Abstract
Scope
Elemental enteral nutrition (EEN) decreases gut-associated lymphoid tissue (GALT) function, including fewer Peyer’s patch lymphocytes, lower levels of the tissue Th2 cytokines and mucosal transport protein polymeric immunoglobulin receptor (pIgR), leading to lower luminal sIgA levels. Since we recently demonstrated cranberry proanthocyanidins (PACs) maintain the Th2 cytokine IL-4 when added to EEN, we hypothesized the addition of PACs to EEN would normalize other GALT parameters and maintain luminal levels of sIgA.
Methods and Results
ICR mice were randomized (12/group) to receive Chow, EEN, or EEN+PACs (100 mg/kg body weight) for 5 days, starting 2 days after intra-gastric cannulation. Ileum tissue was collected to measure IL-4 by ELISA, pIgR by western blot, and phosphorylated STAT6 by microarray. Intestinal wash fluid was collected to measure sIgA by western blot. Compared with Chow, EEN significantly decreased tissue IL-4, Phosphorylated STAT6, and pIgR. The addition of PACs to EEN prevented these alterations. Compared with Chow, EEN resulted in significantly lower levels of luminal sIgA. The addition of PACs to EEN increased luminal sIgA levels compared to EEN alone.
Conclusions
This study suggests the addition of PACs to EEN may support GALT function and maintain intestinal sIgA levels compared with EEN alimentation alone.
Keywords: Proanthocyanidins, Elemental Enteral Nutrition, Secretory IgA, JAK-STAT
INTRODUCTION
Elemental enteral nutrition (EEN) is a useful therapy option for conditions requiring a reduced residual diet, including inflammatory bowel diseases and pancreatitis [1–3]. Decreased dietary bulk and complexity provided with EEN attenuates mucosal agitation and painful symptoms. Unfortunately, reduced dietary complexity, such as provided with EEN or parenteral nutrition (PN), alters the structure and function of the gut-associated lymphoid tissue (GALT). Ultimately, reduced dietary complexity manifests as decreased secretory immunoglobulin-A (sIgA) in the gut lumen compared to enteral feeds [4–7]. sIgA is the primary protective compound of acquired immunity secreted by the host mucosa, which among other notable functions exclude enteric bacteria from attachment to the host [8, 9]. EEN also results in increased bacterial translocation and decreased microbiome diversity [9, 10]. To address EEN induced susceptibilities various interventions, including the use of natural products, have been investigated to provide anti-inflammatory and protective effects in the gut [7]. Since our established feeding model employing intra-gastric administered EEN results in the reproducible loss of intestinal (and respiratory) sIgA, this work investigated whether a class of natural compounds isolated from cranberries, proanthocyanidins (PACs), support mucosal protection by stimulating luminal sIgA levels when added to EEN.
PACs are a class of polyphenols that are found in many dietary sources, including fruits, tea, chocolate, and wine [11, 12] that epidemiological studies suggest may prevent the onset of chronic pathologies, such as cardiovascular disease and cancer [13, 14]. Interestingly, greater than 95% of ingested PACs remain in the gut lumen during transit, limiting their interaction with systemic compartments and making benefits of PACs ingestion upon health unique compared with other polyphenolics. We recently investigated the effect of adding PACs to EEN in an animal model and observed increased tissue Th2 cytokines levels, including IL-4 and IL-13 [15]. Increased IL-4 tissue levels have also been observed in experimental colitis following PACs supplementation [16].
Our previous work demonstrates reduced luminal sIgA levels following EEN or parenteral nutrition is multifactorial, including fewer lymphocyte numbers in both Peyer’s patches (PP) and lamina propria compartments; suppressed T helper 2 cytokines (Th2), IL-4 and IL-10, in the lamina propria; and reduced expression of mucosal pIgR, which is the primary transport protein for sIgA [17–20]. Expression of pIgR is regulated in part through the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, a cytokine signaling cascade used to transduce a wide array of cellular events [21–24]. IL-4 binds the IL-4 receptor-α inducing intracellular STAT6-phosphoyrlation, dimerization, and migration into the cell nucleus targeting transcription products, including pIgR [25]. Since EEN decreases sIgA levels and our recent work suggest PACs support intestinal Th2 cytokines, we hypothesized that the addition of physiological PACs doses [12] to EEN would support GALT function and luminal sIgA compared with EEN alimentation alone.
MATERIALS AND METHODS
PAC Preparation and Characterization
The PAC preparation and characterization for use in experimental diets for this study were previously published [26]. Briefly, non-depectinized cranberry presscake was ground with liquid nitrogen and extracted with 70% acetone three times (Fisher Scientific, Fair Lawn, NJ). Acetone was removed by evaporation and the aqueous suspension was solubilized in ethanol (Decon Labs Inc., King of Prussia, PA), followed by centrifugation to eliminate ethanol insoluble material. Cranberry presscake crude extract was loaded on a Sephadex LH-20™ (GE Healthcare, Uppsala, Sweden) column and PAC were isolated by sequential elution with ethanol, ethanol/methanol (1:1) and 80% acetone. Acetone in the last fraction that contained PAC was removed by evaporation under vacuum and re-solubilized in methanol (Fisher Scientific, Fair Lawn, NJ). The total phenolic content of the PAC fraction was determined by Folin-Ciocalteu method and reported as Gallic acid equivalents (GAE).
