Abstract
A diet containing high levels of saturated fat and cholesterol is detrimental to many aspects of health and is known to lead to obesity, metabolic syndrome, heart disease, diabetes, and cancer. However, the effects of a diet rich in saturated fat and cholesterol on the brain are not currently well understood. In order to determine direct effects of a high saturated fat and cholesterol diet upon fetal hippocampal tissue, we transplanted hippocampal grafts from embryonic day 18 rats to the anterior eye chamber of 16-month-old host animals that were fed either a normal rat chow diet or a 10% hydrogenated coconut oil + 2% cholesterol diet (HFHC diet) for 8 weeks. One eye per rat received topical application of an IL-1 receptor antagonist (IL-1Ra, Kineret®) and the other served as a saline control. Results revealed that the HFHC diet led to a marked reduction in hippocampal transplant growth, and detrimental effects of the diet were alleviated by the IL-1 receptor antagonist IL-1Ra. Graft morphology demonstrated that the HFHC diet reduced organotypical development of the hippocampal neuronal cell layers, which was also alleviated by IL-1Ra. Finally, grafts were evaluated with markers for glucose transporter expression, astrocytes, and activated microglia. Our results demonstrate significant effects of the HFHC diet on hippocampal morphology, including elevated microglial activation and reduced neuronal development. IL-1Ra largely blocked the detrimental effects of this diet, suggesting a potential use for this agent in neurological disorders involving neuroinflammation.
Keywords: Cholesterol, Saturated fat, Transplantation, Hippocampus, Inflammation
INTRODUCTION
Intracranial transplantation of fetal neurons or engineered cell lines has been proposed as a potential treatment for neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) [see, e.g., Torrente et al. (64)]. However, a major limitation in this method is graft survival in a compromised environment, such as the aged brain (14,41,46,71). The most prominent risk factor for both AD and PD is aging, and it is well known that the aged brain poses special challenges for successful grafting of cells (10,14,24,25,46). The specific reasons why the aged brain is less permissive for successful survival of grafted cells have not yet been revealed, but several factors, such as increased inflammatory response, reduced growth factor production, and reduced cellular support systems, have been reported (3, 14,24–26,46,60,69–72).
Specific alterations in grafts to aged hosts compared to young hosts could include delayed and altered vascular development, increased and prolonged activation of microglial cells following the grafting procedure, as well as reduced growth and development of neuronal components of the grafts. Studies from our laboratory (69) and others (7,11,55,75) have developed strategies to improve graft survival. For example, our laboratory has previously found that supplementation with brain-derived neurotrophic factor (BDNF) or with blueberries to aged hosts following intraocular transplantation improves graft survival and increases development of new neurons (69,71). These data demonstrate that dietary factors may elicit major effects on development of brain tissue. However, detrimental effects of high-fat diets have not been explored in terms of brain development.
The “Western Diet” is well known in our society for causing cardiovascular disease, obesity, type 2 diabetes, and stroke (12,19,31). It is a diet that is high in saturated fat, cholesterol, trans fat (hydrogenated oils), and low in fiber, fruits and vegetables (15). Previous studies have shown that a high-fat/high-cholesterol diet (HFHC) results in significant circulatory elevations in cholesterol and an altered low to high density lipoprotein ratio (LDL/HDL ratio) (30,51,52). Further, investigators have found that circulating cytokines, in particular interleukin-(IL-1) and IL-6 are also elevated, both in humans and in rodents after a prolonged intake of cholesterol and saturated fat-enriched diets (38,48,61), suggesting that inflammatory pathways may be involved in detrimental effects observed following exposure to this diet. Although previous work has functions such as learning and memory (36,49,73,74), it is not known how these effects are exerted. Cholesterol does not cross the blood–brain barrier (BBB), and most of the brain cholesterol is produced within the brain itself (66,67). However, it is well known that proinflammatory cytokines can cross the BBB and exert effects on both microglial cells and neurons within the brain parenchyma (17, 42,62). Therefore, we hypothesize that damage from the HFHC diet is due, at least in part, to an inflammatory response in the periphery that leads to an inflammatory response in the central nervous system.
In order to further analyze specific effects of a HFHC diet on development of the hippocampus, we isolated fetal hippocampus from the rest of its neural networks and transplanted it to the anterior eye chamber of middle- aged recipient rats. Grafts transplanted to the anterior eye chamber become vascularized in less than 2 weeks (26,34,65) and, therefore, the grafts are perfused with growth factors, cytokines, and many other molecules circulating in the host animal’s blood. For this experiment, we chose to graft developing hippocampal tissue to middle-aged recipient rats, because we have previously shown that survival of grafted tissue is compromised when grafted to aged or middle-aged hosts (72). We hypothesized that the increased systemic cytokines as a result of the aging process and the HFHC diet would give rise to further detriment in the development of hippocampal grafts.
To test our hypothesis that the damaging effects of a HFHC diet are at least in part due to inflammation, we also applied the IL-1 receptor antagonist (Amgen Kineret®, anakinra). Kineret is typically used in humans to treat rheumatoid arthritis (28) and is known to cross the BBB (13). Since Kineret is a common treatment for rheumatoid arthritis, has no known major side effects, and has defined pharmacological effects (32), we chose this drug to examine whether blocking an inflammatory cascade in grafted tissues would prevent damaging effects of the HFHC diet. IL-1 is a major player in the immune response not only due its own actions, but the fact that downstream signaling through NF-κB also increases expression of proinflammatory cytokines and chemokines (9,53,54). Endogenous IL-1 receptor antagonist (IL-1Ra) has also been defined as an anti-inflammatory agent (1). The aims of the present study were twofold: 1) to explore effects of a HFHC diet upon hippocampal development and 2) to determine whether the age-related detriment of hippocampal development in aged hosts fed a high-fat diet could be alleviated by IL-1Ra.
MATERIALS AND METHODS
Animals and Diets
Fourteen-month-old female Fischer 344 rats (Harlan, Indianapolis, IN, USA) were given 1 week to acclimate to the vivarium and then randomly divided into two dietary treatment groups (n = 6 rats per group). Rats were fed a control rat chow diet or HFHC diet for 8 weeks. The control diet consisted of standard rat chow (8656 Harlan Teklad Sterilizable Rodent Diet; Harlan). This diet delivered 13% calories from fat (soybean oil), 53% calories from carbohydrate, 34% calories from protein, and contained a vitamin/mineral mix. The treatment diet consisted of 10% hydrogenated coconut oil and 2% cholesterol by weight (“Custom Diet D2-AIN93 without choline bitartrate and with 2% cholesterol”) manufactured by MP Biomedicals (Solon, OH, USA). This diet delivered about 45% calories from fat, 35% calories from carbohydrate, 20% calories from protein, and contained a vitamin/mineral mix. This diet was previously used by our laboratory and we have shown that it elevated cholesterol and triglyceride levels (33). Total body weights and food consumption were measured weekly throughout the experiment. Animal protocols were approved by the Medical University of South Carolina Institutional Care & Use Committee and carried out according to guidelines from the National Institutes of Health (NIH).
Dissection and Transplantation
Pregnant Fischer 344 dams (Harlan) were euthanized with an overdose of isofluorane and their fetuses were removed at embryonic day 18. The fetal brain was rapidly removed and the hippocampal formation was dissected. The hippocampal formation was further cut into several pieces along the longitudinal axis, each piece containing the CA1–CA3 and dentate gyrus, as described previously (29). Fetal tissue pieces were incubated with either sterile saline or an IL-1Ra solution (see below) for 30 min prior to grafting. Graft recipient rats (14-month-old female Fischer 344 rats) were anesthetized with ketamine (Ketaset, 80 mg/kg, IP) and xylazine (Rompun, 12 mg/kg, IP) and their pupils were dilated with one drop of 1% atropine solution on the cornea to facilitate grafting, and to avoid damage to the iris vasculature during the grafting procedure. A razor blade slit through the cornea was made in the lateral angle of the eye, and 1 × 1 mm pieces of fetal hippocampal tissue were slowly injected into the anterior eye chamber using a modified glass syringe according to our previously published protocol (5,29). Animals recovered under heat lamps and also received topical treatment with an analgesic agent, proparacaine ophthalmic solution (0.05 %), onto the cornea every hour for 4 h after the transplant surgery.
IL-1Ra Treatment
The IL-1Ra, Kineret, was purchased from Amgen (anakinra, Thousand Oaks, CA, USA). The drug is manufactured for use in humans with rheumatoid arthritis and is provided in 1-ml single-use prefilled glass syringes. The syringes contain 0.67 ml (100 mg) of anakinra as well as 1.29 mg sodium citrate, 5.48 mg sodium chloride, 0.12 mg disodium EDTA, and 0.70 mg polysorbate 80 in water for injection, pH 6.5. Before transplantation, half of the hippocampal grafts were incubated for 30 min in the anakinra solution (100 mg/0.67 ml) while the other half were incubated in sterile saline (control). One piece of either IL-1Ra-treated or vehicle-treated tissue was grafted into alternate eyes of the same animal, so that each rat served as an internal control for the locally applied treatment paradigm. For the next 6 weeks, graft recipients received a topical application (once every week) on the outside of the cornea with either 1 drop of IL-1Ra solution (8 mg dosage) or sterile saline. Hence, the IL-1Ra-treated grafts were first incubated for 30 min, and then received topical application of the same treatment once weekly for 6 weeks. Topical application of cytokines or trophic factors onto the cornea of rats with intraocular grafts has proven to be an effective treatment paradigm for administration of drugs to hippocampal grafts for several decades in our research group (23,70).
Measurement of Graft Growth In Oculo
In previous work from our laboratory and others (5,29), it has been shown that the grafted tissue will first grow to the height of the anterior eye chamber, and then it grows laterally. Because the height of the anterior eye chamber is approximately 1 mm, the measurements we are reporting using the caliper (length × width) can be estimated to reveal the volume of the graft. Grafts were measured using a protocol as described previously [see Gerhardt et al. (29) for methodological details].
