Abstract
It is unknown whether autophagy activity is altered in insulin resistant podocytes and whether autophagy could be a therapeutic target for diabetic nephropathy (DN). Here we used shRNA transfection to knockdown the insulin receptor (IR) gene in cultured human immortalized podocytes as an in vitro insulin resistant model. Autophagy related proteins LC3, Beclin, and p62 as well as nephrin, a podocyte injury marker, were assessed using western blot and immunofluorescence staining. Our results show that autophagy is suppressed when podocytes lose insulin sensitivity and that treatment of rapamycin, an mTOR specific inhibitor, could attenuate insulin resistance induced podocytes injury via autophagy activation. The present study deepens our understanding of the role of autophagy in the pathogenesis of DN.
Keywords: Diabetic nephropathy, Autophagy, Insulin resistance, Rapamycin
Introduction
Diabetic nephropathy (DN) is the leading cause of end-stage kidney disease (ESRD) worldwide. It is also a major devastating complication of diabetes mellitus (DM), with up to 40% of diabetic patients experiencing this problem (Shi & Hu, 2014). With the rapidly increasing prevalence of DM being a major global health issue, it becomes more and more important to find therapeutic interventions directed at preventing the development and progression of DN.
The natural history of DN is dominated by progressive albuminuria, and podocytes are key components of the ultrafiltration system in the glomeruli. Moreover, podocyte number and morphology have been proved to be predictors of DN progression. This makes the glomerular podocyte an attractive early target cell. Molecular mechanisms involved in the etiology and progression of DM and its complications have been studied intensively. Among them, insulin resistance was proved to be a critical one. In glomeruli of obese and diabetic rats, insulin sensitivity was reduced (Mima et al., 2011). Previous studies have shown that podocytes are insulin responsive cells in glomeruli (Madhusudhan et al., 2015; Tejada et al., 2008), and a loss of podocyte insulin sensitivity in perfused glomerulus results in an albuminuric phenotype even under normal glycemic conditions (Coward et al., 2005). Thus, insulin signaling in podocytes is essential for normal glomerular function. This has been proved by studies that show specific deletion of podocyte insulin receptor (IR) causes significant proteinuria and glomerulosclerosis in mice (Welsh et al., 2010). However, how podocyte inuslin resistance leads to podocytes injury remains unclear.
Autophagy is an intracellular catabolic process by which aggregates and malfunctioned organelles are degraded to maintain intracellular homeostasis (Levine & Ranganathan, 2010). Defects in autophagy have been closely associated with many human diseases, including cancer, neurodegenerative disorders. Accumulating evidence suggests that regulation of autophagy system may become a new therapeutic option for treatment of DN (Kume et al., 2014). In both genetic and dietary mouse models of obesity and insulin resistance, decreased autophagy in hepatic cells has been observed (Yang et al., 2010). Moreover, in obese animals, the activation of autophagy could be protective metabolic abnormalities (He et al., 2012). However, it is worthy of notice that modulation of autophagy in DM varies in different cell types. In pancreatic β-cells, autophagy is activated due to peripheral insulin resistance, whereas autophagy is inhibited in the hepatic cells of Type 2 diabetes (T2D) mice with insulin resistance (Rovira-Llopis et al., 2015). Whether the process of autophagy in insulin resistant podocytes is altered is still an open question. There are also uncertainties about whether autophagy participates in the insulin resistance mediated podocyte injury. The present study aims at evaluating the role of autophagy in diabetic nephropathy with focus on podocyte insulin resistance. We hypothesize that blunted autophagy activity in insulin resistant podocyte is one of the mechanisms that accounts for the podocytes injury in the pathogenesis of diabetic nephropathy. To test our hypothesis, IR knockout podocytes were used as an in vitro insulin resistant model.
Materials and Methods
Cell culture
Conditionally immortalized human podocytes were obtained from professor Fan Yi of Shan Dong University. The cells were maintained in RPMI 1640 medium (HyClone, South Logan, UT, USA) containing 10% heat-inactivated fetal calf serum (Hyclone, USA) and 100 U/ml penicillin (Hyclone, USA) in the presence of 5% CO2 as described (Saleem et al., 2002). The cells were cultured in 33 °C to sustain podocyte proliferation, then podocytes were cultured at 37 °C for 10–14 days to induce differentiation. All experiments were performed on passages 10–14 differentiated podocytes in the present study. Insulin (200 nM) was added 30 min before cells were harvested.
