Abstract
Objective:
To explore the potential causative genes of paroxysmal hypnogenic dyskinesia (PHD), which was initially considered a subtype of paroxysmal dyskinesia and has been recently considered a form of nocturnal frontal lobe epilepsy (NFLE).
Methods:
Eleven patients with PHD were recruited. Mutations in proline-rich region transmembrane protein-2 (PRRT2), myofibrillogenesis regulator 1 (MR-1), solute carrier family 2, member 1 (SLC2A1), calcium-activated potassium channel alpha subunit (KCNMA1), cholinergic receptor, nicotinic, alpha 4 (CHRNA4), cholinergic receptor, nicotinic, beta 2 (CHRNB2), cholinergic receptor, nicotinic, alpha 2 (CHRNA2), and potassium channel subfamily T member 1 (KCNT1) were screened by direct sequencing.
Results:
Two PRRT2 mutations were identified in patients with typical PHD. A mutation of c.649dupC (p.Arg217ProfsX8) was identified in a patient with PHD and his father who was diagnosed with paroxysmal kinesigenic dyskinesia. An additional mutation of c.640G>C (p.Ala214Pro) was identified in a sporadic patient and his asymptomatic mother. No mutations were found in the other screened genes.
Conclusions:
The present study identified PRRT2 mutations in PHD, extending the phenotypic spectrum of PRRT2 and supporting the classification of PHD as a subtype of paroxysmal dyskinesia but not NFLE. Based on the results of this study, screening for the PRRT2 mutation is recommended in patients with PHD.
Paroxysmal hypnogenic dyskinesia (PHD) is characterized by paroxysmal involuntary dystonic, choreoathetoid, and ballistic attacks during sleep without triggers.1–3 PHD was initially considered a subtype of paroxysmal dyskinesia (PD), which also includes paroxysmal kinesigenic dyskinesia (PKD), paroxysmal nonkinesigenic dyskinesia (PNKD), and paroxysmal exercise-induced dyskinesia (PED). However, PHD is distinguished from the other subtypes of PD by the characteristic of nocturnal attacks without triggers. To date, proline-rich region transmembrane protein-2 (PRRT2) has been proven to be a common causative gene for the 3 subtypes of PD.4,5 Mutations in solute carrier family 2, member 1 (SLC2A1), myofibrillogenesis regulator 1 (MR-1), and calcium-activated potassium channel alpha subunit (KCNMA1) have been proven to cause PED and PNKD.6,7 However, the genetic causes of PHD remain unknown.
From another perspective, the high frequency and short duration of nocturnal attacks of PHD make it challenging to differentiate from epileptic seizures.8 Ictal alterations of diffuse irregular sharp waves or slowing with anterior predominance on EEG have been recorded in some patients with PHD.9 Therefore, PHD is speculated to be a form of nocturnal frontal lobe epilepsy.10 Mutations in CHRNA4 (cholinergic receptor, nicotinic, alpha 4), CHRNB2 (cholinergic receptor, nicotinic, beta 2), CHRNA2 (cholinergic receptor, nicotinic, alpha 2),11–14 and KCNT1 (potassium channel subfamily T member 1)15,16 have been identified in patients with autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE). In the present study, we screened 11 patients with PHD for mutations in PRRT2, MR-1, SLC2A1, KCNMA1, CHRNA4, CHRNB2, CHRNA2, and KCNT1. Two PRRT2 mutations were identified, suggesting that PHD is potentially a subtype of PD.
METHODS
Participants.
Eleven patients with PHD were recruited from a cohort of 108 patients with PD who were diagnosed and followed for more than 2 years in the Second Affiliated Hospital of Guangzhou Medical University. The collected clinical data included semiology and evolution of the disorder, family history, and results of general and neurologic examinations. Brain MRI was performed to exclude symptomatic PD. Video-EEG monitoring that included hyperventilation, intermittent photic stimulation, and sleep recordings was obtained. All EEGs were reviewed by 2 qualified electroencephalographers. Any disagreements about EEGs were resolved through discussion or consulting a third electroencephalographer when necessary.
