Abstract
Findings from studies of metformin use with risk of cancer incidence and outcome provide mixed results; with few studies examined associations by recency of diabetes diagnosis or duration of medication use. Thus, in the Women’s Health Initiative, we examined these associations and further explored whether associations differ by recency of diabetes and duration of metformin use. Cox regression models were used to estimate hazard ratios (HR) and their 95% confidence intervals. Diabetes was associated with higher risk of total invasive cancer (HR, 1.13; p < 0.001) and of several site-specific cancers (HR, 1.2–1.4, and up to over twofold). Diabetes was also associated with higher risk of death from cancer (HR, 1.46; p < 0.001). There was no overall difference in cancer incidence by diabetes therapy (p = 0.66). However, there was a lower risk of death from cancer for metformin users, compared to users of other medications, relative to women without diabetes, overall (HRs, 1.08 vs. 1.45; p = 0.007) and for breast cancer (HRs, 0.50 vs. 1.29; p = 0.05). Results also suggested that lower cancer risk associated with metformin may be evident only for a longer duration of use in certain cancer sites or subgroup populations. We provide further evidence that postmenopausal women with diabetes are at higher risk of invasive cancer and cancer death. Metformin users, particularly long-term users, may be at lower risk of developing certain cancers and dying from cancer, compared to users of other anti-diabetes medications. Future studies are needed to determine the long-term effect of metformin in cancer risk and survival from cancer.
Keywords: diabetes, metformin, invasive cancer, incidence, mortality
Diabetes mellitus and cancer are both relatively common diseases with incidence increasing worldwide. Epidemiological evidence suggests that diabetes alters risk of various cancers and that cancer mortality is increased in individuals with diabetes.1 The underlying mechanisms linking diabetes and cancer have not been fully elucidated, but insulin resistance and hyperinsulinemia have been proposed.2 Higher circulating levels of insulin can promote the growth of tumors through a direct effect on epithelial tissues by interacting with insulin-like growth factor (IGF) family receptors, or indirectly by affecting the levels of IGFs, sex hormones and adipokines.3 Metformin, an insulin sensitizer, reduces gluconeogenesis in the liver and fosters glucose uptake by peripheral tissues, leading to lower blood glucose, insulin resistance and circulating insulin levels by activation of AMP-activated protein kinase (AMPK) and inhibition of mammalian target of rapamycin (mTOR) signaling pathway.4 Thus, it is reasonable to expect that the diabetes and cancer association could be affected by the type of anti-diabetic therapy. Indeed, in some studies, the cancer risk differs by diabetes therapy category with higher risk for individuals on insulin or sulfonylureas and lower risk for those on metformin.5 However, most studies have had limited power to examine associations with less common cancers and often have limited follow-up. Moreover, current studies have been conducted mostly in diabetic patients, and few large studies have examined the effect of metformin on cancer incidence and mortality in a population that includes both non-diabetic and diabetic participants. In addition, few studies have defined cancer risk by recency of diabetes diagnosis or duration of medication use. Lastly, in many cases, findings of previous studies were not stratified by sex, thus the associations were not clearly investigated in women. Furthermore, risk of several cancers, such as breast and ovarian cancer, are increased in women, especially in obese women who are postmenopausal. Therefore, it is important to study the potential benefits of metformin on cancer risk and mortality in postmenopausal, elderly women.6
Therefore, we examined associations of diabetes and metformin use with cancer risk and mortality overall and by cancer site among women participating in the Women’s Health Initiative (WHI), a large multicenter prospective cohort study of postmenopausal women. We hypothesized that 1) diabetes is associated with a higher cancer risk and death overall and with certain cancer types; and 2) metformin use is associated with a lower cancer risk and death whereas other anti-diabetes medication use is associated with an increased risk. The unique data in WHI, a large prospective cohort study of 145,826 postmenopausal women with high-quality data and long-term follow-up, allow a comprehensive evaluation of these study hypotheses.
Material and Methods
Study population
The WHI is a large, multicenter study designed to advance understanding of the determinants of major chronic diseases in postmenopausal women,7 which includes three clinical trials (CT) and an observational study (OS), with details described previously.8,9 Briefly, eligible were postmenopausal women between 50 and 79 years of age, accessible for follow-up, expected to live in the same geographic area for 3 years, with minimum life expectancy of 3 years and were recruited at 40 US clinical centers between 1993 and 1998. A woman of eligible age, 50 to 79 years old, was considered postmenopausal if she had experienced no vaginal bleeding for 6 months (12 months for 50 to 54 years old), had had a hysterectomy, or had ever used postmenopausal hormones. After the initial WHI study period ended on March 31, 2005, participants are reconsented to participate in the first (2005–2010) and second extension (2010–2015). Protocols had institutional review board approval from all clinical centers, and women provided written informed consent.
The study population includes all CT (N = 68,132) and OS (N = 93,676) women. Of the 161,808 women, we excluded a total of 15, 982 women with one or more of the following: prior cancer (n = 14,849), bilateral mastectomy (n = 774), report of diabetic coma (n = 125), diabetes diagnosed at younger than age 21 (to exclude likely type 1 diabetes; n = 140), those with missing baseline diabetes information (n = 102), or no follow-up (n = 692), leaving 145,826 women for these analyses.
Data collection
Study implementation details have been published previously.8 Briefly, participants attended a baseline screening visit, during which they completed self-administered questionnaires that collected information on demographics, reproductive, medical and family history, and various lifestyle factors such as physical activity. Height, weight, and waist and hip circumference, measured by trained clinic staff, were used to determine body mass index (BMI) and waist-to-hip ratio (WHR).
WHI participants were asked to bring all medications to their clinic visits. Clinic interviewers then entered each medication name directly from the containers into a computer-driven system that assigned drug codes using Medi-Span software (First DataBank, San Bruno, CA) and recorded durations of use reported by participants. These medication inventories were collected at baseline and at Years 1, 3, 6 and 9 for the CT and Year 3 for the OS during the WHI study period. Women participating in extended follow-up were again asked to complete the medication inventory by mail. All these data were then used to construct a participant’s use of anti-diabetes medications over time, with details described in the Supporting Information.
Identification of women with diabetes
At baseline, participants were asked “Did a doctor ever say that you had sugar diabetes or high blood sugar when you were not pregnant?” During the study, by self-administered medical history questionnaires, they were asked “Since the date given on this form, has a doctor prescribed any of the following pills or treatments?” Choices included “pills for diabetes” and “insulin shots for diabetes.” This self-reported medical history was updated semiannually in the CT and annually in the OS, and annually for all participants during extended follow-up. In addition to self-reported medical histories at baseline and during the study, medication inventories as described above were also used to identify women with diabetes. Thus, in this study, diagnosis of diabetes were not based on medical record review, rather they were determined by ongoing direct query and review of the use of anti-diabetic medication, which has been shown to be a favorable approach in identifying women with diabetes.10,11
Specifically, a time-dependent variable was coded: (0) Non-diabetics; (1) Diabetic—users of metformin medications; (2) Diabetic—users of other known non-metformin anti-diabetes medications; (3) Diabetic—unknown medication; incident diabetes based on medical history occurred prior to treatment reported in the medication inventory; (4) Diabetic—untreated; no anti-diabetes medication in medication inventory. Because type of diabetes treatment could only be determined from the medication inventories, women with diabetes identified by their medical history were initially grouped as “(3) diabetic— unknown medication” and later grouped by treatment, coded (1) or (2), after subsequent completion of the medication inventory. To investigate diabetes status (yes/no) categories (1), (2), (3) and (4) were combined and compared to (0) non-diabetics.
