Abstract
Short-term memory (STM) has generally been thought to be independent of the medial temporal lobe (MTL) in contrast to long-term memory (LTM). Prodromal Alzheimer's disease (AD) is a condition in which the MTL is a major early focus of pathology and LTM is thought disproportionately affected relative to STM. However, recent studies have suggested a role for the MTL in STM, particularly hippocampus, when binding of different elements is required. Other work has suggested involvement of extrahippocampal MTL structures, particularly in STM tasks that involve item-level memory. We examined STM for individual objects, locations, and object-location conjunctions in amnestic mild cognitive impairment (MCI), often associated with prodromal AD. Relative to age-matched, cognitively normal controls, MCI patients not only displayed impairment on object-location conjunctions but were similarly impaired for non-bound objects and locations. Moreover, across all participants, these conditions displayed dissociable correlations of cortical thinning along the long axis of the MTL and associated cortical nodes of anterior and posterior MTL networks. These findings support the role of the MTL in visual STM tasks and the division of labor of MTL in support of different types of memory representations, overlapping with findings in LTM.
Keywords: Alzheimer's disease, hippocampus, memory, mild cognitive impairment, perirhinal cortex
Introduction
Alzheimer's disease (AD) involves the accumulation of the hallmark pathologic substrates of amyloid plaques and neurofibrillary tangles (NFTs) and leads to progressive decline in cognitive and functional status (Brun and Gustafson 1976; Arnold et al. 1991; McKhann et al. 2011). Generally, episodic, or long term (LTM), memory is accepted to be the initial and most salient domain of cognitive impairment in AD consistent with the initial involvement of NFTs in medial temporal lobe (MTL) structures and perhaps amyloid deposition within the default mode network, tightly linked to episodic memory function (Braak and Braak 1991; Buckner et al. 2005; Dubois et al. 2007).
Short-term memory (STM) is generally thought less affected during the prodromal (i.e., mild cognitive impairment [MCI]) and mild stages of AD. This profile is consistent with a large body of literature, which has suggested sparing of short-term and working memory in individuals with lesions limited to the MTL despite profound impairments of episodic memory (Wickelgren 1968; Warrington and Baddeley 1974; Cave and Squire 1992). Indeed, the paradigmatic MTL amnesic patient HM displayed a striking dissociation between his ability to retain information over brief, undistracted periods of time, but complete loss of this information when active rehearsal was disrupted (Squire and Wixted 2011).
However, recent work has suggested that memory for certain stimuli after delays within the traditional range of STM paradigms (<15 s) may be dependent on the integrity of MTL structures (Olson et al. 2006; Race et al. 2013). In particular, several groups have argued that STM tasks that require the binding of different elements, such as an item in a context, are dependent on the MTL (Hannula et al. 2006; Olson et al. 2006; Finke et al. 2008; Yee et al. 2014). For example, Olson and colleagues reported that memory for the binding of an object with a location on a 3 × 3 grid (conjunction condition) after delays of 1 and 8 s was associated with disproportionate impairment in hippocampal amnesics relative to memory for single features (i.e., objects or locations). Indeed, when carefully matching for “working memory load,” amnesic patients performed normally relative to age-matched controls on the single feature condition suggesting a selective role of the MTL in relational STM.
Alternatively, other work has suggested that the MTL may also support item-level representations in STM depending on the nature of the stimuli. For example, both functional imaging and lesion-based human studies have argued for the role of extrahippocampal MTL structures, such as the perirhinal cortex (PRC), in the maintenance of face stimuli in STM (Ranganath and D'Esposito 2001; Olsen et al. 2009; Race et al. 2013). Further, work in monkeys suggested the necessity of the PRC in STM for novel objects (Zola-Morgan et al. 1989). Some have argued that specialization of MTL representations within STM is analogous to several models of LTM (Ranganath 2010; Race et al. 2013). These models generally argue that the PRC and parahippocampus (PHC) support the memory of unitized items or objects and contextual details, respectively, whereas the hippocampus binds these elements together. Evidence for this in the STM domain is still somewhat limited, but as noted earlier a number of studies have found that interitem or item–context relations are particularly impaired in individuals with hippocampal lesions, as well as being associated with hippocampal activation during functional imaging studies (Mitchell, Johnson, Raye, D'Esposito 2000; Hannula and Ranganath 2008). Alternatively, Race and colleagues reported that recognition of novel faces after 8-s and 15-s delays were only impaired in amnesics whose MTL lesions extended into extrahippocampal MTL regions (Race et al. 2013). Nonetheless, it is worth noting that not all work has been consistent with the role of the hippocampus and MTL structures in STM, and it has been argued that those tasks that are impaired in amnesic patients are generally due to a reliance on LTM (Shrager et al. 2008; Baddeley et al. 2010; Jeneson et al. 2012).