Knockdown of insulin receptor (IR) by shRNA transfection
Selected shRNA lentivirus vector (pGMLV-SC1) against IR or negative control shRNA with eGFP were designed and purchased from Genomeditech Company (Shanghai, China). The transfection was performed as previously described (Xu et al., 2014). Figure 1 shows the successful downregulation of IR by IR shRNA transfection. Under the experimental conditions, we routinely obtain 80% downregulation of IR (Fig. 1). Thus, IR shRNA transfection was used as the in vitro model to study the role of autophagy in insulin resistant podocytes.
Figure 1. Successful downregulation of IR by IR shRNA transfection.
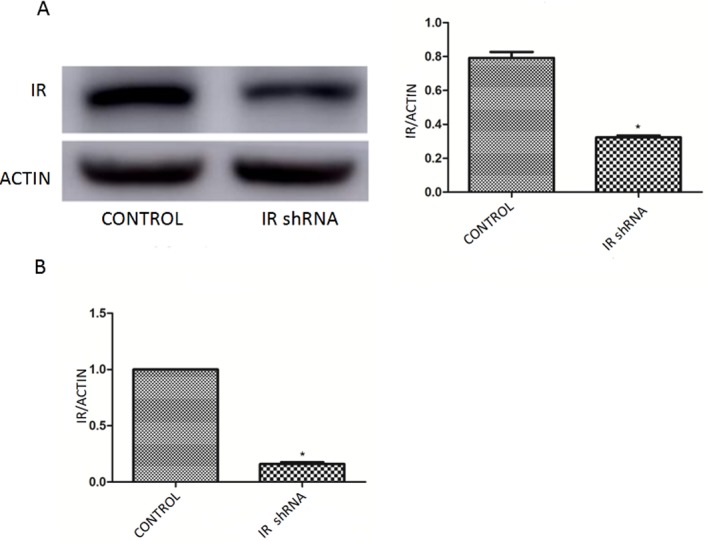
(A) Western blots of IR after transfected with IR shRNA. (B) qRT-PCR analysis showed a 80% knockdown efficiency of IR after transfected with IR shRNA.
Real time quantitative PCR (RT-qPCR)
Total RNA was isolated from podocyte and reverse transcribed to cDNA by PrimeScript™ RT Reagent Kit with gDNA Eraser (Takara). Real time PCR was performed using the light cycler 480 (Roche Diagnostics) with SYBR Premix Ex Taq™ II (Takara). The knockdown efficiency of the IR was confirmed by real time quantitative PCR (RT-qPCR) using sequence-specific primers for IR (forward 5′- GGAGCTGTCCTAGGTGCTGTTTC -3′ and reverse 5′- CTTGTGTCAGTTCCCACAGCTTC -3′), which were designed by TaKaRa. The expression levels of IR were normalized to β-actin expression level (forward 5′- TGGC ACCCAGCACAATGAA -3′ and reverse 5′- CTAAGTCATAGTCCGCCTAGAAGCA -3′).
Western blot analysis
The procedure of western blot analysis was carried out as described previously (Delfin et al., 2011). The following primary antibodies were used: antibodies for SQSTM1/p62 (5114; Cell Signaling), LC3B (ab48394; Abcam), Beclin-1 (SC-11427; Santa Cruz), IR (sc-711; Santa Cruz) and nephrin (ab58968; Abcam) Primary antibody against β-actin and horseradish peroxidase-conjugated secondary antibodies were from ZSGB-BIO.
Immunofluorescence staining
The immunofluorescence staining was performed using protocol modified from previous publication (Ning et al., 2011). Briefly, cells were fixed in 4% paraformaldehyde for 10 min at room temperature, then washed and permeabilized using PBS containing 0.3% Triton X-100, followed by blocking with 5% BSA in PBS. 1:100 dilution primary antibodies were added to the cells. After incubating overnight at 4 °C, cells were washed. 10 µg/ml Alexa Fluor488 or Alexa Fluor594 secondary antibodies were added and incubated for 30 min. Images were acquired by immunofluorescence microscopy (Nikon Ti-S, Tokyo, Japan).