PHD was diagnosed according to Lugaresi and Demirkiran's descriptions.1,3,8 Diagnoses were classified as typical PHD and atypical PHD. A diagnosis of typical PHD was made if a patient presented with paroxysmal dyskinetic movements (including choreoathetosis, ballistic, and dystonia) predominantly while sleeping and if the duration of the attack was shorter than 1 minute. Atypical PHD was characterized by 1 of the following features: the duration of attacks was longer than 1 minute, attacks occurring at wakefulness were more frequent than or at the same frequency as those during sleep, and any generalized tonic-clonic seizures (GTCS) after dyskinesia. Exclusion criteria were as follows: (1) presence of focal neurologic deficits; (2) abnormal brain imaging; (3) dyskinetic attacks secondary to a clear etiology or lesion, such as brain tumor, cerebrovascular disease, metabolic disturbances, and endocrine disorders; and (4) clinical and EEG manifestations of frontal lobe epilepsy, such as asymmetric extremities tonic movement, paroxysmal fear, loss of consciousness, and ictal or interictal epileptic discharges in the frontal lobe region.
Molecular analysis.
Blood samples were obtained from the probands, their parents, and other family members when available. Genomic DNA was extracted from peripheral blood using a QuickGene DNA whole-blood kit L (Fujifilm, Tokyo, Japan). Mutations in PRRT2 were screened using the methods described in our previous study.5 Mutations of SLC2A1, MR-1, KCNMA1, CHRNA4, CHRNB2, CHRNA2, and KCNT1 genes were screened by PCR and direct Sanger sequencing on an ABI3730 XL sequencer (Applied Biosystems, Foster City, CA) using the primers designed according to the sequences available through GenBank (table e-1 at Neurology.org/ng). Parental DNA was used to determine the origin of the mutation. Six hundred healthy volunteers were recruited as controls.
Standard protocol approvals, registrations, and patient consents.
The ethics committee of the hospital approved the study protocol, and written informed consent was obtained from the participants or their guardians.
RESULTS
Genetic findings.
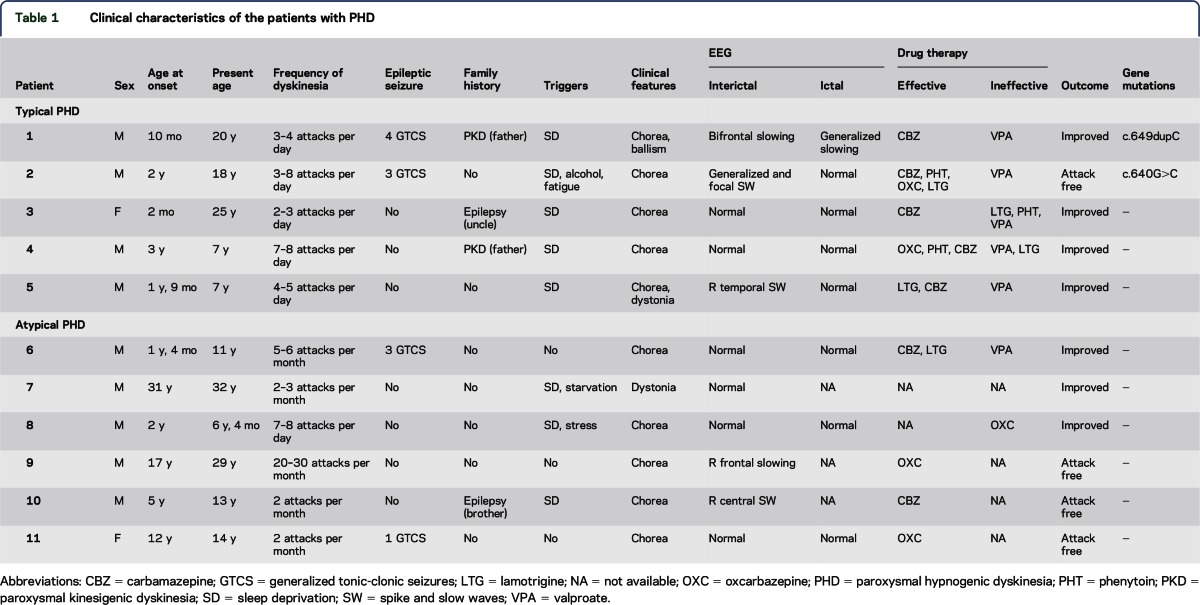
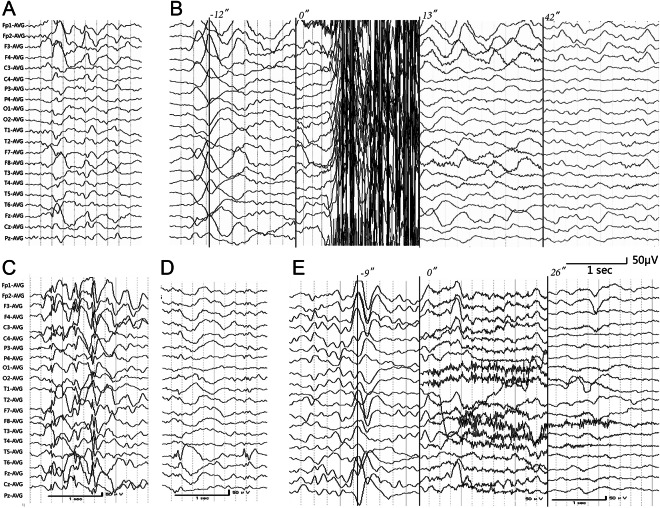