An aliquot of the cranberry presscake PAC fraction was diluted tenfold and a sample was injected onto a Waters Spherisorb® 10 μm ODS2 RP-18 column. The solvents for elution were trifluoroacetic acid/water (0.1%) and methanol. The HPLC system consisted of a Waters automated gradient controller, two Waters 501 HPLC pumps, and a Rheodyne 7125 manual injector. The elution was monitored by a Waters 996 diode array detector using Waters Millennium software for collecting and analyzing three-dimensional chromatograms.
An aliquot of the cranberry presscake PAC fraction was mixed with 2,5-dihydroxybenzoic acid (Aldrich, Milwaukee, WI) and the mixture was applied onto a MALDI-TOF MS stainless steel target and dried at room temperature. Mass spectra were collected on a Bruker Reflex II MALDI-TOF-MS (Billerica, MA) equipped with delayed extraction and a N2 laser (337 nm) in order to characterize the range in degree of polymerization (DP) and nature of interflavan bonds in the cranberry PAC. All preparations were analyzed in the positive ion linear and reflectron mode to detect [M+Na]+ and [M+K]+ molecular ions. MALDI-TOF MS is ideally suited for characterizing PAC because, unlike electrospray ionization in which multiple charge molecular ions create very complex spectral peaks that are often difficult to interpret, this mass spectral technique produces only a singly charged molecular ion for each parent molecule [27]
Animals
The Animal Care and Use Committee of the University of Wisconsin-Madison and Middleton Veterans Administration Hospital, Madison approved all animal experimental protocols. Male Institute of Cancer Research (ICR) mice were purchased through Harlan (Indianapolis, IN) and housed in an American Association for Accreditation of Laboratory Animal Care-accredited conventional facility on the V.A Williamson Hospital Campus. Mice were acclimatized for one week in an environment controlled for temperature and humidity with a 12/12-hour light/dark cycle. Mice were housed 5 per covered/filtered box and fed ad libitum chow (LabDiet, PMI Nutrition International, St. Louis, MO) and water for 1 week prior to initiation of study protocol. After entering study protocol mice were housed individually in metal cages with wire grid floors to prevent coprophagia and bedding ingestion.
Experimental Design
Male ICR mice, ages 6 to 8 weeks, were randomized to Chow with a gastric catheter (n = 12), intragastric elemental nutrition (EEN) (n=12) via gastrostomy, or EEN + PACs via gastrostomy (100 mg/kg body weight (EEN+PACs)) (n=12). Animals were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and acepromazine (10 mg/kg). Catheters were tunneled subcutaneously from the gastrostomy site over the back and exited mid tail. Mice were partially immobilized by tail fixation to protect the catheter during infusion. This technique does not induce significant physical or biochemical stress as was previously shown [28].
Catherized mice were connected to infusion pumps and allowed recovery for 48 hours while receiving 4 mL/day saline (0.9%) and ad libitum chow (Agway Inc., Syracuse, NY) and water. Following the recovery period experimental diets were given. Chow mice continued to receive 0.9% saline at 4 mL/day as well as ad libitum chow and water throughout the study. The EEN solution includes 6.0% amino acids, 35.6% dextrose, electrolytes, and multivitamins, with a non-protein calorie/nitrogen ratio of 126.1 (527.0 kJ/g Nitrogen). This value meets the calculated nutrient requirements of mice weighing 25 to 30 g [29]. EEN and EEN + PAC fed mice received solution at 4 mL/day (day 1), 7 mL/day (day 2) and 10 mL/day (days 3–5) as well as ad libitum water throughout the study.
After 5 days of feeding (7 days post-catherterization), mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and acepromazine (10 mg/kg), and exsanguinated via left axillary artery transection. The small intestine was removed and the lumen rinsed with 20 mL Hanks Balanced Saline Solution (HBSS, Bio Whittaker, Walkersville, MD). The luminal rinse was centrifuged at 2,000 x g for 10 min and supernatant aliquots were frozen at −80°C for sIgA analysis. Tissue samples were taken by removing a 3 cm segment of ileum excluding PPs. PP lymphocytes were assessed by counting on a hemocytometer. Samples were frozen in liquid N2 and stored at −80°C until processing or fixed in 4% paraformaldehyde overnight, transferred to 70% ethanol, and stored at 4°C for immunohistochemistry.
Peyer’s Patch Lymphocytes
The Peyer’s patch (PP) from the entire length of the SI were removed into 1.5 mL tubes of CMF-HBSS. PP were strained through 100-μm mesh with a total volume of 15 mL CMF-HBSS. The effluent was collected and spun at 1700 rpm at 5°C for 10 min. The supernatant was removed and the pellet resuspended in 15 mL CMF-HBSS; this step was repeated. Cells were counted on a hemocytometer with trypan blue.
Tissue Cytokine Quantitative Analysis
The flash-frozen small intestine segment from each animal was homogenized in RIPA lysis buffer (Upstate, Lake Placid, NY) containing 1% protease inhibitor cocktail (P8340, Sigma-Aldrich, St. Louis, MO). The homogenate was kept on ice for 30 min prior to centrifugation at 16,000 x g for 10 min at 4°C. The supernatant was then stored at −20°C until analysis. Prior to storage, the protein concentration of the supernatant was determined by the Bradford method using BSA as a standard.
Concentration of IL-4 was determined in the supernatant using solid phase sandwich ELISA kits (BD Biosciences, San Diego, CA), according to manufacturer’s instructions. The absorbance at 450 nm was determined using a Vmax Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA). The IL-4 concentrations in the samples were determined by using a 4-parameter logistic fit standard curve (SOFTmax PRO software; Molecular Devices; Sunnyvale, CA) and normalized to total tissue protein content.