Electron microscopy
After treatments, podocytes were trypsinized and collected into centrifuge tubes after washing by PBS. Then, the cells were fixed by 3% glutaraldehyde at 4 °C, dehydrated by dimethylketone. After embedment in Epon-812, the samples were cut into ultrathin sections (70 nm). Uranium acetate and plumbum citrate were used to dye the ultrathin sections. The samples were observed with JEM-100sX electron microscopy.
Statistics
Data are presented as means ± SD unless stated otherwise. Data were analyzed using repeated measurement ANOVA followed by t test when appropriate with two tailed p-values < 0.05 considered statistically significant. GraphPad Prism software (version 5) was used for data analysis.
Results
Autophagy activity was down-regulated in IR-knockdown podocytes
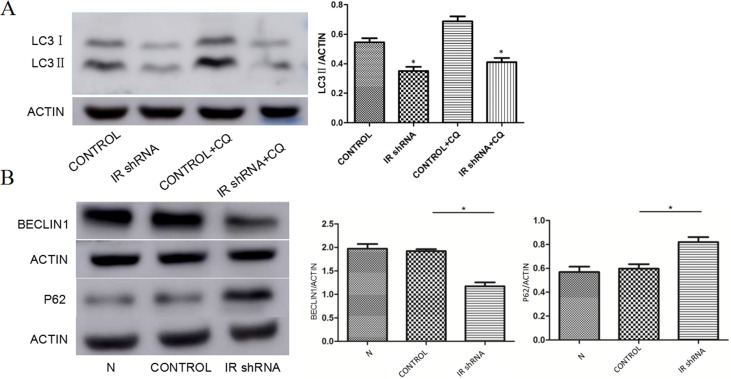
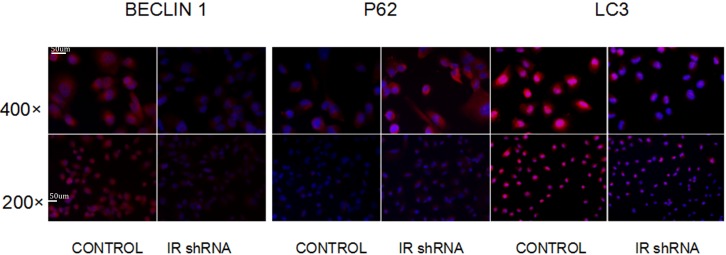
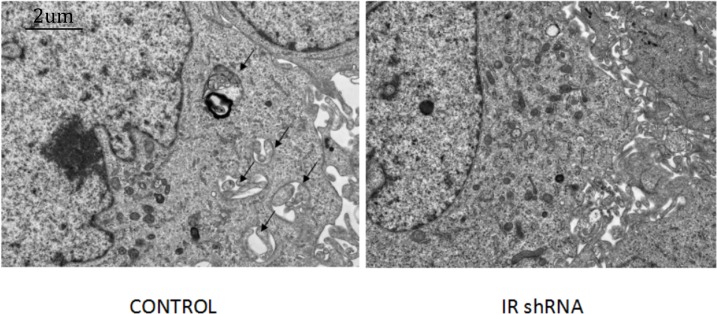
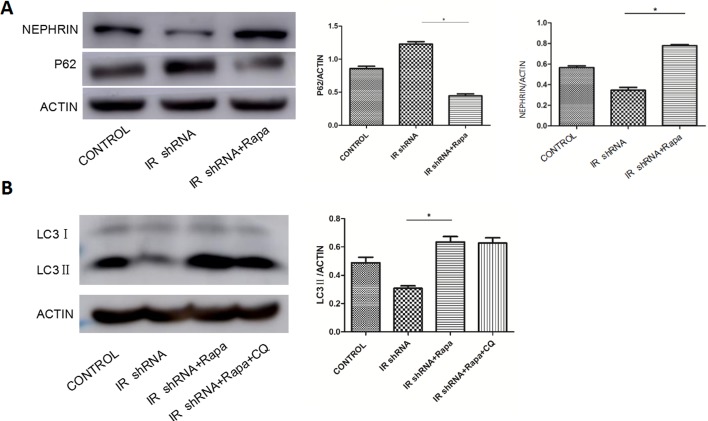
To investigate autophagy activity under insulin resistant conditions, we examined the changes of autophagy related protein abundances in IR deficient podocytes (Fig. 1). Figure 2 shows the western blots of Beclin1, p62 and LC3II, indicating that autophagy was downregulated in IR knockdown podocytes. During the autophagosome formation, LC3-I is processed into a lapidated LC3-II form. The LC3-II/LC3-I ratio is considered as a marker of auophagosome formation. Chloroquine (CQ) was used as the lysosomal activity blocker to evaluate the autophagic flux. Compared with control group, a significant less LC3-II was detected in the IR-knockdown cells (Fig. 2A). Similar to the LC3 protein, western blots showed a decreased level of Beclin1 (a marker for autophagosome initiation complex) (Fig. 2B). Compared to control cells, IR knockdown podocytes showed significant increased expression of p62, indicating decreased autophagy activity (Fig. 2B). Figure 3 shows the immunofluorescence of Beclin1, p62 and LC3. Consistent with the western blot results, the immunofluorescence staining of Beclin1 and p62 also indicates a decreased autophagy activity in IR-knockdown podocytes. Figure 4 shows the electron microscopy images of control and IR knockdown podocytes. There were significantly less autophagosomes in IR knockdown podocytes compared to control cells.