Of the 11 patients with PHD, 5 were diagnosed as typical PHD and 6 as atypical PHD (table 1). Two PRRT2 mutations were identified in 2 patients with typical PHD. An insert mutation, c.649dupC (p.Arg217ProfsX8), was identified in patient 1 and his father with PKD (figure 1A). This was a hot-spot mutation of PRRT2 resulting in a stop codon and has previously been identified in the other 3 subtypes of PD.17–22 Another mutation, c.640G>C (p.Ala214Pro), was detected in patient 2 and his asymptomatic mother (figure 1B). This mutation was a missense mutation and has previously been reported in patients with PKD.23,24The mutation was predicted to be “probably damaging” by PolyPhen (score = 0.999) and “deleterious” by SIFT (score = 0). The 2 mutations were not found in 600 normal controls. The amino acid sequence alignment of the PRRT2 family showed that A214 and R217 were highly conserved from various species (figure 1C). No mutations in the coding exons of MR-1, SLC2A1, KCNMA1, CHRNA4, CHRNB2, CHRNA2, or KCNT1 were identified in any of the 11 patients with PHD.
Table 1.
Clinical characteristics of the patients with PHD
Figure 1. Genetic data on the patients with paroxysmal hypnogenic dyskinesia (PHD) with gene mutations in PRRT2.
(A) The pedigree (left) and PRRT2 sequence (right) of the patient with mutation of c.649dupC (patient 1). The patient carried the heterozygous mutation of the PRRT2 gene c.649dupC (p.Arg217ProfsX8), inherited from his father with paroxysmal kinesigenic dyskinesia (PKD). (B) The pedigree (left) and PRRT2 sequence (right) of the patient with mutation c.640G>C (patient 2). The patient carried the heterozygous mutation of the PRRT2 gene c.640G>C (p.Ala214Pro), inherited from his asymptomatic mother. (C) The amino acid sequence alignment of the PRRT2 family showed the evolutionary conservation of the residues. Ala214 and Arg217 were highly conserved in various species.
Clinical presentation of the patients with PRRT2 mutations.
Patient 1 was a 24-year-old man who presented with episodic dyskinesia since the first year of life. He experienced ballistic or choreoathetoid attacks while sleeping. The attacks lasted 10–60 seconds and were usually exacerbated by sleep deprivation. The patient was aware during the attacks. The frequency was 5 to 8 times per year and then increased to daily after the age of 13. He had 4 GTCS during wakefulness without any triggers after the age of 15. His father had PKD attacks since the age of 12, and the attacks remitted spontaneously at the age of 41.