JAK-STAT Profiling by the JAK-STAT Antibody Microarray
The Phospho Explorer antibody microarray (Full Moon Biosystems Inc, Sunnyvale, CA), contains 42 antibodies. Each of the antibodies has six replicates that are printed on coated glass microscope slide, along with multiple positive and negative controls. The antibody array experiment was performed according to established protocol [30]. In brief, ileum tissue lysates (n=8/group) were biotinylated with Antibody Array Assay Kit. The antibody microarray slides were first blocked in a blocking solution for 30 min at room temperature, rinsed with Milli-Q grade water for 5 min, and dried with compressed nitrogen. The slides were then incubated with the biotin-labeled cell lysates (~80 μg protein) in coupling solution at room temperature for 2 hours. The array slides were washed 5 times with 1X Wash Solution and rinsed extensively with Milli-Q grade water before detection of bound biotinylated proteins using Cy3-conjugated streptavidin. The slides were scanned on a GenePix 4000 scanner and the images were analyzed with GenePix Pro 6.0 (Molecular Devices, Sunnyvale, CA). The fluorescence signal of each antibody was obtained from the fluorescence intensity of this antibody spot after subtraction of the blank signal (spot in the absence of antibody), and we used the signal of the phosphorylated protein to GAPDH housekeeping protein expression.
Analysis of pIgR expression by Western Blot
Solubilized protein from small intestinal tissue homogenate was denatured at 95°C for 10 min with sodium dodecylsulfate and β-mercaptoethanol, and 20 μg of protein from each sample was separated in a denaturing 10% polyacrylamide gel by electrophoresis at 150V for 1 hour at room temperature. Proteins were transferred to a PVDF membrane, and western blot was performed as previously described [31]. Densitometric measurements of protein bands were analyzed and quantified with the NIH Image J software. pIgR standard (Cat 2800, R&D, Minneapolis, MN) was used to compare multiple gels. The combined value of the 120 kda and 94 kda bands was determined for the quantitation of the pIgR protein expression in sample.
Analysis of IgA by Western Blot
Luminal wash IgA was measured by western blot since we observed the addition of PACs to control animal luminal wash samples rapidly decreases sensitivity and total signal measured by IgA ELISA (unpublished observation), likely through the complexation between PACs with proteins [32]. 4 μL of luminal fluid was denatured at 95°C for 10 min with sodium dodecylsulfate and β-mercaptoethanol. Proteins were separated in a denaturing 10% polyacrylamide gel by electrophoresis at 150 V for 1 hour at room temperature and transferred to polyvinylidene fluride membrane using tris-glycine buffer plus 20% methanol at 80 V for 50 min at 4°C. The membrane was blocked with 5% nonfat dry milk prepared in TBS-Tween for 1 hour at room temperature with constant agitation. Membranes were incubated with goat anti-mouse IgA, α-chain specific (Sigma-Aldrich, St. Louis, MO) diluted 1:7,000 for 1 hour at room temperature with constant agitation. Then, membranes were washed and incubated with stabilized donkey anti-goat IgA-HRP conjugated secondary diluted 1:20,000 for 1 hour at room temperature. After washing, membranes were incubated with HRP substrate (Super Signal West Femto maximum sensitivity substrate; Pierce, Rockford, IL) for 5 min and bands were detected using photographic film. Densitometric measurements of immunoglobulin α-chain protein bands (~55 kDa) were analyzed and quantified with the NIH Image J software. IgA heavy chain standard (M-1421, Sigma-Aldrich) was used to normalize across multiple gels.
Statistical analysis
Experimental values were compared using analysis of variance (ANOVA) and Fisher protected least significance difference (PLSD) corrected for multiple comparisons, with α = 0.05 considered significant (Statview 5.0.1, SAS, Cary, NC). Numerical results are presented as mean ± standard deviation of the mean.
RESULTS
PAC characterization by HPLC and MALDI-TOF MS
The cranberry presscake PAC eluted as two unresolved peaks that had absorbance at 280 nm and minor absorbance at 520 nm due to the presence of covalently linked anthocyanin-proanthocyanidin pigments. No peaks were observed with an absorbance max typical of the other classes of cranberry polyphenolic compounds (anthocyanins, hydroxycinnamic acids, and flavonols). The poorly resolved chromatogram at 280 nm is due to structural heterogeneity of cranberry presscake PAC [27].
Reflectron mode MALDI-TOF MS showed masses that correspond to PAC with at least 1A-type interflavan bond in trimers to undecamers. MALDI-TOF MS linear mode spectra had m/z peaks that correspond to cranberry presscake PAC with a range of 3 to 23 degrees of polymerization. The spectra also contained m/z peaks that correspond to covalently linked anthocyanin-proanthocyanidin molecules, ranging from monomers to heptamers (data not shown).
