Abstract
Purpose
Radiographic followup after pyeloplasty for the correction of ureteropelvic junction obstruction is not well defined in children. We characterize trends in frequency and modality of postoperative imaging after open and minimally invasive pediatric pyeloplasty.
Materials and Methods
Using the MarketScan® database, we identified patients 0 to 18 years old undergoing pyeloplasty between 2007 and 2013. Followup imaging was classified as functional (diuretic renography, excretory urography) or nonfunctional (ultrasound, computerized tomography, magnetic resonance imaging). We excluded patients with less than 24 months of postoperative enrollment in MarketScan. Multivariate logistic regression was performed to determine associations between demographic variables and imaging use patterns.
Results
We identified 926 patients with a mean ± SD followup of 3.6 ± 1.3 years, of whom 30% underwent minimally invasive pyeloplasty. Overall 5.9% of patients had no postoperative imaging available. Within the first 6 months postoperatively 853 patients (91%) underwent at least 1 imaging study and 192 (24%) underwent renography. Within the first 12 months postoperatively 91% of patients underwent at least 1 imaging study, most commonly ultrasound. After 12 months almost a third of the patients were not followed with imaging. Of the 71% undergoing imaging most underwent ultrasound. Younger age and female gender were independently associated with frequent imaging (at least yearly) on multivariate logistic regression.
Conclusions
Following pediatric pyeloplasty there is variation in modality and frequency of imaging followup. The majority of patients are followed with renal ultrasound, with less frequent use of functional imaging. Almost a third of patients do not undergo followup imaging after 1 year.
Keywords: diagnostic imaging, hydronephrosis, kidney, pediatrics, ureteral obstruction
Success after pyeloplasty for the repair of ureteropelvic junction obstruction in children has been routinely defined by a combination of clinical and radiographic criteria. Despite high reported success rates,1 there are no established recommendations to guide imaging surveillance modality, timing or duration after pediatric pyeloplasty.
A recent study among privately insured adults revealed that 1 of 8 did not undergo postoperative imaging after pyeloplasty.2 Overall duration of imaging was shorter than expected, with approximately half of patients undergoing imaging after 1 year. We hypothesize that in children there is wide variation in imaging protocols. We characterize trends in frequency and modality of followup imaging after open and minimally invasive pediatric pyeloplasty.
METHODS
Data Source
The MarketScan database contains longitudinal records from United States employer based commercial health plans.3 The data set contains more than 196 million unique patients from 1995 onward. In addition to outpatient visits, the database includes records of approximately 50% of all hospital discharges in the United States. Socioeconomic data, including income and ethnicity, are not available. Patients are deidentified from the database. Institutional review board approval was not obtained for this study.
Sample Population
Patients undergoing pyeloplasty from 2007 to 2013 were identified using CPT (Current Procedural Terminology) codes 50400 (pyeloplasty), 50405 (complicated pyeloplasty-congenital kidney abnormality, secondary pyeloplasty, solitary kidney, calycoplasty) and 50544 (laparoscopic pyeloplasty). Patients were excluded if they were 19 years or older at surgery or if there was less than 24 months of enrollment data after the index surgery so that insurance coverage status was maintained in the postoperative period. This criterion was applied to ensure that if there was lack of imaging, it was not due to change in insurance status.
Patient and Hospital Characteristics
Characteristics evaluated included age at surgery, gender, number of comorbidities, use of a minimally invasive approach, year of surgery, hospital region, insurance status (HMO or nonHMO) and length of stay. Age in years was divided into the categories 0 to 2, 3 to 6, 7 to 13 and 14 to 18. Use of a minimally invasive approach was identified by CPT code 50544. Length of stay was divided into the categories 1 to 2, 3 to 5 and 6 or more days. Secondary interventions, including stent/drain procedures, endoscopic correction and repeat pyeloplasty, were identified using CPT and ICD-9-CM codes (see supplementary Appendix, http://jurology.com/).