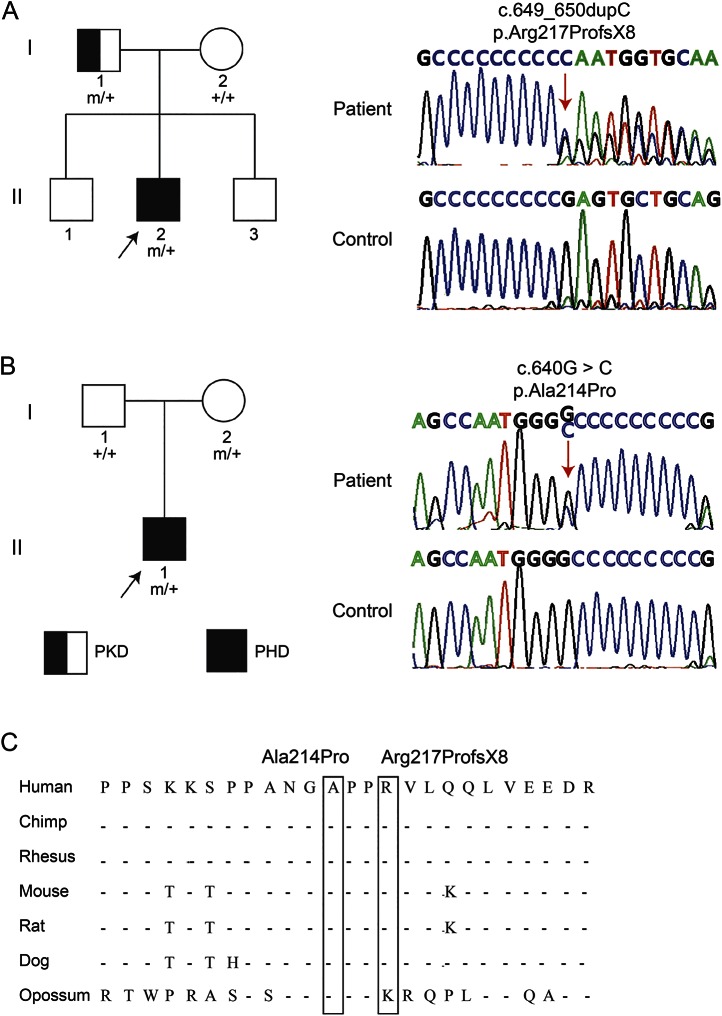
EEG monitoring performed at the age of 20 showed interictal intermittent high-voltage delta slow waves in the bifrontal region (figure 2A). An attack was recorded during non-REM sleep (NREM) stage II, which manifested as sudden stiffening and massive jerk movements of extremities lasting for 12 seconds. Then the patient fell asleep again (video 1). The ictal EEG showed muscle artifacts in all channels and then postictal high-voltage slow waves, dominant in the bifrontal lobe, lasting until the 180th second and followed by NREM-IV (figure 2B).
Figure 2. EEG changes in the patients with paroxysmal hypnogenic dyskinesia with PRRT2 mutations.
(A) Interictal EEG of patient 1 obtained at the age of 20 years showed irregular delta activities in the bifrontal lobe, more dominant in the right frontal lobe. (B) Ictal EEG of patient 1. The attack started with rhythmic convulsions during non-REM sleep (NREM) stage II accompanied by muscle artifacts on EEG recording. It lasted 12 seconds, followed by slow waves in the bifrontal lobe, and returned to the sleeping background (NREM-IV) 1 minute later. (C) Interictal EEG of patient 2 obtained at the age of 10 years showed irregular generalized spikes and slow waves and (D) focal spike and waves in the right posterior temporal lobe. (E) Ictal recording of patient 2 showed that the patient awakened suddenly from NREM-II and presented irregular bilateral arm and leg choreoathetoid movements, accompanied by movement artifacts and generalized low- and medium-voltage theta slowing. The movements lasted 26 seconds, and the slowing continued for 1 minute.
The patient did not respond to valproate (VPA, 15 mg/kg/d). Carbamazepine (CBZ, 12 mg/kg/d) decreased the frequency of attacks from 5 to 8 times per day to 1 to 2 times per month.
Patient 2 was an 18-year-old man who presented with choreoathetoid movements during sleep since the age of 2. He experienced uncontrollable extremities and a bizarre sequence of movements with awareness at a frequency of 1 to 12 times per night. The attacks occurred predominantly during sleep but occasionally during wakefulness with precipitating factors, including fatigue, stress, sleep deprivation, and alcohol. He had 3 GTCS during daytime between ages 6 and 8.
His mother carried the same mutation and did not present any dyskinetic symptoms. There was no family history of paroxysmal disorders in his family.
Video-EEG monitoring at age 9 showed irregular generalized spikes, slow waves (figure 2C), and focal spike slow waves in the right posterior temporal lobe interictally (figure 2D). One episode was recorded, which was a sudden wake up accompanied by choreoathetoid movement of the extremities and lasted for 26 seconds (video 2). He maintained consciousness during the event. The ictal EEG showed an arousal from NREM-II sleep followed by movement artifacts and generalized slowing that lasted about 1 minute (figure 2E).
The patient did not respond to VPA (30 mg/kg/d). CBZ, phenytoin, or oxcarbazepine (OXC) monotherapy markedly reduced the attacks from more than 10 times per night to 2 to 3 times per night. At the age of 17, the attacks were controlled by a combination of lamotrigine (2.5 mg/kg/d) and OXC (20 mg/kg/d).