In light of the potential role of MTL structures in STM, we examined the performance of patients with amnestic MCI and cognitively normal (CN) older adults on a task that required maintenance of either individual features (i.e., objects or locations) versus conjunctions (i.e., object-location bindings) after a delay (8 s) consistent with the STM domain. MCI is often conceptualized as a transitional state between normal aging and clinical dementia. While the diagnostic category of MCI is heterogeneous with regard to underlying etiology, a vast literature supports the notion that patients with amnestic MCI are enriched in those with underlying prodromal AD. The earliest NFT pathology of AD involves the transentorhinal cortex (i.e., the medial wall of the PRC), followed by the entorhinal cortex and then the CA1 subfield of the hippocampus proper (Arnold et al. 1991; Braak and Braak 1991). Both pathological and structural imaging data support the notion that, by the time that individuals are symptomatic and meet criteria for MCI designation, they tend to display such pathology in both extrahippocampal MTL and hippocampal structures (Petersen et al. 2006; Pluta et al. 2012; Augustinack et al. 2013).
As such, we anticipate that if the MTL only plays a role in STM for relational information, there should be disproportionate impairment of MCI patients in the conjunction condition, as was seen in amnesics with hippocampal lesions (Olson et al. 2006). Alternatively, if object or item-level information is supported by the PRC, we would also expect impairment on the feature condition for objects. In addition to behavioral measurement, we related performance on the experimental task to the measures of cortical thickness across all participants to determine whether the neural substrates supporting these different representations (i.e., objects vs. conjunctions) dissociate. Finally, we related performance on this STM task to performance on a long-term, recognition memory task that estimates the integrity of recollection and familiarity (Wolk et al. 2008, 2013). Recollection is thought to reflect associative, contextual memories whereas familiarity is conceptualized as an acontextual item memory (Yonelinas 2002; Eichenbaum et al. 2007). If the neural mappings of LTM overlap with that of STM, we would predict that performance on the conjunction condition would correlate more strongly with recollection whereas the object condition would correlate more strongly with familiarity.
Methods
Participants
Forty-three CN older adults (mean age: 71.7 ± 9.0 [SD] years; mean education 16.1 ± 2.9 [SD] years) and 31 adults with a diagnosis of a-MCI (mean age: 72.4 ± 7.5 [SD] years; mean education 16.2 ± 2.9 [SD] years) participated in the study (1 additional CN adult and 3 a-MCI patients were excluded due to performance on the experimental task suggesting poor understanding of the study instructions). All participants were recruited from the Penn Memory Center (PMC), which includes individuals in the University of Pennsylvania's Alzheimer's Disease Center. Each participant underwent an extensive evaluation, including medical history and physical examination, neurological history and examination, and psychometric testing. Clinical diagnosis was determined by a consensus group of neurologists, neuropsychologists, and psychiatrists at the PMC.
Diagnosis of a-MCI was made essentially following the criteria of Peterson and others (Petersen 2004; Winblad et al. 2004). Patients had to have a memory complaint, generally intact cognitive functioning and activities of daily living, objective evidence of memory impairment on cognitive testing, and not qualify for diagnosis of dementia. There was no strict cutoff based on the degree of memory impairment, but generally patients performed greater than 1.5 SDs below age-adjusted means on verbal and/or nonverbal memory tests. Patients with a-MCI included those with isolated memory impairment (i.e., single domain, n = 14) and those with involvement of other aspects of cognition (i.e., multiple domain, n = 17). Control participants did not exhibit significant cognitive complaints, performed normally on age-adjusted cognitive measures, and were designated by the consensus group as ‘normal’.