Serum Total Cholesterol and Triglyceride Levels
Serum was centrifuged and stored at −20°C until analysis. Serum cholesterol and triglyceride levels were analyzed using the SYNCHRON® Systems kits [see Granholm et al. (33) for details].
Morphological Evaluation of Hippocampal Grafts
After the hippocampal grafts were allowed to develop in the anterior eye chamber for 6 weeks, the host rats (n = 6 animals per dietary group) were sacrificed with an overdose of isofluorane, and the intraocular grafts were removed. As done previously in our laboratory [see, e.g., Willis et al. (69) for details], grafts were immersion fixed overnight in 4% paraformaldehyde prepared in 0.1 M phosphate-buffered saline (PBS) and then transferred to 30% sucrose in PBS. Grafts were sectioned on a cryostat (Zeiss-Microm) to a thickness of 10 µm and immediately mounted on glass slides. Sections were arranged using a systematic random design, such that every 15th section was placed on the same slide.
Hematoxylin-eosin (H&E) staining was performed on the grafted tissue in order to observe gross morphology. The first section in a series was randomly picked, where after every 15th section was stained (see above). Sections were incubated in xylene followed by decreasing concentrations of ethanol in order to dehydrate the tissue. Slides were then incubated in Mayer’s hemalum (Sigma, St. Louis, MO, USA) for 4 min, followed by tap water and then incubation in eosin (Sigma) for 1 min. Finally, slides were differentiated in 95% ethanol, washed in absolute ethanol and xylene, and coverslipped in Permount (Fisher Scientific, Pittsburgh, PA, USA).
Further analysis of the tissue was achieved using immunofluorescence and the following antibodies: glial fibrillary acidic protein (GFAP, Chemicon, Billerica, MA, USA, concentration 1:1000), OX-6 (RT1B class II monomorphic for activated microglia, Serotec, Raleigh, NC, USA, concentration 1:1000), IL-1 receptor associated kinase (IRAK, Abcam, Cambridge, MA, USA, concentration 1:100), and glucose transporter 1 (Glut-1, Chemicon, concentration 1:1000). Slides were briefly washed in PBS and then blocked in 5% normal goat serum (NGS) for 1 h. Slides were washed again in PBS and then incubated with the primary antibody overnight at 4°C in a humidifying chamber. After a series of washes (3 × 10 min) in PBS, the slides were incubated with the secondary antibody, directed against the appropriate species (conjugated to AlexaFluor 488 or AlexaFluor 594, Molecular Probes, Eugene, OR, USA) for 1 h in the humidifying chamber. Sections were washed again in PBS and then coverslipped in PVA/DABCO (polyvinyl alcohol mounting medium with DABCO®, antifading agent). Grafts were visualized using a Nikon Optiphot fluorescence microscope equipped with a Nikon camera.
Densitometry
Semiquantitation of the immunofluorescence was performed on every 15th section through the fetal hippocampal grafts. Images were first captured on a Nikon Optiphot fluorescence microscope equipped with a Nikon camera using a 20× lens, and then converted to black and white in preparation for densitometry measurements. Since the hippocampal grafts are small (maximum thickness 1 mm), they are not suitable for unbiased stereology assessment. A randomized area density measurement system has therefore been employed by our group in the past, in order to assess the presence of specific neuronal or glial markers in the grafted tissue [see, e.g., Willis et al. (70,72) for further details]. The Nikon Elements Image Software (NIS Elements) was used to measure densitometry. This software program measures gray scale values within the range of 0–256, with 0 representing white and 256 black. Measurements were performed blinded and the values from the images were averaged to obtain mean per animal. A threshold was applied to the image determined based on morphology. Once the threshold was determined for a specific section, a set rectangular region of interest (ROI, the same across groups) was placed over the section and measured multiple times throughout the section (depending on the size of the graft). The area covered by thresholding was measured (pixels) and compared between groups. This method of semiquantitation allowed us to measure the area covered by Glut-1-ir, GFAP-ir, and IRAK-ir. Semiquantitative analysis was also performed on OX-6 immunofluorescence by counting all OX-6-positive cells. Raw images of each graft were captured and converted to black and white, after which all the cells in each section were counted.
Statistical Analysis
A one-way ANOVA with Fisher’s post hoc analysis was used in order to determine whether there were significant differences in body weights, food consumption, triglyceride levels, and cholesterol levels between treatment groups. Changes in graft growth were examined using random effects models (SAS Institute Inc., Cary, NC, USA) on measurements from baseline and at the 21- and 35-day follow-up points using diet (control, HFHC) and treatment (saline, IL-1Ra) as between-subjects factors. The random effects models provide the same basic information as repeated measures ANOVAs, but are more flexible with unbalanced follow-up assessments like those used here.
RESULTS
Weight and Food Consumption
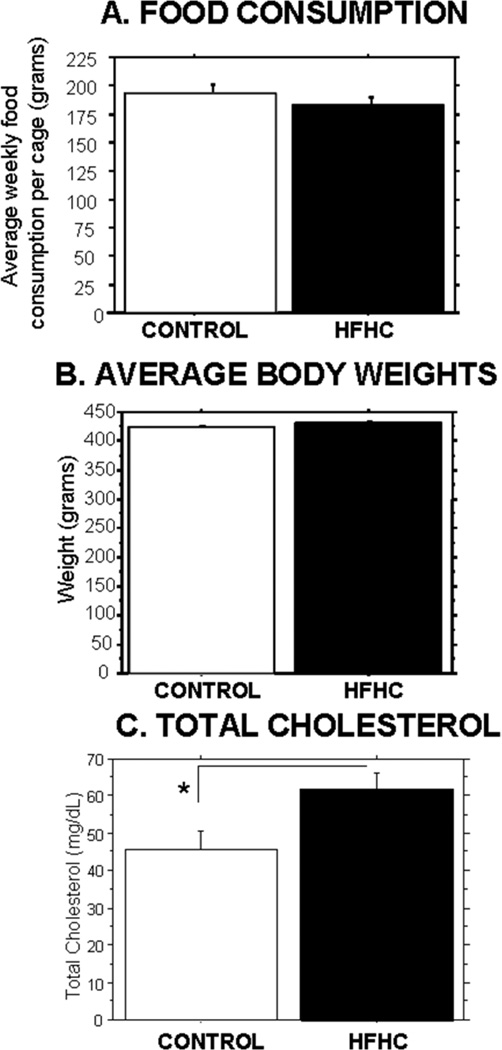
Animals were fed either a control rat chow diet (n = 6) or HFHC diet (n = 6) for a total of 8 weeks, 2 weeks prior to grafting and 6 weeks postgrafting until sacrifice. Food consumption as measured in grams per week per cage (2 animals per cage) revealed no significant differences (p = 0.6054) between the two groups. Body weights were recorded weekly, and average body weights during the 8-week study revealed no significant differences (p = 0.4648) between the two dietary treatment groups (see Fig. 1).
Figure 1.
Food consumption and average body weights. Host animals were fed a high-fat, high-cholesterol (HFHC) diet (n = 6 at start of experiment) or control diet (n = 6 at start of experiment) for 8 weeks. There were no significant differences in food consumption per cage (A) or average body weight (B) between the two dietary groups at any point during the experiment. (C) Total cholesterol. Serum total cholesterol was measured post sacrifice. HFHC-treated animals (n = 5) had a significantly higher total cholesterol level compared to control-treated animals (n = 4; p = 0.0423).
Triglyceride and Cholesterol Levels
Serum triglyceride levels and total cholesterol levels were compared between all groups at the end of the study (6 weeks postgrafting and 8 weeks following initiation of the diet treatments). No significant group differences were revealed by an overall one-way ANOVA for the triglyceride measurements (data not shown, p = 0.2595). However, animals that received the HFHC diet exhibited significantly elevated cholesterol levels (p = 0.0423) compared to the control-fed animals (35% higher cholesterol levels in serum following the HFHC diet) (Fig. 1C).