Comparison of clinical features of typical and atypical PHD.
Because PRRT2 mutations were detected in typical cases but not in atypical cases, we analyzed their clinical difference to isolate the potential clinical biomarkers for PRRT2 mutations. The demographic and clinical features of the cases are summarized in table 1. As defined in the diagnosis criteria, attacks in typical cases have a shorter duration and are predominantly nocturnal. Typical cases tend to have an earlier onset (1.55 ± 4.88 years, ranging from 2 months to 3 years in typical cases vs 11.4 ± 4.64 years, ranging from 1 year 4 months to 31 years in atypical cases) and more frequent family history of PKD (2/5 vs 0/6 in typical and atypical cases, respectively), but no statistical significances were detected. There was no significant difference between typical and atypical cases with regard to trigger factors, concurrency of GTCS, EEG abnormalities, or family history of epilepsy.
Regarding the response to antiepileptic drug therapy, patients with PHD generally had good responses to antiepileptic drugs with the action of sodium channel blocking, especially CBZ. Typical cases tended to have lower attack-free rates with monotherapy than atypical cases (0/5 vs 3/5, respectively), and 1 typical case achieved remission with a combination of OXC and lamotrigine. VPA was applied in 6 cases and was ineffective in all of them.
DISCUSSION
PHD is a paroxysmal disorder with unknown etiology. In the present study, PRRT2 mutations were identified in patients with PHD, which established an association between the PRRT2 gene and PHD and expanded the phenotypic spectrum of PRRT2 mutations. This study also highlights that PHD is a genetic disorder similar to other types of PD such as PKD, PNKD, and PED.
PRRT2 is a presynaptic protein that potentially plays an important role in exocytosis and neurotransmitter release.25 Mutations in the PRRT2 gene have recently been proven to be a major cause of PD. In the present study, an insert mutation c.649dupC and a missense mutation c.640G>C (p.Ala214Pro) were identified in the patients with PHD. The mutation c.649dupC causes frameshift and a premature stop codon (p.Arg217ProfsX8) and leads to loss of function and haploinsufficiency. This mutation is a hot-spot mutation and is responsible for 57% of the cases with different phenotypes.20 The missense mutation p.Ala214Pro changed a highly conserved amino acid residue and potentially damages the protein (score = 0 by SIFT and score = 0.999 by PolyPhen). It was also previously reported in PKD cases,23,24supporting its possible role in pathogenesis. A recent study has shown that truncated or missense mutation could affect glutamate signaling and glutamate receptor activity through their weakened interaction with SNAP25, resulting in increased glutamate release and subsequent neuronal hyperexcitability.26 However, the mutations c.649dupC and c.640G>C are present at frequencies of 0.004626 and 0.004827, respectively, in East Asians (ExAC database), raising suspicion about their pathogenicity. It is noted that p.Ala214Pro was detected in the asymptomatic mother of the patient with PHD. In our previous study, incomplete penetrance was observed in 26.5% of PD individuals with PRRT2 mutations, including those with truncated mutations, and even led to recessive inheritance.5 Incomplete penetrance was reported to be 18% in families with benign familial infantile convulsion.27 Our recent study on SCN1A has demonstrated that lower penetrance was associated with fewer pathogenic mutations.28It is therefore possible that PRRT2 mutations were relatively less pathogenic, explaining their frequencies in the general population.
In the present study, c.649dupC was identified in the patient with PHD and his father with PKD. The mutation c.649dupC was previously detected in patients with PKD, PED, PNKD, and other paroxysmal disorders.17–22 Similarly, the missense mutation p.Ala214Pro was also identified in patients with PKD previously.23,24 The striking pleiotropic phenotypic expression of the PRRT2 mutation indicates that the PRRT2 genotype likely does not predict clinical phenotype. The underlying mechanism of phenotype variations should be examined in ways other than genotype of PRRT2. Clinically, PHD shares similar clinical characteristics with other subtypes of PD, especially PKD, such as dyskinetic symptoms, short duration of attacks, and good response to sodium channel blockers. The present study suggests that PHD may have a similar genetic etiology to the other types of PD. However, each of the PD subtypes differs with regard to precipitating factors. Sudden movement is the most common trigger observed in PKD, and prolonged exercise usually induces attacks in patients with PED. Our previous study demonstrated that 4 of the 9 children with infantile convulsions and paroxysmal choreoathetosis had attacks during feeding,5 suggesting that suction is a possible kinesigenic trigger in infants. In the present study, the majority of dyskinetic attacks occurred during NREM, suggesting that NREM is potentially a trigger for PHD symptoms. Future studies should focus on detecting internal and external precipitating factors in patients with PD and identifying the relationship between triggers and attacks.