Peyer’s Patch Lymphocytes
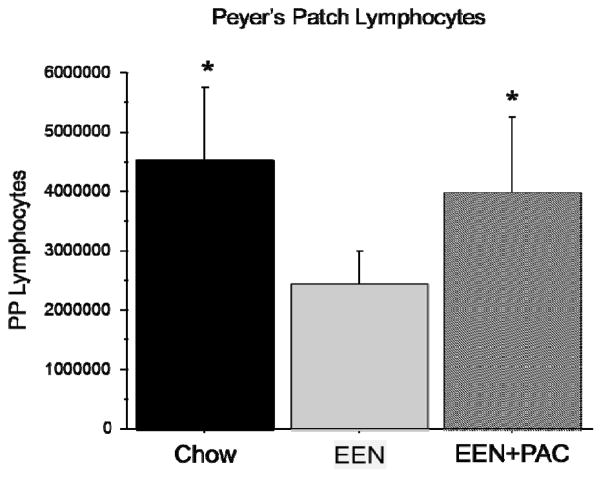
Compared with Chow (4.533×106 ± 1.226 ×106 cells), EEN significantly lowered PP lymphocytes (2.428 ×106 ± 0.574 ×106 cells, P < 0.0001) [Figure 1]. Compared with EEN alone, PP lymphocytes were significantly higher in EEN+PAC (3.957 ×106 ± 1.291 ×106 cells, P < 0.001). There were no significant differences between Chow and EEN+PAC (P = 0.19).
Figure 1.
The total number of Peyer’s Patch lymphocytes in Chow, EEN, and EEN+PAC fed mice. * P < 0.001 vs EEN.
Ileum Tissue IL-4
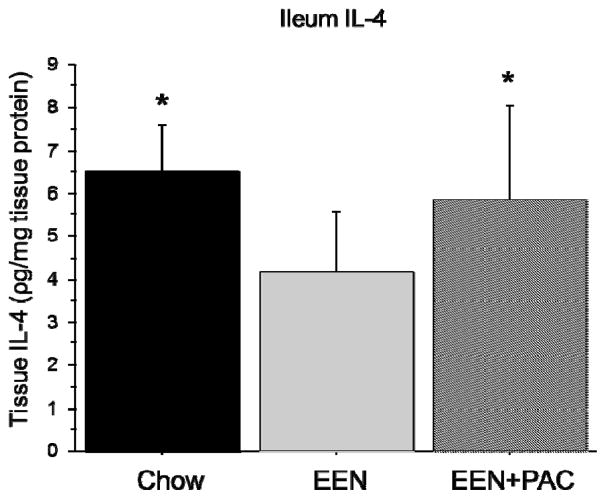
Compared with Chow (6.5 ± 1.11 pg/mg protein), EEN significantly lowered ileum IL-4 (4.15 ± 1.44, P < 0.01) [Figure 2]. Compared with EEN alone, ileum IL-4 was significantly higher in EEN+PAC (5.8 ± 2.2, P < 0.05). There were no significant difference between the level of ileum IL-4 between Chow and EEN+PAC (P = 0.42).
Figure 2.
Ileum tissue IL-4 levels in Chow, EEN, and EEN+PAC fed mice. * P < 0.05 vs EEN.
Ileum Tissue Phosphorylated STAT6
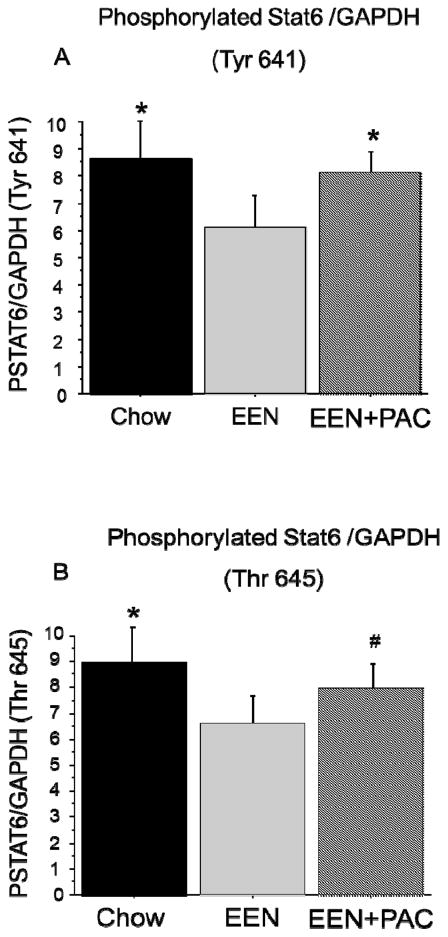
Phosphorylated STAT6 (PSTAT6) was measured at two phosphorylation sites, Tyrosine 641 (Tyr641) and Threonine 645 (Thr645), and normalized to GAPDH expression. Compared with Chow (8.66 ± 1.5 PSTAT6 (Tyr641)/GAPDH), PSTAT6 at Tyr641 site was significantly reduced with EEN (6.08 ± 1.3, P < 0.001). The addition of PAC to EEN significantly elevated PSTAT6 at Tyr 641 (8.11 ± 0.7, P < 0.01). There was no difference between Chow and EEN+PAC (P = 0.37) [Figure 3A]. Similarly, compared with Chow (8.97 ± 1.6 PSTAT6 (Thr645)/GAPDH), PSTAT6 at Thr645 was significantly lower with EEN (6.60 ± 1.0, P < 0.01). The addition of PAC to EEN significantly elevated PSTAT6 at Thr645 (7.99 ± 0.9, P < 0.05), however, there were no significant difference between Chow and EEN+PAC (P = 0.13) [Figure 3B].
Figure 3.
(A) Phosphorylated STAT6 (Tyr 641) ileum tissue levels in Chow, EEN, and EEN+PAC fed mice. (B) Phosphorylated STAT6 (Tyr 645) ileum tissue levels in Chow, EEN, and EEN+PAC fed mice. * P < 0.01 vs EEN. # P < 0.05 vs EEN.
