Charcot-Marie-Tooth neuropathy type 4 (CMT4) comprises a large group of genetically heterogeneous progressive sensory motor neuropathies characterized by autosomal recessive inheritance. Among these, CMT4B includes 3 forms related to genes of the myotubularin family, namely CMT4B1 (MTMR2), CMT4B2 (MTMR13/SBF2), and CMT4B3 (MTMR5/SBF1).
Only 2 CMT4B3 families have been reported to date. In the original Korean family, 3 siblings showed a homogeneous phenotype of pure sensory motor demyelinating neuropathy with focally folded myelin sheaths, closely resembling CMT4B1 and CMT4B2. All patients had onset of distal atrophy and weakness in upper and lower limbs, decreased vibration and position sense, areflexia, and pes planus in the first decade, with a slow progression to loss of ambulation in the fifth decade of life. None had cognitive impairment, dysmorphic features, or obvious extraneurologic syndromic manifestations.1
In the second SBF1-mutated family, from Saudi Arabia, the 3 affected siblings presented a more complex syndromic phenotype. Sensory motor polyneuropathy was associated with progressive microcephaly, intellectual disability, syndactyly, and multiple cranial nerve involvement, which resulted in ophthalmoparesis, absence of pupil reactivity to light, mild facial weakness, swallowing difficulties, and dysarthria. There was distal muscle wasting and weakness but no pes cavus. Brain MRI showed unspecific diffuse brain atrophy.2
We further expand the phenotypic spectrum of SBF1-associated CMT to include “fork and bracket” syndrome, a peculiar condition that we previously described in a Syrian family.3 The 2 affected siblings from this family were recently reassessed, and whole-exome sequencing was performed in the proband. Only the SBF1 homozygous p.L335P mutation survived the filtering pipeline (e-Methods and figure e-1 at Neurology.org/ng). The 2 siblings shared relevant features with the Saudi Arabian family, including early-onset progressive microcephaly, multiple cranial nerve neuropathies, and moderate to severe intellectual disability. Moreover, the sister recently developed a severe oromandibular dystonia that impaired mouth closure, making it difficult to eat and speak. In contrast to CMT4B1, CMT4B2, and the pure neuropathic form of CMT4B3, which are all characterized by demyelinating neuropathy with focally folded myelin sheaths, both families presented a predominantly axonal sensory motor neuropathy with evidence of denervation, markedly reduced amplitude of action potentials, and relatively preserved nerve conduction velocities (table e-1). However, there were also clinical differences, as proprioception, touch, and temperature sensations were largely spared in the 2 Syrian siblings and they both had joint laxity and thumb sign but no syndactyly. Furthermore, their brain MRI showed peculiar anomalies at the pontine and mesencephalic level described as the “fork and bracket sign” (figure e-2),3 presumably related to the presence of degenerated fiber bundles of oculomotor and facial nerves, which were not reported in the Saudi Arabian family (see table e-2 for a detailed phenotypic comparison among the 3 families).
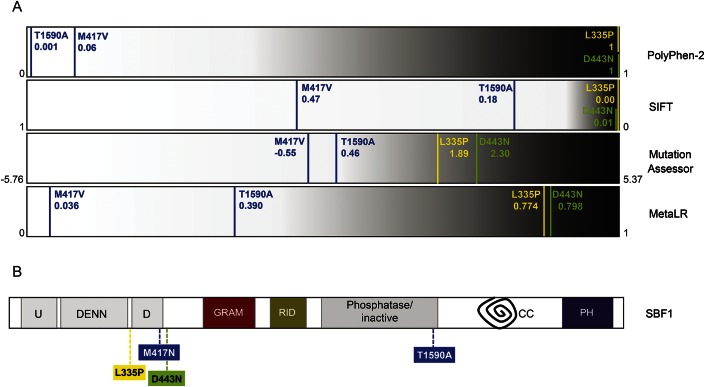
There seem to be relevant genotype–phenotype correlations in CMT4B3, as the Korean patients with pure demyelinating neuropathy were compound heterozygous for 2 missense variants, both predicted as benign or tolerated by most prediction software, suggesting a mild impact on the protein. On the contrary, the 2 families with severe syndromic presentation carried missense mutations that were consistently predicted to be deleterious for the protein structure or function (figure 1A).
Figure 1. Prediction of pathogenicity and protein localization of SBF1 mutations.