Figure 2. Autophagy was down-regulated after knockdown of IR.
(A) After 200 nM insulin stimulation for 30 min, the expression of LC3ll were decreased in cells transfected with IR shRNA with or without 50 uM chloroquine (CQ), compared with control shRNA. ∗P < 0.05 vs. control shRNA. (B) After 200 nM insulin stimulation for 30 min, the expression of BECLIN1 were decreased, but P62 was up-regulated in cells transfected with IR shRNA, compared with control shRNA. ∗P < 0.05 vs. control shRNA.
Figure 3. Immunofluorescence of autophagy markers shows that autophagy was down-regulated after knockdown of IR.
After 200 nM insulin stimulation for 30 min, the staining of BECLIN1 and LC3ll was decreased but P62 was enhanced in cells transfected with IR shRNA, compared with cells transfected with control shRNA.
Figure 4. Electronic microscopy analysis of podocyte shows that autophagy was down-regulated after knockdown of IR.
After 200 nM insulin stimulation for 30 min, the number of autophagosomes in cells transfected with IR shRNA was decreased compared with cells transfected with control shRNA. Black arrows indicate autophagosomes. Magnification ×15,000.
IR deficiency induced podocyte injury in vitro
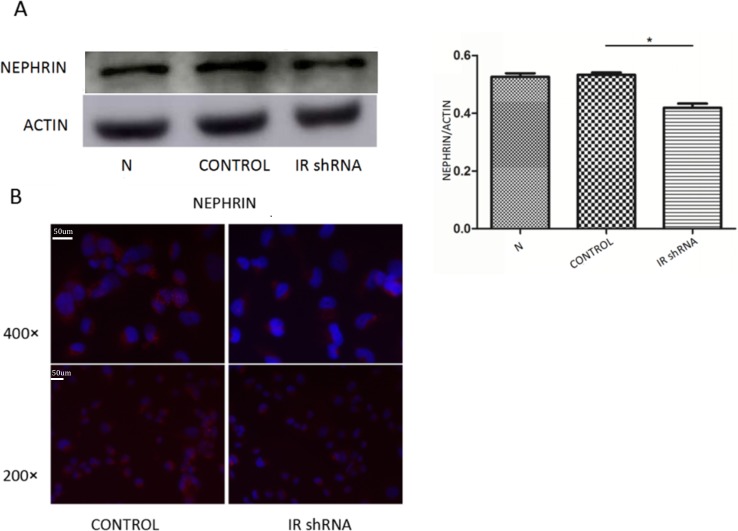
Nephrin has been proved to be critical for the action of insulin on podocytes (Coward et al., 2007) and its expression has been used as the marker of podocyte integrity. Figure 5 shows the nephrin expression levels in control and IR knockdown podocytes. Both western blot (Fig. 5A) and immunofluorescence staining (Fig. 5B) of nephrin show that IR-knockdown podocytes had a significant decrease of nephrin expression level, indicating podocyte injury induced by insulin resistance.
