Abstract
Context:
Although the primary cause of premature hair graying (PHG) is considered to be genetic, certain environmental factors also play a role. Trace element deficiencies such as Vitamin B12, Vitamin D3, and calcium may also be associated with PHG. However, India-specific data are relatively sparse.
Aims:
The present study aimed at identifying factors associated with PHG in Indian patients.
Settings and Design:
A case–control study was conducted at a trichology clinic in Bengaluru between October 2013 and April 2014 with a total of 37 cases of PHG and 37 age- and gender-matched controls.
Materials and Methods:
A total of 100 subjects were investigated for various parameters such as hemoglobin, serum ferritin, zinc, copper, calcium, Vitamin B12, and Vitamin D after obtaining informed consent.
Statistical Analysis Used:
Chi-square test was used to compare proportions between groups. Means were compared between groups using Student's t-test.
Results:
Serum ferritin levels were lower in patients with PHG as compared to the control group and the differences were statistically significant (P < 0.001). Furthermore, as compared to the controls, patients with PHG had lower serum Vitamin B12 levels (P < 0.001). Individuals with PHG had significantly lower levels of high-density lipoprotein cholesterol (HDL-C) as compared to the control group (P < 0.001). Significant proportions of patients with PHG had a sedentary lifestyle and admitted to having irregular eating habits.
Conclusion:
PHG is associated with low serum ferritin, Vitamin B12, and HDL-C levels in Indian patients aged <25 years. However, studies with large sample sizes may be required to conclusively define these putative associations.
Keywords: Hair graying, premature hair graying, trace elements, Vitamin D
INTRODUCTION
Hair is important to an individual's physical appearance and self-perception.[1] Aging of hair follicle refers to reduced melanocyte function and decreased hair production.2 Hair graying is a natural age-associated feature.3 The term premature hair graying. (PHG) is used when it occurs before the age of 20 in whites, 25 in Asians, and 30 in Africans. Although the primary cause of PHG may be genetic, studies have also reported the role of autoimmune disorders, smoking, and trace element deficiencies.[4]
Sparse data in Indian settings prompted us to investigate the factors associated with PHG among Indian patients through a case–control study.
MATERIALS AND METHODS
A 6-month long case–control study was conducted between October 2013 and April 2014. The study was approved by the Institutional Ethics Committee. A total of 50 subjects (aged <25 years) with PHG were included in the study. In this study, PHG was defined as 5 or more gray hair in the scalp in the age group between 15 and 25 years.[4] A densitometer was used to count the number of gray hair in temporal, parietal, vertex, and occipital areas. The study also included 50 age-matched controls. Controls included patients visiting our clinic for hair problems other than PHG. A signed informed consent was obtained from all the participants in the study.
This study assessed the association of PHG with abnormal hemoglobin (Hb), serum ferritin, trace element, lipid parameters, glycemic parameters, and thyroid-stimulating hormone (TSH). Furthermore, the association of sedentary lifestyle and irregular food habits with PHG was also assessed.
Hb was estimated by cell counter method. TSH, serum ferritin, Vitamin B12, and Vitamin D were analyzed by electrochemiluminescence immunoassay. Serum zinc and copper were determined by colorimetric method. Fasting blood sugar (FBS) and fasting insulin were determined using glucose hexokinase reagent and chemiluminescence immunoassay, respectively. The homeostatic model assessment-estimated insulin resistance (HOMA-IR) was calculated with a cut-off value of 1.7. High-density lipoprotein cholesterol (HDL-C) (by direct enzymatic method), low-density lipoprotein (LDL) (derived), very-LDL (derived), and triglycerides (using GPO Trinder reagent) were all examined in the fasting state.
Sedentary lifestyle was defined as subjects with no or irregular physical activity. Sedentary lifestyle was measured by eliciting self-reporting from patients. In this study, irregular eating habits were defined as a deviation from regular meal timing for 1 h or more at least once per week. Questions regarding the practice of skipping meals were worded to detect subjects who omitted noncorresponding meals of the day (i.e., not the same meal every day). This included questions regarding regularity of meals, frequency and duration of any changes to usual meal timing, variation in the amount of food eaten, and practice of skipping meals. Subjects who missed the same meal each day were considered to have a regular meal pattern consisting of one less meal per day.
