Juvenile amyotrophic lateral sclerosis (jALS) is characterized by progressive upper and lower motor neuron degeneration leading to facial muscle spasticity, spastic dysarthria, and spastic gait with an early onset (before 25 years old). Unlike adult-onset amyotrophic lateral sclerosis (ALS), patients with jALS tend to have slower progression of motor neuron disease and prolonged survival to a normal life expectancy. Mutations in FUS gene have been reported in jALS,1 including p.P525L mutation that has been consistently associated with early onset and aggressive presentation.2 Here, we report a patient carrying p.P525L FUS mutation and experiencing an aggressive course of ALS presenting with dysphonia and diplopia.
The patient was a 21-year-old woman who initially reported progressive dysphonia followed by the development of diplopia. She was previously healthy with no known family history of neuromuscular disorders. Four months after her initial presentation, she developed bilateral ptosis and weakness of her upper extremities. Her weakness was initially subtle in the right deltoids (4+/5 strength), with ptosis and dysphonia being her primary concerns. MRI of brain and cervical spine were reported as normal. Six months after her initial presentation, she developed a viral upper respiratory tract infection and a new head droop and dyspnea, which prompted her intubation only days after her hospitalization. Repeat brain and cervical spine MRI were negative; however, EMG disclosed lower motor neuron dysfunction with no sensory involvement. She received several courses of IV immunoglobulin and plasma exchange with no clinical improvement. The condition of the patient continued to worsen, and she was transferred to our institution for further workup. Clinically, she was alert, with no obvious signs of cognitive impairment. Examination of the cranial nerves revealed limited upgaze and substantially slowed saccades. Extraocular (EO) movements were restricted in all directions, and she reported diplopia in all extremes of vision. She had bilateral ptosis and facial, tongue, and sternocleidomastoid weakness with extensive fasciculations. Motor examination revealed spasticity of the lower extremities, with decreased bulk in the upper extremities and diffuse fasciculations. She had substantial proximal weakness in the upper extremities, with no observable movement in deltoids, but somewhat greater strength in her hands and lower extremities. There were brisk reflexes in all 4 extremities, with downgoing plantar reflexes. The rest of the neurologic examination was unremarkable.
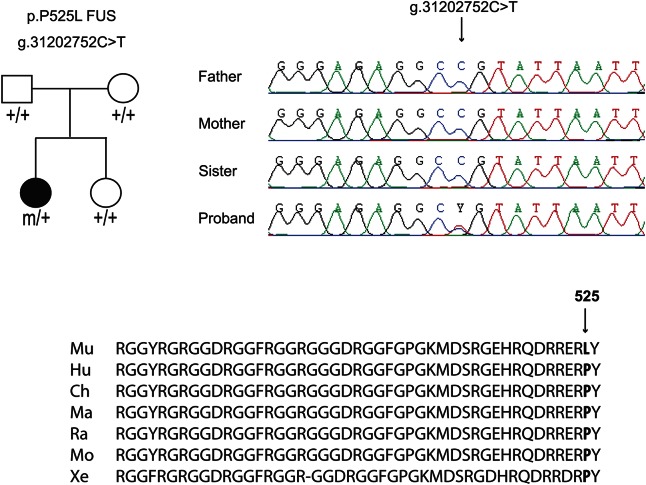
Structural, inflammatory, toxic, paraneoplastic, and other causes were ruled out. A repeat EMG was performed and revealed active denervation with intact sensory nerve conductions. The p.P525L FUS mutation was identified in the patient by Sanger sequencing, and segregation analysis across the family showed that the mutation appeared de novo (figure, appendix e-1 at Neurology.org/ng).
Figure. Pedigree of the family and segregation analysis of the FUS p.P525L mutation.
Nucleotide and protein positions are according to NM_004960 from GRCh37/hg19 on the positive DNA strand (chromosome 16). Mu = Mutant; Hu = Human; Ch = Chimpanzee; Ma = Macaque; Ra = Rat; Mo = Mouse; Xe = Xenopus.