Graft Growth
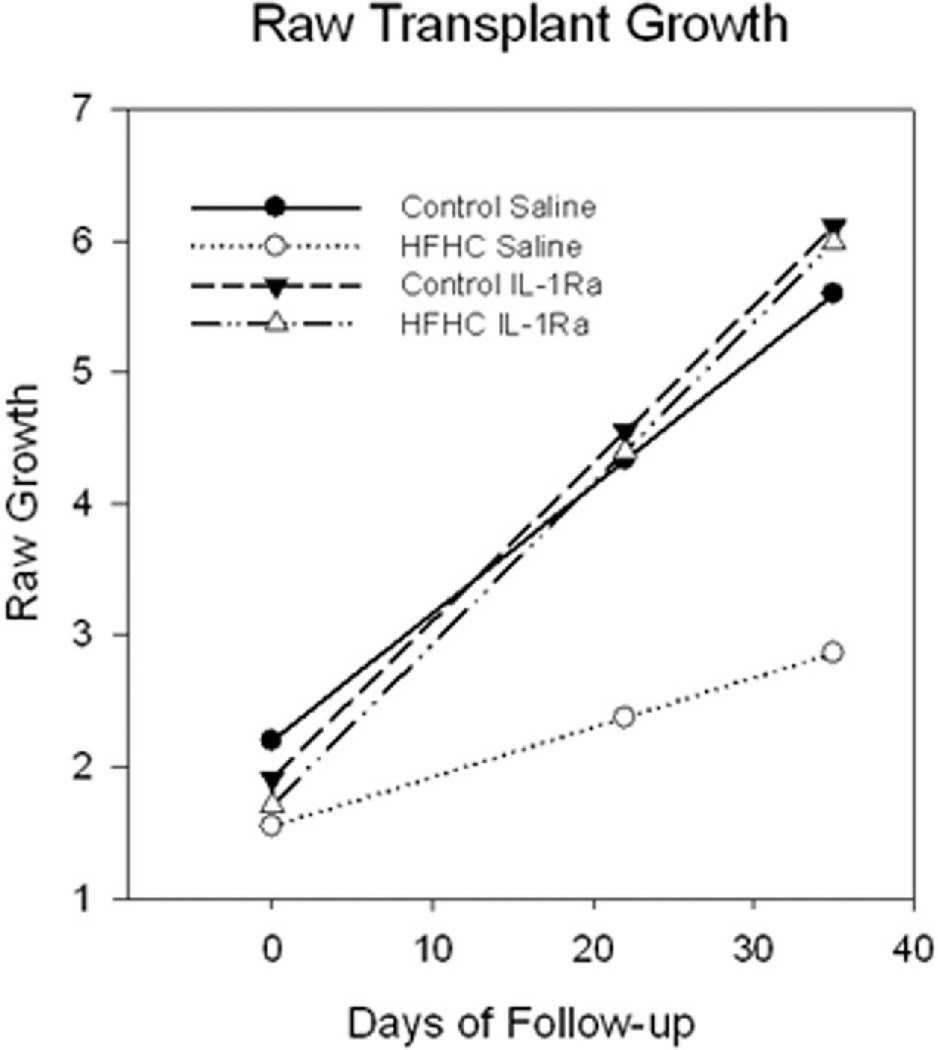
The volume of hippocampal grafts was measured after 3 and 5 weeks postgrafting in the anterior chamber of the eye using a caliper through the cornea of lightly anesthetized recipient rats [see Gerhardt et al. (29) for methodological details]. Vascularization was also assessed at the time of graft measurements using a light microscope through the translucent cornea. Blood pools formed first and then blood vessels developed about 2 weeks postgrafting, which is consistent with previous studies from our laboratory (72). Hippocampal grafts that were transplanted to HFHC diet-treated recipient rats and received saline (HFHC + S) grew significantly less compared to all other grafts. The estimated means for the grafts across the four groups are shown in Figure 2. An analysis with all groups failed to produce a statistically significant three-way interaction for time × diet × treatment. However, the grafts in the HFHC group treated with saline clearly showed less growth over the follow-up period. Separate analyses were conducted within each diet group comparing the changes in graft growth as a function of treatment. In the control group, there was evidence for statistically significant graft growth over the follow-up period (est. = 0.097 mm3 per week, SE = 0.036, p = 0.014), but no time × treatment interaction. Grafts into control diet-treated hosts that were treated with saline (Control + S) or IL-1Ra (Control + IL-1Ra) grew to comparable sizes, with the saline-treated slightly smaller (reaching average final sizes of 5.5 and 6 mm3, respectively). For the HFHC group, the time was not statistically significant (p = 0.094), but the interaction with treatment was statistically significant (p = 0.011). Further analyses within each treatment group indicated that the animals treated with saline failed to exhibit statistically significant growth over the follow-up period (est. = 0.038, SE = 0.018, p = 0.061), whereas the group treated with IL-1Ra did exhibit statistically significant growth over time (est. = 0.122, SE = 0.024, p < 0.001). The HFHC-fed and IL-1Ra-treated grafts (HFHC + IL-1Ra) grew to a similar size as the tissue grafted into control diet-treated recipient rats (about 6 mm3), whereas the grafts in the HFHC saline group only reached an average size of 2.5 mm3 (Fig. 2). These results suggest that the blockade of the IL-1 receptor may have diminished detrimental effects of the HFHC diet on hippocampal graft growth in oculo. Moreover, the 95% CI (0.068–0.177) for the estimate of growth from this group did not contain the time estimate for the HFHC saline group (est. = 0.038), but did contain the estimates for the groups on the control diet treated with saline or IL-1Ra (est. = 0.097, est. = 0.119, respectively), indicating that the growth for the HFHC group treated with IL-1Ra was statistically different from the HFHC saline group, but no different from either of the control conditions. Lastly, further illustration of the differences in average graft growth between the groups is presented in histological sections in Figure 3 (see below).
Figure 2.
Growth of hippocampal grafts throughout the experiment (6 weeks) as measured by volume. Note that grafts were the same size at the start of the experiment, but the HFHC saline grafts were significantly smaller compared to all other groups at the end of the experiment. On the other hand, HFHC interleukin (IL)-1Ra grafts were comparable in size to control-treated grafts (both groups n = 5). The HFHC animals treated with saline (n = 5) failed to exhibit statistically significant growth over the follow-up period (est. = 0.038, SE = 0.018, p = 0.061), whereas the group treated with IL-1Ra (n = 5) did exhibit statistically significant growth over time (est. = 0.122, SE = 0.024, p < 0.001).
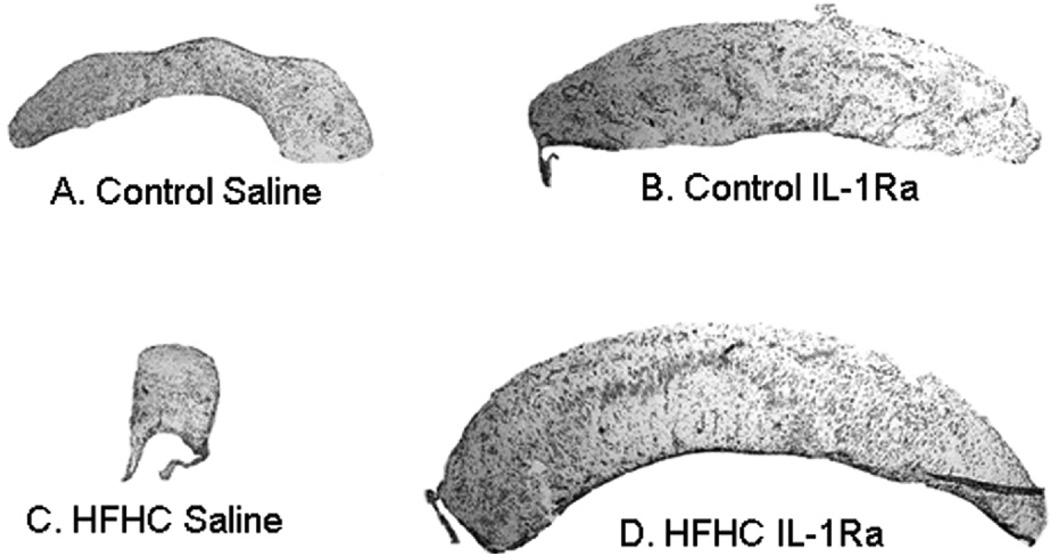
Figure 3.
Overall graft morphology. Hematoxylin-eosin staining was performed on hippocampal transplants. Note the difference in size, shape, and organization between HFHC-treated (C, D) and control-treated (A, B) as well as IL-1Ra-treated (B, D) and saline-treated (A, C). Graft growth and development was significantly stunted for the HFHC saline-treated graft (C), but rescued with IL-1Ra administration (D).
Graft Morphology
In addition to the significant differences in graft growth between the groups reported above, there were also marked histological differences as observed with the routine staining. H&E staining was employed and revealed both general and layer-specific alterations in hippocampal morphology as a result of the HFHC diet treatment (Figs. 3 and 4). HFHC + S grafts exhibited small, scattered neurons, with limited if any formation of the neuronal cell layers organotypic for the hippocampal CA1 in situ (Figs. 3C and 4C). For each of the treatment groups except for the HFHC + S, neurons appeared large, had a distinct nucleolus, and exhibited morphological resemblance to hippocampal pyramidal neurons in situ. Control + IL-1Ra and HFHC + IL-1Ra grafts exhibited the most robust, developed neurons as well as the most organotypical general organization into distinct layers within the grafted tissue. As is obvious from the overall images shown in Figure 3, three of the four groups demonstrated a stratified organization, with several areas containing layers of large organotypical pyramidal-shaped neurons, resembling hippocampal layers seen in situ. In general, layers were not observed within HFHC + S grafts, suggesting not only a lack of growth as a result of the diet, but also a lack of migration of newly formed neurons into organotypical cell layers in the hippocampus in situ.
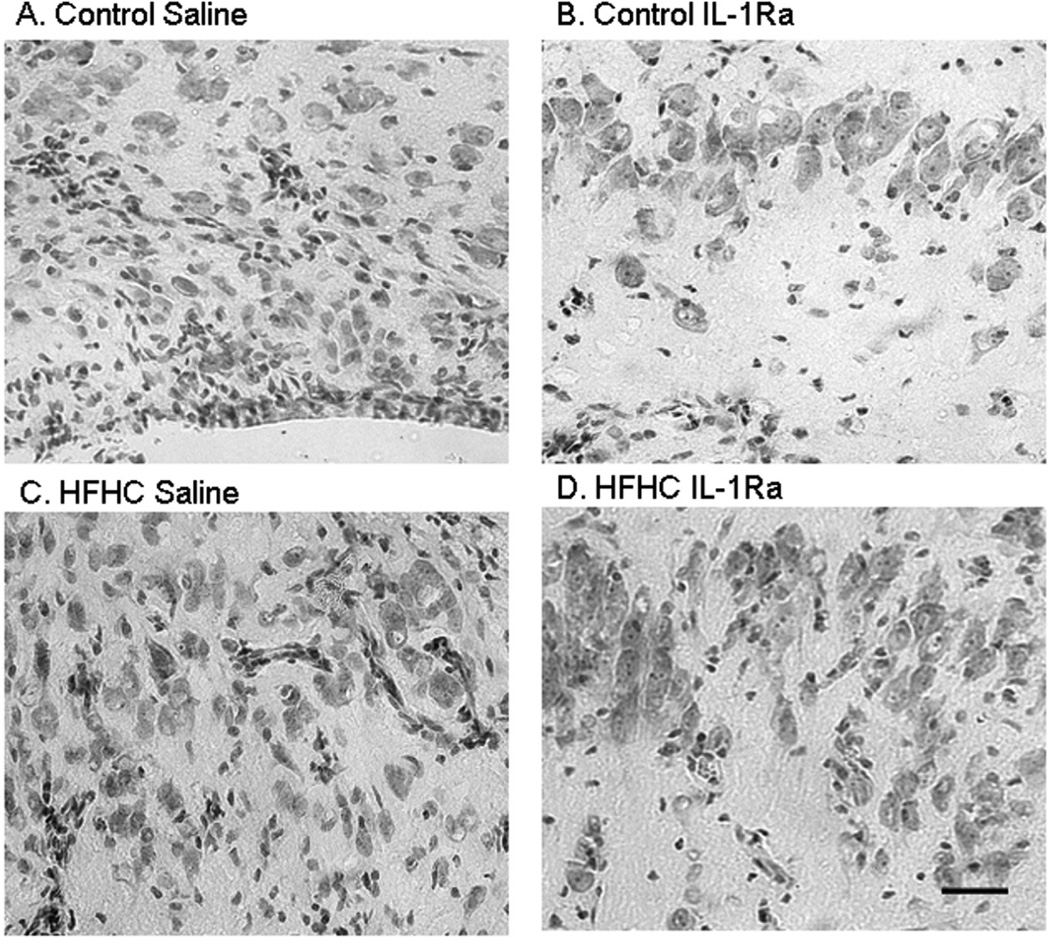
Figure 4.