(A) Predicted pathogenicity of SBF1 missense mutations according to 4 distinct software programs (PolyPhen-2, SIFT, Mutation Assessor, and MetaLR). For each software program, predicted pathogenicity is represented as a spectrum of increasing severity, from white (tolerated/benign variants) to black (deleterious variants). SBF1 mutations are reported with vertical lines of different colors (blue: Korean family; green: Saudi Arabian family; yellow: Syrian family) (see e-Methods for more details). (B) Schematic structure of SBF1 protein and site of mutations. Abbreviation of domains is as follows: DENN = differentially expressed in neoplastic vs normal cells domain, made by the 3 modules uDENN (U), DENN, and dDENN (D); GRAM = glucosyltransferase, Rab-like GTPase activators and myotubularins; RID = Rac-induced recruitment domain; phosphatase = inactive catalytic domain of tyrosine and dual-specificity phosphatase; CC = coiled coil domain; PH = pleckstrin homology domain.
SBF1 is part of the myotubularin family, a large and highly conserved group of ubiquitously expressed phosphatidylinositol 3-phosphatases encompassing catalytically active (including MTMR2) and inactive (including SBF1 and SBF2) enzymes that share a core of protein domains.4 Most MTMR2 mutations are truncating or missense changes that drastically reduce phosphatase activity, suggesting loss of function of the protein as the key mechanism leading to CMT4B1. Both SBF1 and SBF2 proteins interact directly with MTMR2 in the cytosol, markedly increasing its enzymatic activity5; the impairment of this interaction, possibly related to protein absence, subcellular mislocalization, or functional changes of the interacting C-terminus domains, is a likely mechanism to explain the polyneuropathy associated with mutations in both genes. However, the severe syndromic phenotype shown by 2 SBF1-mutated families calls for additional explanations.
Of note, both mutations causative of syndromic CMT4B3 fall within the DENN domain (figure 1B), shared only by SBF1 and SBF2 among myotubularins.4 This domain was implicated in membrane trafficking and endosome function5 as well as in regulation of the proteins' subcellular localization, which suggests that it may confer additional functions to SBF1 and SBF2 besides interaction with MTMR2.6 However, an SBF2 deletion abolishing the whole D-DENN module caused nonsyndromic demyelinating neuropathy in a Turkish family.7 This phenotypic variability may relate to yet unknown differences between SBF1 and SBF2 in their function and/or tissue expression pattern or to a more deleterious impact of missense mutations on the protein structure and function.
Supplementary Material
Footnotes
Supplemental data at Neurology.org/ng
Author contributions: M.R.: whole-exome sequencing experiments, data analysis, and manuscript writing; C.F.M.: data analysis and validation of mutations; T.M.: bioinformatics data analysis; A.M.: clinical assessment of patients and critical revision of the manuscript for important intellectual content; E.M.V.: data analysis, manuscript writing. All authors read and approved the manuscript.
Study funding: Supported by the European Research Council (ERC Starting Grant 260888).
Disclosure: Drs. Romani, Mehawej, and Mazza report no disclosures. Dr. Mégarbané has served on the editorial board of the European Journal of Medical Genetics. Dr. Valente has received funding for travel and/or speaker honoraria from Allergan, has received research support from the Italian Ministry of Health, European Community FP7 Program, European Research Council, Italian Ministry of University and Research, and Telethon Foundation Italy. Go to Neurology.org/ng for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Nakhro K, Park JM, Hong YB, et al. SET binding factor 1 (SBF1) mutation causes Charcot-Marie-Tooth disease type 4B3. Neurology 2013;81:165–173. [DOI] [PubMed] [Google Scholar]
- 2.Alazami AM, Alzahrani F, Bohlega S, Alkuraya FS. SET binding factor 1 (SBF1) mutation causes Charcot-Marie-tooth disease type 4B3. Neurology 2014;82:1665–1666. [DOI] [PubMed] [Google Scholar]
- 3.Mégarbané A, Dorison N, Rodriguez D, Tamraz J. Multiple cranial nerve neuropathies, microcephaly, neurological degeneration and “fork and bracket sign” in the MRI: a distinct syndrome. Am J Med Genet A 2010;152A:2297–2300. [DOI] [PubMed] [Google Scholar]
- 4.Laporte J, Bedez F, Bolino A, Mandel JL. Myotubularins, a large disease associated family of cooperating catalytically active and inactive phosphoinositides phosphatases. Hum Mol Genet 2003;12:R285–R292. [DOI] [PubMed] [Google Scholar]
- 5.Marat AL, Dokainish H, McPherson PS. DENN domain proteins: regulators of Rab GTPases. J Biol Chem 2011;286:13791–13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Firestein R, Cleary ML. Pseudo-phosphatase Sbf1 contains an N-terminal GEF homology domain that modulates its growth regulatory properties. J Cell Sci 2001;114:2921–2927. [DOI] [PubMed] [Google Scholar]
- 7.Senderek J, Bergmann C, Weber S, et al. Mutation of the SBF2 gene, encoding a novel member of the myotubularin family, in Charcot-Marie-Tooth neuropathy type 4B2/11p15. Hum Mol Genet 2003;12:349–356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.