Abstract
Chlamydia pneumoniae is a community-acquired bacterial pathogen that has been strongly associated with exacerbation of atherosclerosis. We evaluated the role of CD8+ T cells in the C57BL/6J mouse model of C. pneumoniae-induced atherosclerosis. Groups of 4- to 6-week-old male wild-type C57BL/6J (WT) mice and mice with a gene deficiency in CD8α (CD8 KO mice) were infected with C. pneumoniae and fed a high fat (HF) diet. Serum antibody response and serum cholesterol were comparable between infected CD8 KO and WT mice. However, infected CD8 KO mice displayed significantly reduced atherosclerotic plaque lesions on day 100 compared to infected WT mice, at a level comparable to both uninfected WT and CD8 KO mice fed the HF diet. Moreover, repletion of CD8 KO mice with WT CD8+ T cells (1 × 107 cells/mouse intravenously) at the time of infection reverted atherosclerotic plaque lesions to WT levels. These results demonstrate that CD8+ T cells play an important role in mediating C. pneumoniae-induced exacerbation of atherosclerotic pathology.
Keywords: vaccine, respiratory infection, resurgence, immune memory
This study uses the mouse model to demonstrate the role of CD8 T cells, an immune cell type, in causation of cardiovascular diseases by the human pathogen Chlamydia pneumoniae.
Graphical Abstract Figure.
This study uses the mouse model to demonstrate the role of CD8 T cells, an immune cell type, in causation of cardiovascular diseases by the human pathogen Chlamydia pneumoniae.
Chlamydiae are obligate intracellular bacteria that cause chronic inflammatory pathologies following infection in humans and animals. Specifically, genital Chlamydia trachomatis infections induce upper female reproductive tract pathologies whereas respiratory C. pneumoniae infections have been strongly associated with exacerbation of atherosclerosis (Campbell and Kuo 2004; Campbell and Rosenfeld 2015). We have recently shown using the mouse model that Chlamydia-induced female upper reproductive tract pathologies are mediated by TNF-α-producing CD8+ T cells (Murthy et al. 2011; Manam, Nicholson and Murthy 2013; Manam et al. 2014). CD8+ T cells that produce TNF-α also have been shown recently to induce atherosclerotic pathology in mice with a gene deficiency in apolipoprotein E (Kyaw et al. 2013). Apart from cellular and physical factors that lead to plaque formation in the walls of large- and medium-sized arteries, environmental factors such as microbial infections have been shown to play an important role in this disease (Ross 1999). Specifically, respiratory C. pneumoniae infection in wild-type (WT) C57BL/6J mice fed a high-fat (HF) diet induces significant enhancement of atherosclerotic lesions compared to HF-fed animals not infected with C. pneumoniae (Campbell and Rosenfeld 2015).
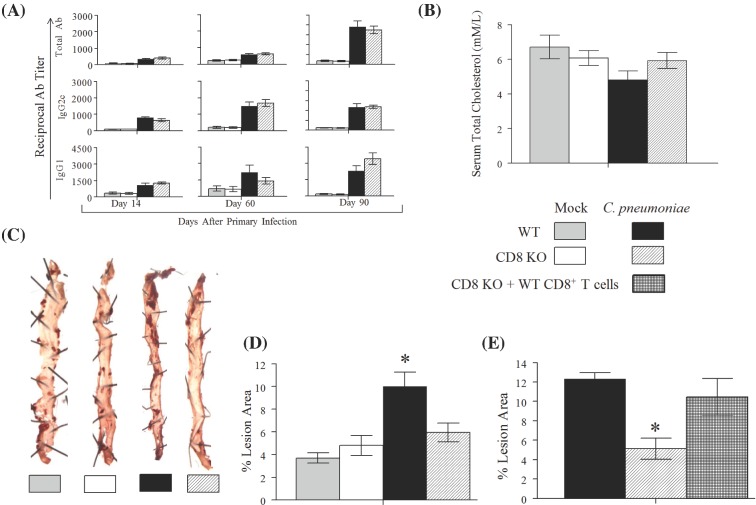