Cancer endpoint ascertainment
Along with diabetes history, self-report of diagnosis of any cancer other than nonmelanoma skin cancers was also updated semiannually in the CT and annually in the OS during the study period, and annually during extended follow-up. Cancer self-reports were verified by medical record and pathology report review at the clinical centers by centrally trained physician adjudicators, and then confirmed centrally at the Clinical Coordinating Center (CCC). Cancer death and cancer death site were ascertained using medical records reviewed at the CCC.12
Statistical analysis
Baseline characteristics of women with diabetes were compared to those of women without diabetes using the Chi-square test. Similarly, baseline characteristics of women with diabetes treated using metformin either as monotherapy or combined with other diabetes medications were compared to those of women with treated diabetes not using metformin.
Cox regression models with a time-dependent categorical exposure variable, described above, were used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for invasive cancer incidence and mortality. The Cox proportional hazard analyses were adjusted for the following baseline covariates: age, race/ethnicity, education, smoking, physical activity, aspirin use, history of hyperlipidemia, duration of hormone therapy (HT) use, BMI and WHR. Analyses were repeated after excluding BMI and WHR from the model because obesity is the most common shared risk factor for both diabetes and cancer. This allowed us to examine the extent to which obesity may be a confounder of the association between diabetes and cancer risk. The baseline hazard functions were allowed to vary by age (10-year group), study participation (four hormone therapy trial randomization arms, the dietary trial randomization arms, or enrollment into the OS), hysterectomy status and enrollment in WHI extensions (I/II; time-dependent). Models were fit for each outcome, where time of event was defined as time from enrollment to the first relevant clinical event, death, or withdrawal from the study, whichever came first.
In the primary analyses, HRs and 95% CIs were reported for women with diabetes compared with women without diabetes. HRs and 95% CIs were also reported for diabetes patients treated with metformin (vs. non-diabetes) and patients using other medications (vs. non-diabetes). For both primary analyses, a 1 degree-of-freedom test of statistical significance was used to compare women with diabetes to those of women without diabetes and to compare metformin therapy to the other known treatment group. To limit the number of tests, p-values were generated only for the ten summary endpoints (total invasive cancer, the eight grouped invasive cancer sites and cancer death). We used forest plots to present HRs and 95% CIs by individual invasive cancer site.
In exploratory analyses, we further examined cancer risk by recency of diabetes diagnosis, defined by whether diabetes was present at baseline (prevalent) or occurred during follow-up (incident). Duration of exposure to metformin use or other non-metformin medication use was also modeled as a time-dependent exposure (<3.5, 3.5–<=5 and > 5years).13 Only CT participants were included for the duration analysis because medication inventories were not collected frequently enough among OS participants to compute a reliable cumulative exposure.
All analyses were conducted by using SAS software, version 9.3 (SAS Institute, Cary, NC) and R software version 2.15 (R Foundation for Statistical Computing, Vienna, Austria). Statistical tests were two-sided and considered statistically significant for p ≤ 0.05.
Results
The distributions of various participant characteristics by diabetes status and anti-diabetes therapy at baseline are summarized in Table 1. Of the 145,826 postmenopausal women in the study, 8,484 women (5.8%) had diabetes at baseline. Among women with diabetes, 1,100 women (13.0%) were treated with metformin, 4,106 (48.4%) were treated with other anti-diabetes medications, and 3,278 (38.6%) women were either untreated or treatment unknown at baseline. Women with diabetes were older and were more likely to be a minority, less well educated, less physically active and more obese. Fewer significant differences were observed between women with diabetes based on treatment (metformin vs. other). Metformin users tended to be white, use aspirin and medications for hyperlipidemia, have not had a hysterectomy and have greater central adiposity.
Table 1.
Baseline characteristics of participants by diabetes status and therapy at baseline in the WHI CT and OS participants (N = 145,826)
| Characteristics | Diabetes status | Participants with diabetes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No (n=137,342) | Yes (n=8 484) | p1 | Metformin (n=1,100) | Other meds (n=4,106) | p2 | |||||
| N | % | N | % | N | % | N | % | |||
| Age group at screening, y | <0.001 | 0.40 | ||||||||
| 50–59 | 47,089 | 34.3 | 2,198 | 25.9 | 258 | 23.5 | 1,038 | 25.3 | ||
| 60–69 | 61,393 | 44.7 | 4,168 | 49.1 | 566 | 51.5 | 2,039 | 49.7 | ||
| 70–79 | 28,860 | 21.0 | 2,118 | 25.0 | 276 | 25.1 | 1,029 | 25.1 | ||
| Race/ethnicity | <0.001 | <0.001 | ||||||||
| White | 114,580 | 83.4 | 5,492 | 64.7 | 706 | 64.2 | 2,494 | 60.7 | ||
| Black | 11,439 | 8.3 | 1,851 | 21.8 | 207 | 18.8 | 1,083 | 26.4 | ||
| Hispanic | 5,349 | 3.9 | 557 | 6.6 | 90 | 8.2 | 256 | 6.2 | ||
| American Indian | 520 | 0.4 | 107 | 1.3 | 12 | 1.1 | 60 | 1.5 | ||
| Asian/Pacific Islander | 3,562 | 2.6 | 332 | 3.9 | 63 | 5.7 | 137 | 3.3 | ||
| Unknown | 1,892 | 1.4 | 145 | 1.7 | 22 | 2.0 | 76 | 1.9 | ||
| Education | <0.001 | 0.26 | ||||||||
| 0–8 years | 2,039 | 1.5 | 326 | 3.9 | 41 | 3.8 | 160 | 3.9 | ||
| Some high school | 4,677 | 3.4 | 615 | 7.3 | 74 | 6.8 | 322 | 7.9 | ||