Figure 5. Nephrin expression was down-regulated after knockdown of IR.
(A) WB showed after 200 nM insulin stimulation for 30 min, the expression of NEPHRIN was down-regulated in cells transfected with IR shRNA, compared with cells transfected with control shRNA ∗P < 0.05 vs. control shRNA. (B) Immunofluorescence demonstrated that after 200 nM insulin stimulation for 30 min, NEPHRIN staining was decreased in cells transfected with IR shRNA, compared with cells transfected with control shRNA.
Rapamycin ameliorated insulin resistance induced podocyte injury via autophagy activation
Rapamycin has been clinically used to inhibit rejection after organ transplantation as a FDA approved immunosuppressive drug. It works by binding to mammalian target of rapamycin (mTOR) and inhibiting the activity of mTOR protein kinase (Hartford & Ratain, 2007). Rapamycin has been shown to induce autophagy in many cell types and species (Huber, Walz & Kuehn, 2011). Figure 6 shows the p62, LC3II and nephrin level in IR-deficient podocytes with or without the treatment of rapamycin. DMSO was used as the control group since rapamycin was resolved in DMSO. Compared to the DMSO control group, IR-deficient podocytes treated with rapamycin showed elevated autophagy activity indicated by increased LC3II expression. Meanwhile, nephrin expression was restored by rapamycin treatment. Our results indicate that podocyte injury under insulin resistant conditions was ameliorated by rapamycin treatment via activation of autophagy.
Figure 6. Rapamycin (RAPA) activated autophagy in IR-knockdown podocytes and increased the expression of Nephrin.
(A) After 10 uM Rapamycin (RAPA) stimulation for 2 h, the expression of p62 in IR-knockdown podocytes were decreased while NEPHRIN was up-regulated compared with cells without RAPA. ∗P < 0.05 vs. IR shRNA.(B) After 10 uM Rapamycin (RAPA) stimulation for 2 h the expression of LC3ll in IR-knockdown podocytes was up-regulated in cells with or without CQ compared with cells without RAPA. ∗P < 0.05 vs. IR shRNA.
Discussion
The main findings of the current study are that under insulin resistant conditions, autophagy activity in podocytes was suppressed in vitro; Treatment of rapamycin, an mTOR specific inhibitor, could attenuate insulin resistance induced podocytes injury via autophagy activation.
Our results show that autophagy related proteins such as Beclin1 and LC3 were down regulated and p62 was increased in IR-knockdown podocytes. Thus, our data indicates decreased autophagy activity is induced in podocytes under insulin resistant conditions. It has been demonstrated previously that autophagy plays a renoprotective role in the kidney. Podocyte-specific autophagy deficient mice generated by Atg5 gene deletion have glomerular lesions accompanied by podocytes apoptosis and albuminuria with aging (Mizushima et al., 2004). Greatly increased susceptibility to glomerular disease was exhibited in mice lacking Atg5 gene in podocytes. These findings underscore the importance of autophagy regulation as a critical mechanism to maintain podocyte homeostasis. With the evidence of autophagy in kidney health and disease accumulating, studies to investigate the role of autophagy in DN have attracted intensive interests. Histone deacetylase 4 inhibition could ameliorates podocyte injury and attenuates glomerulopathy in DN, and the maintenance of autophagy in podocytes was suggested to be the mechanism underlying (Wang et al., 2014). In patients and rats with diabetes, insufficient podocyte autophagy was observed histologically. Moreover, podocyte-specific autophagy-deficient mice developed podocytes loss and massive proteinuria in a high-fat diet-induced diabetes model that usually presented with minimal proteinuria (Tagawa et al., 2016). These all underline the role of autophagy in podocytes in DN pathogenesis. Our study contributes to the understanding of how autophagy activity responses to insulin resistance with further detailed mechanisms to be investigated.