Ileum Tissue pIgR
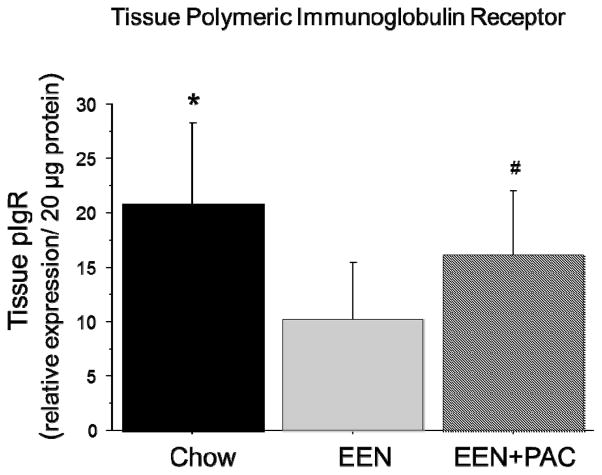
EEN (10.23 ± 5.23) lowered tissue pIgR (relative concentration/ 20 ug protein) compared with Chow (20.71 ± 7.63, P < 0.001) [Figure 4]. PAC+EEN (16.13 ± 5.97, P < 0.03) levels of tissue pIgR were significantly higher than EEN alone. There were no significant difference between Chow and EEN+PAC (P = 0.08).
Figure 4.
Ileum tissue levels of polymeric immunoglobulin receptor (pIgR) in Chow, EEN, and EEN+PAC fed mice. * P < 0.05 vs EEN. # P < 0.05 vs EEN.
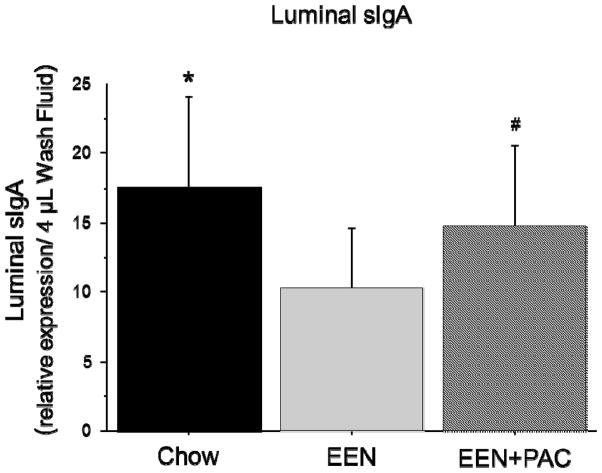
Luminal sIgA
Compared with Chow (17.62 ± 6.52), the level of luminal sIgA (relative concentration/ 4 uL luminal wash) was significantly lower following EEN (10.33 ± 4.23, P < 0.001) [Figure 5]. The addition of PAC to EEN (14.67 ± 5.86, P < 0.05) significantly elevated luminal sIgA compared with EEN alone. There was no significant difference between EEN+PAC and Chow (P = 0.15).
Figure 5.
Concentration of secretory IgA in small intestine luminal wash samples in Chow, EEN, and EEN+PAC fed mice. * P < 0.01 vs EEN. # P < 0.05 vs EEN.
DISCUSSION
EEN allows alimentation to patients with contraindication to normal feeding by administering a liquid diet directly into the gastrointestinal tract [33]. EEN formulas are usually used in clinical conditions involving intestinal or pancreatic inflammation [2]. Our previous work demonstrates the administration of a glucose-amino acid infusion (EEN) administered via gastrostomy decreases several aspects of GALT function, including fewer PP and lamina propria lymphocytes; reduced tissue IL-4 and IL-10; pIgR, the sIgA mucosal transport protein; and decreased levels of luminal sIgA [4–7, 34]. Unfortunately, these changes result in increased susceptibility to infection and inflammation since sIgA is the primary protective molecule of specific (acquired) immunity that is secreted onto mucosal surfaces [34, 35]. sIgA opsinizes bacteria, preventing their attachment to the mucosa [36], and reduces virulent expression in enteric pathogens [37]. Consistent with its negative effect on luminal sIgA, EEN also increases mucosal barrier permeability and decreases microbiome diversity [10, 35]. Since EEN is the only enteral formula tolerated in certain patients, EEN supplements that improve host immune and barrier function are of particular value. In this work, we investigated the effect of PACs, isolated from cranberry press cake, upon GALT function leading to the release of sIgA in the intestinal lumen.
PACs are complex oligomeric polyphenolic compounds distributed in fruits, including grapes, cranberries, and apples, and other foods and beverages such as chocolate and wine [11, 12]. Epidemiological data suggest PACs may have beneficial health effects by preventing multiple chronic diseases [38]. However, PACs do not appear to leave the gut lumen for a variety of reasons, including non-hydrolysable bonds between flavan-3-ol monomeric units and their ability to complex both dietary and endogenous proteins. Further, PAC oligomers range in degree of polymerization from 3 to 25+ and therefore have higher molecular weight than other common plant polyphenols. Due to these characteristics, rodent models demonstrate greater than 95% of PACs remain in the intestinal lumen during transit through the gastrointestinal tract [39, 40], and a recent human study demonstrated ingested PACs do not contribute to circulating flavanol levels [41]. These observations suggest PACs may exert beneficial health effects through their interaction at the gut mucosa.