| High school diploma/GED | 23,286 | 17.1 | 1,777 | 21.1 | 229 | 21.0 | 906 | 22.2 | ||
| School after high school | 51,377 | 37.7 | 3,413 | 40.6 | 428 | 39.2 | 1,646 | 40.4 | ||
| College degree or higher | 54,943 | 40.3 | 2,285 | 27.2 | 319 | 29.2 | 1,045 | 25.6 | ||
| Smoking status | 0.39 | 0.007 | ||||||||
| Never | 69,516 | 51.3 | 4,342 | 52.2 | 545 | 50.3 | 2,131 | 53.0 | ||
| Past | 56,673 | 41.8 | 3,417 | 41.0 | 480 | 44.3 | 1,604 | 39.9 | ||
| Current | 9,419 | 6.9 | 566 | 6.8 | 59 | 5.4 | 287 | 7.1 | ||
| Body mass index, kg/m2 | <0.001 | 0.77 | ||||||||
| <25 | 49,485 | 36.3 | 1,114 | 13.2 | 115 | 10.5 | 418 | 10.2 | ||
| 25–<30 | 47,964 | 35.2 | 2,381 | 28.3 | 310 | 28.3 | 1,112 | 27.2 | ||
| ≥30 | 38,712 | 28.4 | 4,924 | 58.5 | 671 | 61.2 | 2,551 | 62.5 | ||
| Alcohol consumption | <0.001 | 0.15 | ||||||||
| Non/past drinker | 37,904 | 27.8 | 4,832 | 57.6 | 692 | 63.8 | 2,568 | 63.3 | ||
| <1 drink/week | 45,448 | 33.3 | 2,411 | 28.8 | 303 | 27.9 | 1,073 | 26.4 | ||
| 1–14 drinks/week | 47,557 | 34.9 | 1,034 | 12.3 | 84 | 7.7 | 387 | 9.5 | ||
| >14 drinks/week | 5,425 | 4.0 | 105 | 1.3 | 6 | 0.6 | 29 | 0.7 | ||
| Recreational physical activity3 | <0.001 | 0.13 | ||||||||
| No activity | 20,194 | 15.5 | 1,787 | 22.0 | 229 | 20.9 | 936 | 24.0 | ||
| Some activity | 52,344 | 40.1 | 3,764 | 46.3 | 515 | 47.1 | 1,856 | 47.6 | ||
| 2–<4 episodes per week | 23,379 | 17.9 | 1,233 | 15.2 | 183 | 16.7 | 540 | 13.9 | ||
| 4+ episodes per week | 34,574 | 26.5 | 1,352 | 16.6 | 167 | 15.3 | 566 | 14.5 | ||
| Dietary energy (kcal) | 0.03 | 0.30 | ||||||||
| <1,200 | 34,096 | 25.6 | 2,203 | 27.2 | 289 | 27.7 | 1,108 | 28.3 | ||
| 1,200–<1,500 | 28,581 | 21.4 | 1,652 | 20.4 | 223 | 21.4 | 783 | 20.0 | ||
| 1,500–<2,000 | 39,078 | 29.3 | 2,094 | 25.9 | 281 | 26.9 | 968 | 24.7 | ||
| ≥2,000 | 31,630 | 23.7 | 2,138 | 26.4 | 250 | 24.0 | 1,053 | 26.9 | ||
| Family history of cancer | 86,894 | 66.1 | 5,057 | 63.7 | 0.04 | 643 | 63.2 | 2,395 | 62.7 | 0.84 |
| Family history of adult diabetes | 41,744 | 32.0 | 5,104 | 65.4 | <0.001 | 684 | 68.2 | 2,589 | 68.6 | 0.60 |
| Duration of diabetes, y | N/A | 0.44 | ||||||||
| <5 y | 0 | 3,242 | 39.6 | 386 | 37.1 | 1,403 | 35.9 | |||
| 5–<10 | 0 | 1,955 | 23.9 | 287 | 27.6 | 1,024 | 26.2 | |||
| ≥10 | 0 | 2,986 | 36.5 | 367 | 35.3 | 1,482 | 37.9 | |||
| Aspirin | 28,278 | 20.6 | 2,223 | 26.2 | <0.001 | 334 | 30.4 | 1,112 | 27.1 | 0.04 |
| NSAIDs | 46,395 | 33.8 | 3,361 | 39.6 | <0.001 | 476 | 43.3 | 1,684 | 41.0 | 0.16 |
| History of high cholesterol requiring pills | 17,807 | 13.0 | 2,303 | 27.1 | <0.001 | 387 | 35.2 | 1,163 | 28.3 | <0.001 |
| Hysterectomy at randomization | 54,472 | 39.7 | 4,117 | 48.6 | <0.001 | 487 | 44.3 | 2,020 | 49.2 | 0.02 |
| Enrollment into CEE trial | 0.76 | 0.06 | ||||||||
| Active CEE | 4,552 | 8.4 | 482 | 11.7 | 74 | 15.2 | 253 | 12.5 | ||
| Placebo | 4,596 | 8.4 | 492 | 12.0 | 55 | 11.3 | 282 | 14.0 | ||
| Not enrolled w/o uterus | 45,324 | 83.2 | 3,143 | 76.3 | 358 | 73.5 | 1,485 | 73.5 | ||
| Enrollment into CEE+MPA trial | 0.98 | 0.74 | ||||||||
| Active CEE+MPA | 7,813 | 9.4 | 478 | 11.0 | 71 | 11.6 | 231 | 11.1 | ||
| Placebo | 7,439 | 9.0 | 459 | 10.5 | 69 | 11.3 | 217 | 10.4 | ||
| Not enrolled w/uterus | 67,553 | 81.6 | 3,425 | 78.5 | 472 | 77.1 | 1,636 | 78.5 | ||
| Enrollment into DM trial | 0.97 | 0.76 | ||||||||
| Comparison | 26,195 | 19.1 | 1,688 | 19.9 | 208 | 18.9 | 859 | 20.9 | ||
| Intervention | 17,507 | 12.7 | 1,123 | 13.2 | 132 | 12.0 | 553 | 13.5 | ||
| Not enrolled | 93,640 | 68.2 | 5,673 | 66.9 | 760 | 69.1 | 2,694 | 65.6 | ||
| Bilateral oophorectomy | 24,924 | 18.6 | 1,785 | 22.0 | 0.99 | 212 | 20.2 | 863 | 22.0 | 0.86 |
| Unopposed estrogen use status | <0.001 | 0.15 | ||||||||
| Never used | 88,876 | 64.8 | 5,699 | 67.2 | 763 | 69.4 | 2,841 | 69.2 | ||
| Past user | 16,918 | 12.3 | 1,183 | 14.0 | 142 | 12.9 | 581 | 14.2 | ||
| Current user | 31,457 | 22.9 | 1,593 | 18.8 | 195 | 17.7 | 683 | 16.6 | ||
| Estrogen + progesterone use status | <0.001 | 0.69 | ||||||||
| Never used | 99,328 | 72.3 | 7,188 | 84.7 | 928 | 84.4 | 3,564 | 86.8 | ||
| Past user | 11,843 | 8.6 | 474 | 5.6 | 64 | 5.8 | 203 | 4.9 | ||
| Current user | 26,126 | 19.0 | 820 | 9.7 | 107 | 9.7 | 338 | 8.2 | ||
| Duration of HRT use, y | <0.001 | 0.09 | ||||||||
| None | 58,599 | 42.7 | 4,650 | 54.8 | 621 | 56.5 | 2,387 | 58.1 | ||
| < 5 | 30,386 | 22.1 | 1,761 | 20.8 | 221 | 20.1 | 865 | 21.1 | ||
| 5–< 10/y | 17,916 | 13.0 | 740 | 8.7 | 105 | 9.5 | 305 | 7.4 | ||
| 10–< 15 | 13,252 | 9.6 | 514 | 6.1 | 54 | 4.9 | 231 | 5.6 | ||
| 15+ | 17,185 | 12.5 | 819 | 9.7 | 99 | 9.0 | 318 | 7.7 | ||
| Mammogram in the last 2 years | 111,173 | 83.5 | 6,601 | 81.5 | 0.30 | 853 | 81.4 | 3,167 | 80.7 | 0.64 |
| Age at menopause, y | <0.001 | 0.77 | ||||||||
| <45 | 28,144 | 21.7 | 2,267 | 29.2 | 285 | 27.6 | 1,088 | 29.3 | ||
| 45–54 | 83,614 | 64.6 | 4,340 | 55.8 | 596 | 57.6 | 2,051 | 55.2 | ||
| >54 | 17,705 | 13.7 | 1,169 | 15.0 | 153 | 14.8 | 576 | 15.5 | ||
| Age at menarche, y | <0.001 | 0.39 | ||||||||
| <12 | 29,458 | 21.5 | 2,317 | 27.5 | 317 | 29.1 | 1,122 | 27.5 | ||
| 12–13 | 75,561 | 55.2 | 4,343 | 51.5 | 565 | 51.8 | 2,103 | 51.5 | ||
| ≥14 | 31,775 | 23.2 | 1,779 | 21.1 | 209 | 19.2 | 860 | 21.1 | ||
| Age at first birth, y | <0.001 | 0.42 | ||||||||
| Never preg/No term preg | 16,070 | 12.9 | 913 | 12.4 | 124 | 13.1 | 421 | 11.8 | ||
| <20 | 16,973 | 13.6 | 1,563 | 21.2 | 198 | 20.9 | 788 | 22.1 | ||
| 20–29 | 81,417 | 65.4 | 4,317 | 58.5 | 552 | 58.3 | 2,077 | 58.3 | ||
| 30+ | 10,099 | 8.1 | 587 | 8.0 | 73 | 7.7 | 277 | 7.8 | ||
| Waist/hip ratio, baseline, mean (SD) | 0.81 | (0.1) | 0.87 | (0.1) | <0.001 | 0.89 | (0.1) | 0.88 | (0.1) | <0.001 |
Test of association between baseline characteristic and diabetes status (no vs. yes) adjusted for age, race/ethnicity, education, BMI and hysterectomy status.