Podocyte insulin resistance is associated with glomerular podocyte dysfunction. In this study, downregulated autophagy activity was demonstrated to participate in podocyte injury. Since insulin resistance is a prevalent metabolic feature in DM, it is difficult to answer the question that which happens first, the onset of insulin resistance or alterations of autophagy. High-fructose feeding mice showed disruption of autophagy in the liver, which appeared as an early event preceding the onset of insulin resistance. Drugs able to restore the autophagic flux could indeed prevent insulin resistance (Wang et al., 2015). Importantly, a recent study found that autophagy regulates muscle glucose homeostasis and increase insulin sensitivity (Tam & Siu, 2014). However, most of the works about autophagy and insulin resistance were done in adipose tissue, skeletal muscles or liver, and what the relationship in the kidney remains unknown. Our results show that after IR knockdown in podocyte, there are decreased autophagy activities. With the current study, we report a decreased autophagy that was poised in an in vitro setting of insulin resistance. Hence, a reciprocal interaction between podocyte insulin resistance and autophagy activity should be proposed. Taken together, among mechanisms that might be altered in podocytes under insulin resistant conditions, autophagy seems to play a role. Other mechanisms such as oxidative stress and endoplasmic reticulum (ER) stress are also implicated. Recently, it has been shown that ER stress (Madhusudhan et al., 2015) and mitochondrial function (Ising et al., 2015) are modulated by insulin signaling. In adipocytes in vitro, endoplasmic reticulum (ER) stress causes insulin receptor (IR) down-regulation, which accounts for insulin resistance. While inhibition of autophagy could alleviate the ER stress induced IR down-regulation (Zhou et al., 2009). It is also proposed that in diabetes, autophagy system is activated in response to ER stress induced insulin resistance (Zhang et al., 2015). These results indicate that autophagy regulation in DN is a rather complicated process. The genes and signaling pathways that link autophagy and insulin resistance as well as podocyte injury in vivo need to be delineated.
Our results showed that with manipulation, the activation of autophagy could protect the podocyte from injuries induced by insulin resistance. Podocyte insulin resistance has been considered to play a role in genesis and propagation of DN. The study of insulin signaling pathways in podocyte has gained considerable momentum recently. Our results show that treatment of rapamycin could attenuate insulin resistance induced podocyte injury via autophagy activation. Rapamycin is an mTOR specific inhibitor, while mTOR is an evolutionarily conserved serine/threonine kinase. The role of rapamycin in DN has been studied in a large spectrum. Several recent studies have shown that mTORC1 signaling is highly activated in podocytes of diabetic kidneys in human beings and animals. Studies by others show that rapamycin ameliorated renal hypotrophy in mice model of diabetes (Sakaguchi et al., 2006; Sataranatarajan et al., 2007). Our previous study also found that rapamycin attenuated high glucose induced lipotoxicity and epithelial-to-mesenchymal transition (EMT) via autophagy activation in proximal tubular cells (Xu et al., 2015). Taken together, the present study further provides evidence for use of rapamycin to treat DN.
In summary, we found under insulin resistant conditions, autophagy activity in podocytes is downregulated in vitro, and activation of autophagy could prevent insulin resistance induced podocyte injury. The present study deepens our understanding of the role of autophagy in the pathogenesis of DN. However, since the development of DM and/or DN is a very complex process, autophagy may have impact on each stage in the pathogenesis. Future research is demanded to further clarify the roles of autophagy, which will provide more evidences for autophagy to be a therapeutic target to prevent or alleviate development of DN.
Supplemental Information
Funding Statement
This work was performed in the central laboratory supported by Shandong Provincial Hospital Affiliated to Shandong University and supported by the National Natural Science Foundation of China (81200610, 81441106, 81570654 to QW, 81471007 to WX, 81500553 to YX, 81470498 to LC), Grant BS2015YY018 from Shandong Doctoral Foundation of China to YX, and Grant 201311022 from Jinan Science and Technology Developing Project to WX. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Ying Xu conceived and designed the experiments, analyzed the data, wrote the paper, reviewed drafts of the paper.
Qi Zhou performed the experiments, analyzed the data, prepared figures and/or tables, reviewed drafts of the paper.
Wei Xin contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Zhaoping Li and Liyong Chen contributed reagents/materials/analysis tools.
Qiang Wan conceived and designed the experiments, reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
The research in this article did not generate any raw data.