Investigations have shown that PACs and precursors exert antioxidant and non-specific antimicrobial functions in the gut [38] and are capable of palliating chemically-induced colitis and ulceration while increasing Th2 cytokines, including IL-4, in gastrointestinal tissue [42–44]. In vitro studies demonstrate that intra-epithelial γδ T lymphocytes from the intestine respond to PACs with activation and cell proliferation that was dependent upon the PAC degree of polymerization [45]. The importance of molecular weight has also been demonstrated in vitro, with blood mononuclear cells, where higher molecular weight flavonoids induced greater cytokine production, including IL-4, than correspondingly lower molecular weight fractions [46]. These observations suggest PACs may play an influential role in context of mucosal barrier physiology and immunity, but also that the characteristics of the PACs are important when investigating their effects. Accordingly, we previously characterized the PACs used in this experiment [26], which showed the PACs ranged from 3–26 degrees of polymerization. This analysis allows for the characterization and reliable reproduction of chromatographic fractions for use in experimental treatments and future studies.
The expression of pIgR is regulated through IL-4 stimulation of the nuclear factor STAT-6, a member of the JAK/STAT signaling cascade [21, 47]. STAT-6, in part, regulates luminal sIgA through regulation of the mucosal transport protein pIgR [19]. We established the importance of STAT-6 during parenteral nutrition with lack of enteral stimulation showing that lower IL-4 levels correlated levels of phosphorylated STAT-6, pIgR, and luminal sIgA. Administration of exogenous cytokines that stimulate STAT-6 phosphorylation during parenteral nutrition significantly increased levels of pIgR expression and luminal IgA levels, suggesting a cause and effect relationship [3]. In this work, EEN decreased intestinal tissue levels of IL-4 and phosphorylated STAT-6, correlated with decreased pIgR and luminal sIgA. The addition of PACs to EEN at physiological levels (100 mg GAE/kg body weight) resulted in increased tissue IL-4, STAT-6 phosphorylation, pIgR, and luminal sIgA, supporting our hypothesis that PACs may influence health by interacting with GALT function.
Previous work demonstrated that polyphenolic supplementation, including curcumin or a polyphenolic rich diet, increases sIgA levels when added to normal diets [48, 49]. However, this is the first to demonstrate PAC supplementation may improve luminal sIgA during EEN. PACs posed a significant challenge for accurately quantifying luminal sIgA, since PACs form complexation with endogenous and dietary proteins, including immunoglobulins, through hydrophobic and hydrogen bonding interaction [32]. During our analysis we observed that the addition of small concentrations of PACs to luminal wash fluid from control animals rapidly decreased the detectable levels of sIgA via ELISA quantification (unpublished observation). For this reason, measurement of luminal sIgA in this study was achieved by first denaturing and reducing intestinal wash fluid samples with heat, sodium dodecyl sulfate, and β-mercaptoethanol and performing western blot analysis to detect the sIgA heavy chain directly. Future work with PACs should take the complexation and masking effect into consideration when investigating intestinal sIgA.
One limitation to this study is we do not have evidence of whether PACs stimulate the GALT directly, such as through PP or intra-epithelial lymphocyte interactions at the mucosa, or if changes to the gastrointestinal luminal environment during the PAC addition to EEN are responsible for GALT stimulation. Other work in our laboratory demonstrated the addition of PACs to EEN maintains microbiome diversity in the gut lumen compared to the reduced diversity that both we and others have observed in EEN alone [35]. Future work aims to determine the effect of PACs upon leukocytes from GALT compartments, including the PP, intra-epithelial space, and lamina propria to assess the potential role of these cells.
In summary, this work supports the hypothesis that decreased enteral stimulation, such as EEN or parenteral feeding, suppresses GALT function – including total PP and lamina propria lymphocytes numbers, Th2 cytokine levels, and the mucosal sIgA transport protein, pIgR - that leads to reduced luminal sIgA levels. Consistent with the hypothesis of the current study, that PACs may provide immunoprotective effects through interactions with the GALT and intestinal mucosa, the supplementation of physiological doses of PACs to EEN elevated GALT function and luminal sIgA compared to EEN feeding alone. This study suggests moderate levels of PACs may be beneficial when added to enteral diets by promotion of adaptive immune function.
Acknowledgments
The project described was also supported by Award Number I01BX001672 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development. The contents of this article do not represent the views of the Veterans Affairs or the United States Government. This project was also supported by the Reed Research Group Multi-donor Fund.