Radiographic Followup
CPT and ICD-9-CM codes were used to identify postoperative imaging studies, which included abdominal and renal ultrasound, abdominal CT, abdominal MRI, renography and IVP (supplementary Appendix http://jurology.com/). Imaging type was categorized as functional (renogram or IVP) or nonfunctional (ultrasound, CT or MRI). CT and MRI can provide information on renal function and excretion. However, the billing codes do not allow determination of whether delayed contrast imaging was used. Therefore, in this study these tests were categorized as nonfunctional imaging.
Statistical Analysis
Associations between demographic variables and at least annual radiographic followup were determined using unadjusted and adjusted multivariate logistic regression. Stepwise regression was used to determine inclusion variables for the final adjusted model. Exploratory analysis using the chi-square test was performed to determine univariate associations between demographic variables and lack of imaging after pyeloplasty. Statistical analysis was performed using Stata®, version 12.1, with 2-sided p value less than 0.05 considered statistically significant.
RESULTS
A total of 926 children were identified with a mean ± SD followup of 3.6 ± 1.3 years. Patient demographics are listed in table 1. A minimally invasive approach was used in 10.2% of patients 0 to 2 years, 14.6% of those 3 to 6, 31.8% of those 7 to 13 and 43.4% of those 14 to 18 years old (30% of patients overall). Complicated pyeloplasty (CPT 50405) was performed in 43% of patients. A secondary procedure was required in 13.7% of patients.
Table 1.
Patient demographics
| No. Pts (%) | |
|---|---|
| Age group (yrs): | |
| 0–2 | 403 (43.5) |
| 3–6 | 157 (17.0) |
| 7–13 | 194 (20.9) |
| 14–18 | 172 (18.6) |
| Gender: | |
| Male | 625 (67.5) |
| Female | 301 (32.5) |
| Operative approach: | |
| Open | 652 (70.4) |
| Minimally invasive | 274 (29.6) |
| Surgery yr: | |
| 2007 | 152 (16.4) |
| 2008 | 192 (20.7) |
| 2009 | 200 (21.6) |
| 2010 | 213 (23.0) |
| 2011 | 156 (16.8) |
| 2012 | 13 (1.4) |
| Region: | |
| Northeast | 169 (18.3) |
| North central | 260 (28.1) |
| South | 285 (30.8) |
| West | 183 (19.8) |
| Unknown | 29 (3.1) |
| HMO: | |
| Yes | 781 (84.3) |
| No | 125 (13.5) |
| Unknown | 20 (2.2) |
| Length of stay (days): | |
| 1–2 | 703 (75.9) |
| 3–5 | 200 (21.6) |
| 6+ | 23 (2.5) |
| Need for secondary procedure(s): | |
| No | 799 (86.2) |
| Yes | 127 (13.7) |
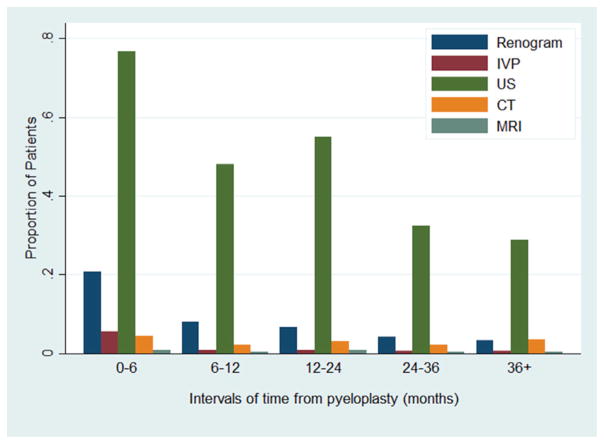
Figure 1 shows the proportion of patients undergoing imaging studies by postoperative time interval. Overall 5.9% of patients underwent no postoperative imaging. In the first 12 months 91% underwent at least 1 imaging study, of which ultrasound was most commonly performed. The most common interval during which renography was performed was 0 to 6 months (192 of 816 patients, 14%). After 12 months 29% of all children did not undergo further imaging.