Neuronal morphology of hippocampal transplants. Hematoxylin-eosin staining was performed on hippocampal transplants. Note the difference in neuron size and formation of neuronal layers. Control/IL-1Ra grafts (B) exhibited large neurons and clear formation of a layer. HFHC/IL-1Ra grafts (D) had a similar organization with distinct organotypical cell layers. However, HFHC/Saline grafts (C) had small and scattered neurons without any evidence of organotypical cell layers observed in the hippocampus in situ. Scale bar: 10 µm.
Vascularization of Grafts
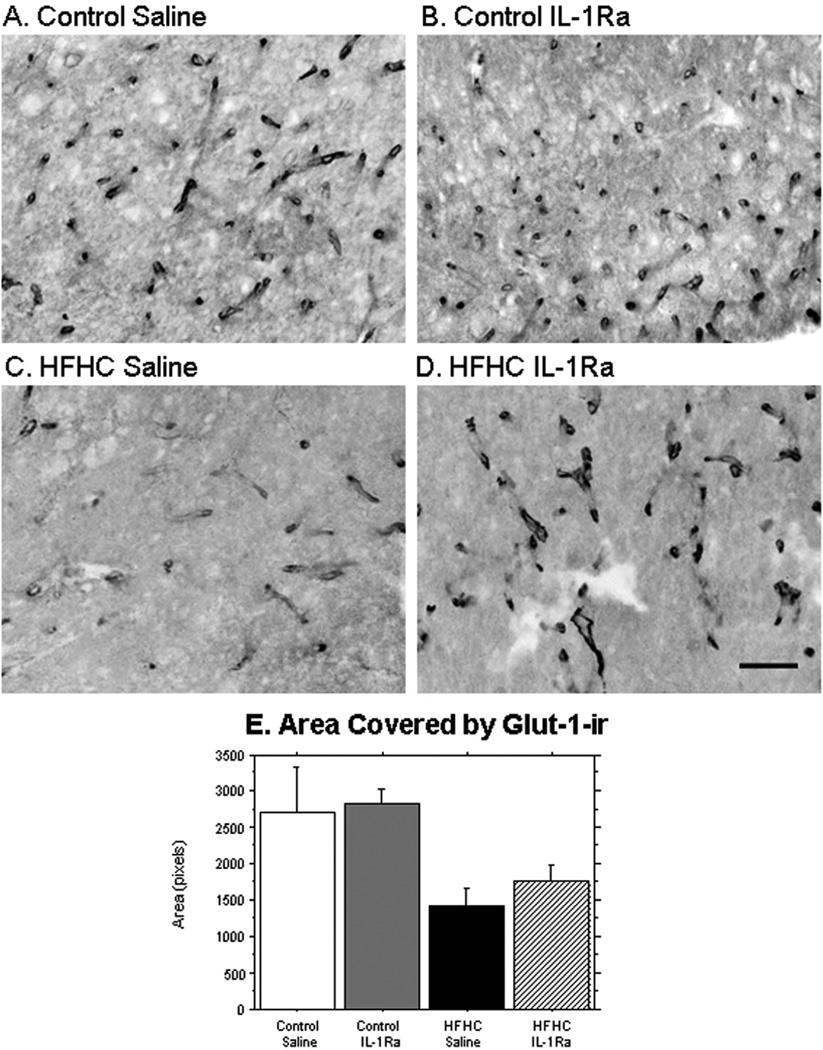
Vascularization of the grafts, as analyzed by glucose transporter-1 immunoreactivity (Glut-1-ir) revealed a greater Glut-1 staining in blood vessels in both of the control-treated grafts compared to grafts grown in the HFHC diet-treated recipient rats. The significant decrease in Glut-1 staining density observed in both HFHC + S and HFHC + IL-1Ra (Fig. 5E) suggests that the HFHC diet either affected protein levels of Glut-1 within each vessel, or significantly reduced the rate of vascularization within the grafted hippocampal tissues. A close inspection of the images shown in Figure 5 suggests that both of these scenarios in combination may be true. The fact that the IL-1Ra did not significantly restore Glut-1 staining density to control diet values suggests that there are factors involved in the detrimental effects of the HFHC diet other than IL-1, affecting the vascular development in the hippocampal tissue. Further studies including assessment of BBB markers as well as an in-depth morphological assessment of the vascularization process would determine factors involved for HFHC diets effects on intraocular grafts.
Figure 5.
Vascularization of grafts. Distribution of microvessels within the grafted tissues was evaluated using an antibody directed against glucose transporter-1 (Glut-1). Control saline (n = 4) and control IL-1Ra-treated grafts (n = 2) exhibited increased Glut-1 immunostaining compared to HFHC-treated grafts (saline n = 3; IL-1Ra, n = 4), manifested both as a denser structure of vessels stained with Glut-1 and enhanced staining within each blood vessel (A, B). Control grafts (saline and IL-1Ra) versus HFHC (saline and IL-1Ra) had a significantly greater area covered by Glut-1-ir (p = 0.018). Scale bar: 20 µm.
Astrocyte Densitometry
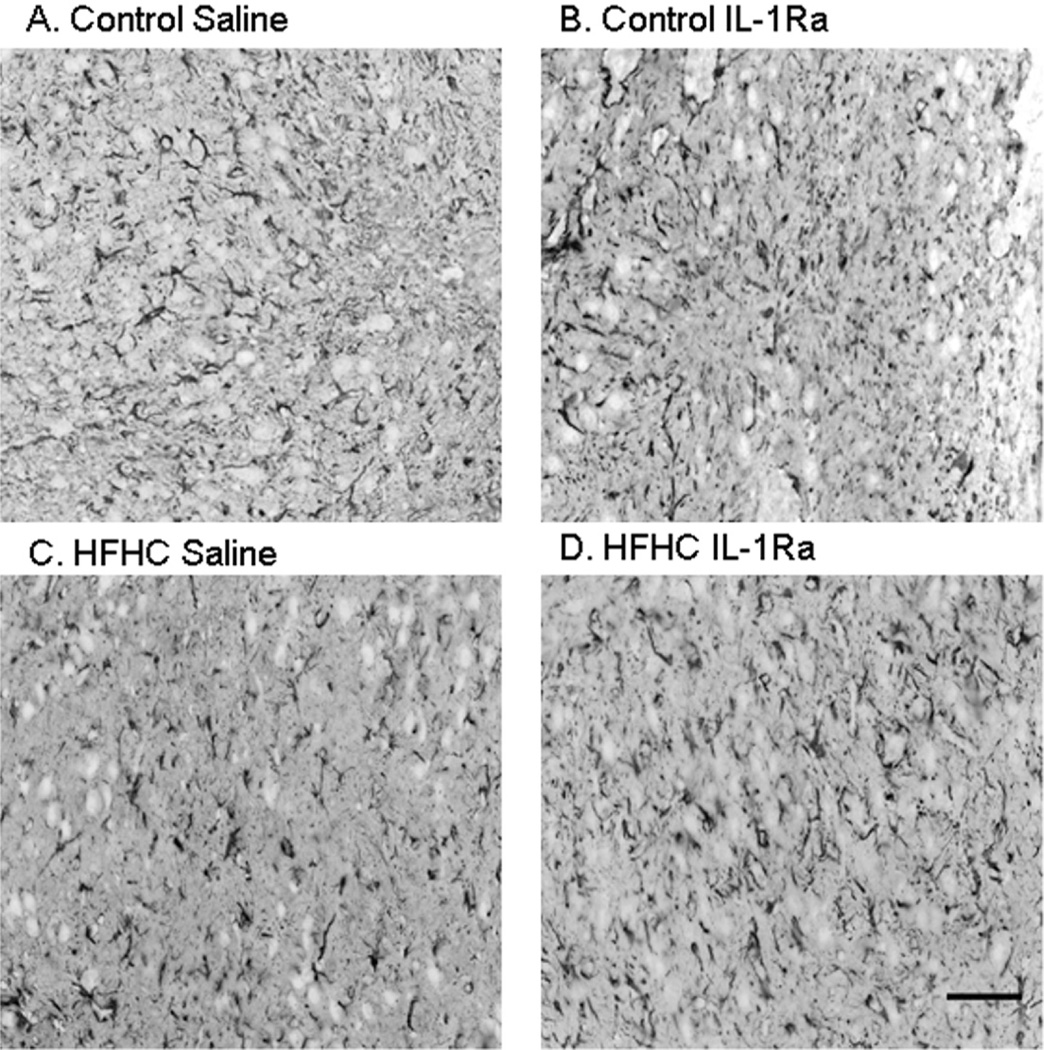
Astrocyte morphology and densitometry were analyzed in order to determine the level of astrogliosis in hippocampal grafts exposed to the four different treatments (fluorescent images are displayed in black and white and as inverted images in order to better visualize morphology). Typically, astrocytes are necessary for providing a support system for the brain including repair and metabolic support. Due to rapid changes in these grafts during their development and the stress following transplantation, it was important to examine any possible changes in astrocytic morphology. The density of an astrocyte marker (the intermediate filament GFAP) was measured for all groups and no significant differences were found (p = 0.2754) (Fig. 6), suggesting that there was no significant astrogliosis occurring in the grafted tissues, at least not at the time of sacrifice. However, these findings do not exclude a possible role for astrocytes in initiation of inflammatory processes occurring at earlier time points or later time points following HFHC diet treatment during hippocampal development.