References
- Coward et al. (2007).Coward RJ, Welsh GI, Koziell A, Hussain S, Lennon R, Ni L, Tavare JM, Mathieson PW, Saleem MA. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes. 2007;56:1127–1135. doi: 10.2337/db06-0693. [DOI] [PubMed] [Google Scholar]
- Coward et al. (2005).Coward RJ, Welsh GI, Yang J, Tasman C, Lennon R, Koziell A, Satchell S, Holman GD, Kerjaschki D, Tavare JM, Matheieson PW, Saleem MA. The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005;54:3095–3102. doi: 10.2337/diabetes.54.11.3095. [DOI] [PubMed] [Google Scholar]
- Delfin et al. (2011).Delfin DA, Xu Y, Peterson JM, Guttridge DC, Rafael-Fortney JA, Janssen PM. Improvement of cardiac contractile function by peptide-based inhibition of NF-kappaB in the utrophin/dystrophin-deficient murine model of muscular dystrophy. Journal of Translational Medicine. 2011;9:68–78. doi: 10.1186/1479-5876-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartford & Ratain (2007).Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clinical Pharmacology and Therapeutics. 2007;82:381–388. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- He et al. (2012).He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, Walz & Kuehn (2011).Huber TB, Walz G, Kuehn EW. mTOR and rapamycin in the kidney: signaling and therapeutic implications beyond immunosuppression. Kidney International. 2011;79:502–511. doi: 10.1038/ki.2010.457. [DOI] [PubMed] [Google Scholar]
- Ising et al. (2015).Ising C, Koehler S, Brahler S, Merkwirth C, Hohne M, Baris OR, Hagmann H, Kann M, Fabretti F, Dafinger C, Bloch W, Schermer B, Linermann A, Bruning JC, Kurschat CE, Muller RU, Wiesner RJ, Langer T, Benzing T, Brinkkoetter PT. Inhibition of insulin/IGF-1 receptor signaling protects from mitochondria-mediated kidney failure. EMBO Molecular Medicine. 2015;7:275–287. doi: 10.15252/emmm.201404916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume et al. (2014).Kume S, Yamahara K, Yasuda M, Maegawa H, Koya D. Autophagy: emerging therapeutic target for diabetic nephropathy. Seminars in Nephrology. 2014;34:9–16. doi: 10.1016/j.semnephrol.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Levine & Ranganathan (2010).Levine B, Ranganathan R. Autophagy: snapshot of the network. Nature. 2010;466:38–40. doi: 10.1038/466038a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusudhan et al. (2015).Madhusudhan T, Wang H, Dong W, Ghosh S, Bock F, Thangapandi VR, Ranjan S, Wolter J, Kohli S, Shahzad K, Heidel F, Krueger M, Schwenger V, Moeller MJ, Kalinski T, Reiser J, Chavakis T, Isermann B. Defective podocyte insulin signalling through p85-XBP1 promotes ATF6-dependent maladaptive ER-stress response in diabetic nephropathy. Nature Communications. 2015;6 doi: 10.1038/ncomms7496. Article 6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima et al. (2011).Mima A, Ohshiro Y, Kitada M, Matsumoto M, Geraldes P, Li C, Li Q, White GS, Cahill C, Rask-Madsen C, King GL. Glomerular-specific protein kinase C-beta-induced insulin receptor substrate-1 dysfunction and insulin resistance in rat models of diabetes and obesity. Kidney International. 2011;79:883–896. doi: 10.1038/ki.2010.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima et al. (2004).Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Molecular Biology of the Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning et al. (2011).Ning H, Lin G, Lue TF, Lin CS. Mesenchymal stem cell marker Stro-1 is a 75 kd endothelial antigen. Biochemical and Biophysical Research Communications. 2011;413:353–357. doi: 10.1016/j.bbrc.2011.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira-Llopis et al. (2015).Rovira-Llopis S, Diaz-Morales N, Banuls C, Blas-Garcia A, Polo M, Lopez-Domenech S, Jover A, Rocha M, Hernandez-Mijares A, Victor VM. Is autophagy altered in the leukocytes of type 2 diabetic patients? Antioxidants & Redox Signaling. 2015;23:1050–1056. doi: 10.1089/ars.2015.6447. [DOI] [PubMed] [Google Scholar]