Test of association between baseline characteristic and diabetes treatment (metformin vs. other medication) adjusted for age, race/ethnicity, education, BMI and hysterectomy status.
Episodes per week of moderate or strenuous physical activity of ≥20 minutes duration.
Over a median (IQR) of 15.0 (9.1 to 16.9) years of follow-up among a total of 1,935,060 person-years, 24,796 women were diagnosed with diabetes and 16,248 were diagnosed with invasive cancer. Diabetes status was associated with a higher risk of total invasive cancer (HR, 1.13; 95% CI, 1.07, 1.19; p < 0.001; Fig. 1) and higher risk of cancers of the digestive organs and peritoneum (HR, 1.37; 95% CI, 1.23, 1.53; p < 0.001), including higher colon cancer risk (HR, 1.24; 95% CI, 1.06, 1.46) and higher risk (over twofold) of liver and pancreas cancers. Compared to women without diabetes, women with diabetes had a higher risk of reproductive tract cancer (HR, 1.22; 95% CI, 1.05, 1.42; p = 0.01), particularly endometrial cancer (HR, 1.36, 95% CI, 1.12, 1.65). There was also a suggestion for a higher risk of the overall category of malignant neoplasms of lymphatic and hematopoietic tissue (p = 0.07), particularly for non-Hodgkin lymphoma (HR, 1.26; 95% CI, 1.02, 1.55). Breast cancer risk was not higher in women with diabetes (HR, 1.02; 95% CI, 0.93, 1.11), but when BMI and WHR were not included as covariates in the model there was a higher risk (HR, 1.11; 95% CI, 1.01, 1.20). Women with diabetes were more likely to die from invasive cancer overall (HR, 1.46; 95%CI, 1.34, 1.60; p < 0.001) and from colorectal cancer (HR, 1.44; 95% CI, 1.06–1.96) compared to women without diabetes.
Figure 1.
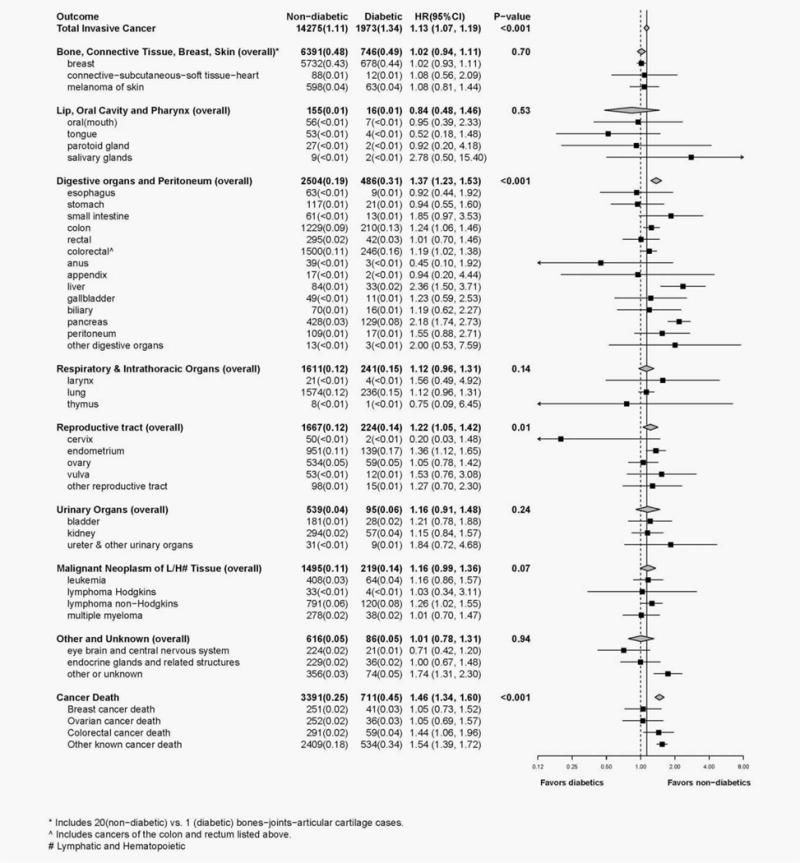
Forest plot displays number of cases, annualized percentages and multivariable adjusted hazard ratios (95% CI) for the risk of cancer associated with diabetes status (participants with diabetes vs. participants without diabetes). Hazard ratios were obtained from Cox proportional hazards models that were adjusted for the baseline covariates of age, race/ethnicity, education, smoking, physical activity, aspirin use, history of hyperlipidemia, duration of HT use, BMI and WHR; baseline hazard functions were allowed to vary by age (10-year group), study participation (four hormone therapy trial randomization arms, the dietary trial randomization arms, or enrollment into the OS), hysterectomy status and enrollment in WHI extensions (I/II; time-dependent). Diabetes status was modeled as a time-dependent exposure variable and participants were censored if their medication inventory became out-of-date (i.e., more than 3.5 years old); participants were allowed to re-enter the model upon completion of a current medication inventory. P-values, for the ten summary endpoints, correspond to a 1 degree-of-freedom test of significance for the estimated hazard ratios.
In women with diabetes as compared with risk in non-diabetic women, risk of total invasive cancer did not differ between metformin users (HR, 1.13; 95% CI, 1.04–1.23) and users of other medications (HR, 1.10; 95% CI, 1.00–1.21; p = 0.66; see Fig. 2). In addition, in women with diabetes, the risk of cancer death differed significantly between metformin users and non-users (p = 0.007). Compared to women without diabetes, women with diabetes who used medications other than metformin were at significantly higher risk of dying of cancer (HR, 1.45; 95% CI, 1.25, 1.69), while women with diabetes who used metformin were not (HR, 1.08; 95% CI, 0.91, 1.28). Further, results suggested a differential risk of death from breast cancer (p = 0.05) for metformin users (HR, 0.50; 95% CI, 0.22, 1.13) compared to women with diabetes on other medications (HR, 1.29; 95% CI, 0.71, 2.33).