For the purposes of this study, each subject completed the following psychometric battery within 3 months of the experimental paradigm: Mini-Mental Status Exam (MMSE [Folstein et al. 1975]); Digit Span subtest of the Wechsler Adult Intelligence Scale III (Wechsler 1987); category fluency (animals) (Spreen and Strauss 1998); Consortium to Establish a Registry for Alzheimer's Disease Word List Memory (WLM) test (Morris et al. 1989); Trail Making Test (TMT) A and B (Reitan 1958); and a 30-item version of the Boston Naming Test (BNT [Kaplan et al. 1983]).
Inclusion criteria for all participants included age between 55 and 89, more than 7 years of education, and English speaking at an early age. Participants were excluded if they had a history of clinical stroke, traumatic brain injury, alcohol or drug abuse/dependence, prior electroconvulsive therapy, and any significant disease or medical/psychiatric condition thought to impact neuropsychological performance. The study was approved by the Institutional Review Board of the University of Pennsylvania.
Materials and Design
The design of the experimental task was adapted from previous studies (Mitchell, Johnson, Raye, Mather, et al. 2000; Olson et al. 2006). The stimuli consisted of 237 Snodgrass and Vanderwort-renderings of common objects and animals (Rossion and Pourtois 2004). Stimuli were presented within a 3 × 3 black grid on a white background. Stimuli were shown without replication.
Equipment and Procedure
Participants were tested individually on a laptop computer. For each trial, they studied 3 objects, each in a different grid position. After a short, uninterrupted delay, they then performed a recognition memory task on either prior object presentation (“object” condition), location presentation (“location” condition), or object-location presentation (“conjunction” condition). The task was programmed and run using E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA, USA).
For all trials, regardless of condition, a blank grid appeared on the screen for 500 ms to signal the beginning of each study sequence. Three stimuli were then presented sequentially for 1 s each in 1 of 9 randomly chosen locations within the grid except that locations were never repeated during any three-item study sequence. After the study sequence, the screen went blank for 8 s of delay period. This was then followed by a recognition memory test probe in which participants made an “old” or “new” response based on whether the test item was 1 of the 3 previously studied stimuli. The test probe remained on the screen until a response was recorded; the screen then cleared for 500 ms before the next trial.
The object and location conditions were intermixed for a total of 48 trials (24 objects; 24 locations). To ensure that the participants were attempting to retain both object and location information, the specific recognition memory decision was not presented until after the 8-s delay. After which a “Remember Object” or “Remember Location” prompt appeared for 1 s prior to the test probe. For object trials, a single object stimulus was presented in the center of the grid that was either previously studied or not (12 studied; 12 unstudied). Alternatively, for the location condition, a filled black circle was presented in one of the grid locations, either at the site of one of the previously presented 3 study stimuli or at a different location (12 studied; 12 unstudied). Trial type order was randomized.
The conjunction condition was performed separately as its own block (24 trials). The study portion of the task was identical to the object/location condition. Participants were again asked to remember object and location information of each presented stimulus for the uninterrupted 8 s of delay period in which the screen went blank. After the delay, a “Remember Object + Location” prompt appeared on the screen for 1 s. This was then followed by the test probe. The test stimulus consisted of one of the previously studied objects and one of the previously studied grid locations of the preceding study stimuli. Again, participants made an “old” or “new” designation. Correct “old” responses were for objects presented in the same location as at study whereas correct “new” responses were for test items in which the test probe object was rearranged with the location of one of the other 2 stimuli for that trial. Half the trials were intact object-location mappings and half were re-arranged (12 trials each).
In all cases, the object/location condition occurred prior to the conjunction condition so that testing conditions were the same for all individuals. Each block was preceded by 3 practice trials. For each condition, ‘hits’ (i.e., correct “old” endorsements) and ‘false alarms’ (i.e., incorrect “new” endorsements for unstudied objects/locations or object-location conjunction) were calculated. To account for differences in baseline false alarm rates independent of response bias, a measure of discrimination (d′) derived from signal detection theory was calculated (Yonelinas et al. 1995; Davidson et al. 2006).
