Abstract
Cigarette smoke (CS)-exposed mice have been used to model airway inflammation and emphesema in humans; however, the impact of exposure duration, sex, and strain differences in susceptibility to progression of airway inflammation and to emphesema are poorly investigated. This study was designed to determine the association between inflammation and emphysema by exposing 2 strains of mice, C3H/HeN (C3H) and C57BL/6 (Bl/6), to filtered air (FA) or CS for 10, 16, or 22 weeks. Both genders and strains of CS-exposed mice developed pulmonary inflammation as characterized by cell counts in the bronchoalveolar lavage fluid (BALF) and the levels of matrix metalloproteinases (MMPs) in the BALF. CS exposure caused persistently higher number of BALF macrophages in C3H compared to BL/6 mice, while more BALF neutrophils and persistently higher MMP-2 and MMP-9 levels were observed in BL/6 mice. The mean linear intercept (Lm) increased progressively by 26%, 33%, and 55% at 10, 16, and 22 weeks, respectively, in CS-exposed C3H mice compared to the matched air controls. In BL/6 mice, although CS exposure also increased the Lm compared to FA controls, no further increase in Lm beyond the levels observed at 16 weeks of exposure was observed by 22 weeks. These findings suggest that extent of inflammation is not associated with severity of emphysema and underscores the importance of carefully selecting the mouse strains and endpoints when exploring effective treatments for emphesema.
Keywords: cigarette smoke, mouse strains, metalloprotease, alveolar destruction
Exposure to cigarette smoke (CS) is the major risk factor for the development of chronic obstructive pulmonary disease (COPD) (Lo Tam Loi et al., 2013; Vestbo et al., 2013). The pathology of COPD includes 2 major pathologies: (1) chronic bronchitis that involves mucous cell hyperplasia in the airway epithelia and in the mucus-producing submucosal glands and (2) emphysema that involves the destruction of alveolar septa resulting in larger and irregularly shaped air spaces distal to terminal bronchioles (Kim and Criner, 2012; MacNee, 2005).
Several mechanisms have been proposed to play a role in the pathogenesis of CS-induced emphysema. These include airway inflammation (Celli et al., 2012), T-lymphocyte induced immunity (O'Shaughnessy et al., 1997), alveolar cell loss through apoptosis due to oxidant-antioxidant imbalance (Bowler et al., 2004; Demedts et al., 2006; Nyunoya et al., 2011), protease- antiprotease imbalance due to increasd numbers of neutrophils and macrophages producing proteases such as neutrophil elastase (NE) and matrix metalloprotinases (MMPs) that destroy the matrix proteins of the lung (Gadek et al., 1979). However, the relative contribution of airway inflammation to severity of emphesema remains unclear.
Mouse models have proven useful in identifying the role of MMP-12 in CS-induced emphysema (Hautamaki et al., 1997) that was later validated as one of the susceptibility genes for COPD in humans (Hunninghake et al., 2009). Further, different mouse strains show differences in susceptibility to CS-induced emphysema. The severity of CS-induced emphysema depends on the mouse strain, thus, resembling the variation in susceptibility of human smokers to developing COPD. For example, mice of the B6C3F1 hybrid strain that are commonly used in toxicology and carcinogenesis bioassays develop a mild form of emphesema after exposure to CS for 7 months (March et al., 1999a), whereas the more susceptible A/J mice develop more severe pulmonary emphysema when exposed over a duration of 15 weeks (March et al., 2006). In this study, we wanted to investigate which of the parental inbred strains of the B6C3F1 hybrid, namely C3H/HeN (C3H) and C57BL/6 (BL/6) mice, might confer susceptibility or resistance to CS-induced airway inflammation and emphysema. Therefore, we exposed C3H and BL/6 mice either to filtered air (FA) as control or to CS for 10, 16, or 22 weeks. Airway inflammation by total and differential cell counts in bronchoalveolar lavage fluid (BALF), activities of MMP-2 and MMP-9 in the lungs, and induction of emphysema by histopathology and computer-assisted morphometry were evaluated. Results show that the BL/6 strain was more resistant to developing emphysema compared to the C3H strain because of a progressive response to CS-induced emphysema. More severe emphesema in C3H mice was associated with persistently higher number of BALF macrophages in comparison to BL/6 mice, while more numerous BALF neutrophils and persistently higher MMP levels were found in the more resistant BL/6 mice compared with the C3H mice. These findings show that extent of CS-induced emphysema does not correlate with number of neturophils but with number of macrophages.
MATERIALS AND METHODS
Animals and exposures
All procedures were conducted under protocols approved by an Institutional Animal Care and Use Committee at LRRI, a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Male and female C57Bl/6 (BL/6) and C3H/HeN (C3H) mice (Charles River Laboratories, Raleigh, North Carolina), at 8–10 weeks of age, were exposed in whole-body exposure chambers to mainstream CS from 1R3 research cigarettes (University of Kentucky Tobacco Research and Development Center, Lexington, Kentucky) or to filtered air (FA, control), as was previously described for exposure of A/J mice (March et al., 2006). Mice were acclimated to CS by exposing to 100 mg TPM/m3 (CS-100) during the first week followed by 250 mg TPM/m3 (CS-250) for 6 hours/day, 5 days/week, for a total exposure period of 10, 16, and 22 weeks.