Figure 1.
Imaging use through time after pyeloplasty by imaging type. Patients may have undergone multiple types of imaging in same period.
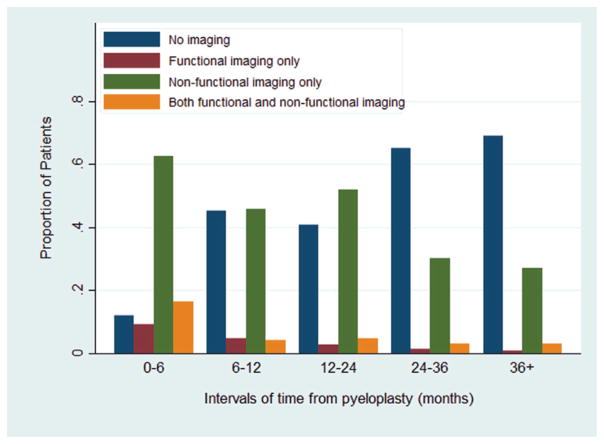
Figure 2 demonstrates the proportion of patients undergoing functional and/or nonfunctional imaging by postoperative time interval. Use of functional imaging, including IVP and renography, was most common during the 0 to 6-month interval (26%). However, nonfunctional imaging was more commonly used overall. The proportion of patients with no imaging during each interval increased from 12% in the 0 to 6-month period to 69% after 3 years.
Figure 2.
Imaging use through time after pyeloplasty by functional (renogram, IVP) or nonfunctional (ultrasound, CT, MRI) imaging type.
On univariate analysis year of surgery, geographic region, HMO insurance status, length of stay and need for secondary procedures were not statistically significantly associated with frequent performance of imaging (at least yearly) after pyeloplasty. On multivariate analysis patients 7 to 13 years old (HR 0.52, 95% CI 0.35–0.77) or 14 to 18 years old (HR 0.30, 95% CI 0.20–0.47) were less likely to undergo at least annual imaging compared to those 0 to 2 years old (table 2). Use of a minimally invasive approach was associated with not undergoing imaging at least yearly on univariate analysis (HR 0.70, 95% CI 0.52–0.94). However, this finding did not remain significant on multivariate analysis (HR 1.10, 95% CI 0.77–1.57). Female gender was independently associated with a greater likelihood of undergoing imaging at least yearly (HR 1.52, 95% CI 1.11–2.08).
Table 2.
Association between demographic factors and at least yearly radiographic followup
| No. Annual Screening (%)
|
Unadjusted OR (95% CI)* | Multivariate OR (95% CI)† | ||
|---|---|---|---|---|
| Yes | No | |||
| Age (yrs): | ||||
| 0–2 | 304 (49) | 99 (33) | Reference | Reference |
| 3–6 | 111 (18) | 46 (15) | 0.79 (0.52–1.19) | 0.78 (0.51–1.18) |
| 7–13 | 121 (19) | 73 (24) | 0.54 (0.37–0.78) | 0.52 (0.35–0.77) |
| 14–18 | 88 (14) | 84 (28) | 0.34 (0.23–0.50) | 0.30 (0.20–0.47) |
| Gender: | ||||
| Male | 407 (65) | 218 (72) | Reference | Reference |
| Female | 217 (35) | 84 (28) | 1.38 (1.02–1.87) | 1.52 (1.11–2.08) |
| Operative approach: | ||||
| Open | 455 (73) | 197 (65) | Reference | Reference |
| Minimally invasive | 169 (27) | 105 (35) | 0.70 (0.52–0.94) | 1.1 (0.77–1.57) |
Values in boldface represent significant ORs (p <0.05).
Year of surgery, geographic region, HMO insurance status, length of stay and need for secondary procedures did not show statistically significant associations with annual screening status.
Adjusted for age, gender and operative approach.