In this study, we evaluated whether CD8+ T cells mediate C. pneumoniae-induced exacerbation of atherosclerosis in the HF-diet-fed C57BL/6J mouse model (Campbell et al. 2005). We used male mice with a gene deficiency in CD8α (CD8 KO) on a C57BL/6J background and C57BL/6J WT mice both purchased from Jackson Laboratories (Bar Harbor, ME). The mice were housed in the animal facility at Midwestern University (MWU). Food and water was provided ad libitum, and all procedures were performed in accordance with an approved protocol (number 2291) by the Institutional Animal Care and Use Committee at MWU. Groups (n = 10) of CD8 KO and WT mice were either infected intranasally with 1 × 107 IFU of C. pneumoniae AR39 (C. pneumoniae; obtained from Dr. Lee Ann Campbell, University of Washington, Seattle) in 10 μl of sterile 1X phosphate-buffered saline (PBS) or were mock infected with sterile 1X PBS on days 0, 14 and 28. All groups of mice were fed the HF (15% fat, 1.25% cholesterol and 0.5% cholate) diet from day 0 until the end of the experiment. The four groups of mice were bled on days 14, 60 and 90. Sera was prepared and analyzed for total antibody (Ab), IgG2c, IgG1 against C. pneumoniae, as described previously (Manam et al. 2013). Chlamydia pneumoniae infected WT and CD8 KO mice displayed progressively increasing titers of total Ab, IgG2c and IgG1 with the antibody levels being comparable between the two groups (Fig. 1A). Furthermore, as expected, uninfected WT and CD8 KO mice did not display detectable levels of antibody against C. pneumoniae. This confirmed that the infected and mock-infected groups responded appropriately to the inoculations. On day 90 after primary inoculation, serum cholesterol levels were analyzed using a kit (Catalog # ab65390, Abcam, Cambridge, MA) and found to be comparable among all four groups of animals (Fig. 1B). These results suggest that neither C. pneumoniae infection nor absence of CD8+ T cells directly affected serum cholesterol levels.
Figure 1.
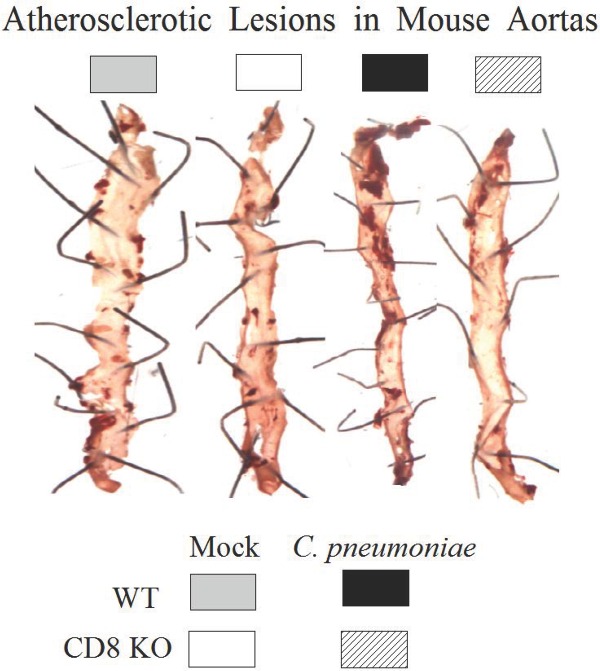
Role of CD8+ T cells in C. pneumoniae-induced atherosclerosis. (A–D) Groups (n = 10) of WT and CD8 KO were fed the HF diet and infected with C. pneumoniae or mock-infected with PBS on days 0, 14 and 28. (A) Anti-C. pneumoniae total Ab, IgG2c and IgG1 were evaluated on days 14, 60 and 90 after primary inoculation. Mean ± SEM in the group of reciprocal antibody titer corresponding to 50% maximal binding is shown. (B) On day 90, serum total cholesterol was measured and the mean ± SEM per group is shown. (C) Representative atherosclerotic lesions detected using en face oil red O staining in aortic tissues on day 100 following primary inoculation. (D) The percentage of lesion area to total aortic tissue luminal surface area was calculated for each mouse and mean ± SEM per group is shown. *Significant (p ≤ 0.05, ANOVA) difference between the indicated group and all others. (E) Groups (n = 6) of WT mice, CD8 KO mice and CD8 KO mice repleted with WT CD8+ T cells were HF fed and infected with C. pneumoniae on days 0, 14 and 28. On day 100 following primary inoculation, atherosclerotic lesions were quantified and the mean ± SEM per group is shown. *Significant (p ≤ 0.05, ANOVA) difference between the indicated group and all others.