Necropsy and BALF analysis
Mice were killed with an intraperitoneal (i.p.) injection of a barbiturate euthanasia solution and exsanguination. Tracheas were cannulated, left mainstem bronchi were temporarily clamped, and right lungs were lavaged 3 times with 0.5 ml phosphate buffered saline (PBS). The bronchoalveolar lavage fluid (BALF) was assessed for the number of inflammatory cells and differential cell types were evaluated on a GIEMSA-stained cytocentrifuge preparation. The activity of matrix metalloproteinases (MMPs) was assessed by zymography in the noncellular BAL supernatant fraction as described (March et al., 2002; Nyunoya et al., 2011; Seagrave et al., 2004). Conditioned medium from HT1080 cells was run on each gel, and the MMP-9 signal from this sample was used to normalizing all other signals.
Histopathology
Histopathology procedures were performed as described previously (March et al., 2005). Briefly, mice were killed as described above and lungs from mice designated for morphometry of emphysema were fixed by constant pressure inflation with 4% buffered paraformaldehyde. The volume of fixed whole lungs (VL) was determined, and left lung lobes were trimmed, processed for histology, sectioned, and stained with hematoxylin and eosin (H&E). Sections of lungs were qualitatively assessed by light microscopy for the severity of emphysema described as the irregular expansion of alveolar air spaces due to alveolar septal destruction, such that alveoli of normal size were often juxtaposed to greatly expanded air spaces. Morphometry for intercept- and point-counting was used to quantify emphysematous alveolar air space expansion. The most commonly used indicator of alveolar air space size, the mean linear intercept (Lm, in µm), was calculated. The Lm was corrected for histoprocessing shrinkage by dividing it by the group-average linear shrinkage factor. From point counting, fractional volume densities of alveolar septa (VVspt) and alveolar air space (VVair) were also measured and multiplied by VL to yield total volume of a particular component for the lung.
Statistical analysis
Comparisons for the morphometry and BALF data were made by the general linear models analysis of variance procedure (ANOVA; SAS Institute, Inc., Cary, North Carolina) for 4 independent variables (exposure, strain, gender, duration). Following this initial analysis, data were separated by gender and analyzed by ANOVA for up to 3-way interactions among exposure, strain, and duration of exposure. Data were tested for normal distribution (Kolmogorov-Smirnov test) and equal variance (Levene’s median test or 2-sample F test). Where the tests failed (P < .05), data were transformed by ranking prior to ANOVA. Interactions of the independent variables were of particular interest. For example, the effect of an interaction between exposure and duration might have suggested progressive worsening of the disease process over time. In order to assess pairwise differences between individual exposure-strain-duration groups post hoc to ANOVA, a least squares means procedure was done with the Tukey-Kramer adjustment for multiple comparisons, and the significance level was set at P < .05.
RESULTS
Differences in the Number of Inflammatory Cells in the BALF of CS-Exposed BL/6 and C3H Mice
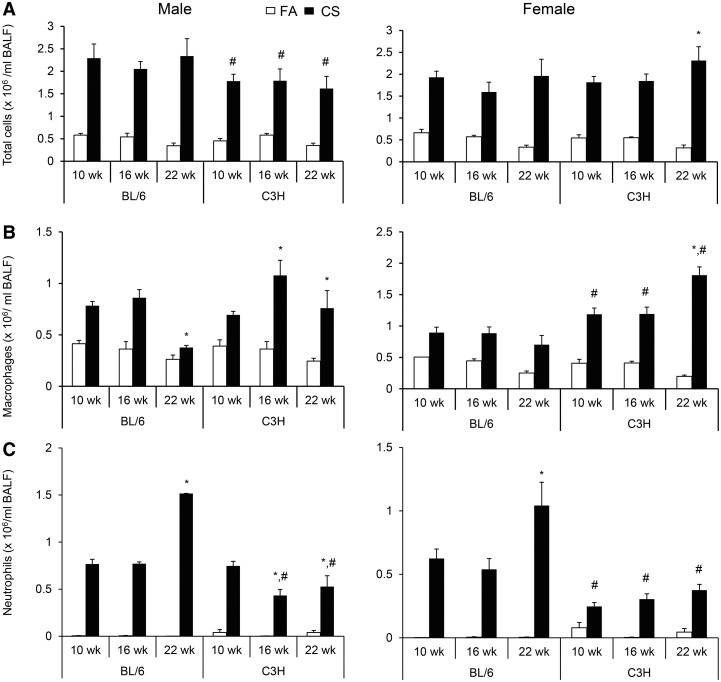
CS exposure increased the number of total cells/ml BAL in both BL/6 and C3H mice of both genders (P < .05, Fig. 1A). While the total number of BALF cells in CS-exposed male mice remained similar through 10–22 weeks in both BL/6 and C3H strains, male C3H compared to BL/6 mice had fewer number of cells at each time point. In female C3H mice, the total number of BALF cells increased significantly from 10 to 22 weeks of exposure, whereas no progressive increase was observed in female BL/6 mice.
FIG. 1.
Numbers of (A) total inflammatory cells, (B) macrophages, (C) and neutrophils in the BALF of male and female BL/6 and C3H mice after 10, 16, and 22 weeks of CS exposure. The number of inflammatory cells (total cells per ml BALF), macrophages and neutrophils in both genders and strains of mice after 10, 16, and 22 weeks of CS exposure compared to filtered-air exposed controls were quantified from cytospins that were stained with GIEMSA (FA, P < .05). Data (n = 5–6 mice per group) were rank-transformed prior to ANOVA. There were significant interactions between Strain and Exposure (P = .03, males; ANOVA) and between Exposure and Duration (P = .005, males; P < .0001, females). Significant differences by pair-wise tests between groups (P < .05) are indicated by symbols above the bars as follows: *within strain and gender, significant difference from 10-week CS exposure; #gender- and duration-matched difference between C3H and BL/6 strains.