Recollection and Familiarity Task
An additional behavioral paradigm was performed to estimate recollection and familiarity. This task is a variant of the ‘Process Dissociation Procedure (PDP)’ and was previously described in prior reports involving some of the current cohort (Wolk et al. 2008, 2011, 2013). In brief, subjects studied unrelated word pairs. At test they were shown “intact” pairs, “rearranged” pairs in which each word was previously studied, and “novel” pairs in which neither word was previously studied. They were instructed to make an “Old/New” decision, but to only endorse intact pairs as “Old.” Using the language of the PDP, intact pairs are considered the ‘included’ items. Rearranged pairs, or the ‘excluded’ items, produce a condition in which recollection opposes familiarity. As each word of the rearranged pair had been studied previously, these items would be associated with familiarity, driving the subject to incorrectly endorse the pair as “Old.” However, the contextual retrieval of recollection would allow the subject to recall that the words had a different associate at study and correctly endorse the pair as “New.” Based on the rate of “Old” endorsements to these classes of items, one can calculate estimates of recollection (R) and familiarity (F) based on the following: R = p(included) − p(excluded); F = p(excluded)/(1 − R). To account for differences in base rates of false alarms (“Old” responses to novel words), familiarity was calculated using discrimination (d′) measure.
Image Acquisition
A subset of participants underwent MRI scanning (33 CN adults, 27 MCI patients). This group did not significantly differ from the larger behavioral sample on demographic and standard testing. MRI scans were acquired on a 3T Siemens Trio scanner at the Hospital of the University of Pennsylvania. A standard T1-weighted (MPRAGE) whole brain scan was acquired with the following parameters (TR/TE/TI = 1600/3.87/950 ms, 15° flip angle, 1.0 × 1.0 × 1.0 mm3 resolution, acquisition time 5:13).
Image Processing
Subject's structural MRI was preprocessed using the N4ITK tool (Tustison et al. 2010) to minimize intensity inhomogeneity. Tissue segmentation was performed using the Atropos tool (Avants et al. 2011), generating gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) probability maps. These tissue probability maps were then used to measure GM thickness using the DiReCT algorithm (Das et al. 2009), which uses a diffeomorphic mapping between the GM–WM and GM–CSF interface to estimate local thickness of the GM sheet.
Each subject's structural MRI was also mapped to a population template described in (Tustison et al. 2014) using high-dimensional diffeomorphic registration implemented in the ANTs tool (Avants et al. 2008). These mappings were applied to the subject's GM thickness maps providing voxel-wise thickness values for each subject in a common image space. Isotropic spatial smoothing was performed with a Gaussian kernel of width 3 mm.
Statistical Analysis of Imaging Data
A general linear model was constructed at each voxel in the template image space, with estimated local thickness as the dependent variable. Explanatory variables consisted of 1 of the 3 behavioral measures, age, and education. In an analysis across the entire cohort (MCI, CN), the statistical parametric maps of the t-statistic for main effect of the respective behavioral measure were further analyzed to correct for multiple comparisons. Specifically, the threshold-free cluster enhancement method (Smith and Nichols 2009) implemented in the FSL toolkit (Smith et al. 2004) was used to define clusters of significant effect, followed by family-wise error rate (FWER) correction based on permutation-based clustering (Nichols and Holmes 2002). An additional analysis explored the relationship between cortical thickness and behavioral performance on the 3 STM conditions in the a-MCI group alone.
Statistical Analysis of Behavioral Data
Statistical analyses were performed in a standard manner using SPSS 20.0. In general, group differences were determined using t-tests and analysis of variance (ANOVA). The Greenhouse–Geisser correction procedure was used for repeated-measures factors with greater than 1 numerator degree of freedom. Correlation coefficients were calculated for determining the relationship between measures of recollection and familiarity and conditions of the STM task.
Results
Demographic and Psychometric Data
Demographic and psychometric data are presented in Table 1. The groups did not differ with regard to age or education. While the overall degree of cognitive impairment of the a-MCI group was relatively mild based on the MMSE (27.8), they performed significantly worse than that of the CN group (29.4) (t72 = 5.6; P < 0.001). As a point of reference, the mean MMSE from the Alzheimer's Disease Neuroimaging Initiative a-MCI cohort was 27.0 (Petersen et al. 2010). As expected, the a-MCI group was significantly impaired on tests of memory relative to the CN participants. Nonmemory cognitive measures were also affected consistent with the multi-domain status of many of these patients but were generally impaired to a lesser degree and often were within 1 standard deviation of the control group.
Table 1.
