. Effects of aqueous extract of Ocimum gratissimum on hematological parameters of Wistar rats. Biokemistri. 2008;20:33–7. [Google Scholar]
- 30.Agbai EO, Nwafor A, Ugwu FN. The hematological action of ethanol extracts of Gongronema latifolium and Ocimum gratissimum in alloxan induced diabetic rats. Int J Adv Pharm Biol Chem. 2014;3:235–40. [Google Scholar]
- 31.Thomas N, Okem UE, Imelda NW, Eghosa IE, Paschal CC, Chisolu UA, et al. Methanolic crude leaf extract of Ocimum gratissimum reverses phenylhydrazine-induced anemia in albino Wistar rats. Niger J Exp Clin Biosci. 2013;1:23–7. [Google Scholar]
- 32.Di Naso FC, Simões Dias A, Porawski M, Marroni NA. Exogenous superoxide dismutase: Action on liver oxidative stress in animals with streptozotocin-induced diabetes. Exp Diabetes Res 2011. 2011 doi: 10.1155/2011/754132. 754132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelm MA, Nair MG, Strasburg GM, DeWitt DL. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine. 2000;7:7–13. doi: 10.1016/S0944-7113(00)80015-X. [DOI] [PubMed] [Google Scholar]
- 34.Kwon E, Ahn C. Low hemoglobin concentration is associated with several diabetic profiles. Korean J Intern Med. 2012;27:273–4. doi: 10.3904/kjim.2012.27.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevens PE. Anaemia, diabetes and chronic kidney disease: Where are we now? J Ren Care. 2012;38(Suppl 1):67–77. doi: 10.1111/j.1755-6686.2012.00281.x. [DOI] [PubMed] [Google Scholar]
- 36.Yip R. Significance of an abnormally low or high hemoglobin concentration during pregnancy: Special consideration of iron nutrition. Am J Clin Nutr. 2000;72(1 Suppl):272S–9S. doi: 10.1093/ajcn/72.1.272S. [DOI] [PubMed] [Google Scholar]
- 37.Raji Y. Effects of bioactive principles from stem bark extract of Quassia amara, Quassin and 2-methoxycanthine-6-one, on haematological parameters in albino rats. Niger J Physiol Sci. 2010;25:103–6. [PubMed] [Google Scholar]