On exploratory analysis age 14 to 18 years was the only factor associated with lack of imaging after pyeloplasty (p <0.001). In this age group 14.5% of patients did not undergo imaging, compared to 4.2% of those 0 to 2 years, 1.9% of those 3 to 6 years and 5.2% of those 7 to 13 years old. On univariate analysis gender, minimally invasive approach, year, region, HMO status and length of stay were not associated with lack of imaging.
DISCUSSION
We identified variations in the intensity and type of imaging followup after pediatric pyeloplasty. Of the patients 5.9% did not undergo following imaging after the index surgery, and almost a third were not followed radiographically beyond 1 year according to data captured in MarketScan. Ultrasound is the most common imaging modality used after pyeloplasty, while a small but sizable proportion of patients undergo functional studies, most commonly renography. Younger children and girls are more likely to undergo routine followup.
Older children are less likely to undergo imaging after pyeloplasty, although it is not clear why. It is possible that younger children have more scheduled interaction with health care providers, or that imaging is performed less routinely after use of a minimally invasive approach, which is more common in older children. Furthermore, imaging may be performed more frequently in younger children because they are less able to articulate the presence of symptoms. It is also possible that younger children, particularly those less than 2 years, were operated on as a result of prenatal screening findings and did not initially present with symptoms. These children would be less likely to experience flank pain or other pain symptoms in the event of treatment failure, so clinicians may have used imaging as the window into success. Older children may have been first diagnosed with obstruction based on symptoms such as pain. Thus, absence of pain postoperatively may have led clinicians to minimize postoperative imaging.
Contemporary series on pediatric pyeloplasty have revealed high success rates, although differing protocols exist regarding imaging surveillance (table 3).4–20 This variation in post-pyeloplasty imaging impacts health care expenditures and affects the amount of radiation exposure in children. To our knowledge, there has been no formal study evaluating the cost-effectiveness of routine followup imaging after pyeloplasty. Such a study would need to account for the sequelae of symptomatic failures and the costs associated with silent obstruction, including chronic kidney disease.
Table 3.
Selected contemporary series on pediatric pyeloplasty including at least 50 cases
| References | Approach (No.) | Age (yrs) | Followup (mos) | Definition of Success | % Success | Imaging Followup Protocol |
|---|---|---|---|---|---|---|
| Chacko et al4 | Laparoscopic transperitoneal (52) | Mean 4.3 | Mean 20 | Absence of symptoms + improved hydronephrosis (US) or improved drainage (RS) | 98 | – |
| Maheshwari et al5 | Laparoscopic transperitoneal (82) | Mean 7.1 | Mean 41.6 | Absence of obstruction on RS | 92 | – |
| Szavay et al6 | Laparoscopic transperitoneal (70) | Median 1.7 | Median 24 | – | 98 | US every 3 mos; MAG3 at 3 mos, 12 mos |
| Sweeney et al7 | Laparoscopic transperitoneal (112) | Mean 9.4 | Mean 15.3 | Symptoms and/or radiographic evidence of obstruction | 97 | – |
| Olsen et al8 | Robotic retroperitoneal (67) | Mean 7.9 | Median 12.1 | Absence of symptoms and/or decreased intrarenal pelvis anteroposterior diameter (US), or unchanged or improved differential function (RS) | 94 | US + RS at 3 mos, 12 mos |
| Minnillo et al9 | Robotic transperitoneal (155) | Mean 10.5 | Mean 31.7 | Improved symptoms, improved hydronephrosis (US) and/or improved drainage parameters (RS) | 96 | US at 1 mo, 3 mos, then yearly; MAG3 if recurrent symptoms or not improved on US |