Figure 2.
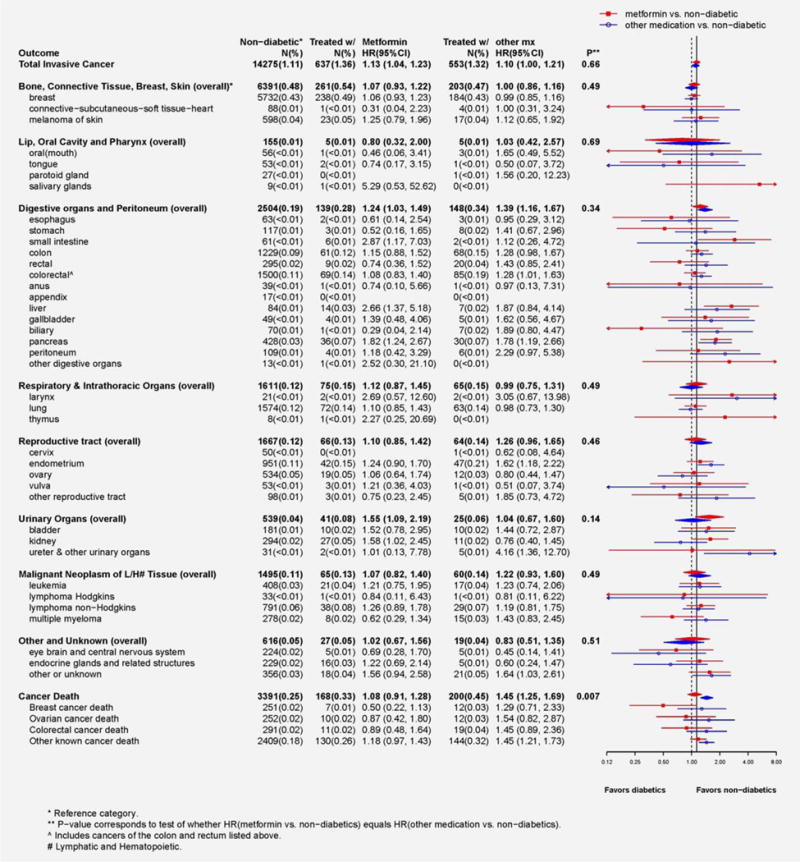
Forest plot displays number of cases, annualized percentages and multivariable adjusted hazard ratios (95% CI) for the risk of cancer associated with diabetes treatment (users of metformin vs. participants without diabetes; users of other known non-metformin anti-diabetes medications vs. participants without diabetes). Hazard ratios were obtained from multivariable adjusted Cox proportional hazard models that were described above. P-values, for the ten summary endpoints, correspond to a 1 degree-of-freedom test of significance for whether the estimated hazard ratios differ.
Overall, no significant findings were observed in the secondary analyses. Invasive cancer risk was not influenced by duration of use for metformin or other anti-diabetes medications (Table 2). There was a suggestion, however, longer metformin use was associated with lower breast cancer risk (p-trend = 0.04). We also found no significant differences between prevalent and incident diabetes for risk of invasive cancer and for risk of cancer death (data not shown). However, our results suggested that among women with incident diabetes there was a trend of decreasing risk of cancer death with increasing duration of metformin use, but a trend of increasing risk for other anti-diabetes medication use (p < 0.001; Table 2).
Table 2.
Multivariable1 adjusted effects of Diabetes Treatment (Metformin or other diabetic medication vs. non-diabetic) on Invasive Cancer Types and Cancer death by Duration in the Women’s Health Initiative CT
| Main effect Duration (years) | Non-diabetic | Metformin use | Other diabetic medication use | p-diff3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | HR | (95% CI) | p2 | N | % | HR | (95% CI) | p2 | ||
| Total invasive cancer | 7,335 | (1.11) | 405 | (1.38) | 1.11 | (0.99, 1.24) | 342 | (1.38) | 1.13 | (1.00, 1.27) | |||
| <3.5 | 225 | (1.43) | 1.17 | (1.01, 1.35) | 0.27 | 136 | (1.16) | 1.04 | (0.87, 1.24) | 0.58 | 0.24 | ||
| 3.5–≤5 | 68 | (1.33) | 1.09 | (0.84, 1.40) | 74 | (1.71) | 1.41 | (1.10, 1.80) | |||||
| >5 | 112 | (1.32) | 1.02 | (0.83, 1.25) | 132 | (1.51) | 1.11 | (0.91, 1.34) | |||||
| Bone, connective tissue and skin | 3,194 | (0.47) | 159 | (0.52) | 1.00 | (0.84, 1.20) | 131 | (0.51) | 1.11 | (0.92, 1.34) | |||
| <3.5 | 94 | (0.58) | 1.14 | (0.91, 1.43) | 0.05 | 57 | (0.47) | 1.03 | (0.77, 1.36) | 0.94 | 0.17 | ||
| 3.5–≤5 | 25 | (0.47) | 1.00 | (0.67, 1.51) | 29 | (0.65) | 1.56 | (1.08, 2.26) | |||||
| >5 | 40 | (0.45) | 0.74 | (0.52, 1.07) | 45 | (0.49) | 0.99 | (0.72, 1.36) | |||||
| Invasive breast cancer | 2,839 | (0.42) | 146 | (0.48) | 1.01 | (0.84, 1.22) | 120 | (0.47) | 1.14 | (0.93, 1.38) | |||
| <3.5 | 88 | (0.54) | 1.18 | (0.94, 1.49) | 0.04 | 52 | (0.43) | 1.06 | (0.79, 1.42) | 0.79 | 0.20 | ||
| 3.5–≤5 | 21 | (0.39) | 0.93 | (0.59, 1.44) | 28 | (0.63) | 1.67 | (1.14, 2.43) | |||||
| >5 | 37 | (0.42) | 0.76 | (0.52, 1.11) | 40 | (0.44) | 0.98 | (0.70, 1.37) | |||||
| Lip, oral cavity and pharynx | 83 | (0.01) | 3 | (0.01) | 0.74 | (0.22, 2.42) | 4 | (0.02) | 1.35 | (0.48, 3.80) | |||
| <3.5 | 1 | (0.01) | 2 | (0.02) | |||||||||
| 3.5–≤5 | 0 | (0.00) | 0 | (0.00) | |||||||||
| >5 | 2 | (0.02) | 2 | (0.02) | |||||||||
| Digestive organs and peritoneum | 1,345 | (0.19) | 94 | (0.30) | 1.30 | (1.03, 1.64) | 97 | (0.37) | 1.49 | (1.18, 1.87) | |||
| <3.5 | 54 | (0.32) | 1.41 | (1.05, 1.91) | 0.31 | 34 | (0.28) | 1.23 | (0.85, 1.76) | 0.22 | 0.11 | ||
| 3.5–≤5 | 18 | (0.32) | 1.35 | (0.81, 2.27) | 21 | (0.46) | 1.76 | (1.09, 2.86) | |||||
| >5 | 22 | (0.24) | 1.07 | (0.69, 1.67) | 42 | (0.45) | 1.66 | (1.19, 2.33) | |||||
| Respiratory and intrathoracic organs | 860 | (0.12) | 52 | (0.16) | 1.24 | (0.91, 1.70) | 36 | (0.14) | 0.83 | (0.56, 1.24) | |||
| <3.5 | 30 | (0.18) | 1.44 | (0.96, 2.14) | 0.35 | 16 | (0.13) | 1.03 | (0.61, 1.77) | 0.42 | 0.96 | ||
| 3.5–≤5 | 8 | (0.14) | 1.02 | (0.48, 2.17) | 5 | (0.11) | 0.52 | (0.17, 1.61) | |||||
| >5 | 14 | (0.15) | 1.06 | (0.59, 1.89) | 15 | (0.16) | 0.75 | (0.39, 1.46) | |||||
| Reproductive tract | 828 | (0.12) | 40 | (0.13) | 1.10 | (0.79, 1.54) | 39 | (0.15) | 1.26 | (0.88, 1.80) | |||
| <3.5 | 20 | (0.12) | 1.09 | (0.69, 1.71) | 0.95 | 13 | (0.11) | 0.98 | (0.55, 1.74) | 0.28 | 0.46 | ||
| 3.5–≤5 | 7 | (0.13) | 1.15 | (0.54, 2.43) | 9 | (0.20) | 1.51 | (0.71, 3.20) | |||||
| >5 | 13 | (0.14) | 1.11 | (0.62, 1.98) | 17 | (0.18) | 1.49 | (0.87, 2.56) | |||||
| Urinary organs | 293 | (0.04) | 25 | (0.08) | 1.45 | (0.92, 2.28) | 14 | (0.05) | 1.06 | (0.60, 1.87) | |||
| <3.5 | 5 | (0.03) | 0.55 | (0.20, 1.48) | <0.001 | 6 | (0.05) | 1.19 | (0.52, 2.69) | 0.38 | 0.004 | ||
| 3.5–≤5 | 4 | (0.07) | 1.07 | (0.34, 3.38) | 4 | (0.09) | 1.77 | (0.65, 4.79) | |||||
| >5 | 16 | (0.17) | 3.04 | (1.76, 5.25) | 4 | (0.04) | 0.61 | (0.19, 1.92) | |||||
| Malignant Neoplasm of L/H4 Tissue | 769 | (0.11) | 35 | (0.11) | 0.87 | (0.60, 1.27) | 37 | (0.14) | 1.24 | (0.88, 1.75) | |||
| <3.5 | 20 | (0.12) | 0.96 | (0.59, 1.56) | 0.44 | 13 | (0.11) | 1.04 | (0.60, 1.82) | 0.61 | 0.36 | ||
| 3.5–≤5 | 7 | (0.13) | 0.97 | (0.43, 2.17) | 9 | (0.20) | 1.64 | (0.81, 3.32) | |||||
| >5 | 8 | (0.09) | 0.67 | (0.31, 1.41) | 15 | (0.16) | 1.27 | (0.76, 2.15) | |||||