Figure 6.
Astrocytic morphology of hippocampal transplants. Astrocyte morphology and densitometry were evaluated using antibodies directed against glial fibrillary acidic protein (GFAP). No significant differences in morphology or density of GFAP-immunoreactive elements were observed between the four groups, at least not at this time point postgrafting. Scale bar: 20 µm.
IRAK Densitometry
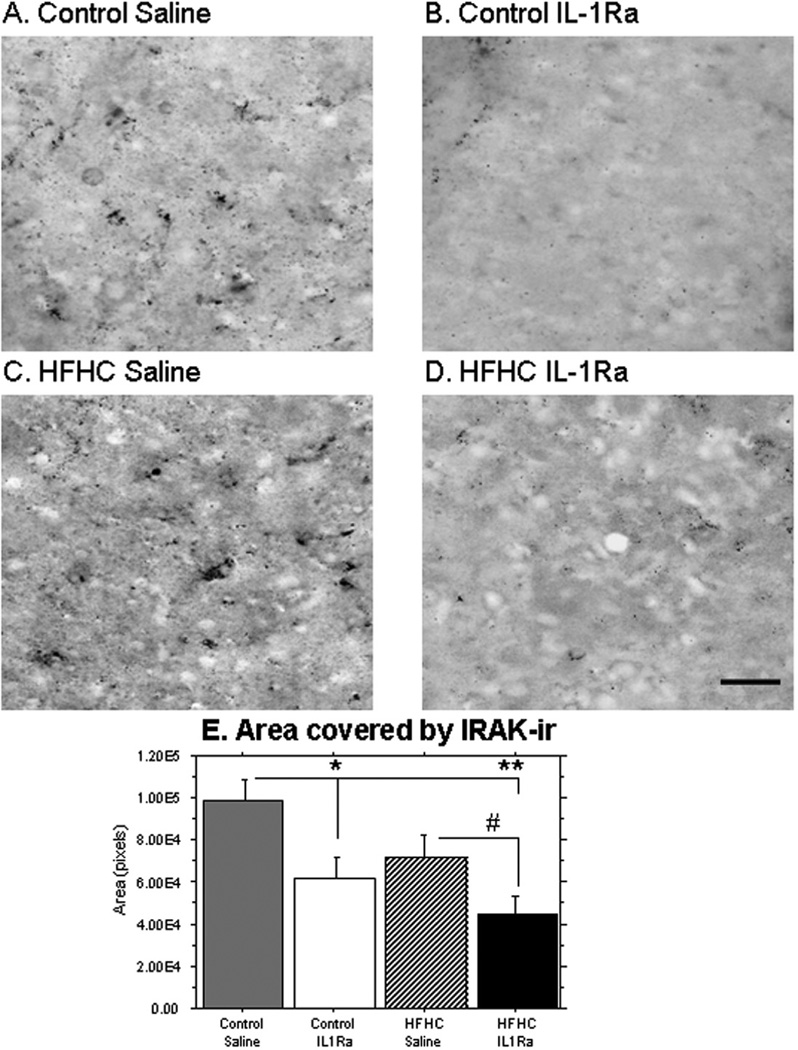
In order to assess the specific effects of the IL-1Ra treatment upon IL-1 function in the grafted tissues, we used an antibody directed against IRAK, which is one of the first molecules recruited to the receptor upon stimulation by its agonist (IL-1). The data shown in Figure 7 revealed a significant increase in IRAK-ir in HFHC + S grafts, compared to their IL-1Ra-treated counterparts (control + IL-1Ra was significantly lower than control + S, p = 0.0183; HFHC + IL-1Ra was approaching significance compared to HFHC + S, p = 0.0596; HFHC + IL-1Ra was significantly lower than control + S, p = 0.0010). These data demonstrated that IL-1Ra treatment reduced IRAK staining in both control and HFHC diet-treated recipient rats. Even though inflammation is involved in the detrimental hippocampal growth and development shown here, other elements such as those affecting the vascularization may also play prominent roles.
Figure 7.
IRAK distribution. An antibody to IL-1 receptor associated kinase (IRAK) was used in order to visualize effects of the HFHC and the IL-1Ra upon IL-1 signaling in grafted hippocampal tissues. As can be seen here, saline-treated grafts exhibited a significant increase in IRAK immunostaining (A, C), compared to the IL-1Ra-treated grafts (B, D). The IRAK staining appeared to be localized both intracellularly, especially in the HFHC group (C), and diffusely throughout the tissues. Densitometry (E) confirmed that IL-1Ra significantly reduced IRAK staining in both diet groups [control caline (n = 5) is significantly greater than control IL-1Ra (n = 4), p = 0.018; HFHC saline (n = 5) is significantly greater than HFHC IL-1Ra (n = 5), p = 0.059; control saline is significantly greater than HFHC IL-1Ra, p = 0.0010]. Scale bar: 20 µm.
Activated Microglia
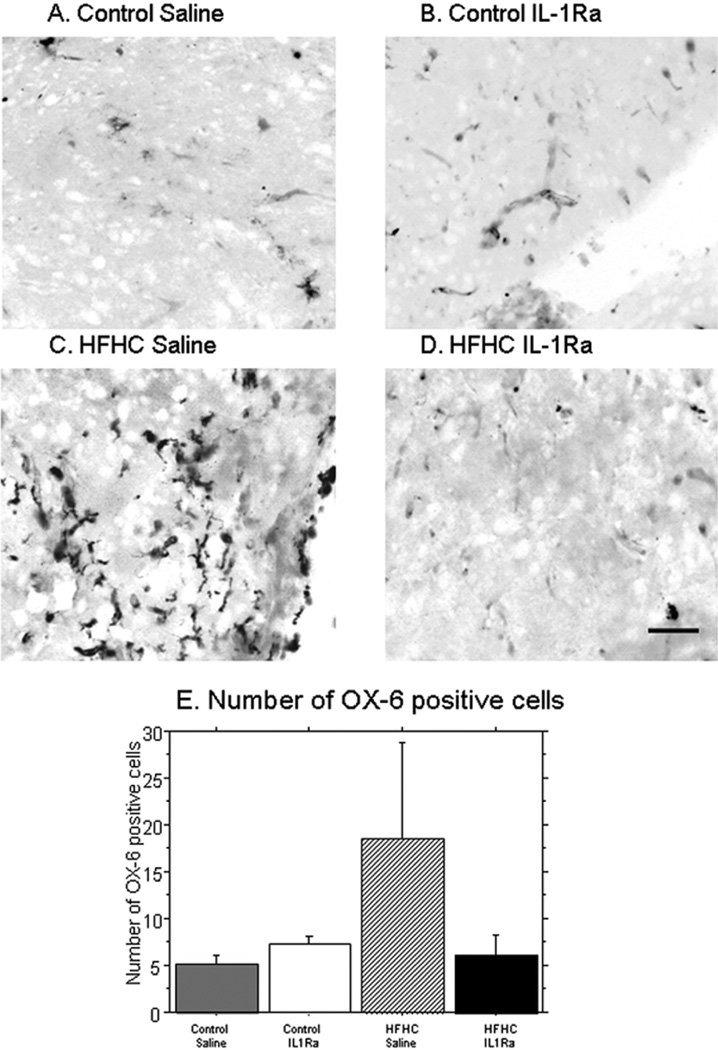
Lastly, we examined activated microglia in the grafted tissues using an antibody (OX-6 or RT1B, Serotec) that labels major histocompatibility complex II-positive cells. This antibody has been utilized to label activated microglial cells in grafted hippocampal tissues in previous work from our group (71) and reliably labels inflammatory activation of resident microglial cells in brain tissue. The number of OX-6-positive cells were counted and their morphology was examined. Both control + S and control + IL-1Ra revealed small numbers of OX-6-positive cells per section (control + IL-1Ra = 7 ± 1.5, control + S = 5 ± 1.5). Grafts to HFHC diet-treated recipients, on the other hand, exhibited two to three times as many cells per section (HFHC + S = 18 ± 20) (Fig. 8). The HFHC + IL-1Ra grafts exhibited a reduction in the number of OX-6-positive microglial cells to levels observed in grafts on the control diet (HFHC + IL-1Ra = 6 ± 5). In addition to differences in the number of activated microglia found for each group, alterations in microglial morphology were observed. All grafts exhibited at least a few microglial cells; however, the HFHC grafts also exhibited cells that appeared more reactive (amoeboid microglia) with shorter processes and very thick cell bodies, highly stained with the OX-6 antibody. The other groups, while displaying activated microglia, had more ramified morphology with longer processes and less OX-6 staining compared to the HFHC + S grafts. The difference in microglial morphology of the four groups is demonstrated in Figure 8A–D.
Figure 8.
OX-6: Activated and reactive microglia. An antibody to MHC class II-positive cells (OX-6, RT1B) was used in order to examine the distribution of activated microglial cells in the hippocampal grafts. As can be seen in (C), HFHC diet saline grafts exhibited the greatest number of OX-6-positive cells compared to all other groups. Densitometry (E) confirmed these findings, and revealed a significant increase in OX-6 immunolabeled microglial cells in this group compared to the other three groups. Control + IL-1Ra grafts (n = 3) had 7 ± 1.5 activated microglia, control saline (n = 3) had 5 ± 1.5 activated microglia, HFHC + IL-1Ra (n = 5) had 6 ± 5 activated microglia, and HFHC saline (n = 5) had 18 ± 20 activated microglia. Scale bar: 20 µm.