On day 100 after primary inoculation, mice were euthanized and tissues perfused with sterile 1× PBS followed by 2% paraformaldehyde. The arch of the aorta and descending aorta was dissected. The dissected tissues were split open longitudinally to expose the lumen and were stained en face with Oil Red O stain to identify atherosclerotic plaques, as described previously (Chen et al. 2010). Images were acquired using a Bioreader-FZ-beta ELISPOT instrument (Biosys, Germany) with inverted optics. WT mice infected with C. pneumoniae displayed increased number of Oil red O-stained plaques compared to the other groups, whereas plaques in uninfected WT and infected or uninfected CD8 KO mice were similar to each other (representative tissues shown in Fig. 1C). We further quantified the ratio of the area covered by the atherosclerotic lesions to the total surface area of aortic tissue using ImageJ software (National Institutes of Health, USA). The percent lesion area was calculated using the formula: (pixels in oil red O-stained region) × 100/(pixels in the entire surface area of aortic tissue). As shown in Fig. 1D, infected WT mice displayed significantly enhanced lesion area compared to both uninfected WT and CD8 KO mice. Importantly, infected CD8 KO mice displayed significantly reduced atherosclerotic lesions compared to infected WT mice, and at a level comparable to both uninfected WT and CD8 KO mice. These results suggest that CD8+ T cells contribute to C. pneumoniae-induced atherosclerosis.
The observation that the absence of CD8+ T cells correlates with pathology does not per se prove that CD8+ T cells mediate the pathology. To confirm this hypothesis, we conducted a second experiment which included three groups (n = 6 per group) of mice. One group each of WT and CD8 KO mice were infected with C. pneumonaie and fed the HF diet. A third group of CD8 KO mice, HF fed and infected with C. pneumoniae were repleted with WT CD8+ T cells at the time of primary inoculation. Donor CD8+ T cells were prepared from spleens of naïve WT mice as described previously (Manam et al. 2014). Briefly, single-cell suspensions of splenocytes were subjected to magnetic bead-based negative selection (Stemcell Technologies) for enrichment of CD8+ T cells. The enriched population was determined to contain at least 95% CD3+CD8+ T cells. The enriched population of CD8+ T cells was injected (1 × 107 cells/mouse in 100 μl of 1X sterile PBS) intravenously into recipient CD8 KO animals. Atherosclerotic pathology was measured on day 100 following primary inoculation as described above. As shown in Fig. 1E, HF-fed C. pneumoniae-infected CD8 KO mice displayed significantly reduced atherosclerotic pathology compared to similarly treated WT animals confirming our previous observation. Importantly, CD8 KO mice repleted with WT CD8+ T cells displayed significant enhancement in atherosclerotic pathology compared to CD8 KO mice, and at a level comparable to that of WT animals. These results demonstrate that CD8+ T cells mediate the C. pneumoniae-induced atherosclerotic pathology.