| Other and Unknown | 407 | (0.06) | 26 | (0.08) | 1.19 | (0.77, 1.85) | 15 | (0.06) | 0.82 | (0.47, 1.44) | |||
| <3.5 | 14 | (0.08) | 1.20 | (0.67, 2.16) | 0.98 | 5 | (0.04) | 0.71 | (0.29, 1.72) | 0.54 | 0.64 | ||
| 3.5–≤5 | 4 | (0.07) | 1.12 | (0.41, 3.03) | 3 | (0.07) | 0.71 | (0.17, 2.85) | |||||
| >5 | 8 | (0.09) | 1.23 | (0.57, 2.64) | 7 | (0.07) | 1.02 | (0.45, 2.31) | |||||
| Cancer death | 1,854 | (0.26) | 102 | (0.32) | 1.00 | (0.80, 1.25) | 124 | (0.47) | 1.46 | (1.20, 1.78) | |||
| <3.5 | 49 | (0.29) | 1.01 | (0.74, 1.37) | 0.92 | 40 | (0.32) | 1.21 | (0.87, 1.69) | 0.17 | 0.41 | ||
| 3.5–≤5 | 19 | (0.34) | 0.93 | (0.57, 1.51) | 24 | (0.52) | 1.59 | (1.05, 2.41) | |||||
| >5 | 34 | (0.37) | 1.04 | (0.72, 1.51) | 60 | (0.63) | 1.64 | (1.24, 2.17) | |||||
| Cancer death (incident only) | 1,854 | (0.26) | 45 | (0.26) | 0.82 | (0.59, 1.14) | 51 | (0.54) | 1.57 | (1.17, 2.11) | |||
| <3.5 | 28 | (0.27) | 0.98 | (0.65, 1.47) | 0.07 | 24 | (0.40) | 1.30 | (0.85, 1.98) | <0.001 | <0.001 | ||
| 3.5–≤5 | 8 | (0.25) | 0.67 | (0.32, 1.41) | 9 | (0.52) | 1.39 | (0.72, 2.70) | |||||
| >5 | 9 | (0.25) | 0.60 | (0.27, 1.34) | 18 | (0.99) | 2.47 | (1.50, 4.07) | |||||
The Cox proportional hazard analyses were adjusted for the baseline covariates of age, race/ethnicity, education, smoking, physical activity, aspirin use, history of hyperlipidemia, duration of HT use, BMI and WHR; baseline hazard functions were allowed to vary by age (10-year group), study participation (four hormone therapy trial randomization arms, the dietary trial randomization arms, or enrollment into the OS), hysterectomy status and enrollment in WHI extensions (I/II; time-dependent).
p-value corresponds to a test of trend for duration of metformin use or other known diabetic medication.
p-value corresponds to test of whether trend for duration of metformin use differs from trend of other known medication use.
lymphatic and Hematopoietic.
Discussion
In this large prospective cohort of postmenopausal women, women with diabetes, compared to women without diabetes, had a higher risk of total invasive cancer, cancers of digestive organs and peritoneum, and reproductive organs, including cancers of the colon, liver, pancreas, endometrium and non-Hodgkin lymphoma. Overall, cancer risks in women with diabetes did not differ by diabetes therapy (metformin vs. other). However, a higher risk for overall cancer mortality was observed for women with diabetes treated with medications other than metformin compared to women without diabetes, but not in women with diabetes using metformin. Our results also suggested that long duration of metformin use was associated with a decreased risk of overall cancer mortality among women with incident diabetes.
In this large prospective cohort study, our findings that women with diabetes had a 13% higher risk of cancer compared to women without diabetes provide further evidence that diabetes is associated with increased cancer risk. This finding is similar to results from a recent meta-analysis.1 Significantly higher risks for several site-specific cancers were observed in our study and these findings are consistent with several meta-analyses, showing the highest risks (over twofold) for cancers of liver and pancreas,14,15 and a modestly higher risk (1.2 to1.4-fold) for colon, non-Hodgkin lymphoma and endometrial cancer.16–19 Despite the strong evidence for a diabetes-cancer association, mechanisms involved in this association are not completely understood, especially for the site-specific cancer relationship. There are several biologic factors, though, that have been proposed linking diabetes and cancer risk, including insulin resistance and hyperinsulinemia, as the most frequently proposed hypotheses, and other related mechanisms include hyperglycemia, oxidative stress, increased hormones and inflammatory cytokines.20,21
There has been a question whether any diabetes-cancer association mainly represents shared risk factors by both diseases, such as obesity and physical inactivity, or whether diabetes itself with its associated metabolic profile of insulin resistance, hyperglycemia and hyperinsulinemia directly mediates cancer risk.18 In the current analyses, after adjustment for many potential confounding variables, including obesity and physical activity, diabetes remained a risk factor for overall cancer risk and risk of certain cancers. However, in the current analyses among postmenopausal women, while diabetes was also associated with higher breast cancer risk, as suggested in a previous meta-analysis,22 adjustment for overall (BMI) and abdominal obesity (WHR) markedly attenuated the association. It is possible that obesity is a particularly strong confounder for the association between diabetes and breast cancer, as obesity is a well-established risk factor for breast cancer in postmenopausal women.23,24 Another fact that also has to be considered is that many complex factors shared by diabetes and many cancers, due to common underlying pathophysiological mechanisms, are interrelated, such as obesity, especially abdominal obesity-insulin resistance, making it difficult to differentiate their individual contributions to overall risk.