DISCUSSION
In this study, we explored the effects of a high saturated fat and cholesterol diet on survival of fetal hippocampal grafts to middle-aged hosts. Graft growth measurements throughout the 6-week experiment revealed stunted growth of fetal hippocampal tissue when transplanted to the host animals fed the HFHC diet compared to control. In addition, grafts to HFHC animals that were treated topically with IL-1Ra grew to approximately three times the size of HFHC saline-treated grafts. H&E staining revealed that grafts transplanted to the HFHC-fed host animals exhibited a reduced organotypical development of cells into hippocampal layers compared to the grafts transplanted to control-fed subjects as well as the IL-1Ra-treated HFHC hosts. Differences in the distribution of the vascular marker Glut-1 observed between dietary groups suggested that HFHC diet affected vascularization in the hippocampal grafts; here, the IL-1Ra topical application did not appear to improve conditions, suggesting that other factors may also be involved. Interestingly, no significant differences were found for astrocyte densitometry. Finally, significant differences were found in the number of activated microglia and IRAK distribution. HFHC-fed hosts exhibited transplants with a significant increase in activated microglia compared to all other groups. For both the HFHC-fed and control-fed hosts, IL-1Ra topical administration reduced IRAK activity compared to saline administration, strongly suggesting that the IL-1 receptor blocker crossed the BBB and exerted effects on IL-1 signaling in the grafted tissue. Taken together, the HFHC diet was shown to exert detrimental effects on hippocampal growth and development. Factors contributing to this effect include, but are not limited to, vascularization and inflammation.
The HFHC Diet Altered Fetal Hippocampal Growth
Previous studies from our laboratory and others have shown stunted growth of fetal brain tissue grafts or embryonic stem cells to aged host animals compared to young hosts (59,60,69,72). Furthermore, we have shown that dietary supplementation with blueberries improves the growth of hippocampal transplants to aged host animals, and reduces both astrogliosis and microglial activation in the grafts, suggesting that either antioxidant or anti-inflammatory properties of blueberries reversed most observed effects of the aged mileu on grafted fetal tissues (69). Not only did this study reveal altered graft growth, but it also exhibited reduced activated microglia following blueberry supplementation. This is support for our notion that inflammation may play an important role in development of brain tissue grafts. In a study by Das et al., neocortex from various stages of embryos was transplanted to the cerebellum of host rats. It was found that as the age of the tissue increased, the survival and growth of that tissue following transplantation decreased. Therefore, not only does the age of the host animal affect graft growth but the age of the graft is important as well (18). A study on the growth of the hippocampus was found to be best using tissue at embryonic days 16 and 18 (18,44). These results are similar to our earlier work, suggesting E18 to be the most appropriate age for hippocampal grafting to the anterior chamber of the eye as well (29). Previous work has suggested that hippocampal growth and development is affected by: 1) vascularization (37), 2) proinflammatory cytokines (39), and 3) growth factor production in the host and the grafted tissues (4,70). However, studies have not been undertaken previously regarding the effects of HFHC diets upon hippocampal growth and development. Our findings in the present study demonstrate that not only graft growth was altered by our treatment, but neuronal development and organization was affected as well (see below). Potential mechanisms for the massive loss of graft growth and organization observed in HFHC-treated hosts were explored using different neuronal and glial markers. Here, we see inhibition of hippocampal transplant growth due to two detrimental factors: age of the host and HFHC diet. These findings may impact individuals receiving both neuronal and other grafted tissues and could lead to potential treatment therapies to enhance graft survival.
Further studies from our laboratory have used high-fat diets in vivo for short-term (8 weeks) (33) and long-term (6 months) treatment (currently unpublished), particularly in the aged rat. Similar findings to the intraocular transplant model were found such as increased microgliosis and altered Glut-1 immunoreactivity. For example, a study in which isocaloric diets were administered including a high saturated fat diet (lard), high-cholesterol diet (soybean oil with 2% added cholesterol), and high-trans fat diet (hydrogenated coconut oil) compared to a mainly unsaturated fat diet (soybean oil), revealed detrimental effects of each treatment diet after just 8 weeks. This study was designed in order to determine which aspect of the “Western diet” was the most detrimental to serum lipid profiles and hippocampal morphology. While the diets revealed varying degrees of effects, each of the diets altered the lipid profile, increased microgliosis in the hippocampus, and altered Glut-1 immunoreactivity. Therefore, each component of the “Western diet” led to detrimental effects and changes in the hippocampus (Freeman et al., Nutritional Neuroscience, in press). When all of these factors were combined, such as in the HFHC diet, greatest effects were observed.
Altered Organization Accompanies the Reduced Growth
In the studies by Willis et al. (69–72) as well as our current study, stunted graft growth was also accompanied by altered hippocampal organization and neuronal development. It has been previously reported by our laboratory and others that host animal age affects organotypical development of neurons and glia in the grafted tissues (25,69). Based on previous studies demonstrating significant effects of the HFHC diet used here upon hippocampal function in middle-aged hosts (33), we decided to utilize the same age for hosts in the current study. Further disruption to cellular organization was observed when middle-aged hosts were fed the HFHC diet, with most of the grafts in this group exhibiting no organization into layers, and smaller, scattered neurons throughout the grafted tissue. A more organotypical development of hippocampal neurons was found when these grafts received topical treatment with the IL-1 receptor antagonist IL-1Ra. This is similar to the previous findings by Willis et al. (69,71,72) in which blueberry supplementation rescued effects on organization due to transplantation to aged hosts. Our findings presented here certainly suggest that host dietary intake can have significant detrimental effects on migration of neurons in the hippocampus into the appropriate layers. Since IL-1Ra gave rise to a significantly higher order in hippocampal layers, we suggest that elevated proinflammatory cytokines in middle-aged and HFHC-treated rats gain access to the grafted tissue and affect development.
Vascularization of Hippocampal Grafts
As can be observed in Figure 5, the HFHC diet reduced the area covered by Glut-1 immunoreactivity compared to the control diet. While these results were not statistically significant for diet × treatment, once the data were collapsed we found the HFHC diet (saline and IL-1Ra) to have significantly less Glut-1-ir compared to control (saline and IL-1Ra; data not shown, p = 0.018). The density of Glut-1 staining within each vessel as well as the overall density of Glut-1-ir vessels was reduced by the HFHC diet. It is known that with aging, many proangiogenic factors are increased such as vascular endothelial growth factor, basic fibroblast growth factor, and transforming growth factor-β (63) while antiangiogenic factors decrease (27,56). However, effects of a high-fat diet on brain vascularization, especially in the hippocampus, have not been well studied. The proinflammatory cytokine IL-1β has been shown to play a significant role for BBB permeability and subsequent neuroinflammation following systemic inflammation (43) and it was therefore interesting that IL-1Ra treatment of HFHC-treated grafts had no apparent effect on Glut-1-labeled vessels, certainly suggesting that other factors are involved in the vascular development in grafts to HFHC-treated hosts. Glut-1 is present in brain blood vessel walls in the fetal rat at E13, and increases concomitantly during development to adulthood (8). As blood vessels become more mature, the absolute amount of Glut-1 per vessel profile increases, while the vessel size narrows with thinner walls as the brain matures. It is therefore possible that the altered Glut-1-ir observed is the result of delayed vascular development, even though further proof for this needs to be generated in future studies. Further analysis of BBB integrity is also necessary as this may be an important component in both age-associated and diet-associated alterations to hippocampal growth and development according to our previous studies (34,72).
Astrogliosis in Hippocampal Grafts
Contrary to findings in the previous study from our laboratory using an intraocular transplant model (71) and others (24,26) in which astrogliosis increased with age of hosts, astrocytes as measured by GFAP were not increased further in HFHC-fed host grafts, at least not at the postgrafting time point examined here. Typically, astrocyte activation is associated with neurodegeneration and goes hand in hand with microglial activation as both influence each other through various mechanisms (2,16, 20). Studies have also shown factors that can reduce astrocyte activation such as trophic factor stimulation (6,57) and blueberry supplementation (71). We found a significant increase in microglial activation and perhaps astrogliosis would have been observed following a longer postgrafting time.
Increased Microglial Activation From the HFHC Diet
Lastly, significant microglial activation was observed in grafts from the HFHC-fed host rats compared to all other groups. Previous studies from our laboratory have shown increased microglial activation in aged host rats (69), as well as middle-aged rats treated with the HFHC diet (33). Increased microglial activation in the hippocampus with aging has been connected with decreased neurogenesis (22,40,47), demonstrating a role for microglial cells in age-related neuronal loss and proposing a plausible biological mechanism for observed effects on neuronal migration and development in the present study. To our knowledge, no other studies have investigated diet-induced microglial activation in the developing hippocampus. However, studies in the hypothalamus support our current findings that a high-fat diet can induce an inflammatory response (58,68) and increase activated microglia (35). Another interesting finding in our current study was the reversal of microglial activation with IL-1Ra administration; HFHC diet grafts that also received IL-1Ra had a comparable number of activated microglia to both control groups whereas the HFHC diet grafts with saline had about five times as many. Earlier studies have utilized dietary interventions to reduce microglial activation with aging or inflammation, and support our findings. Blueberry supplementation (71) as well as docosahexanoic acid (DHA) have both been shown to reduce microglial activation in rodent models of degeneration (21).
In order to test the effects of the IL-1Ra, IL-1 receptor (IL-1R) activation was examined using an antibody to IRAK. IRAK is one of the first molecules recruited to the IL-1R complex when its agonist (IL-1) is bound and, therefore, can provide evidence for intracellular IL-1 signaling. This is a novel marker for brain tissue, which has been utilized for peripheral tissues (45,50). The fact that no significant differences were found for IRAK activity between the HFHC and control diets suggests that factors other than IL-1 are also involved in the HFHC-induced effects on transplant growth and organization, even though IL-1Ra had significant effects on hippocampal transplant growth. However, IL-1Ra administration significantly reduced IRAK activity for both groups compared to saline, demonstrating that the IL-1 receptor inhibitor crossed the BBB into grafted hippocampus and affected IL-1 signaling. Because HFHC-diet grafts that received IL-1Ra had reduced microglial activation, improved growth and neuronal organization, it is likely that inflammation is playing an essential role in the damaging effects observed here with a high-fat diet.