The results of this study have several important implications. First, this study joins a short list of studies that have explored and demonstrated the role of CD8+ T cells in atherosclerosis. It was shown recently that TNF-α- and perforin-producing CD8+ T cells contribute significantly to atherosclerotic pathologies in the apolipoprotein E-deficient mice (ApoE KO mice) which spontaneously develop atherosclerosis (Kyaw et al. 2013). Our study confirms and extends that observation by showing a similar role of this cell type within a C57BL/6J WT mice fed a HF diet and infected with C. pneumoniae. Notably, we did not find any differences in CD8 KO mice and WT mice not infected with C. pneumoniae, suggesting that the mechanisms by which CD8+ T cells play a role in infection-mediated atherosclerotic development in WT mice may be different from that in ApoE KO mice; that possibility needs to be explored further. Second, CD8+ T cells had been correlatively implicated in pathogenesis of both ocular and reproductive tract following C. trachomatis and/or C. muridarum infections (Kimani et al. 1996; Van Voorhis 1996, 1997; Igietseme et al. 2009), and we demonstrated recently that TNF-α−producing and TNF receptor 2-bearing CD8+ T cells mediate oviduct pathology in mice following intravaginal C. muridarum infection (Murthy et al. 2011; Manam et al. 2014). The current study confirms and extends our understanding of the role of CD8+ T cells in chronic pathogenesis following chlamydial infections by adding to the list a different species of Chlamydia which is a human pathogen, a different site of infection and a different chronic pathology. Chlamydia muridarum is a mouse pathogen and has minimal tropism for human individuals. However, C. trachomatis which infects humans can be cleared by innate immune responses in mice (Morrison et al. 2011; Sturdevant and Caldwell 2014) making it difficult to evaluate the role of adaptive immune responses in this model. Additionally, C. trachomatis infections do not induce the same high level of pathology as C. muridarum in the oviduct leaving a smaller window in the phenotype to resolve pathogenic mechanisms. In this context, C. pneumoniae is a human pathogen and can be used in mice to induce a significant degree of atherosclerosis over and above that induced by the HF diet. This allows us to resolve the contribution of various immune components, including those of adaptive immunity, to Chlamydia-induced chronic pathology. Therefore, the C. pneumoniae-atherosclerosis model in mice provides an avenue to compare and contrast mechanisms of immunopathogenesis discovered using the C. muridarum mouse model. Third, infectious agents have been thought to contribute to atherosclerotic development either via direct infection of vasculature or via infection elsewhere that causes systemic elevations in inflammatory cytokines and other acute phase proteins and indirectly causes atherosclerotic pathologies (Campbell and Rosenfeld 2015). The results of the current study demonstrating the role of CD8+ T cells provide a new perspective in a pathogen-induced atherosclerosis scenario. CD8+ T-cell responses that are induced following the respiratory infection may target microbial antigens that reach the vasculature in an antigen-specific fashion. In this regard, a number of studies have detected nucleic acid or antigen of a variety of organisms in atherosclerotic plaques, but the identification of cultivable organisms has been rare (Rosenfeld and Campbell 2011). Taken in context of the results from the current study, we hypothesize that microbial components and antigens originating from primarily infected tissues can disseminate extracellularly or within cells such as macrophages (Rupp et al. 2009) and home to the vasculature and serve as targets for attack by antigen-specific CD8+ T cells. Such a scenario could occur alongside or in lieu of productive infection in the vasculature, or the need for systemic elevation of cytokines or other acute phase proteins. In summary, we have demonstrated that CD8+ T cells mediate C. pneumoniae-mediated atherosclerosis and provided potentially important insights into microbe-induced atherosclerotic development.
FUNDING
This work was supported by Midwestern University Faculty Start-up Fund, NIH Grant 1R15AI101920, and American Heart Association Midwest Affiliate Scientist Development Grant 13SDG17310011 to AKM. This work also was supported partially by Biomedical Sciences Master's Thesis Research funds to MTZ.
Conflict of interest. None declared.