Overall, BALF macrophages were increased by CS exposure in both BL/6 and C3H mice of both genders (P < .05, Fig. 1B). However, the number of macrophages decreased in male and remain unchanged in female BL/6 mice from 10 to 22 weeks CS exposure, but showed an increase in both male and female C3H mice, with female C3H mice having greater number of macrophages than female BL/6 mice at each time of exposure. Similar to macrophage numbers, neutrophils numbers were also increased by CS exposure in both strains of mice at all time points (P < .05, Fig. 1C). However, unlike the number of macrophages, neutrophils were more numerous in BALF from CS-exposed BL/6 mice at 16 and 22 weeks for male mice, and 10, 16, and 22 weeks for female mice than their C3H counterparts. The number of neutrophils increased in both male and female BL/6 mice and decreased in male C3H mice. Although no significant differences were observed between time points, there was a positive linear correlation between duration of exposure and number of BAL neutrophils in female C3H mice (r = 0.998). The number of lymphocytes increased by CS exposure in both male and female mice but only at the 16-week time point and this increase was independent of gender or strain (P = .002, data not shown).
Differences in MMP-2 and MMP-9 Activities in the BALF of CS-Exposed BL/6 and C3H Mice
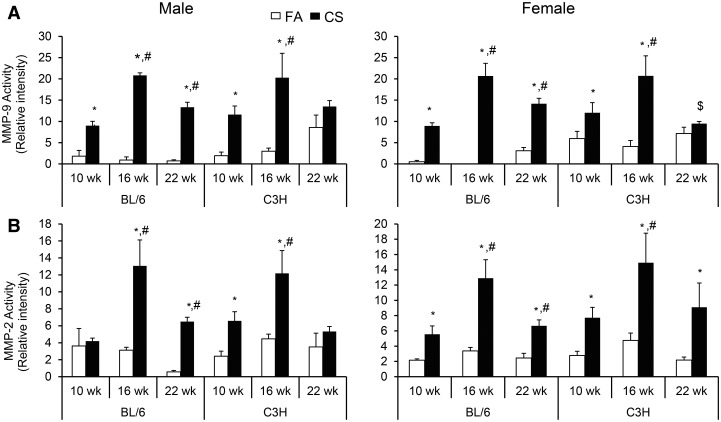
CS-induced increased MMP levels in both sexes at all time points relative to the FA-exposed animals (Fig. 2; Supplementary Fig. S1A–S1C). The MMPs analyzed included a high molecular weight doublet, and bands migrating with the expected molecular weights for MMP-2, pro-MMP-2, and MMP-9. Pro-MMP-9 was generally not detected, and the bands corresponding to Pro-MMP-2 were generally relatively weak (Supplementary Fig. S1C). The high molecular weight forms most likely represent complexes of MMP-9 and pro-MMP-9 with neutrophil gelatinase-associated lipocalin (NGAL, also known as LC2) (Di Carlo, 2013). The increases in relative activity of MMP-9 compared with the FA-exposed control groups (up to 23-fold at 16 weeks) were generally much greater than the activity for MMP-2 and pro-MMP-2 (2 to 4-fold for most sex/time conditions). The high molecular weight forms were often undetectable in the control animals, but increased substantially in the CS-exposed animals. Of particular interest was the strong increase in the high molecular weight forms observed in the CS-exposed male and female BL/6 mice at the 22-week time point. Although there was a general increase in all MMP forms in both male and female BL/6 mice from 10 to 22 weeks CS exposure, MMP levels decreased or remained unchanged over the exposure period in C3H mice, except for the high molecular weight form in female C3H mice that showed an increase at 22 weeks compared with the levels observed at 10 weeks (Supplementary Fig. S1B).
FIG. 2.
Activity of matrix metaloprotinase (A) (MMP)-9 and (B) MMP-2 in the cell free-BALF of BL/6 and C3H mice after 10, 16, and 22 weeks of of CS exposure. MMPs were assessed by zymography in the non-cellular supernatant fraction as described in Materials and Methods. Conditioned media from HT1080 cells was included on each zymogram, and the MMP signal from this samples were used to normalize all other signals. Data (n = 5–6 mice per group) were rank-transformed prior to ANOVA. Significant differences by pair-wise tests between groups (P < .05) are indicated by symbols above the bars as follows: *duration-, gender- and strain-matched differences from FA controls; #gender- and strain-matched differences from 10-week CS exposure; $duration- and gender-matched differences between CS-exposed C3H and BL/6 strains.
Differences in Extent of Emphysema in CS-Exposed BL/6 and C3H Mice
Exposure to CS for longer periods caused irregular, multifocal enlargement of air spaces throughout the parenchyma such that alveoli of normal size were often juxtaposed to large, expanded air spaces (Fig. 3A and B). The organization of parenchymal acini, i.e. alveolar ducts branching from terminal bronchioles and peripheral alveolar saccules, was often disrupted or unrecognizable. Alveolar septal fragmentation, blunting, and folding were noted in scattered foci and often associated with exudates of macrophages and neutrophils among cell debris in the alveolar air spaces as described previously (March et al., 1999b, 2006). Emphysema in the CS-exposed BL/6 mice was minimal to mild, while emphysema in C3H mice was more severe and graded as mild to moderate with evidence of progressive worsening with increasing exposure duration. Both male and female CS-exposed C3H mice had emphysema throughout 10 to 22 weeks of CS exposure, while emphesematous changes in CS-exposed C57Bl/6 mice were particularly severe in males (Fig. 3A and D).