The data were analyzed using Statistical Package for the Social Sciences version 22.0 (SPSS Inc., Chicago, IL, USA). The mean ± standard deviation (SD) and number and percentage of participants were tabulated. Means were compared across groups using t-test, and proportions were compared with Karl Pearson's Chi-square test. Statistical significance was set at P < 0.05.
RESULTS
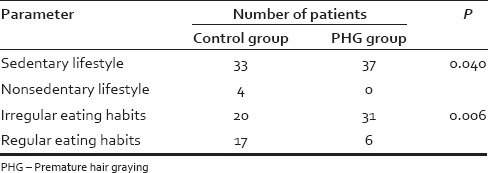
A total of 100 subjects were assigned to the study. Mean age (SD) was 22.8 (1.7) and 22.8 (1.8) in the case and control group, respectively. After matching controls for age and gender, data from a total of 74 records (14 females and 60 males) were analyzed. Table 1 presents the mean (SD) of laboratory parameters such as Hb, serum ferritin, and trace elements observed in the case and control groups. Mean serum ferritin levels were 34.4 ng/mL in women with PHG as compared to 75.78 ng/mL in controls (P = 0.014). Mean serum ferritin levels were 73.68 ng/mL in men with PHG as compared to 126.16 ng/mL in controls (P = 0.001). The mean (SD) of lipid parameters and TSH observed in the case and control groups are shown in Table 2. Table 3 presents the mean (SD) of glycemic parameters such as FBS, fasting insulin, and HOMA-IR in the case and control groups. The significant association of sedentary lifestyle and irregular food habits with PHG is shown in Table 4.
Table 1.
Mean (standard deviation) of hemoglobin, serum ferritin, and trace elements in premature hair graying and control groups
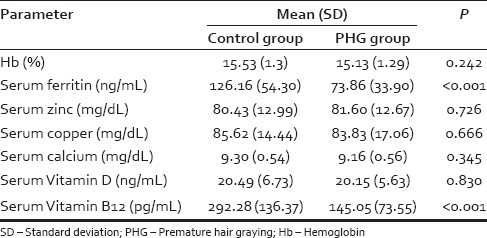
Table 2.
Mean (standard deviation) of lipid parameters and thyroid-stimulating hormone in premature hair graying and control groups
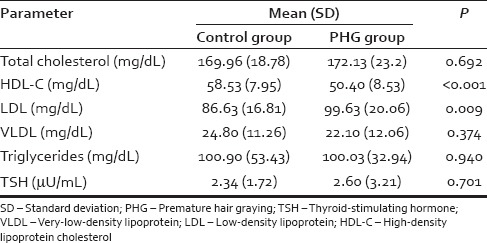
Table 3.
Mean (standard deviation) of glycemic parameters in premature hair graying and control groups
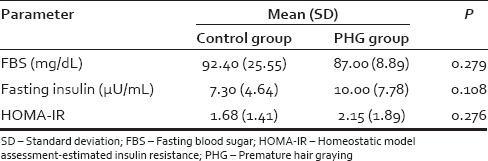
Table 4.
Lifestyle and food habits in premature hair graying and control groups
DISCUSSION
Skin and hair play an important role in human outlook. Length of the hair, its color, and style are of immense value to an individual's physical appearance and self-perception.[1] Aging of hair comprises two important components, namely, weathering of hair shaft and aging of hair follicle. Weathering of hair shaft involves degeneration of hair fiber that progresses from the root to the tip. Aging of hair follicle refers to reduced melanocyte function (known as graying) and decreased hair production.[2]
Hair graying (or canities) is considered to be a natural age-associated feature.[3] A recent study that was carried out challenging the “50” rule of thumb (at least 50% of individuals have 50% gray hair by the age of 50 years) reported that the global range of individuals having 50% gray hair by the age of 50 years was between 6% and 23%.[5]
Hair color being one of the most conspicuous phenotypes in human beings, it ranges from black, brown, and blonde-to-red.[6] The two important components that are responsible for this diversity of hair color include the quantity and ratio of black-brown melanin and reddish-brown pheomelanin.[4] This diversity of hair pigmentation in human beings ranges within physiological variations. However, it differs among the three major ethnic populations, namely, Asians, Africans, and Caucasians.[6]
Although the primary cause of PHG is considered to be genetic, autoimmune disorders such as vitiligo, pernicious anemia, autoimmune thyroid disorders, and Werner's syndrome are also shown to be causative. Few studies have also reported that environmental factors (such as ultraviolet light and climate), smoking, drugs, deficiencies of trace elements, and nutritional deficiencies also play a role in PHG.[4]
Studies have postulated that iron affects melanogenesis.[4] In the current study, serum ferritin levels were low in individuals with PHG as compared to the controls (P < 0.001) despite normal Hb levels in both the groups.