The preponderance of evidence from observational studies suggests those with diabetes treated with metformin have lower cancer risk in comparison to those with diabetes using other therapies.25–28 In the current analyses, we found no significant difference in cancer incidence by diabetes therapy, but found higher cancer mortality compared to women without diabetes only in women with diabetes treated with medications other than metformin. There was no significant difference in cancer risk with metformin compared to non-metformin therapy in the few randomized clinical trials available, as shown in the two recent meta-analyses, although these trials were not primarily designed to investigate the effects of these drugs and that may have affected the results.29,30 Interestingly, in our cumulative exposure analysis among CT participants from whom medication use data were collected repeatedly during the long-term follow-up period, results suggested a pattern of reduced breast cancer risk associated with long duration of metformin use. Metformin use, which decreases insulin levels and insulin resistance and results in weight loss,31,32 could reverse several adverse metabolic findings associated with obesity and diabetes in postmenopausal women. Although the mechanisms by which metformin may mediate anti-cancer effects are not completely understood, its role in insulin signaling, energy balance, glucose and fat metabolism through activation of the AMPK and inhibition of mTOR pathways, is thought to be involved.3,33
Although it is not entirely clear, the inconsistent findings on the metformin-cancer association between ours and other observational studies could be due to various differences, including study design, study populations, how the metformin exposure is collected and defined, sample size, incomplete adjustment of potential confounders and time for follow-up. First, compared to many other studies, our study population is limited to postmenopausal women. Second, as pointed out in a recent systemic review,28 some observational studies were retrospective in nature, and recall bias could be an issue. Third, some studies were based on medical records or insurance data, such that exposure data were not directly collected. Lastly, the metformin benefits related to cancer risk from some of the observational studies could also be partly as a result of time-related bias by not classifying and analyzing metformin exposure over time properly, as discussed recently by Suissa et al.30,34 It is also critical that participants’ medication usage history be collected often enough to capture and reflect general practice trends; we found that the percentage of treated diabetics who used metformin increased year by year from 21.1% at baseline to 32.2, 44.6, 54.7 and 61.5% in years 1, 3, 6 and 9 of the study and remained about 67% at years 12 through 16 during our follow-up extension. Thus, failure to collect exposure data at multiple time points and construct a reasonable approximation to participants’ medication use could introduce bias. Similar to our study, as discussed by Suissa, several recent studies reported no reduced cancer risk among metformin users when they used time-dependent variables in the analyses.35–37
Considering cancer mortality, a preexisting diagnosis of diabetes was associated with increased risk of overall cancer mortality and colorectal cancer-specific mortality after adjustment for obesity variables, which is generally consistent with the literature as shown in the meta-analyses.1,38 Metformin use has been associated with reduced cancer-related mortality compared to use of other anti-diabetes therapy such as insulin therapy.39–42 We found that the increased cancer mortality associated with diabetes was most apparent among women treated with other medications, with little risk among those who used metformin, and the risk significantly differed between metformin use and use of other medications among diabetes patients. Our results also suggested that long duration of metformin use was associated with a decreased risk of overall cancer mortality among women with incident diabetes. Cancer mortality depends on both cancer incidence and survival, and a change in incidence and/or survival is likely to affect mortality. Emerging evidence suggests that compared to other anti-diabetes medications, metformin use is associated with better survival in diabetes patients with cancer, including ovarian, endometrial, colorectal and breast cancer.43–47 It is possible that the differential effects of these drugs on cancer survival contribute to the differences in cancer mortality between the two treatment groups. In contrast, metformin use, especially for longer duration, may be associated with improved survival, contributing to our finding of a trend of reduced cancer-related death among long-term metformin users. Alternatively, women with diabetes that can be controlled by fewer drugs, like metformin users, may be likely to differ from those who require more drugs and intensive treatment, for example, with less comorbidity, and that may influence risk of cancer, cancer survival and ultimately cancer mortality, although in our cohort, baseline characteristics of metformin users and non-users were generally comparable.
This study has several strengths. We examined associations of diabetes, recency of diabetes, medication with risk of cancer and cancer mortality overall and by cancer site simultaneously in a large prospective cohort study, thereby mitigating issues of recall or selection bias that are encountered in retrospective studies. Our sample size was large (145,826 women) and we had a long-term follow-up (median: 15-year). Detailed data were collected by trained interviewers on exposures at baseline and updated during the follow-up visits and on various risk factors such as obesity and physical activity, enabling us to adjust for these important covariates in our analyses. Cancer diagnosis and cancer outcomes by site were reviewed locally and then confirmed by centrally trained physician adjudicators, minimizing information bias. Information on diabetes status and medication use were collected not only at baseline and updated during the study and extension follow-up, thus cox regression analysis with time-dependent exposure variables was modeled to estimate the risk, limiting the potential time-related bias. Despite these, our analyses were still limited by the small number of certain rarer cancers occurring in diabetes women, especially when stratified by medication use. This also limited our ability to examine effects of more detailed diabetes therapy other than metformin, and information on cancer treatments is unavailable. Finally, we included only postmenopausal women, which may have limited generalizability of our findings to premenopausal women or men. Nevertheless, our data from a large cohort of postmenopausal, elderly women could be valuable for future studies.
In summary, in this large prospective cohort study, we provide further evidence that postmenopausal women with diabetes are at higher risk of cancer, cancer mortality and certain site-specific cancers. In contrast to many prior observational studies, we found limited evidence to support a potential anticancer effect for metformin. There was a suggestion that the lower cancer risk associated with metformin may be evident only for a longer duration of use, in certain cancer sites or population subgroups. Interestingly, unlike other anti-diabetes medications, metformin was not associated with increased cancer mortality, suggesting that it may be associated with improved survival in women with cancer. Ongoing and future studies are needed to provide additional evidence to help determine the effects of various anti-diabetes medications, specifically the potential anti-tumor activity of metformin, in relation to cancer risk and survival from cancer.
Supplementary Material
What’s new?
Insulin resistance and excess circulating insulin are likely suspects underlying the link between diabetes mellitus and cancer. Hence, metformin therapy, which sensitizes tissues to insulin, may have a role in the prevention or management of diabetes-associated cancer. Here, among diabetic postmenopausal women, long-term metformin use was associated with a reduced risk of death from cancer. This benefit was not observed among women who took other antidiabetes medications. Overall cancer risk was found to be similar across diabetes therapies, though possible metformin-mediated anticancer effects may become apparent only after long duration of use.