FIG. 3.
Effects of exposure to CS and filtered air (FA), duration of exposure (10, 16 and 22 weeks), mouse strain (C3H and BL/6), and mouse gender on the development of emphysema. Representative micrographs of the left lungs of (A) male and (B) female mice exposed to CS or FA were processed, sectioned, and stained with hematoxylin and eosin (H&E). Scale bar, 200 µm. (C) The mean linear intercept (Lm, µm) showing interactions among strain, exposure, and duration (P < .0001) and (D) strain, exposure, and gender (P < .05, 4-way ANOVA). Significant differences between individual groups (n = 6–8 mice per group, Tukey-Kramer pair-wise tests, P < .05) are indicated by symbols above the bars as follows: for (C), *duration-, and strain-matched differences from FA controls; #strain-matched differences from 10-week CS exposure; $duration-matched differences between CS-exposed C3H and BL/6 strains, and for (D), *strain- and gender-matched differences from FA groups; #gender-matched differences between CS-exposed C3H and C57Bl/6 strains.
Morphometric Quantification of Extent of Emphysema in CS-Exposed BL/6 and C3H Mice
Morphometry for alveolar air space enlargement corroborated the histopathology. The mean linear intercept (Lm) was assessed as a measure of alveolar air-space size (Fig. 3C and D). For the 4 independent variables (exposure to CS or FA, duration of exposure, mouse strain, and gender), ANOVA yielded significant 3-way interactions among strain, exposure, and duration (P < .0001, Fig. 3C) and among strain, exposure, and gender (P < .05, Fig. 3D). No significant difference between the strains was observed at 10 weeks but at both 16 and 22 weeks of CS exposures, C3H mice had greater enlargement of alveolar air space compared to BL/6 mice. The mean linear intercept (Lm) increased progressively by 26%, 33%, and 55% at 10, 16, and 22 weeks, respectively, in CS-exposed C3H mice compared to the matched air controls. Thus, worsening of emphesema between 16 to 22 weeks was observed in C3H but not in BL/6 mice. Age-related enlargement of alveoli was seen in FA and CS-exposed mice for both genders. However, the significant effect of an interaction between duration and exposure suggested that alveolar enlargement was different than expected from either aging or CS exposure alone.
To determine whether CS-induced emphysema may be worse in female mice, other morphometric parameters including the total volume of alveolar septa (VVspt × VL) and alveolar air space (VVair × VL) were analyzed with separation of the data by gender (Table 1). No gender-related differences were observed for those parameters. However, as observed in previous studies (March et al., 2002; March et al., 2005), CS caused a significant increase in the VVair × VL of approximately 1.2-fold in males and 1.3-fold in females when averaged over the entire exposure period (Table 1). The magnitude of increase in VVair × VL was similar to the CS-induced increase in VL. The volume of alveolar septa (VVspt × VL) was also increased by CS exposure, and in males this increase was similar to the increase in VL (1.2-fold, averaged over the whole exposure period). The VVspt × VL in females increased only slightly (1.1-fold) consistent with the enlargement of VL being primarily due to increased parenchymal air space rather than an increase in septal tissue.
TABLE 1.
Morphometric parameters (mean ± SD) of emphysema in mice exposed to CS for varying durations
| Strain → | BL/6 |
C3H |
Effectsa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration → | 10 week |
16 week |
22 week |
10 week |
16 week |
22 week |
|||||||
| Exposure → | FA | CS | FA | CS | FA | CS | FA | CS | FA | CS | FA | CS | |
| Male | |||||||||||||
| No. per group | 8 | 7 | 8 | 8 | 10 | 10 | 8 | 8 | 7 | 7 | 10 | 7 | |
| Lung weight (mg)* | 164 ± 27 | 230 ± 28 | 158 ± 21 | 240 ± 16 | 243 ± 16 | 302 ± 24 | 150 ± 17 | 224 ± 26 | 164 ± 23 | 243 ± 45 | 169 ± 18 | 339 ± 52 | E, D |
| VL (cm3)* | 1.32 ± 0.08 | 1.56 ± 0.13 | 1.28 ± 0.14 | 1.57 ± 0.06 | 1.44 ± 0.11 | 1.65 ± 0.13 | 1.38 ± 0.08 | 1.81 ± 0.18 | 1.52 ± 0.11 | 1.98 ± 0.35 | 1.53 ± 0.16 | 2.26 ± 0.14 | E, S, D |
| VVspt × VL (cm3) | 0.22 ± 0.02 | 0.21 ± 0.03 | 0.19 ± 0.03 | 0.20 ± 0.04 | 0.18 ± 0.03 | 0.21 ± 0.03 | 0.17 ± 0.05 | 0.23 ± 0.02 | 0.17 ± 0.02 | 0.19 ± 0.03 | 0.17 ± 0.03 | 0.20 ± 0.03 | E, D |
| VVair × VL (cm3)* | 0.97 ± 0.06 | 1.17 ± 0.13 | 0.94 ± 0.08 | 1.11 ± 0.07 | 1.09 ± 0.09e | 1.26 ± 0.10e | 1.04 ± 0.08d | 1.01 ± 0.05d | 1.17 ± 0.07d,e | 1.57 ± 0.33d,e | 1.19 ± 0.20d | 1.82 ± 0.16d | E, SxD |