The cells of hair follicle are considered to be rapidly dividing cells. However, proliferation of these cells depends on the synthesis of DNA, which is further dependent on sufficient supply of Vitamin B12.[7] Vitamin B12 facilitates stabilization of the initial anagen phase of hair follicle.[8] In the current study, serum Vitamin B12 levels were significantly low in individuals with PHG as compared to the controls (P < 0.001).
The current study reported that individuals with PHG had significantly lower levels of HDL-C as compared to the control group (P < 0.001). Studies with large sample sizes may be required to conclusively define this association.
Bhat et al. reported that serum calcium levels and Vitamin D3 levels were significantly lower in patients with PHG.[4] The current study could not identify any such association. However, Vitamin D levels were found to be lower in both the case and control groups.
The current study did report a few interesting findings that were significantly different between patients with PHG and the control group. A significant number of patients with PHG had a sedentary lifestyle as compared to those who enjoyed a nonsedentary lifestyle (P = 0.006). The current study also reported that a significant proportion of patients with PHG admitted to irregular eating habits as compared to those with regular eating habits (P < 0.001). Studies with large sample sizes may be required to conclusively define any such putative associations.
Zayed et al. reported that there exists a significant association between PHG and cigarette smoking.[9] The current study did report an association between PHG and smoking. This study also reported that there was no significant association between PHG and consumption of alcohol and stress.
CONCLUSION
It appears that PHG is associated with lower serum ferritin, Vitamin B12, and HDL-C levels in Indian patients aged <25 years. However, studies with larger sample sizes may be required to conclusively define these putative associations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pandhi D, Khanna D. Premature graying of hair. Indian J Dermatol Venereol Leprol. 2013;79:641–53. doi: 10.4103/0378-6323.116733. [DOI] [PubMed] [Google Scholar]
- 2.Trüeb RM. Pharmacologic interventions in aging hair. Clin Interv Aging. 2006;1:121–9. doi: 10.2147/ciia.2006.1.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trüeb RM. Oxidative stress in ageing of hair. Int J Trichology. 2009;1:6–14. doi: 10.4103/0974-7753.51923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhat RM, Sharma R, Pinto AC, Dandekeri S, Martis J. Epidemiological and investigative study of premature graying of hair in higher secondary and pre-university school children. Int J Trichology. 2013;5:17–21. doi: 10.4103/0974-7753.114706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panhard S, Lozano I, Loussouarn G. Greying of the human hair: A worldwide survey, revisiting the ‘50’ rule of thumb. Br J Dermatol. 2012;167:865–73. doi: 10.1111/j.1365-2133.2012.11095.x. [DOI] [PubMed] [Google Scholar]
- 6.Ito S, Wakamatsu K. Diversity of human hair pigmentation as studied by chemical analysis of eumelanin and pheomelanin. J Eur Acad Dermatol Venereol. 2011;25:1369–80. doi: 10.1111/j.1468-3083.2011.04278.x. [DOI] [PubMed] [Google Scholar]
- 7.Volkov I, Press Y, Rudoy I. Vitamin B12 could be a “master key” in the regulation of multiple pathological processes. J Nippon Med Sch. 2006;73:65–9. doi: 10.1272/jnms.73.65. [DOI] [PubMed] [Google Scholar]
- 8.Krugluger W, Stiefsohn K, Laciak K. Vit B 12 activates wnt-pathway in human hair follicle by induction of β catenin and inhibition of glycogen synthase kinase 3 transcription. J Cosmet Dermatol Sci Appl. 2011;1:25–9. [Google Scholar]
- 9.Zayed AA, Shahait AD, Ayoub MN, Yousef AM. Smokers’ hair: Does smoking cause premature hair graying? Indian Dermatol Online J. 2013;4:90–2. doi: 10.4103/2229-5178.110586. [DOI] [PMC free article] [PubMed] [Google Scholar]