The nocturnal and movement features of PHD and ADNFLE attacks are similar and difficult to differentiate. The present study identified mutations in PRRT2 but not in ADNFLE-associated genes in patients with PHD. This suggests that PHD is potentially PD but not ADNFLE by nature. It should also be considered that the clinical characteristics presented in the patients with PHD in the present study, such as dyskinetic attacks not followed by GTCS, no discharges in the frontal region, attacks during NREM-II to NREM-IV, and a lack of ictal epileptic patterns in EEG, may be helpful in distinguishing PHD from ADNFLE. In contrast, the seizures in ADNFLE may occur at any stage of sleep, and there is evidence of localized discharges in the frontal region.
The 2 patients with PRRT2 mutations also exhibited occasional GTCS, suggesting a susceptibility to seizures. Previously, occasional, mild, and benign epileptic seizures were observed in patients with PD with PRRT2 mutations, such as benign family infant convulsions, febrile seizures, absence seizures, and occasional GTCS.5,22,25,27,29,30 Taken together, this suggests that PRRT2 mutations are potentially associated with an increased neuronal excitability and susceptibility to seizures as opposed to a distinct type of epilepsy, such as ADNFLE.
In a previous study, PRRT2 mutations were commonly clustered in the patients with epilepsy with onset in the first year of life.31 In the present study, PRRT2 mutations were identified in the 2 patients with PHD with relatively earlier age at onset (age of 10 months and 1 year). Therefore, patients with earlier onset may be an important clue to address PRRT2 gene testing.
Although PRRT2 mutations were identified in the patients with PHD in this study, it is not clear whether PHD is PD or epilepsy. The etiology for the patients without PRRT2 mutation is still unknown. Recently, more causative genes associated with epilepsy and PD were identified, such as DEPDC5 mutations in familial cases with the symptoms of ADNFLE.32,33 Further and wider genetic screening/testing should be performed in patients with PHD without PRRT2 mutation to find potential causative genes.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge the participants for their cooperation. They are grateful to He Shanheng Charity Foundation for contributing to the development of this institute.
GLOSSARY
- ADNFLE
autosomal dominant nocturnal frontal lobe epilepsy
- CBZ
carbamazepine
- CHRNA2
cholinergic receptor, nicotinic, alpha 2
- CHRNA4
cholinergic receptor, nicotinic, alpha 4
- CHRNB2
cholinergic receptor, nicotinic, beta 2
- GTCS
generalized tonic-clonic seizures
- NREM
non-REM sleep
- OXC
oxcarbazepine
- PD
paroxysmal dyskinesia
- PED
paroxysmal exercise-induced dyskinesia
- PHD
paroxysmal hypnogenic dyskinesia
- PKD
paroxysmal kinesigenic dyskinesia
- PNKD
paroxysmal nonkinesigenic dyskinesia
- PRRT2
proline-rich region transmembrane protein-2
- SLC2A1
solute carrier family 2, member 1
- VPA
valproate
Footnotes
Supplemental data at Neurology.org/ng
AUTHOR CONTRIBUTIONS
Dr. Xiao-Rong Liu: design and conceptualization of the study, analysis and interpretation of data, drafting the manuscript. Dr. Dan Huang: analysis and interpretation of data. Dr. Jie Wang: analysis and interpretation of data. Dr. Hui Sun: analysis and interpretation of data. Dr. Yi-Fan Wang: analysis and interpretation of data. Dr. Bin Tang: analysis and interpretation of data. Dr. Wen Li: analysis and interpretation of data. Dr. Jin-Xing Lai: analysis and interpretation of data. Dr. Na He: analysis and interpretation of data, drafting the manuscript. Dr. Mei Wu: analysis and interpretation of data. Dr. Tao Su: analysis and interpretation of data, revising the manuscript. Dr. Heng Meng: analysis and interpretation of data. Dr. Yi-Wu Shi: analysis and interpretation of data. Dr. Bing-Mei Li: analysis and interpretation of data, revising the manuscript. Dr. Bei-Sha Tang: analysis and interpretation of data. Dr. Wei-Ping Liao: design and conceptualization of the study, analysis and interpretation of data, drafting the manuscript.