Demographic and psychometric data
| CN (n = 43) | MCI (n = 31) | sd MCI (n = 14) | md MCI (n = 17) | |
|---|---|---|---|---|
| Age (years) | 71.7 (9.0) | 72.4 (7.5) | 75.1 (6.9) | 70.1 (7.4) |
| Education (years) | 16.1 (2.9) | 16.2 (2.9) | 17.3 (2.7) | 15.4 (2.9) |
| Gender (% female) | 58.1 | 48.4 | 35.7 | 58.8 |
| ApoE4 carrier status (%) | 27.8 | 50.0 | 55.6 | 46.2 |
| MMSE | 29.4 (0.9) | 27.8 (1.5)** | 28.4 (1.5) | 27.4 (1.4) |
| WLM immediate recall | 23.1 (3.6) | 16.6 (4.8)** | 18.8 (5.7) | 16.3 (5.7) |
| WLM delayed recall | 8.0 (1.8) | 3.9 (2.0)** | 4.4 (2.0) | 3.6 (2.0) |
| Digit span forwards | 7.3 (0.9) | 6.9 (1.1) | 6.9 (1.4) | 6.9 (0.8) |
| Digit span backwards | 5.2 (1.3) | 4.8 (1.2) | 5.2 (1.1) | 4.5 (1.2) |
| TMT A (s) | 31.7 (11.3) | 42.5 (30.8)* | 34.8 (10.5) | 48.8 (39.9) |
| TMT B (s) | 68.2 (21.8) | 124.4 (69.3)** | 98.2 (36.2) | 145.9 (82.7) |
| Category fluency (animals) | 21.3 (5.4) | 16.4 (5.2)** | 19.3 (4.6) | 13.9 (4.6) |
| BNT | 28.6 (1.7) | 26.9 (3.8)* | 28.3 (1.4) | 25.7 (4.7) |
Note: WLM immediate recall is the sum of the 3 immediate memory trials. Note that 2 MCI and 10 CN adults did not complete the digit span task and 9 MCI and 7 CN adults did not have ApoE data available. *P < 0.05; **P < 0.01, relative to controls.
Experimental Paradigm
Performance on the working memory task is displayed in Table 2. As can be observed, CN adults performed better than a-MCI patients not only in the conjunction condition but also for location and object discrimination. Despite the poorer performance of the a-MCI group, their discrimination of studied and unstudied stimuli was far from chance levels. A repeated-measures ANOVA on the proportion of old responses for factors of condition (object, location, and conjunction), study status (studied, unstudied), and group (CN, a-MCI) was calculated. Significant main effects of study status (F1,72 = 1161.2, P < 0.001), due to greater old endorsements for studied than unstudied items, group (F1,72 = 16.7, P < 0.001), and condition (F1,72 = 45.8, P < 0.001) were observed. Study status interacted with both group (F1,72 = 34.4, P < 0.001) and condition (F2,144 = 7.3, P < 0.001), the former reflecting the poorer discrimination in the a-MCI group, and the latter due to somewhat better overall discrimination in the location condition. A condition × group interaction was also observed (F2,144 = 4.3, P < 0.05), likely reflecting a disproportionate reduction in old endorsements in the a-MCI group for the object relative to other conditions. Notably, follow-up ANOVAs in each group yielded significant main effects of study status consistent with well-above chance discrimination in both groups (CN: [F1,42 = 2808.7, P < 0.001]; a-MCI: [F1,30 = 177.9, P < 0.001]).
Table 2.
Working memory task performance
| CN (n = 43) | MCI (n = 31) | Cohen's d | |
|---|---|---|---|
| Object hits | 0.85 (0.16) | 0.62 (0.24)** | |
| Object false alarms | 0.02 (0.04) | 0.06 (0.07)* | |
| Location hits | 0.94 (0.07) | 0.80 (0.21)** | |
| Location false alarms | 0.06 (0.07) | 0.13 (0.16)* | |
| Conjunction hits | 0.92 (0.07) | 0.77 (0.18)** | |
| Conjunction false alarms | 0.10 (0.10) | 0.21 (0.15)* | |
| Object discrimination (d′) | 2.83 (0.64) | 1.86 (0.96)** | 1.19 |
| Location discrimination (d′) | 2.97 (0.58) | 2.17 (1.05)** | 0.94 |
| Conjunction discrimination (d′) | 2.64 (0.68) | 1.74 (1.08)** | 1.00 |
Note: *P < 0.01; **P < 0.001.