Abbreviations
- EEN
Elemental Enteral Nutrition
- GALT
gut-associated lymphoid tissue
- Th2
T helper 2
- PP
Peyer’s Patch
- sIgA
secretory immunoglobulin-A
- pIgR
polymeric immunoglobulin receptor
- PACs
Proanthocyanidins
- JAK-STAT
Janus kinase/signal transducer and activator of transcription
- GAE
Gallic acid equivalents
- PN
parenteral nutrition
References
- 1.Isaacs KL, Lewis JD, Sandborn WJ, Sands BE, Targan SR. State of the art: IBD therapy and clinical trials in IBD. Inflamm Bowel Dis. 2005;11(Suppl 1):S3–12. doi: 10.1097/01.mib.0000184852.84558.b2. [DOI] [PubMed] [Google Scholar]
- 2.Curtis C, Kudsk K. Nutrition support in pancreatitis. Surg Clin North Am. 2007;87:1403–1415. viii. doi: 10.1016/j.suc.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Heneghan AF, Pierre JF, Kudsk KA. IL-25 Improves IgA During Parenteral Nutrition Through the JAK-STAT Pathway. Annals of Surgery. 2012 doi: 10.1097/SLA.0b013e318277ea9e. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Kudsk KA, Gocinski B, Dent D, et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44–51. doi: 10.1097/00005373-199507000-00006. discussion 51–42. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Kudsk KA, DeWitt RC, Tolley EA, Li J. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg. 1999;229:662–667. doi: 10.1097/00000658-199905000-00008. discussion 667–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King B, Li J, Kudsk K. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997;132:1303–1309. doi: 10.1001/archsurg.1997.01430360049009. [DOI] [PubMed] [Google Scholar]
- 7.Mosenthal AC, Xu D, Deitch EA. Elemental and intravenous total parenteral nutrition diet-induced gut barrier failure is intestinal site specific and can be prevented by feeding nonfermentable fiber. Crit Care Med. 2002;30:396–402. doi: 10.1097/00003246-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Langkamp-Henken B, Glezer J, Kudsk K. Immunologic structure and function of the gastrointestinal tract. Nutr Clin Pract. 1992;7:100–108. doi: 10.1177/0115426592007003100. [DOI] [PubMed] [Google Scholar]
- 9.Kajiura T, Takeda T, Sakata S, Sakamoto M, et al. Change of intestinal microbiota with elemental diet and its impact on therapeutic effects in a murine model of chronic colitis. Dig Dis Sci. 2009;54:1892–1900. doi: 10.1007/s10620-008-0574-6. [DOI] [PubMed] [Google Scholar]
- 10.Deitch EA, Xu D, Naruhn MB, Deitch DC, et al. Elemental diet and IV-TPN-induced bacterial translocation is associated with loss of intestinal mucosal barrier function against bacteria. Ann Surg. 1995;221:299–307. doi: 10.1097/00000658-199503000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellstrom JK, Torronen AR, Mattila PH. Proanthocyanidins in common food products of plant origin. J Agric Food Chem. 2009;57:7899–7906. doi: 10.1021/jf901434d. [DOI] [PubMed] [Google Scholar]
- 12.Knaze V, Zamora-Ros R, Luján-Barroso L, Romieu I, et al. Intake estimation of total and individual flavan-3-ols, proanthocyanidins and theaflavins, their food sources and determinants in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr. 2011:1–14. doi: 10.1017/S0007114511006386. [DOI] [PubMed] [Google Scholar]
- 13.Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol. 2005;16:77–84. doi: 10.1097/00041433-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 15.JFP, AFH, RPF, DS, et al. JPEN J Parenter Enteral Nutr. 2012 [Google Scholar]
- 16.Li XL, Cai YQ, Qin H, Wu YJ. Therapeutic effect and mechanism of proanthocyanidins from grape seeds in rats with TNBS-induced ulcerative colitis. Can J Physiol Pharmacol. 2008;86:841–849. doi: 10.1139/Y08-089. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Kudsk KA, Gocinski B, Dent D, et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44–51. doi: 10.1097/00005373-199507000-00006. discussion 51–42. [DOI] [PubMed] [Google Scholar]
- 18.Kang W, Gomez F, Lan J, Sano Y, et al. Parenteral nutrition impairs gut-associated lymphoid tissue and mucosal immunity by reducing lymphotoxin Beta receptor expression. Ann Surg. 2006;244:392–399. doi: 10.1097/01.sla.0000234797.42935.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sano Y, Gomez F, Kang W, Lan J, et al. Intestinal polymeric immunoglobulin receptor is affected by type and route of nutrition. JPEN J Parenter Enteral Nutr. 2007;31:351–356. doi: 10.1177/0148607107031005351. discussion 356–357. [DOI] [PubMed] [Google Scholar]
- 20.Sano Y, Gomez F, Hermsen J, Kang W, et al. Parenteral nutrition induces organ specific alterations in polymeric immunoglobulin receptor levels. J Surg Res. 2008;149:236–242. doi: 10.1016/j.jss.2007.12.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 22.Mohr A, Chatain N, Domoszlai T, Rinis N, et al. Dynamics and non-canonical aspects of JAK/STAT signalling. Eur J Cell Biol. 2011 doi: 10.1016/j.ejcb.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Reich NC, Liu L. Tracking STAT nuclear traffic. Nat Rev Immunol. 2006;6:602–612. doi: 10.1038/nri1885. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann SR, Ettinger R, Zhou YJ, Gadina M, et al. Cytokines and their role in lymphoid development, differentiation and homeostasis. Curr Opin Allergy Clin Immunol. 2002;2:495–506. doi: 10.1097/00130832-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Kaetzel C. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev. 2005;206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 26.Feliciano RP, Shea MP, Shanmuganayagam D, Krueger CG, et al. Comparison of Isolated Cranberry ( Vaccinium macrocarpon Ait) Proanthocyanidins to Catechin and Procyanidins A2 and B2 for Use as Standards in the 4-(Dimethylamino)cinnamaldehyde Assay. J Agric Food Chem. 2012;60:4578–4585. doi: 10.1021/jf3007213. [DOI] [PubMed] [Google Scholar]