STUDY FUNDING
Funded by Population and Family Planning Commission of Guangdong Province (Grant No. 2012265), Guangdong Provincial Department of Education (Grant No. 2012KJCX009), Science and Information Technology Bureau of Guangzhou (Guangzhou Science Research Project Grant No. 2014J4100062 and No. 201508020011), National Natural Science Foundation of China (Grant No. 81571273 and 81571274), and The State Key Program of National Natural Science Foundation of China (Grant No. 81130021).
DISCLOSURE
Dr. Xiao-Rong Liu was funded by the Population and Family Planning Commission of Guangdong Province (Grant No. 2012265), Guangdong Provincial Department of Education (Grant No. 2012KJCX009), and the Science and Information Technology Bureau of Guangzhou (Guangzhou Science Research Project Grant No. 2014J4100062). Dr. Yi-Wu Shi is funded by the National Natural Science Foundation of China (Grant No. 81571274). Dr. Bei-Sha Tang has served on the editorial boards of Scientific Reports and Cerebellum & Ataxias and is funded by The State Key Program of the National Natural Science Foundation of China (Grant No. 81130021). Dr. Wei-Ping Liao has served on the editorial board of Seizure and is funded by the National Natural Science Foundation of China (Grant No. 81571273) and Science and Information Technology Bureau of Guangzhou (2014 Guangzhou Science Research Project Grant No. 201508020011). The other authors report no disclosures. Go to Neurology.org/ng for full disclosure forms.
REFERENCES
- 1.Lugaresi E, Cirignotta F. Hypnogenic paroxysmal dystonia: epileptic seizure or a new syndrome? Sleep 1981;4:129–138. [DOI] [PubMed] [Google Scholar]
- 2.Lee BI, Lesser RP, Pippenger CE, et al. Familial paroxysmal hypnogenic dystonia. Neurology 1985;35:1357–1360. [DOI] [PubMed] [Google Scholar]
- 3.Demirkiran M, Jankovic J. Paroxysmal dyskinesias: clinical features and classification. Ann Neurol 1995;38:571–579. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia KP, Schneider SA. Identification of PRRT2 as the causative gene of paroxysmal kinesigenic dyskinesia. Mov Disord 2012;27:707. [DOI] [PubMed] [Google Scholar]
- 5.Liu XR, Wu M, He N, et al. Novel PRRT2 mutations in paroxysmal dyskinesia patients with variant inheritance and phenotypes. Genes Brain Behav 2013;12:234–240. [DOI] [PubMed] [Google Scholar]
- 6.Lee HY, Xu Y, Huang Y, et al. The gene for paroxysmal non-kinesigenic dyskinesia encodes an enzyme in a stress response pathway. Hum Mol Genet 2004;13:3161–3170. [DOI] [PubMed] [Google Scholar]
- 7.Weber YG, Storch A, Wuttke TV, et al. GLUT1 mutations are a cause of paroxysmal exertion-induced dyskinesias and induce hemolytic anemia by a cation leak. J Clin Invest 2008;118:2157–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lugaresi E, Cirignotta F, Montagna P. Nocturnal paroxysmal dystonia. J Neurol Neurosurg Psychiatry 1986;49:375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tinuper P, Cerullo A, Cirignotta F, et al. Nocturnal paroxysmal dystonia with short-lasting attacks: three cases with evidence for an epileptic frontal lobe origin of seizures. Epilepsia 1990;31:549–556. [DOI] [PubMed] [Google Scholar]
- 10.Montagna P. Nocturnal paroxysmal dystonia and nocturnal wandering. Neurology 1992;42:61–67. [PubMed] [Google Scholar]
- 11.Steinlein OK, Mulley JC, Propping P, et al. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet 1995;11:201–203. [DOI] [PubMed] [Google Scholar]
- 12.Tenchini ML, Duga S, Bonati MT, et al. SER252PHE and 776INS3 mutations in the CHRNA4 gene are rare in the Italian ADNFLE population. Sleep 1999;22:637–639. [DOI] [PubMed] [Google Scholar]
- 13.Phillips HA, Favre I, Kirkpatrick M, et al. CHRNB2 is the second acetylcholine receptor subunit associated with autosomal dominant nocturnal frontal lobe epilepsy. Am J Hum Genet 2001;68:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen ZH, Zhai QX, Gui J, et al. Mutational analysis of CHRNB2 and CHRNA2 genes in southern Chinese population with autosomal dominant nocturnal frontal lobe epilepsy [in Chinese]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2011;28:14–18. [DOI] [PubMed] [Google Scholar]