| Female | |||||||||||||
| No. per group | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 7 | 7 | |
| Lung weight (mg)* | 152 ± 24 | 230 ± 28b | 158 ± 27 | 230 ± 23b | 157 ± 21 | 249 ± 14b,c | 162 ± 19 | 210 ± 31b | 150 ± 16 | 241 ± 35b | 146 ± 34 | 271 ± 32b,c | ExD, S |
| VL (cm3)* | 1.22 ± 0.08 | 1.43 ± 0.15f | 1.28 ± 0.06 | 1.57 ± 0.10f | 1.42 ± 0.09 | 1.73 ± 0.08f | 1.23 ± 0.18d | 1.75 ± 0.20d,f | 1.42 ± 0.14d | 1.94 ± 0.28d,f | 1.53 ± 0.13d | 2.22 ± 0.12d,f | E, SxD |
| VVspt × VL (cm3) | 0.18 ± 0.03 | 0.20 ± 0.03 | 0.16 ± 0.03 | 0.19 ± 0.03 | 0.17 ± 0.04 | 0.21 ± 0.03 | 0.17 ± 0.03 | 0.20 ± 0.02 | 0.15 ± 0.04 | 0.19 ± 0.05 | 0.18 ± 0.03 | 0.18 ± 0.02 | E |
| VVair × VL (cm3)* | 0.85 ± 0.09 | 1.06 ± 0.12f | 0.92 ± 0.08 | 1.21 ± 0.09f | 1.03 ± 0.08 | 1.29 ± 0.07f | 0.93 ± 0.17 | 1.38 ± 0.22f,g | 1.13 ± 0.11g | 1.56 ± 0.24f | 1.19 ± 0.12g | 1.83 ± 0.16f,g | ExSxD |
C3H, C3H/HeN mice; BL/6, C57BL/6 mice; FA, filtered air (control); CS, cigarette smoke; VL, lung volume; VVspt × VL, total volume of alveolar septal tissue; VVair × VL, total volume of alveolar air space; Data for parameters marked with an asterisk (*) were transformed by ranking prior to ANOVA because of non-normal distribution and/or unequal variances.
aSignificant effects of exposure (E), strain (S), or duration (D) are indicated (P < .05, 3-way ANOVA). Significant interactions between the independent variables (P < .05) are indicated as products (eg, exposure with duration = ExD). Effects of individual independent variables are fairly obvious (eg, lung weight and VL increased with duration; VVspt × VL decreased with duration, CS exposure increased lung weight, VL, and VVair × VL). Where interactions are present, significant differences between groups by pair-wise tests are indicated by the superscript letters (b through g) applied to values in a row as described below (P < .05, least squares means test).
bDifferent from duration-matched FA-controls (strains combined).
cDifferent from exposure-matched previous time-point (strains combined).
dDifferent from duration-matched BL/6 mice (exposures combined).
eDifferent from strain-matched previous time-point (exposures combined).
fDifferent from strain- and duration-matched FA-control.
gDifferent from exposure- and duration-matched BL/6 mice.
Lung weight (LW) and body weight (BW) were also affected by CS exposure, gender, and duration but without significant interaction among the variables (Table 1; Supplementary Fig. S2). While CS exposure increased LW, the BW in both strains and genders of CS-exposed mice decreased by 15%–20% compared to the corresponding FA controls.
Because CS-induced COPD often involves mucous cell hyperplasia in human COPD, we quantified the number of mucus cells per basal lamina in lung tissue sections that were stained with Alcian Blue and Periodic Acid Schiff (AB and PAS). Both C57Bl/6 and C3H mice showed a statistically nonsignificant increase in the number of mucous cell per basal lamina compared to the FA controls (data not shown).
DISCUSSION
Given the laborious nature of chronic CS-exposure studies, many studies have focused on acute CS-exposure models that lasted for less than 2 weeks to screen for therapeutic targets with the assumption that neutrophilic inflammation predicts the development of emphesema (Stebbins et al., 2010; Vlahos and Bozinovski, 2014). However, inflammatory responses observed under acute CS exposure conditions may not fully reflect the progression and severty of airway inflammation and emphesema. The current study used 2 different strains of mice and different lengths of CS exposure to investigate the impact of duration of exposure and strain and gender of mice on the development of COPD phenotypes.
Macrophages were more numerous in the BALF of C3H than BL/6 mice and increased in number with longer exposure duration, especially in female C3H mice. On the other hand, compared to C3H mice, BL/6 mice had higher number of BALF neutrophils that increased through 10–22 weeks CS exposure. Activated neutrophils and CD8+ lymphocytes are the predominant inflammatory cell types associated with the pathology of CS-induced COPD (Saetta et al., 1998; Tamimi et al., 2012; Zhu et al., 2009), and neutrophils increase progressively in number in the bronchi of nonsmokers to mild/moderate COPD patients to severe COPD patients (Di Stefano et al., 2004). Hence, unlike C3H mice that did not show progressive increases in neutrophils, BL/6 mice may better reflect the development of airway inflammation in human smokers.