REFERENCES
- Campbell LA, Kuo CC. Chlamydia pneumoniae—an infectious risk factor for atherosclerosis? Nat Rev Microbiol. 2004;2:23–32. doi: 10.1038/nrmicro796. [DOI] [PubMed] [Google Scholar]
- Campbell LA, Nosaka T, Rosenfeld ME, et al. Tumor necrosis factor alpha plays a role in the acceleration of atherosclerosis by Chlamydia pneumoniae in mice. Infect Immun. 2005;73:3164–5. doi: 10.1128/IAI.73.5.3164-3165.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LA, Rosenfeld ME. Infection and atherosclerosis development. Arch Med Res. 2015;46:339–50. doi: 10.1016/j.arcmed.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Shimada K, Zhang W, et al. IL-17A is proatherogenic in high-fat diet-induced and Chlamydia pneumoniae infection-accelerated atherosclerosis in mice. J Immunol. 2010;185:5619–27. doi: 10.4049/jimmunol.1001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igietseme JU, He Q, Joseph K, et al. Role of T lymphocytes in the pathogenesis of Chlamydia disease. J Infect Dis. 2009;200:926–34. doi: 10.1086/605411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimani J, Maclean IW, Bwayo JJ, et al. Risk factors for Chlamydia trachomatis pelvic inflammatory disease among sex workers in Nairobi, Kenya. J Infect Dis. 1996;173:1437–44. doi: 10.1093/infdis/173.6.1437. [DOI] [PubMed] [Google Scholar]
- Kyaw T, Winship A, Tay C, et al. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation. 2013;127:1028–39. doi: 10.1161/CIRCULATIONAHA.112.001347. [DOI] [PubMed] [Google Scholar]
- Manam S, Chaganty BK, Evani SJ, et al. Intranasal vaccination with Chlamydia pneumoniae induces cross-species immunity against genital Chlamydia muridarum challenge in mice. PLoS One. 2013;8:e64917. doi: 10.1371/journal.pone.0064917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manam S, Nicholson BJ, Murthy AK. OT-1 mice display minimal upper genital tract pathology following primary intravaginal Chlamydia muridarum infection. Pathog Dis. 2013;67:221–4. doi: 10.1111/2049-632X.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manam S, Thomas JD, Li W, et al. Tumor necrosis factor (TNF) receptor superfamily member 1b on CD8+ T cells and TNF receptor superfamily member 1a on non-CD8+ T cells contribute significantly to upper genital tract pathology following chlamydial infection. J Infect Dis. 2014;211:2014–22. doi: 10.1093/infdis/jiu839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SG, Farris CM, Sturdevant GL, et al. Murine Chlamydia trachomatis genital infection is unaltered by depletion of CD4+ T cells and diminished adaptive immunity. J Infect Dis. 2011;203:1120–8. doi: 10.1093/infdis/jiq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy AK, Li W, Chaganty BK, et al. Tumor necrosis factor alpha production from CD8+ T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infect Immun. 2011;79:2928–35. doi: 10.1128/IAI.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld ME, Campbell LA. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemostasis. 2011;106:858–67. doi: 10.1160/TH11-06-0392. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis–an inflammatory disease. New Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rupp J, Pfleiderer L, Jugert C, et al. Chlamydia pneumoniae hides inside apoptotic neutrophils to silently infect and propagate in macrophages. PLoS One. 2009;4:e6020. doi: 10.1371/journal.pone.0006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturdevant GL, Caldwell HD. Innate immunity is sufficient for the clearance of Chlamydia trachomatis from the female mouse genital tract. Pathog Dis. 2014;72:70–3. doi: 10.1111/2049-632X.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis WC, Barrett LK, Sweeney YT, et al. Analysis of lymphocyte phenotype and cytokine activity in the inflammatory infiltrates of the upper genital tract of female macaques infected with Chlamydia trachomatis. J Infect Dis. 1996;174:647–50. doi: 10.1093/infdis/174.3.647. [DOI] [PubMed] [Google Scholar]
- Van Voorhis WC, Barrett LK, Sweeney YT, et al. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect Immun. 1997;65:2175–82. doi: 10.1128/iai.65.6.2175-2182.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]