| Zhou et al10 | Laparoscopic retroperitoneal (62) | Mean 3 | Mean 24 | – | 98 | IVP + US at 3 mos, 6 mos, then yearly |
| Blanc et al11 | Laparoscopic retroperitoneal (104) | Mean 6.2 | Median 25.2 | Decreased or resolved hydronephrosis (US) + improved drainage with residual less than 50% at 20 mins after furosemide administration with stable differential function (RS) | 96 | US 1 mo after stent removal (6 wks), every 3 mos for 1 yr, then yearly for 5 yrs; MAG3 or magnetic resonance urography if no significant improvement on US, recurrent symptoms or significant preop asymmetrical function |
| Piaggio et al12 | Laparoscopic transperitoneal (37) vs open (41) | Mean 5.1 vs 3.7 | Mean 6.3 vs 24 | Symptom resolution, marked reduction of hydronephrosis (US) or improved drainage curve (RS) | 97 Vs 83 | – |
| Valla et al13 | Laparoscopic retroperitoneal (45) vs open (45) | Mean 5.8 vs 1.8 | Mean 25 vs 38 | Symptom improvement + improved hydronephrosis (US) or renal drainage/function (RS) | 97 Vs 96 | US at 3–6 mos, then yearly; RS at 1 yr |
| Braga et al14 | Laparoscopic transperitoneal (41) vs open (67) | Mean 7.9 vs 8.1 (flank), 7.3 (dorsal) | Mean 28 vs 49 (flank), 47 (dorsal) | Symptom resolution, improved hydronephrosis (US) and/or decreased t1/2 at last clinical appointment (RS) | 95 Vs 96 | Routine US; RS reserved for prolonged, persistent or worsening hydronephrosis and/or recurrent symptoms |
| van der Toorn et al15 | Laparoscopic transperitoneal (57) vs open (57) | Mean 8.1 vs 7.8 | Mean 12 vs 72 min | Symptom resolution, no conversion/reintervention, decreased hydronephrosis (US) and/or improved drainage (RS) | 98 Vs 95 | US + RS in first 50 pts, then renogram omitted if US showed decreased hydronephrosis |
| Garcia-Aparicio et al16 | Laparoscopic transperitoneal (26) vs open (32) | Mean 0.4 | – | Improved hydronephrosis (US) | 100 | US at 1 mo, 6 mos, 12 mos; MAG3 at 6 mos |
| Lee et al17 | Robotic transperitoneal (33) vs open (33) | Mean 7.8 vs 7.6 | Mean 10 vs 21 | Symptom resolution, significant + persistent improvement of hydronephrosis (US) and/or t1/2 less than 10 mins (RS) | 94 Vs 100 | – |
| Sorensen et al18 | Robotic transperitoneal (33) vs open (33) | Mean 9.2 vs 8.2 | Mean 17 vs 19 | Symptom resolution, improved hydronephrosis with renal growth (US) and/or improved drainage (RS) | 97 | – |
| Barbosa et al19 | Robotic transperitoneal (58) vs open (154) | Median 7.2 vs 1.2 | Median 33 vs 31 | Hydronephrosis improved (US) by at least 2 grades (0–5 scale) | 74 Vs 70 | US at 0–6 mos, 6–12 mos, after 12 mos; MAG3 if no improvement on US |
| Riachy et al20 | Robotic transperitoneal (46) vs laparoscopic transperitoneal (18) | Mean 8.8 vs 8.1 | Median 22 vs 43 | Absence of symptoms, hydronephrosis improved by at least 1 grade (US), stable US with symptom resolution or t1/2 less than 10 mins (RS) | 100 Vs 88 | – |
While routine diuretic renograms in the early postoperative period have been shown to predict failure after pyeloplasty in children,21 there is greater awareness regarding minimizing radiation risks related to imaging, particularly in pediatric patients.22 The use of renograms in adults is associated with an effective dose of 1.8 to 3.3 mSv.23 This dose is roughly equivalent to a chest CT in a 5-year-old child.22 In the present study 32% of pediatric patients underwent renography. The concern over radiation dose may explain why this finding contrasts with what has been observed in the adult population after pyeloplasty, where there is greater reliance on renograms, especially in the first year. In a similar study of adults undergoing pyeloplasty renograms comprised 53% and 44% of all imaging tests performed in the 0 to 6-month and 6 to 12-month intervals, respectively.2 Of this population 65% underwent minimally invasive pyeloplasty and 54% underwent imaging after the first year.