Imbalance between proteinases and their inhibitors is considered to be a cause for alveolar destruction in pulmonary emphysema, and previous studies have shown that macrophages are an important source of proteinases. In the current study, we measured the activity of mainly MMP-2 and MMP-9 in BL/6 and C3H mice and found that the activity of both MMPs increase with CS exposure. However, the patterns of MMP-2 and MMP-9 changes showed strain- and CS exposure duration dependence. BL/6 mice maintained higher levels of MMP-2 and MMP-9 activity at 22 weeks compared with 10 weeks whereas in C3H mice the 22 weeks levels of both MMPs decreased to similar or even lower levels than the ones observed at 10 weeks. MMP-2 is synthesized by a wide variety of cells, including fibroblasts, endothelial cells and alveolar epithelial cells, whereas MMP-9 is produced mainly by inflammatory cells such as polymorphonuclear neutrophils, monocytes, macrophages, eosinophils and lymphocytes (reviewed in Corbel et al. (2000)). In this study, no strict correlation was observed between MMP levels and the number of inflammatory cells in the BALF. Similarly, previous studies have established that, although macrophages are a main source of proteinases, their number does not always correlate well with MMP levels (Hautamaki et al., 1997; Delclaux et al., 1997). Other pathways that involve enhanced cell death processes could also be the main drivers for the observed emphysema in the different strains of mice.
Our results show that C3H mice are more susceptible than BL/6 mice in developing CS-induced emphysema. Female C3H mice are particularly susceptible as demonstrated by progressive alveolar air space enlargement with longer duration of CS exposure. Differences among various strains of mice in their responses to CS exposure, particularly in the induction of pulmonary emphysema, has also been reported (Guerassimov et al., 2004). Nevertheless, this study did not find a strict correlations between inflammatory cell numbers or MMP levels and the severity of tissue destruction. C3H mice, with more macrophages and fewer neutrophils in their inflammatory response to CS exposure and lower MMP-2 and MMP-9 activity than BL/6 mice, showed more worsening of tissue distruction over 10–22 weeks of CS exposure. In BL/6 mice, no further destruction of alveolar structures was observed beyond 16 weeks of CS exposure although higher levels of MMP-2, MMP-9 and high molecular weight activity were present at 22 weeks compared with levels observed at 10 weeks. Because MMP-2 and MMP-9 expression did not fully explain the time course of emphysema, it is possible that other protinases were likely involved. Although there are reports on the role for MMP-12 in the proemphysematic responses to CS (Hautamaki et al 1997), we were unable to detect the presence of the protein by Western blotting, or the activity of this enzyme in the lavage fluid by zymography on either gelatin or soluble elastin substrates or by analysis using a fluorogenic substrate. Further, the net activity of the MMPs is the result of a balance between the activated form of the proteins and the presence of the Tissue Inhibitors of Metalloproteinases (TIMPs). Attempts to detect the TIMPs in the lavage fluid by reverse zymography were unsuccessful, and Western blotting detected bands at molecular weights that did not appear to be consistent with the size of target proteins (data not shown). Further studies, possibly using molecular techniques to evaluate the expression of these targets, will be required to begin assessing their roles in CS-induced emphysema in different strains of mice. Other pathways that involve enhanced cell death processes could also be the main drivers for the observed emphysema in the different strains of mice. Despite these limitations, our studies suggest that CS-induced increases in neutrophil numbers and MMPs are correlated with mild tissue destruction, while increases in macrophage numbers correlated with severity and progression of emphesema in mice.
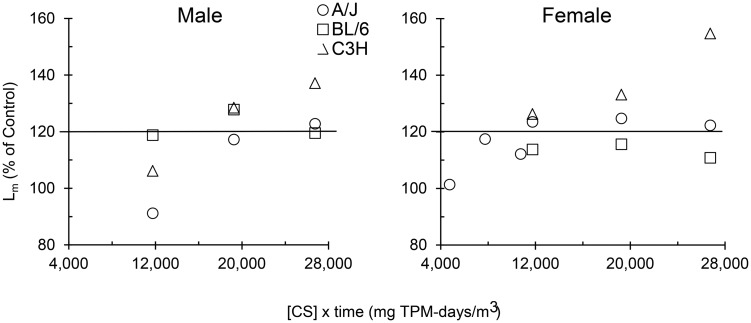
These studies demonstrate that C3H but not BL/6 mice develop more severe emphysema that progressively worsens with increasing length of CS exposure. However, BL/6 mice are the favored strain for genetic engineering and used for CS-induced COPD studies (Hautamaki et al., 1997; Petrache et al., 2005; Yoshida et al., 2010). In previous studies, the A/J mouse has been demonstrated to be more susceptible than other strains to CS-induced emphysema (Foronjy et al., 2005; March et al., 2006), but a direct comparison to C3H mice has not been reported. Exposure to CS can be viewed as a dose-response effect, where “dose” could be equated to the concentration of particulate material in CS atmospheres multiplied by the exposure duration. Comparison of the 3 mouse strains showed that the C3H strain clearly responded with progressively more severe disease to increasing dose of CS (Fig. 4). Part of this pathology may have been a response to an effective dose rather than a dose measured simply as CS partculate concentrations. No comparisons were made on strain-specific pulmonary function, and strain- (or gender-) dependent differences in respiration frequency, pulmonary compliance, and airway resistance, which could have caused different amounts of injurious material to be deposited in the lung parenchyma. However, comparison of the 3 strains of mice clearly indicated that emphesema was more severe in the C3H strain. Our findings also suggest that the resistance to CS-induced airway inflammation and emphysema in the parental inbred strains of the B6C3F1 hybrid may stem from BL/6 mice. QTL (quantitative trait locus) mapping needs to be conducted in the future to identify the chromosomal sites that determine resistance/susceptibility to CS-induced emphysema in mice.