In conclusion, we examined effects of a high-fat diet as well as IL-1Ra administration on hippocampal development. The implications of this study include: 1) a HFHC diet is not only damaging to peripheral systems but the central nervous system as well, 2) a HFHC diet can be damaging to early development and neuronal migration in the hippocampus, and 3) reducing inflammation in the hippocampus may reverse some of these high-fat diet-induced effects.
REFERENCES
- 1.Apostolakis S, Vogiatzi K, Krambovitis E, Spandidos DA. IL-1 cytokines in cardiovascular disease: Diagnostic, prognostic and therapeutic implications. Cardiovasc. Hematol. Agents Med. Chem. 2008;6(2):150–158. doi: 10.2174/187152508783955006. [DOI] [PubMed] [Google Scholar]
- 2.Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J. Neurosci. 2001;21(17):6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beattie MS. Inflammation and apoptosis: Linked therapeutic targets in spinal cord injury. Trends Mol. Med. 2004;10(12):580–583. doi: 10.1016/j.molmed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Berglof E, Stromberg I. Locus coeruleus promotes survival of dopamine neurons in ventral mesencephalon. An in oculo grafting study. Exp. Neurol. 2009;216(1):158–165. doi: 10.1016/j.expneurol.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Björklund H, Hoffer BJ, Palmer MR, Seiger A, Olson L. Survival and growth of neurons with enkephalin-like immunoreactivity in fetal brain areas grafted to the anterior chamber of the eye. Neuroscience. 1983;10(4):1387–1398. doi: 10.1016/0306-4522(83)90120-3. [DOI] [PubMed] [Google Scholar]
- 6.Bjöklund H, Seiger A, Hoffer B, Olson L. Trophic effects of brain areas on the developing cerebral cortex: I. Growth and histological organization of intraocular grafts. Brain Res. 1983;282(2):131–140. doi: 10.1016/0165-3806(83)90091-3. [DOI] [PubMed] [Google Scholar]
- 7.Blandini F, Cova L, Armentero MT, Zennaro E, Levandis G, Bossolasco P, Calzarossa C, Mellone M, Giuseppe B, Deliliers GL, Polli E, Nappi G, Silani V. Transplantation of undifferentiated human mesenchymal stem cells protects against 6-hydroxydopamine neurotoxicity in the rat. Cell Transplant. 2010;19(2):203–217. doi: 10.3727/096368909X479839. [DOI] [PubMed] [Google Scholar]
- 8.Bolz S, Farrell CL, Dietz K, Wolburg H. Subcellular distribution of glucose transporter (GLUT-1) during development of the blood–brain barrier in rats. Cell Tissue Res. 1996;284(3):355–365. doi: 10.1007/s004410050596. [DOI] [PubMed] [Google Scholar]
- 9.Bourke E, Moynagh PN. Antiinflammatory effects of glucocorticoids in brain cells, independent of NF-kappa B. J. Immunol. 1999;163(4):2113–2119. [PubMed] [Google Scholar]
- 10.Brundin P, Björklund A. Survival, growth and function of dopaminergic neurons grafted to the brain. Prog. Brain Res. 1987;71:293–308. doi: 10.1016/s0079-6123(08)61832-4. [DOI] [PubMed] [Google Scholar]
- 11.Castilho RF, Hansson O, Brundin P. Improving the survival of grafted embryonic dopamine neurons in rodent models of Parkinson’s disease. Prog. Brain Res. 2000;127:203–231. doi: 10.1016/s0079-6123(00)27011-8. [DOI] [PubMed] [Google Scholar]
- 12.Chong EW, Sinclair AJ, Guymer RH. Facts on fats. Clin. Experiment. Ophthalmol. 2006;34(5):464–471. doi: 10.1111/j.1442-9071.2006.01250.x. [DOI] [PubMed] [Google Scholar]
- 13.Clark SR, McMahon CJ, Gueorguieva I, Rowland M, Scarth S, Georgiou R, Tyrrell PJ, Hopkins SJ, Rothwell NJ. Interleukin-1 receptor antagonist penetrates human brain at experimentally therapeutic concentrations. J. Cereb. Blood Flow Metab. 2008;28(2):387–394. doi: 10.1038/sj.jcbfm.9600537. [DOI] [PubMed] [Google Scholar]
- 14.Collier TJ, Sortwell CE, Daley BF. Diminished viability, growth, and behavioral efficacy of fetal dopamine neuron grafts in aging rats with long-term dopamine depletion: An argument for neurotrophic supplementation. J. Neurosci. 1999;19(13):5563–5573. doi: 10.1523/JNEUROSCI.19-13-05563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005;81(2):341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 16.Cotrina ML, Nedergaard M. Astrocytes in the aging brain. J. Neurosci. Res. 2002;67(1):1–10. doi: 10.1002/jnr.10121. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol. Psychiatry. 2009;65(4):304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das GD, Hallas BH, Das KG. Transplantation of brain tissue in the brain of rat. I. Growth characteristics of neocortical transplants from embryos of different ages. Am. J. Anat. 1980;158(2):135–145. doi: 10.1002/aja.1001580204. [DOI] [PubMed] [Google Scholar]
- 19.Davis N, Katz S, Wylie-Rosett J. The effect of diet on endothelial function. Cardiol. Rev. 2007;15(2):62–66. doi: 10.1097/01.crd.0000218824.79018.cd. [DOI] [PubMed] [Google Scholar]
- 20.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36(2):180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 21.Ebert S, Weigelt K, Walczak Y, Drobnik W, Mauerer R, Hume DA, Weber BH, Langmann T. Docosahexaenoic acid attenuates microglial activation and delays early retinal degeneration. J. Neurochem. 2009;110(6):1863–1875. doi: 10.1111/j.1471-4159.2009.06286.x. [DOI] [PubMed] [Google Scholar]
- 22.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. USA. 2003;100(23):13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eriksdotter-Nilsson M, Gerhardt G, Seiger A, Hoffer B, Granholm AC. Multiple changes in noradrenergic mechanisms in the coeruleo-hippocampal pathway during aging. Structural and functional correlates in intraocular double grafts. Neurobiol. Aging. 1989;10(2):117–124. doi: 10.1016/0197-4580(89)90020-1. [DOI] [PubMed] [Google Scholar]
- 24.Eriksdotter-Nilsson M, Gerhardt G, Seiger A, Olson L, Hoffer B, Granholm AC. Age-related alterations in noradrenergic input to the hippocampal formation: Structural and functional studies in intraocular transplants. Brain Res. 1989;478(2):269–280. doi: 10.1016/0006-8993(89)91507-2. [DOI] [PubMed] [Google Scholar]
- 25.Eriksdotter-Nilsson M, Olson L. Growth of brain tissue grafts is dependent upon host age. Mech. Ageing Dev. 1989;49(1):1–22. doi: 10.1016/0047-6374(89)90064-x. [DOI] [PubMed] [Google Scholar]
- 26.Eriksdotter-Nilsson M, Skirboll S, Ebendal T, Olson L. Nerve growth factor can influence growth of cortex cerebri and hippocampus: Evidence from intraocular grafts. Neuroscience. 1989;30(3):755–766. doi: 10.1016/0306-4522(89)90167-x. [DOI] [PubMed] [Google Scholar]
- 27.Funaki H, Sawaguchi S, Yaoeda K, Koyama Y, Yaoita E, Funaki S, Shirakashi M, Oshima Y, Shukunami C, Hiraki Y, Abe H, Yamamoto T. Expression and localization of angiogenic inhibitory factor, chondromodulin-I, in adult rat eye. Invest. Ophthalmol. Vis. Sci. 2001;42(6):1193–1200. [PubMed] [Google Scholar]
- 28.Furst DE. Anakinra: Review of recombinant human interleukin- I receptor antagonist in the treatment of rheumatoid arthritis. Clin. Ther. 2004;26(12):1960–1975. doi: 10.1016/j.clinthera.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Gerhardt GA, Palmer MR, Granholm AC. Age-induced changes in single locus coeruleus brain transplants grown in oculo: An in vivo electrochemical study. Neurobiol. Aging. 1991;12(5):487–494. doi: 10.1016/0197-4580(91)90078-x. [DOI] [PubMed] [Google Scholar]
- 30.Ginsberg HN, Kris-Etherton P, Dennis B, Elmer PJ, Ershow A, Lefevre M, Pearson T, Roheim P, Ramakrishnan R, Reed R, Stewart K, Stewart P, Phillips K, Anderson N. Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: The DELTA Study, protocol 1. Arterioscler. Thromb. Vasc. Biol. 1998;18(3):441–449. doi: 10.1161/01.atv.18.3.441. [DOI] [PubMed] [Google Scholar]
- 31.Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006;48(4):677–685. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 32.Goldbach-Mansky R. Blocking interleukin-1 in rheumatic diseases. Ann. NY Acad. Sci. 2009;1182:111–123. doi: 10.1111/j.1749-6632.2009.05159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J. Alzheimers Dis. 2008;14(2):133–145. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granholm AC, Curtis M, Diamond DM, Branch BJ, Heman KL, Rose GM. Development of an intact blood–brain barrier in brain tissue transplants is dependent on the site of transplantation. Cell Transplant. 1996;5(2):305–314. doi: 10.1177/096368979600500219. [DOI] [PubMed] [Google Scholar]
- 35.Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology. 2010;151(4):1622–1632. doi: 10.1210/en.2009-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenwood CE, Winocur G. High-fat diets, insulin resistance and declining cognitive function. Neurobiol. Aging. 2005;26(Suppl. 1):42–45. doi: 10.1016/j.neurobiolaging.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Hallene KL, Oby E, Lee BJ, Santaguida S, Bassanini S, Cipolla M, Marchi N, Hossain M, Battaglia G, Janigro D. Prenatal exposure to thalidomide, altered vasculogenesis, and CNS malformations. Neuroscience. 2006;142(1):267–283. doi: 10.1016/j.neuroscience.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han SN, Leka LS, Lichtenstein AH, Ausman LM, Schaefer EJ, Meydani SN. Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J. Lipid Res. 2002;43(3):445–452. [PubMed] [Google Scholar]