Acknowledgments
We acknowledge the dedicated efforts of investigators and staff at the Women’s Health Initiative (WHI) clinical centers, the WHI Clinical Coordinating Center, and the National Heart, Lung and Blood program office (“short” list available at http://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf.).
Erin S. LeBlanc has received additional research funding from Amgen, Bristol-Meyers Squibb, Merck, and Astrazeneca for unrelated research projects.
Grant sponsors: National Heart, Lung and Blood Institute, National Institutes of Health and U.S. Department of Health and Human Services (WHI program); Grant numbers: HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C and HHSN271201100004C; Grant sponsor: National Institutes of Health (National Cancer Institute; Z.G.); Grant number: K07CA178293
Footnotes
Conflict of Interest: No other disclosures.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Noto H, Tsujimoto T, Sasazuki T, et al. Significantly increased risk of cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Endocr Pract. 2011;17:616–28. doi: 10.4158/EP10357.RA. [DOI] [PubMed] [Google Scholar]
- 2.Joshi S, Liu M, Turner N. Diabetes and its link with cancer: providing the fuel and spark to launch an aggressive growth regime. Biomed Res Int. 2015;2015:390863. doi: 10.1155/2015/390863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samani AA, Yakar S, LeRoith D, et al. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 4.Viollet B, Guigas B, Sanz Garcia N, et al. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122:253–70. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–61. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 6.Maccio A, Madeddu C. Obesity, inflammation, and postmenopausal breast cancer: therapeutic implications. Sci J. 2011;11:2020–36. doi: 10.1100/2011/806787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Women’s Health Initiative Study Group. Design of the women’s health initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 8.Anderson GL, Manson J, Wallace R, et al. Implementation of the women’s health initiative study design. Ann Epidemiol. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 9.Hays J, Hunt JR, Hubbell FA, et al. The women’s health initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 10.Margolis KL, Lihong Q, Brzyski R, et al. Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials. 2008;5:240–47. doi: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chlebowski RT, McTiernan A, Wactawski-Wende J, et al. Diabetes, metformin, and breast cancer in postmenopausal women. J Clin Oncol. 2012;30:2844–52. doi: 10.1200/JCO.2011.39.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the women’s health initiative. Ann Epidemiol. 2003;13:S122. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 13.Bodmer M, Meier C, Krähenbühl S, et al. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33:1304–8. doi: 10.2337/dc09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130:1639–48. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 15.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y, Ben Q, Shen H, et al. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011;26:863–76. doi: 10.1007/s10654-011-9617-y. [DOI] [PubMed] [Google Scholar]
- 17.Kramer HU, Schottker B, Raum E, et al. Type 2 diabetes mellitus and colorectal cancer: meta-analysis on sex-specific differences. Eur J Cancer. 2012;48:1269–82. doi: 10.1016/j.ejca.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Mitri J, Castillo J, Pittas AG. Diabetes and risk of non-hodgkin’s lymphoma: A meta-analysis of observational studies. Diabetes Care. 2008;31:2391–97. doi: 10.2337/dc08-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang ZH, Su PY, Hao JH, Sun YH. The role of preexisting diabetes mellitus on incidence and mortality of endometrial cancer: a meta-analysis of prospective cohort studies. Int J Gynecol Cancer. 2013;23:294–303. doi: 10.1097/IGC.0b013e31827b8430. [DOI] [PubMed] [Google Scholar]
- 20.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: A consensus report. Diabetes Care. 2010;33:1674–85. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher EJ, LeRoith D. Minireview: IGF, insulin, and cancer. Endocrinology. 2011;152:2546–51. doi: 10.1210/en.2011-0231. [DOI] [PubMed] [Google Scholar]
- 22.Col NF, Ochs L, Springmann V, et al. Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res Treat. 2012;135:639–46. doi: 10.1007/s10549-012-2170-x. [DOI] [PubMed] [Google Scholar]
- 23.La Vecchia C, Giordano SH, Hortobagyi GN, et al. Overweight, obesity, diabetes, and risk of breast cancer: interlocking pieces of the puzzle. Oncologist. 2011;16:726–29. doi: 10.1634/theoncologist.2011-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ligibel J. Obesity and breast cancer. Oncology (Williston Park) 2011;25:994–1000. [PubMed] [Google Scholar]
- 25.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–77. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 26.Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–25. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monami M, Colombi C, Balzi D, et al. Metformin and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care. 2011;34:129–31. doi: 10.2337/dc10-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franciosi M, Lucisano G, Lapice E, et al. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;8:e71583. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens RJ, Ali R, Bankhead CR, et al. Cancer outcomes and all-cause mortality in adults allocated to metformin: systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia. 2012;55:2593–603. doi: 10.1007/s00125-012-2653-7. [DOI] [PubMed] [Google Scholar]
- 30.Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care. 2012;35:2665–73. doi: 10.2337/dc12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srinivasan S, Ambler GR, Baur LA, et al. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J Clin Endocrinol Metab. 2006;91:2074–80. doi: 10.1210/jc.2006-0241. [DOI] [PubMed] [Google Scholar]
- 32.Yanovski JA, Krakoff J, Salaita CG, et al. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes. 2011;60:477–85. doi: 10.2337/db10-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suissa S, Azoulay L. Metformin and cancer: mounting evidence against an association. Diabetes Care. 2014;37:1786–88. doi: 10.2337/dc14-0500. [DOI] [PubMed] [Google Scholar]
- 35.Azoulay L, Dell’Aniello S, Gagnon B, et al. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev. 2011;20:337–44. doi: 10.1158/1055-9965.EPI-10-0940. [DOI] [PubMed] [Google Scholar]
- 36.Smiechowski BB, Azoulay L, Yin H, et al. The use of metformin and the incidence of lung cancer in patients with type 2 diabetes. Diabetes Care. 2013;36:124–29. doi: 10.2337/dc12-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mamtani R, Pfanzelter N, Haynes K, et al. Incidence of bladder cancer in patients with type 2 diabetes treated with metformin or sulfonylureas. Diabetes Care. 2014;37:1910–17. doi: 10.2337/dc13-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–87. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 39.Bowker SL, Majumdar SR, Veugelers P, et al. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–58. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 40.Landman GW, Kleefstra N, van Hateren KJ, et al. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33:322–26. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Currie CJ, Poole CD, Jenkins-Jones S, et al. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang P, Li H, Tan X, et al. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37:207–18. doi: 10.1016/j.canep.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Kumar S, Meuter A, Thapa P, et al. Metformin intake is associated with better survival in ovarian cancer: a case-control study. Cancer. 2013;119:555–62. doi: 10.1002/cncr.27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nevadunsky NS, Van Arsdale A, Strickler HD, et al. Metformin use and endometrial cancer survival. Gynecol Oncol. 2014;132:236–40. doi: 10.1016/j.ygyno.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cossor FI, Adams-Campbell LL, Chlebowski RT, et al. Diabetes, metformin use, and colorectal cancer survival in postmenopausal women. Cancer Epidemiol. 2013;37:742–49. doi: 10.1016/j.canep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peeters PJ, Bazelier MT, Vestergaard P, et al. Use of metformin and survival of diabetic women with breast cancer. Curr Drug Saf. 2013;8:357–63. doi: 10.2174/15680266113136660069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin M, Zhou J, Gorak EJ, et al. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: a systematic review and meta-analysis. Oncologist. 2013;18:1248–55. doi: 10.1634/theoncologist.2013-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