The use of routine, repeated renograms in the postoperative period has been challenged.24–27 Pohl et al reported that among patients with a nonobstructive diuretic renogram (t1/2 less than 20 minutes) at 3 months there were no late failures.24 In renal units with improvement in drainage but t1/2 greater than 20 minutes drainage patterns at 12 months continued to improve. When there was no improvement in drainage at 3 months, reoperation may have been required. Psooy et al demonstrated in 77 renal units followed more than 5 years that after a nonobstructive renogram at 1 year subsequent recurrent obstruction is unlikely.25 This latter observation may explain why only 71% of patients underwent any imaging beyond the first year. In this data set performance of renography within the first 6 months postoperatively had no impact on the likelihood of imaging being performed after 1 year.
More recently several groups have questioned whether even early postoperative renography is necessary after pediatric pyeloplasty.28–30 Use of a sentinel ultrasound instead has been advocated to determine if renography is necessary. Almodhen et al reported on 97 patients who underwent 101 pyeloplasties with a mean followup of 4.5 years.28 Of the 91 kidneys with improvement on postoperative ultrasound 2 (2%) exhibited an obstructive pattern on renography, although both spontaneously improved during followup. Hydronephrosis was downgraded in 46 kidneys, and none of these kidneys exhibited an obstructive postoperative scan. Of the 10 kidneys with worsened or no improvement on postoperative ultrasound 4 (40%) had an obstructive renogram, of which 2 were treated with a subsequent procedure.
Cost et al observed similar findings in 49 patients undergoing open pyeloplasty who underwent ultrasound and renography at 3 months.29 Of the 42 children with stable or improved hydronephrosis 41 had stable function and 1 had low function (32% split function) preoperatively but remained stable (21% split function) at longer followup. Of the 7 remaining patients with increased hydronephrosis 2 had worse renal function. Park et al confirmed in 215 patients with at least 5 years of followup that if there was improvement in hydronephrosis, there was no subsequent worsening of hydronephrosis thereafter.30 The initial improvement was observed in 90% of children in the first 12 months. It is the practice of our pediatric institution to obtain ultrasounds within the first 3 months postoperatively and yearly thereafter for 3 to 5 years, and to reserve renograms for patients with worsening hydronephrosis postoperatively.
The purpose of imaging after pyeloplasty is to diagnose obstruction early so that interventions may prevent further nephron loss. Recommendations regarding frequency and type of imaging performed cannot be made based on this study design. We advocate investigation of the cost-effectiveness of post-pyeloplasty imaging and standardization of an imaging protocol after pyeloplasty for ureteropelvic junction obstruction, similar to what already exists for surveillance of oncologic conditions after treatment.
Our findings must be interpreted in the context of the limitations of our study design. Although cases coded as complicated pyeloplasty were known, this data set did not contain specific operative details, such as laterality, case complexity and symptoms. CPT codes do not provide information on the different pyeloplasty approaches beyond simple, complicated or laparoscopic techniques. For instance an uncomplicated robotic pyeloplasty may be coded as either 50400 or 50544. In addition, we expect that around the time of pyeloplasty failure more unscheduled and unanticipated imaging studies are performed, which may confound the estimates of a routine, protocol driven followup. Use of an employer based insurance database may limit the generalizability of our findings, especially in patients without insurance coverage.
CONCLUSIONS
Among a nationally representative pediatric population there is variation in the intensity and type of imaging followup after pyeloplasty. Ultrasound is the most commonly used surveillance modality overall. A nontrivial subset of patients undergoes no postoperative imaging or is lost to followup. Beyond 12 months after pyeloplasty only two-thirds of patients undergo any subsequent imaging. Younger children and girls are more likely to undergo routine imaging, suggesting that there may be different assumptions about the presentation of failure in these groups.