To more directly assess discrimination (d′), a repeated-measures ANOVA with factors of condition (object, location, and conjunction) and group (CN, a-MCI) was performed on the d′ measures. A significant main effect of group (F1,72 = 29.9, P < 0.001) was observed due to the CN adults displaying better discrimination than a-MCI patients. In addition, a main effect of condition was also present (F2,144 = 8.9, P < 0.001) due to a trend toward poorer discrimination in the conjunction condition than object and, in turn, a significant difference in object relative to location (conjunction vs. location: t73 = 4.5, P < 0.001; conjunction vs. object: t73 = 1.7, P < 0.09; object vs. location: t73 = 2.3, P < 0.05). Importantly, there was no condition × group interaction (F2,144 < 1.0) and thus no evidence of a relatively more selective impairment of the conjunction condition in a-MCI. Indeed, a-MCI patients performed significantly worse in each condition (object: t72 = 5.3, P < 0.001; location: t72 = 4.2, P < 0.001; conjunction: t72 = 4.4, P < 0.001) with the largest Cohen's d effect size observed in the object task.
Reaction time data for correct responses in each condition are presented in Table 3. Reaction times appeared to be generally slower across conditions in the a-MCI group. Confirming this impression, an ANOVA with factors of condition (object, location, and conjunction) and group (CN, a-MCI) revealed a main effect of group (F1,72 = 11.8, P < 0.01). An effect of condition was also present (F2,144 = 19.4, P < 0.001) with slowest reaction times in the conjunction condition, likely reflecting, in part, its greater difficulty than the other conditions. Interestingly, there was also a significant interaction between condition × group (F2,144 = 3.3, P < 0.05). This appeared to reflect the fact that the group difference in reaction time was greatest in the object condition, particularly relative to the conjunction task. Indeed, in follow-up ANOVAs comparing each pair of conditions, a significant interaction of condition × group was only observed when comparing the object versus the conjunction task (F1,72 = 4.7, P < 0.05). However, a trend toward significance was also found when comparing location with conjunction (F1,72 = 2.9, P = 0.09).
Table 3.
Reaction times for correct responses
| Reaction time (ms) | CN (n = 43) | MCI (n = 31) |
|---|---|---|
| Object correct responses | 1742.9 (456.2) | 2680.9 (1680.2)* |
| Location correct responses | 1931.0 (556.3) | 2761.4 (1564.5)* |
| Conjunction correct responses | 2389.9 (685.3) | 2951.5 (986.1)* |
Note: *P < 0.01.
Finally, we also examined task performance within the MCI group based on whether participants had single- (sd) or multiple-domain (md) impairment. As expected, the md-MCI patients tended to be more impaired on standard psychometric measures (MMSE: sd vs. md, 28.4 vs. 27.4, t(29) = 1.9, P = 0.06), particularly those involving nonmemory domains (see Table 1). Overall, the sd-MCI group performed at an intermediate level between the md-MCI and CN adults across the 3 conditions. To more formally examine discrimination performance, a repeated-measures ANOVA with factors of condition (object, location, and conjunction) and group (sd and md) was performed on the d’ measures. A significant main effect of group (F1,29 = 6.1, P < 0.05) was present due to generally poorer performance in the md-MCI group compared with those with sd-MCI. As in the overall analysis, there was also a significant effect of condition (F2,58 = 4.6, P < 0.05) due to progressively lower discrimination in the location, object, and conjunction conditions, respectively. There was not an interaction between condition and group (F2,58 = 1.4, P > 0.1). However, it is worth noting that the group difference in the object condition appeared somewhat blunted in absolute terms and was the only condition that did not reach statistical significance in direct comparison (object: sd vs. md, 2.11 vs. 1.65, t(29) = 1.3, P > 0.1; location: sd vs. md, 2.66 vs. 1.77, t(29) = 2.5, P < 0.05; conjunction: 2.24 vs. 1.33, t(29) = 2.5, P < 0.05).