- Sakaguchi et al. (2006).Sakaguchi M, Isono M, Isshiki K, Sugimoto T, Koya D, Kashiwagi A. Inhibition of mTOR signaling with rapamycin attenuates renal hypertrophy in the early diabetic mice. Biochemical and Biophysical Research Communications. 2006;340:296–301. doi: 10.1016/j.bbrc.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Saleem et al. (2002).Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. Journal of the American Society of Nephrology. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- Sataranatarajan et al. (2007).Sataranatarajan K, Mariappan MM, Lee MJ, Feliers D, Choudhury GG, Barnes JL, Kasinath BS. Regulation of elongation phase of mRNA translation in diabetic nephropathy: amelioration by rapamycin. The American Journal of Pathology. 2007;171:1733–1742. doi: 10.2353/ajpath.2007.070412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi & Hu (2014).Shi Y, Hu FB. The global implications of diabetes and cancer. Lancet. 2014;383:1947–1948. doi: 10.1016/S0140-6736(14)60886-2. [DOI] [PubMed] [Google Scholar]
- Tagawa et al. (2016).Tagawa A, Yasuda M, Kume S, Yamahara K, Nakazawa J, Chin-Kanasaki M, Araki H, Araki S, Koya D, Asanuma K, Kim EH, Haneda M, Kajiwaa N, Hayashi K, Ohashi H, Ugi S, Maegawa H, Uzu T. Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes. 2016;65:755–767. doi: 10.2337/db15-0473. [DOI] [PubMed] [Google Scholar]
- Tam & Siu (2014).Tam BT, Siu PM. Autophagic cellular responses to physical exercise in skeletal muscle. Sports Medicine. 2014;44:625–640. doi: 10.1007/s40279-013-0140-z. [DOI] [PubMed] [Google Scholar]
- Tejada et al. (2008).Tejada T, Catanuto P, Ijaz A, Santos JV, Xia X, Sanchez P, Sanabria N, Lenz O, Elliot SJ, Fornoni A. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney International. 2008;73:1385–1393. doi: 10.1038/ki.2008.109. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2014).Wang X, Liu J, Zhen J, Zhang C, Wan Q, Liu G, Wei X, Zhang Y, Wang Z, Han H, Xu H, Bao C, Song Z, Zhang X, Li N, Yi F. Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney International. 2014;86:712–725. doi: 10.1038/ki.2014.111. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2015).Wang H, Sun RQ, Zeng XY, Zhou X, Li S, Jo E, Molero JC, Ye JM. Restoration of autophagy alleviates hepatic ER stress and impaired insulin signalling transduction in high fructose-fed male mice. Endocrinology. 2015;156:169–181. doi: 10.1210/en.2014-1454. [DOI] [PubMed] [Google Scholar]
- Welsh et al. (2010).Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstadt H, Tavare JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJ. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metabolism. 2010;12:329–340. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2014).Xu Y, Huang J, Xin W, Chen L, Zhao X, Lv Z, Liu Y, Wan Q. Lipid accumulation is ahead of epithelial-to-mesenchymal transition and therapeutic intervention by acetyl-CoA carboxylase 2 silence in diabetic nephropathy. Metabolism: Clinical and Experimental. 2014;63:716–726. doi: 10.1016/j.metabol.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2015).Xu Y, Liu L, Xin W, Zhao X, Chen L, Zhen J, Wan Q. The renoprotective role of autophagy activation in proximal tubular epithelial cells in diabetic nephropathy. Journal of Diabetes and its Complications. 2015;29:976–983. doi: 10.1016/j.jdiacomp.2015.07.021. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2010).Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metabolism. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2015).Zhang N, Cao MM, Liu H, Xie GY, Li YB. Autophagy regulates insulin resistance following endoplasmic reticulum stress in diabetes. Journal of Physiology and Biochemistry. 2015;71:319–327. doi: 10.1007/s13105-015-0384-1. [DOI] [PubMed] [Google Scholar]
- Zhou et al. (2009).Zhou L, Zhang J, Fang Q, Liu M, Liu X, Jia W, Dong LQ, Liu F. Autophagy-mediated insulin receptor down-regulation contributes to endoplasmic reticulum stress-induced insulin resistance. Molecular Pharmacology. 2009;76:596–603. doi: 10.1124/mol.109.057067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The research in this article did not generate any raw data.