Abbreviations and Acronyms
- CT
computerized tomography
- HMO
health maintenance organization
- IVP
excretory urography
- MAG3
99mtechnetium mercaptoacetyltriglycine
- MRI
magnetic resonance imaging
- RS
renal scan
- US
ultrasound
References
- 1.Autorino R, Eden C, El-Ghoneimi A, et al. Robot-assisted and laparoscopic repair of ureteropelvic junction obstruction: a systematic review and meta-analysis. Eur Urol. 2014;65:430. doi: 10.1016/j.eururo.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 2.Hsi RS, Holt SK, Gore JL, et al. Trends in followup imaging after adult pyeloplasty. J Urol. 2014;191:1357. doi: 10.1016/j.juro.2013.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danielson E. [Accessed November 11, 2014];Health Research Data for the Real World: The MarketScan® Databases. 2014 Jan; Available at http://truvenhealth.com/Portals/0/Users/031/31/31/PH_134340314_MarketScan_WP_web.pdf.
- 4.Chacko JK, Piaggio LA, Neheman A, et al. Pediatric laparoscopic pyeloplasty: lessons learned from the first 52 cases. J Endourol. 2009;23:1307. doi: 10.1089/end.2009.0057. [DOI] [PubMed] [Google Scholar]
- 5.Maheshwari R, Ansari MS, Mandhani A, et al. Laparoscopic pyeloplasty in pediatric patients: the SGPGI experience. Indian J Urol. 2010;26:36. doi: 10.4103/0970-1591.60441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szavay PO, Luithle T, Seitz G, et al. Functional outcome after laparoscopic dismembered pyeloplasty in children. J Pediatr Urol. 2010;6:359. doi: 10.1016/j.jpurol.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Sweeney DD, Ost MC, Schneck FX, et al. Laparoscopic pyeloplasty for ureteropelvic junction obstruction in children. J Laparoendosc Adv Surg Tech A. 2011;21:261. doi: 10.1089/lap.2010.0155. [DOI] [PubMed] [Google Scholar]
- 8.Olsen LH, Rawashdeh YF, Jorgensen TM. Pediatric robot assisted retroperitoneoscopic pyeloplasty: a 5-year experience. J Urol. 2007;178:2137. doi: 10.1016/j.juro.2007.07.057. [DOI] [PubMed] [Google Scholar]
- 9.Minnillo BJ, Cruz JA, Sayao RH, et al. Long-term experience and outcomes of robotic assisted laparoscopic pyeloplasty in children and young adults. J Urol. 2011;185:1455. doi: 10.1016/j.juro.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, Li H, Zhang X, et al. Retroperitoneoscopic Anderson-Hynes dismembered pyeloplasty in infants and children: a 60-case report. Pediatr Surg Int. 2009;25:519. doi: 10.1007/s00383-009-2369-z. [DOI] [PubMed] [Google Scholar]
- 11.Blanc T, Muller C, Abdoul H, et al. Retroperitoneal laparoscopic pyeloplasty in children: long-term outcome and critical analysis of 10-year experience in a teaching center. Eur Urol. 2013;63:565. doi: 10.1016/j.eururo.2012.07.051. [DOI] [PubMed] [Google Scholar]
- 12.Piaggio LA, Franc-Guimond J, Noh PH, et al. Transperitoneal laparoscopic pyeloplasty for primary repair of ureteropelvic junction obstruction in infants and children: comparison with open surgery. J Urol. 2007;178:1579. doi: 10.1016/j.juro.2007.03.159. [DOI] [PubMed] [Google Scholar]
- 13.Valla JS, Breaud J, Griffin SJ, et al. Retroperitoneoscopic vs open dismembered pyeloplasty for ureteropelvic junction obstruction in children. J Pediatr Urol. 2009;5:368. doi: 10.1016/j.jpurol.2009.02.202. [DOI] [PubMed] [Google Scholar]