- 15.Heron SE, Smith KR, Bahlo M, et al. Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet 2012;44:1188–1190. [DOI] [PubMed] [Google Scholar]
- 16.Moller RS, Heron SE, Larsen LH, et al. Mutations in KCNT1 cause a spectrum of focal epilepsies. Epilepsia 2015;56:e114–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q, Qi Z, Wan XH, et al. Mutations in PRRT2 result in paroxysmal dyskinesias with marked variability in clinical expression. J Med Genet 2012;49:79–82. [DOI] [PubMed] [Google Scholar]
- 18.Marini C, Conti V, Mei D, et al. PRRT2 mutations in familial infantile seizures, paroxysmal dyskinesia, and hemiplegic migraine. Neurology 2012;79:2109–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riant F, Roze E, Barbance C, et al. PRRT2 mutations cause hemiplegic migraine. Neurology 2012;79:2122–2124. [DOI] [PubMed] [Google Scholar]
- 20.Becker F, Schubert J, Striano P, et al. PRRT2-related disorders: further PKD and ICCA cases and review of the literature. J Neurol 2013;260:1234–1244. [DOI] [PubMed] [Google Scholar]
- 21.Ishii A, Yasumoto S, Ihara Y, et al. Genetic analysis of PRRT2 for benign infantile epilepsy, infantile convulsions with choreoathetosis syndrome, and benign convulsions with mild gastroenteritis. Brain Dev 2013;35:524–530. [DOI] [PubMed] [Google Scholar]
- 22.Labate A, Tarantino P, Palamara G, et al. Mutations in PRRT2 result in familial infantile seizures with heterogeneous phenotypes including febrile convulsions and probable SUDEP. Epilepsy Res 2013;104:280–284. [DOI] [PubMed] [Google Scholar]
- 23.Chen WJ, Lin Y, Xiong ZQ, et al. Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat Genet 2011;43:1252–1255. [DOI] [PubMed] [Google Scholar]
- 24.Chen YP, Song W, Yang J, et al. PRRT2 mutation screening in patients with paroxysmal kinesigenic dyskinesia from Southwest China. Eur J Neurol 2014;21:174–176. [DOI] [PubMed] [Google Scholar]
- 25.Nobile C, Striano P. PRRT2: a major cause of infantile epilepsy and other paroxysmal disorders of childhood. Prog Brain Res 2014;213:141–158. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Niu F, Zhu X, et al. PRRT2 mutant leads to dysfunction of glutamate signaling. Int J Mol Sci 2015;16:9134–9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schubert J, Paravidino R, Becker F, et al. PRRT2 mutations are the major cause of benign familial infantile seizures. Hum Mutat 2012;33:1439–1443. [DOI] [PubMed] [Google Scholar]
- 28.Meng H, Xu HQ, Yu L, et al. The SCN1A mutation database: updating information and analysis of the relationships among genotype, functional alteration, and phenotype. Hum Mutat 2015;36:573–580. [DOI] [PubMed] [Google Scholar]
- 29.Lee HY, Huang Y, Bruneau N, et al. Mutations in the gene PRRT2 cause paroxysmal kinesigenic dyskinesia with infantile convulsions. Cell Rep 2012;1:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ono S, Yoshiura K, Kinoshita A, et al. Mutations in PRRT2 responsible for paroxysmal kinesigenic dyskinesias also cause benign familial infantile convulsions. J Hum Genet 2012;57:338–341. [DOI] [PubMed] [Google Scholar]
- 31.Zara F, Specchio N, Striano P, et al. Genetic testing in benign familial epilepsies of the first year of life: clinical and diagnostic significance. Epilepsia 2013;54:425–436. [DOI] [PubMed] [Google Scholar]
- 32.Picard F, Makrythanasis P, Navarro V, et al. DEPDC5 mutations in families presenting as autosomal dominant nocturnal frontal lobe epilepsy. Neurology 2014;82:2101–2106. [DOI] [PubMed] [Google Scholar]
- 33.Dibbens LM, de Vries B, Donatello S, et al. Mutations in DEPDC5 cause familial focal epilepsy with variable foci. Nat Genet 2013;45:546–551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.