FIG. 4.
Comparison of CS-induced emphysema severity (measured by the mean linear intercept [Lm]) in 3 strains of mice relative to the CS dose that was calculated by multiplying CS concentration and exposure duration in mg total particulate material [TPM]-days/m3. Mean values are expressed as a percentage of duration-matched filtered air-exposed control means. Data used for the A/J mice were published previously (March et al., 2006), where mean percentages for females at 4750, 7750, and 10 750 mg TPM-days/m3 correspond to exposure at 100 mg TPM/m3 for 10, 16, and 22 weeks, respectively. Remaining values at 11 750, 19 250, and 26 750 mg TPM-days/m3 correspond to exposure at 250 mg TPM/m3 for 10, 16, and 22 weeks, respectively. The line at 120% of control represents a minimum for clinically significant abnormal air space enlargement in age-matched human lungs (Verbeken et al., 1992).
Individuals chronically exposed to CS have a wide variety of responses ranging from clinically inconsequential inflammatory reactions to chronic bronchitis, or severe emphysema and/or lung carcinogenesis. The specific molecular mechanisms underlying this variation in response to CS exposure are not understood, but aside from dose-response relationships, individual differences in inflammatory response, differences in innate and acquired immunologic responses, and differences in repair capacity of CS-induced destruction by reactive oxygen species are likely genetically inherent. In general, CS does not robustly increase mucous cell hyperplasia in mice, while it readily causes emphysematous changes. The C3H mice may prove to be a useful animal model to investigate the development of emphesema in genetically susceptible individuals. Finally, although both BL/6 and C3H mice could develop pulmonary inflammation upon CS exposure, no strict correlation was observed between neutrophilic inflammation and extent of tissue destruction, whereas only increase in the number of BALF macrophages in C3H mice was correlated with severity and progression of emphesema. This underscores the need for carefully selecting mouse strains when exploring emphesema development and screening for effective therapeutic agents in animal models of human COPD.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
The studies were supproted by grants from the National Institutes of Health (HL68111 and ES015482).
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Lovelace Respiratory Research Institute Histology Laboratoty for assistance in lung tissue processing and staining.
REFERENCES
- Bowler R. P., Barnes P. J., Crapo J. D. (2004). The role of oxidative stress in chronic obstructive pulmonary disease. Copd 1, 255–277. [DOI] [PubMed] [Google Scholar]
- Celli B. R., Locantore N., Yates J., Tal-Singer R., Miller B. E., Bakke P., Calverley P., Coxson H., Crim C., Edwards L. D., et al. (2012). Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am. J. Respir. Critic. Care Med. 185, 1065–1072. [DOI] [PubMed] [Google Scholar]
- Corbel M., Boichot E., Lagente V. (2000). Role of gelatinases MMP-2 and MMP-9 in tissue remodeling following acute lung injury. Brazilian J. Med. Biol. Res. Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica 33, 749–754. [DOI] [PubMed] [Google Scholar]
- Delclaux C., d'Ortho M. P., Delacourt C., Lebargy F., Brun-Buisson C., Brochard L., Lemaire F., Lafuma C., Harf A. (1997). Gelatinases in epithelial lining fluid of patients with adult respiratory distress syndrome. Am. J. Physiol. 272, L442–L451. [DOI] [PubMed] [Google Scholar]
- Demedts I. K., Demoor T., Bracke K. R., Joos G. F., Brusselle G. G. (2006). Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir. Res. 7, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo A. (2013). Evaluation of neutrophil gelatinase-associated lipocalin (NGAL), matrix metalloproteinase-9 (MMP-9) and their complex MMP-9/NGAL in sera and urine of patients with kidney tumors. Oncol. Lett. 5, 1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano A., Caramori G., Ricciardolo F. L., Capelli A., Adcock I. M., Donner C. F. (2004). Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 34, 1156–1167. [DOI] [PubMed] [Google Scholar]
- Foronjy R. F., Mercer B. A., Maxfield M. W., Powell C. A., D'Armiento J., Okada Y. (2005). Structural emphysema does not correlate with lung compliance: lessons from the mouse smoking model. Exp. Lung Res. 31, 547–562. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Crystal R. G. (1979). Cigarette smoking induces functional antiprotease deficiency in the lower respiratory tract of humans. Science 206, 1315–1316. [DOI] [PubMed] [Google Scholar]
- Guerassimov A., Hoshino Y., Takubo Y., Turcotte A., Yamamoto M., Ghezzo H., Triantafillopoulos A., Whittaker K., Hoidal J. R., Cosio M. G. (2004). The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am. J. Respir. Critic. Care Med. 170, 974–980. [DOI] [PubMed] [Google Scholar]
- Hautamaki R. D., Kobayashi D. K., Senior R. M., Shapiro S. D. (1997). Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277, 2002–2004. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. M., Cho M. H., Tesfaigzi Y., Soto-Quiros M. E., Avila L., Lasky-Su J., Stidley C., Melen E., Soderhall C., Hallberg J., et al. (2009). MMP12, lung function, and COPD in high-risk populations. N. Engl J. Med. 361, 2599–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim V., Criner G. J. (2012). Chronic Bronchitis and COPD. American journal of respiratory and critical care medicine. [Google Scholar]