- 39.Hao LY, Hao XQ, Li SH, Li XH. Prenatal exposure to lipopolysaccharide results in cognitive deficits in age-increasing offspring rats. Neuroscience. 2010;166(3):763–770. doi: 10.1016/j.neuroscience.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol. Aging. 2004;25(3):361–375. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 41.Hoffer B, Seiger A, Freedman R, Olson L, Taylor D. Electrophysiology and cytology of hippocampal formation transplants in the anterior chamber of the eye. II. Cholinergic mechanisms. Brain Res. 1977;119(1):107–132. doi: 10.1016/0006-8993(77)90094-4. [DOI] [PubMed] [Google Scholar]
- 42.Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, Perry VH. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2003;74(6):788–789. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jedrzejowska-Szypulka H, Straszak G, Larysz-Brysz M, Karpe J, Marcol W, Olakowska E, Woszczycka-Korczynska I, Lewin-Kowalik J. Interleukin-1beta plays a role in the activation of peripheral leukocytes after blood–brain barrier rupture in the course of subarachnoid hemorrhage. Curr. Neurovasc. Res. 2010;7(1):39–48. doi: 10.2174/156720210790820226. [DOI] [PubMed] [Google Scholar]
- 44.Jensen S, Sorensen T, Moller AG, Zimmer J. Intraocular grafts of fresh and freeze-stored rat hippocampal tissue: A comparison of survivability and histological and connective organization. J. Comp. Neurol. 1984;227(4):559–568. doi: 10.1002/cne.902270407. [DOI] [PubMed] [Google Scholar]
- 45.Kanakaraj P, Schafer PH, Cavender DE, Wu Y, Ngo K, Grealish PF, Wadsworth SA, Peterson PA, Siekierka JJ, Harris CA, Fung-Leung WP. Interleukin (IL)-1 receptor-associated kinase (IRAK) requirement for optimal induction of multiple IL-1 signaling pathways and IL-6 production. J. Exp. Med. 1998;187(12):2073–2079. doi: 10.1084/jem.187.12.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kordower JH, Brundin P. Lewy body pathology in long-term fetal nigral transplants: Is Parkinson’s disease transmitted from one neural system to another? Neuropsychopharmacology. 2009;34(1):254. doi: 10.1038/npp.2008.161. [DOI] [PubMed] [Google Scholar]
- 47.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leite JO, Vaishnav U, Puglisi M, Fraser H, Trias J, Fernandez ML. A-002 (Varespladib), a phospholipase A2 inhibitor, reduces atherosclerosis in guinea pigs. BMC Cardiovasc. Disord. 2009;9:7. doi: 10.1186/1471-2261-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Cao D, Garber DW, Kim H, Fukuchi K. Association of aortic atherosclerosis with cerebral beta-amyloidosis and learning deficits in a mouse model of Alzheimer’s disease. Am. J. Pathol. 2003;163(6):2155–2164. doi: 10.1016/s0002-9440(10)63572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacGillivray MK, Cruz TF, McCulloch CA. The recruitment of the interleukin-1 (IL-1) receptor-associated kinase (IRAK) into focal adhesion complexes is required for IL-1beta -induced ERK activation. J. Biol. Chem. 2000;275(31):23509–23515. doi: 10.1074/jbc.M003186200. [DOI] [PubMed] [Google Scholar]
- 51.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler. Thromb. 1992;12(8):911–919. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- 52.Mensink RP, Zock PL, Katan MB, Hornstra G. Effect of dietary cis and trans fatty acids on serum lipoprotein[a] levels in humans. J. Lipid Res. 1992;33(10):1493–1501. [PubMed] [Google Scholar]
- 53.Moynagh PN. The interleukin-1 signalling pathway in astrocytes: A key contributor to inflammation in the brain. J. Anat. 2005;207(3):265–269. doi: 10.1111/j.1469-7580.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moynagh PN, Williams DC, O’Neill LA. Activation of NF-kappa B and induction of vascular cell adhesion molecule-1 and intracellular adhesion molecule-1 expression in human glial cells by IL-1. Modulation by antioxidants. J. Immunol. 1994;153(6):2681–2690. [PubMed] [Google Scholar]
- 55.Nakao N, Frodl EM, Duan WM, Widner H, Brundin P. Lazaroids improve the survival of grafted rat embryonic dopamine neurons. Proc. Natl. Acad. Sci. USA. 1994;91(26):12408–12412. doi: 10.1073/pnas.91.26.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogata N, Matsuoka M, Imaizumi M, Arichi M, Matsumura M. Decreased levels of pigment epithelium-derived factor in eyes with neuroretinal dystrophic diseases. Am. J. Ophthalmol. 2004;137(6):1129–1130. doi: 10.1016/j.ajo.2003.11.080. [DOI] [PubMed] [Google Scholar]
- 57.Price ML, Hoffer BJ, Granholm AC. Effects of GDNF on fetal septal forebrain transplants in oculo. Exp. Neurol. 1996;141(2):181–189. doi: 10.1006/exnr.1996.0152. [DOI] [PubMed] [Google Scholar]
- 58.Romanatto T, Roman EA, Arruda AP, Denis RG, Solon C, Milanski M, Moraes JC, Bonfleur ML, Degasperi GR, Picardi PK, Hirabara S, Boschero AC, Curi R, Velloso LA. Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J. Biol. Chem. 2009;284(52):36213–36222. doi: 10.1074/jbc.M109.030874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Shetty AK, Rao MS, Hattiangady B. Behavior of hippocampal stem/progenitor cells following grafting into the injured aged hippocampus. J. Neurosci. Res. 2008;86(14):3062–3074. doi: 10.1002/jnr.21764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sortwell CE, Camargo MD, Pitzer MR, Gyawali S, Collier TJ. Diminished survival of mesencephalic dopamine neurons grafted into aged hosts occurs during the immediate postgrafting interval. Exp. Neurol. 2001;169(1):23–29. doi: 10.1006/exnr.2001.7644. [DOI] [PubMed] [Google Scholar]
- 61.Strandberg L, Verdrengh M, Enge M, Andersson N, Amu S, Onnheim K, Benrick A, Brisslert M, Bylund J, Bokarewa M, Nilsson S, Jansson JO. Mice chronically fed high-fat diet have increased mortality and disturbed immune response in sepsis. PLoS One. 2009;4(10):e7605. doi: 10.1371/journal.pone.0007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: Underlying mechanisms. Neuroscience. 2009;158(3):1062–1073. doi: 10.1016/j.neuroscience.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 63.Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, Pang CP, Lam DS. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am. J. Ophthalmol. 2006;141(3):456–462. doi: 10.1016/j.ajo.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 64.Torrente Y, Polli E. Mesenchymal stem cell transplantation for neurodegenerative diseases. Cell Transplant. 2008;17(10–11):1103–1113. doi: 10.3727/096368908787236576. [DOI] [PubMed] [Google Scholar]
- 65.Tuba A, Kalman M, Makarov FN. [The significance of the vascularization of embryonic donor brain for the viability of intraocular transplants in the rat] Morfologiia. 1996;110(4):29–32. [PubMed] [Google Scholar]
- 66.Vance JE, Hayashi H, Karten B. Cholesterol homeostasis in neurons and glial cells. Semin. Cell Dev. Biol. 2005;16(2):193–212. doi: 10.1016/j.semcdb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Vance JE, Karten B, Hayashi H. Lipid dynamics in neurons. Biochem. Soc. Trans. 2006;34(Pt. 3):399–403. doi: 10.1042/BST0340399. [DOI] [PubMed] [Google Scholar]
- 68.Velloso LA, Araujo EP, de Souza CT. Diet-induced inflammation of the hypothalamus in obesity. Neuroimmunomodulation. 2008;15(3):189–193. doi: 10.1159/000153423. [DOI] [PubMed] [Google Scholar]
- 69.Willis L, Bickford P, Zaman V, Moore A, Granholm AC. Blueberry extract enhances survival of intraocular hippocampal transplants. Cell Transplant. 2005;14(4):213–223. doi: 10.3727/000000005783983142. [DOI] [PubMed] [Google Scholar]
- 70.Willis L, Quintero EM, Nelson M, Granholm A. Regulation of trophic factor expression by innervating target regions in intraocular double transplants. Cell Transplant. 2005;14(1):21–29. [PubMed] [Google Scholar]
- 71.Willis LM, Freeman L, Bickford PC, Quintero EM, Umphlet CD, Moore AB, Goetzl L, Granholm AC. Blueberry supplementation attenuates microglial activation in hippocampal intraocular grafts to aged hosts. Glia. 2010;58(6):679–690. doi: 10.1002/glia.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Willis LM, Small BJ, Bickford PC, Umphlet CD, Moore AB, Granholm AC. Dietary blueberry supplementation affects growth but not vascularization of neural transplants. J. Cereb. Blood Flow Metab. 2008;28(6):1150–1164. doi: 10.1038/jcbfm.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winocur G, Greenwood CE. The effects of high fat diets and environmental influences on cognitive performance in rats. Behav. Brain Res. 1999;101(2):153–161. doi: 10.1016/s0166-4328(98)00147-8. [DOI] [PubMed] [Google Scholar]
- 74.Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol. Aging. 2005;26(Suppl. 1):46–49. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 75.Yurek DM, Flectcher AM, Kowalczyk TH, Padegimas L, Cooper MJ. Compacted DNA nanoparticle gene transfer of GDNF to the rat striatum enhances the survival of grafted fetal dopamine neurons. Cell Transplant. 2009;18(10):1183–1196. doi: 10.3727/096368909X12483162196881. [DOI] [PMC free article] [PubMed] [Google Scholar]