- 14.Braga LH, Lorenzo AJ, Bägli DJ, et al. Comparison of flank, dorsal lumbotomy and laparoscopic approaches for dismembered pyeloplasty in children older than 3 years with ureteropelvic junction obstruction. J Urol. 2010;183:306. doi: 10.1016/j.juro.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 15.van der Toorn F, van den Hoek J, Wolffenbuttel KP, et al. Laparoscopic transperitoneal pyeloplasty in children from age of 3 years: our clinical outcomes compared with open surgery. J Pediatr Urol. 2013;9:161. doi: 10.1016/j.jpurol.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Aparicio L, Blazquez-Gomez E, Martin O, et al. Anderson-Hynes pyeloplasty in patients less than 12 months old. Is the laparoscopic approach safe and feasible? J Endourol. 2014;28:906. doi: 10.1089/end.2013.0704. [DOI] [PubMed] [Google Scholar]
- 17.Lee RS, Retik AB, Borer JG, et al. Pediatric robot assisted laparoscopic dismembered pyeloplasty: comparison with a cohort of open surgery. J Urol. 2006;175:683. doi: 10.1016/S0022-5347(05)00183-7. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen MD, Delostrinos C, Johnson MH, et al. Comparison of the learning curve and outcomes of robotic assisted pediatric pyeloplasty. J Urol, suppl. 2011;185:2517. doi: 10.1016/j.juro.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Barbosa JA, Kowal A, Onal B, et al. Comparative evaluation of the resolution of hydronephrosis in children who underwent open and robotic-assisted laparoscopic pyeloplasty. J Pediatr Urol. 2013;9:199. doi: 10.1016/j.jpurol.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Riachy E, Cost NG, Defoor WR, et al. Pediatric standard and robot-assisted laparoscopic pyeloplasty: a comparative single institution study. J Urol. 2013;189:283. doi: 10.1016/j.juro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Neste MG, du Cret RP, Finlay DE, et al. Postoperative diuresis renography and ultrasound in patients undergoing pyeloplasty. Predictors of surgical outcome. Clin Nucl Med. 1993;18:872. doi: 10.1097/00003072-199310000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Stratton KL, Pope JC, IV, Adams MC, et al. Implications of ionizing radiation in the pediatric urology patient. J Urol. 2010;183:2137. doi: 10.1016/j.juro.2010.02.2384. [DOI] [PubMed] [Google Scholar]
- 23.Mettler FA, Jr, Huda W, Yoshizumi TT, et al. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 24.Pohl HG, Rushton HG, Park JS, et al. Early diuresis renogram findings predict success following pyeloplasty. J Urol. 2001;165:2311. doi: 10.1016/S0022-5347(05)66192-7. [DOI] [PubMed] [Google Scholar]
- 25.Psooy K, Pike JG, Leonard MP. Long-term followup of pediatric dismembered pyeloplasty: how long is long enough? J Urol. 2003;169:1809. doi: 10.1097/01.ju.0000055040.19568.ea. [DOI] [PubMed] [Google Scholar]
- 26.van den Hoek J, de Jong A, Scheepe J, et al. Prolonged follow-up after paediatric pyeloplasty: are repeat scans necessary? BJU Int. 2007;100:1150. doi: 10.1111/j.1464-410X.2007.07033.x. [DOI] [PubMed] [Google Scholar]
- 27.Reis LO, Ikari O, Zani EL, et al. Long-term results of Anderson-Hynes pyeloplasty in children: how long follow-up is necessary? Eur J Pediatr Surg. 2014 doi: 10.1055/s-0034-1390018. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Almodhen F, Jednak R, Capolicchio JP, et al. Is routine renography required after pyeloplasty? J Urol. 2010;184:1128. doi: 10.1016/j.juro.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Cost NG, Prieto JC, Wilcox DT. Screening ultrasound in follow-up after pediatric pyeloplasty. Urology. 2010;76:175. doi: 10.1016/j.urology.2009.09.092. [DOI] [PubMed] [Google Scholar]
- 30.Park K, Baek M, Cho SY, et al. Time course of hydronephrotic changes following unilateral pyeloplasty. J Pediatr Urol. 2013;9:779. doi: 10.1016/j.jpurol.2012.10.005. [DOI] [PubMed] [Google Scholar]