- Lo Tam Loi A. T., Hoonhorst S. J., Franciosi L., Bischoff R., Hoffmann R. F., Heijink I., van Oosterhout A. J., Boezen H. M., Timens W., Postma D. S., et al. (2013). Acute and chronic inflammatory responses induced by smoking in individuals susceptible and non-susceptible to development of COPD: from specific disease phenotyping towards novel therapy. Protocol of a cross-sectional study. BMJ Open 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNee W. (2005). Pathogenesis of chronic obstructive pulmonary disease. Proc. Am. Thoracic Soc. 2, 258–266; discussion 290–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March T. H., Barr E. B., Finch G. L., Hahn F. F., Hobbs C. H., Menache M. G., Nikula K. J. (1999a). Cigarette smoke exposure produces more evidence of emphysema in B6C3F1 mice than in F344 rats. Toxicol. Sci. Off. J. Soc. Toxicol. 51, 289–299. [DOI] [PubMed] [Google Scholar]
- March T. H., Barr E. B., Finch G. L., Nikula K. J., Seagrave J. C. (2002). Effects of concurrent ozone exposure on the pathogenesis of cigarette smoke-induced emphysema in B6C3F1 mice. Inhal. Toxicol. 14, 1187–1213. [DOI] [PubMed] [Google Scholar]
- March T. H., Bowen L. E., Finch G. L., Nikula K. J., Wayne B. J., Hobbs C. H. (2005). Effects of strain and treatment with inhaled aII-trans-retinoic acid on cigarette smoke-induced pulmonary emphysema in mice. Copd 2, 289–302. [PubMed] [Google Scholar]
- March T. H., Kolar L. M., Barr E. B., Finch G. L., Menache M. G., Nikula K. J. (1999b). Enhanced pulmonary epithelial replication and axial airway mucosubstance changes in F344 rats exposed short-term to mainstream cigarette smoke. Toxicol. Appl. Pharmacol. 161, 171–179. [DOI] [PubMed] [Google Scholar]
- March T. H., Wilder J. A., Esparza D. C., Cossey P. Y., Blair L. F., Herrera L. K., McDonald J. D., Campen M. J., Mauderly J. L., Seagrave J. (2006). Modulators of cigarette smoke-induced pulmonary emphysema in A/J mice. Toxicol. Sci. Off. J. Soc. Toxicol. 92, 545–559. [DOI] [PubMed] [Google Scholar]
- Nyunoya T., March T. H., Tesfaigzi Y., Seagrave J. (2011). Antioxidant diet protects against emphysema, but increases mortality in cigarette smoke-exposed mice. Copd 8, 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shaughnessy T. C., Ansari T. W., Barnes N. C., Jeffery P. K. (1997). Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am. J. Respir. Critic. Care Med. 155, 852–857. [DOI] [PubMed] [Google Scholar]
- Petrache I., Natarajan V., Zhen L., Medler T. R., Richter A. T., Cho C., Hubbard W. C., Berdyshev E. V., Tuder R. M. (2005). Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat. Med. 11, 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetta M., Di Stefano A., Turato G., Facchini F. M., Corbino L., Mapp C. E., Maestrelli P., Ciaccia A., Fabbri L. M. (1998). CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am. J. Respir. Critic. Care Med. 157, 822–826. [DOI] [PubMed] [Google Scholar]
- Seagrave J., Barr E. B., March T. H., Nikula K. J. (2004). Effects of cigarette smoke exposure and cessation on inflammatory cells and matrix metalloproteinase activity in mice. Exp. Lung Res. 30, 1–15. [DOI] [PubMed] [Google Scholar]
- Stebbins K. J., Broadhead A. R., Baccei C. S., Scott J. M., Truong Y. P., Coate H., Stock N. S., Santini A. M., Fagan P., Prodanovich P., et al. (2010). Pharmacological blockade of the DP2 receptor inhibits cigarette smoke-induced inflammation, mucus cell metaplasia, and epithelial hyperplasia in the mouse lung. J. Pharmacol. Exp. Therapeutics 332, 764–775. [DOI] [PubMed] [Google Scholar]
- Tamimi A., Serdarevic D., Hanania N. A. (2012). The effects of cigarette smoke on airway inflammation in asthma and COPD: therapeutic implications. Respir. Med. 106, 319–328. [DOI] [PubMed] [Google Scholar]
- Verbeken E. K., Cauberghs M., Mertens I., Clement J., Lauweryns J. M., Van de Woestijne K. P. (1992). The senile lung. Comparison with normal and emphysematous lungs. 1. Structural aspects. Chest 101, 793–799. [DOI] [PubMed] [Google Scholar]
- Vestbo J., Hurd S. S., Agusti A. G., Jones P. W., Vogelmeier C., Anzueto A., Barnes P. J., Fabbri L. M., Martinez F. J., Nishimura M., et al. (2013). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Critic. Care Med. 187, 347–365. [DOI] [PubMed] [Google Scholar]
- Vlahos R., Bozinovski S. (2014). Recent advances in pre-clinical mouse models of COPD. Clin. Sci. 126, 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Mett I., Bhunia A. K., Bowman J., Perez M., Zhang L., Gandjeva A., Zhen L., Chukwueke U., Mao T., et al. (2010). Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nat. Med. 16, 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Gadgil A. S., Givelber R., George M. P., Stoner M. W., Sciurba F. C., Duncan S. R. (2009). Peripheral T cell functions correlate with the severity of chronic obstructive pulmonary disease. J. Immunol. 182, 3270–3277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.