On follow-up examination 1.5 years later, the patient remained in a long-term care hospital, ventilated. She was able to follow commands, although her responses were limited to minimal cranial nerve functions only. She had very slow saccades with limitations of her EO movements in all directions. Cranial nerve examination revealed only +2/5 strength in the buccinator muscle and a flicker of tongue movement. The motor examination revealed flaccid tone, profound wasting in all muscle groups, and no movement in the upper and lower extremities, without elicitation of reflexes. Her parents and siblings at the time of follow-up were asymptomatic and otherwise healthy.
This case is an atypical presentation of ALS because of the involvement of the EO muscles, including slow saccades and diplopia. The involvement of EO muscles has been reported in patients with ALS, but it is a rare presentation,3,4 which probably caused the delay in diagnosis. The rapid progression to ventilation only 6 months after the initial presentation is exceptional compared with previously reported cases. This was complicated by a respiratory tract infection, which seemed to accelerate the disease progression. To our knowledge, the association of p.P525L FUS mutation and clinical EO muscle involvement is rare.
This case report strengthens the role of FUS in jALS and the association of the p.P525L mutation with an aggressive course of jALS. Although genetic testing in individuals with no family history is controversial, it is becoming increasingly clear that FUS mutation screening and more precisely p.P525L genotyping of sporadic jALS should be done in clinical practice considering the increasing number of cases reported carrying FUS mutation. Further studies are needed to better assess the prevalence of EO muscle involvement in patients carrying p.P525L FUS mutation and more generally in patients with jALS to determine whether this phenotype could be used as an early diagnostic criterion in aggressive forms of jALS.
Supplementary Material
Acknowledgments
Acknowledgment: The authors thank the patient and her family. In addition, we thank Dr. Ken Mast for his medical contributions, Sandra B. Laurent for her contributions with the maternity and paternity tests and the Sanger sequencing, and Pascale Hince and Dr. Hélène Catoire for their contributions with the preparation of the samples.
Footnotes
Supplemental data at Neurology.org/ng
Author contributions: C. S. Leblond and A. Webber: study concept and design, acquisition of data, analysis and interpretation, and writing the manuscript. Z. Gan-Or, F. Moore, and P. A. Dion: critical revision of the manuscript for important intellectual content. A. Dagher and G. A. Rouleau: study concept and design and critical revision of the manuscript for important intellectual content.
Study funding: No targeted funding reported.
Disclosure: Dr. Leblond is recipient of Tim E. Noël fellowship from ALS Canada. Dr. Webber has served on the scientific advisory board of Genzyme. Dr. Gan-Or has received research support from the Canadian Institute of Health Research, McGill University, and the Michael J. Fox Foundation. Dr. Moore has participated in a clinical trial sponsored by Novartis. Dr. Dagher has served on the editorial board of Neuroimage and has received research support from the Canadian Institutes for Health Research. Dr. Dion reports no disclosures. Dr. Rouleau has received ALS-related funding from the Canadian Institutes of Health Research, the ALS Association, and the ALS Society of Canada. Go to Neurology.org/ng for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Leblond CS, Kaneb HM, Dion PA, Rouleau GA. Dissection of genetic factors associated with amyotrophic lateral sclerosis. Exp Neurol 2014;262:91–101. [DOI] [PubMed] [Google Scholar]
- 2.Conte A, Lattante S, Zollino M, et al. P525L FUS mutation is consistently associated with a severe form of juvenile amyotrophic lateral sclerosis. Neuromuscul Disord 2012;22:73–75. [DOI] [PubMed] [Google Scholar]
- 3.Ahmadi M, Liu JX, Brannstrom T, Andersen PM, Stal P, Pedrosa-Domellof F. Human extraocular muscles in ALS. Invest Ophthalmol Vis Sci 2010;51:3494–3501. [DOI] [PubMed] [Google Scholar]
- 4.Beaufils E, Corcia P, de Toffol B, Praline J. Occurrence of eye movement disorders in motor neuron disease. Amyotroph Lateral Scler 2012;13:84–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.