- 27.Reed JD, Krueger CG, Vestling MM. MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry. 2005;66:2248–2263. doi: 10.1016/j.phytochem.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Sitren HS, Heller PA, Bailey LB, Cerda JJ. Total parenteral nutrition in the mouse: development of a technique. JPEN J Parenter Enteral Nutr. 1983;7:582–586. doi: 10.1177/0148607183007006582. [DOI] [PubMed] [Google Scholar]
- 29.SoLA N, CoA N, Bo A, NRC . The National Academic Press; 1995. [Google Scholar]
- 30.Kang S, Elf S, Lythgoe K, Hitosugi T, et al. p90 ribosomal S6 kinase 2 promotes invasion and metastasis of human head and neck squamous cell carcinoma cells. J Clin Invest. 2010;120:1165–1177. doi: 10.1172/JCI40582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sano Y, Hermsen J, Kang W, Gomez F, et al. Parenteral nutrition maintains pulmonary IgA antibody transport capacity, but not active transport, following injury. Am J Surg. 2009;198:105–109. doi: 10.1016/j.amjsurg.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagerman AE, Butler LG. The specificity of proanthocyanidin-protein interactions. J Biol Chem. 1981;256:4494–4497. [PubMed] [Google Scholar]
- 33.Kudsk K, Stone J, Sheldon G. Nutrition in trauma and burns. Surg Clin North Am. 1982;62:183–192. doi: 10.1016/s0039-6109(16)42644-7. [DOI] [PubMed] [Google Scholar]
- 34.Fukatsu K, Kudsk KA. Nutrition and gut immunity. Surg Clin North Am. 2011;91:755–770. vii. doi: 10.1016/j.suc.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kajiura T, Takeda T, Sakata S, Sakamoto M, et al. Change of intestinal microbiota with elemental diet and its impact on therapeutic effects in a murine model of chronic colitis. Dig Dis Sci. 2009;54:1892–1900. doi: 10.1007/s10620-008-0574-6. [DOI] [PubMed] [Google Scholar]
- 36.Alverdy J. The effect of nutrition on gastrointestinal barrier function. Semin Respir Infect. 1994;9:248–255. [PubMed] [Google Scholar]
- 37.Alverdy JC, Laughlin RS, Wu L. Influence of the critically ill state on host-pathogen interactions within the intestine: gut-derived sepsis redefined. Crit Care Med. 2003;31:598–607. doi: 10.1097/01.CCM.0000045576.55937.67. [DOI] [PubMed] [Google Scholar]
- 38.Cos P, De Bruyne T, Hermans N, Apers S, et al. Proanthocyanidins in health care: current and new trends. Curr Med Chem. 2004;11:1345–1359. doi: 10.2174/0929867043365288. [DOI] [PubMed] [Google Scholar]
- 39.Gonthier MP, Donovan JL, Texier O, Felgines C, et al. Metabolism of dietary procyanidins in rats. Free Radic Biol Med. 2003;35:837–844. doi: 10.1016/s0891-5849(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 40.Reed JD. Nutritional toxicology of tannins and related polyphenols in forage legumes. J Anim Sci. 1995;73:1516–1528. doi: 10.2527/1995.7351516x. [DOI] [PubMed] [Google Scholar]
- 41.Ottaviani JI, Kwik-Uribe C, Keen CL, Schroeter H. Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans. Am J Clin Nutr. 2012;95:851–858. doi: 10.3945/ajcn.111.028340. [DOI] [PubMed] [Google Scholar]
- 42.Wang YH, Yang XL, Wang L, Cui MX, et al. Effects of proanthocyanidins from grape seed on treatment of recurrent ulcerative colitis in rats. Can J Physiol Pharmacol. 2010;88:888–898. doi: 10.1139/y10-071. [DOI] [PubMed] [Google Scholar]
- 43.Yoshioka Y, Akiyama H, Nakano M, Shoji T, et al. Orally administered apple procyanidins protect against experimental inflammatory bowel disease in mice. Int Immunopharmacol. 2008;8:1802–1807. doi: 10.1016/j.intimp.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 44.Chatterjee A, Chatterjee S, Das S, Saha A, et al. Ellagic acid facilitates indomethacin-induced gastric ulcer healing via COX-2 up-regulation. Acta Biochim Biophys Sin (Shanghai) 2012;44:565–576. doi: 10.1093/abbs/gms034. [DOI] [PubMed] [Google Scholar]
- 45.Holderness J, Hedges JF, Daughenbaugh K, Kimmel E, et al. Response of gammadelta T Cells to plant-derived tannins. Crit Rev Immunol. 2008;28:377–402. doi: 10.1615/critrevimmunol.v28.i5.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao T, Van De Water J, Keen CL, Schmitz HH, Gershwin ME. Cocoa procyanidins and human cytokine transcription and secretion. J Nutr. 2000;130:2093S–2099S. doi: 10.1093/jn/130.8.2093S. [DOI] [PubMed] [Google Scholar]
- 47.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 48.Chen CM, Li SC, Lin YL, Hsu CY, et al. Consumption of purple sweet potato leaves modulates human immune response: T-lymphocyte functions, lytic activity of natural killer cell and antibody production. World J Gastroenterol. 2005;11:5777–5781. doi: 10.3748/wjg.v11.i37.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okazaki Y, Han Y, Kayahara M, Watanabe T, et al. Consumption of curcumin elevates fecal immunoglobulin A, an index of intestinal immune function, in rats fed a high-fat diet. J Nutr Sci Vitaminol (Tokyo) 2010;56:68–71. doi: 10.3177/jnsv.56.68. [DOI] [PubMed] [Google Scholar]