Structural Imaging Correlations
We performed linear correlation analysis to examine the relationship between behavioral performance and GM thickness as measured from structural MRI. Significant clusters (FWER, P < 0.05) of positive correlation between performance and thickness across all participants are depicted in Figure 1 using representative slices. All 3 tasks were significantly correlated with some brain regions. The largest significant cluster for the object memory condition was located in the left anterior MTL, including PRC and anterior hippocampus, as seen in the coronal slice through this region. Medial orbitofrontal cortex was also associated with performance in the object condition. In contrast, the conjunction condition was most significantly associated in the posterior cingulate region, as well as right posterior MTL, including the parahippocampus, as seen in the mid-sagittal and right-sagittal slices, respectively. Notably, the significant clusters associated with the object and conjunction conditions had no overlapping voxels, as indicated by the absence of any significant voxels colored yellow in Figure 1. The location condition was associated with significant clusters in the right insular cortex and left superior parietal gyrus; the former cluster overlapped with a significant cluster for the object condition, and the latter cluster overlapped with one for the conjunction condition. Coordinates of the peak voxels of these clusters are included in the Supplementary Table 1.
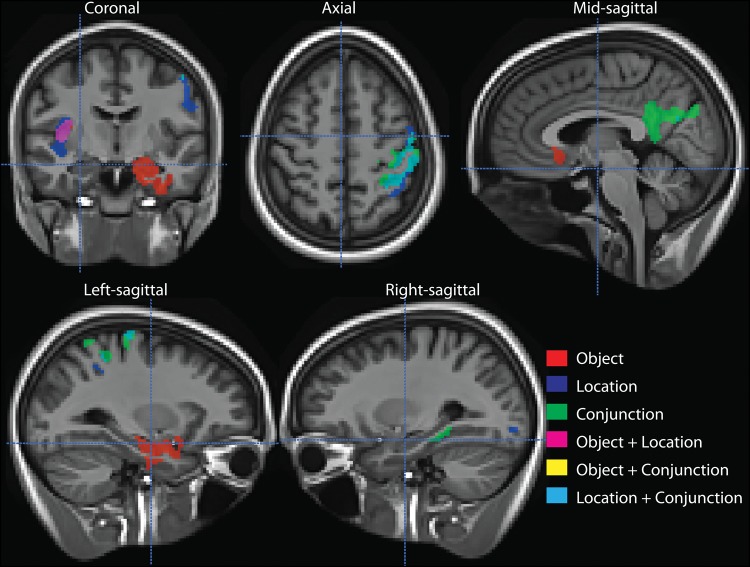
Figure 1.
Significant clusters of positive correlation between cortical thickness and performance in respective cognitive tasks among all participants. Colors indicate individual task correlations and overlapping voxels between clusters of pairs of tasks. Note that there is no yellow voxels indicating no overlap between the object and conjunction conditions. All clusters are corrected at FWER P < 0.05.
To further test the apparent dissociation, particularly with regard to MTL regions, of the object and conjunction conditions, we extracted the mean cortical thickness of the largest clusters associated with each condition. In light of their anatomic location, we will refer to the cluster associated with the object and conjunction condition as “anterior” and “posterior,” respectively. The object condition correlated more strongly with the anterior cluster (r = 0.50, P < 0.0001) than the posterior one (r = 0.34, P < 0.01), including age and education as covariates. Alternatively, the conjunction condition correlated with the posterior cluster (r = 0.52, P < 0.0001) and only marginally with the anterior one (r = 0.24, P < 0.08). However, it is worth noting that cognitive and structural measures tend to covary to different extents in this type of cohort. To further test the degree to which these effects are truly dissociable, we controlled for the shared variance of each condition by covarying for the other. With the conjunction condition as covariate, we found that the object condition still strongly correlated with the anterior cluster (r = 0.47, P < 0.001), but not the posterior cluster (r = 0.00, P > 0.1). Alternatively, with the object condition as covariate, the conjunction condition did not correlate with the anterior cluster (r = −0.15, P > 0.1) but still strongly did so for the posterior cluster (r = 0.41, P < 0.01). In other words, the object and conjunction condition correlate with the respective anterior and posterior clusters, but not vice versa, when shared variance between these measures is removed.
We replicated these findings when we restricted these clusters to the MTL alone using an MTL mask and, thus, producing an isolated anterior MTL and a posterior MTL cluster. Again, we observed a relative dissociation across conditions. Object condition: anterior MTL, r = 0.49, P < 0.0001; posterior MTL, r = 0.27, P < 0.05. Conjunction condition: anterior MTL, r = 0.23, P < 0.09; posterior MTL, r = 0.40, P < 0.01. Including the conjunction condition as covariate resulted in a significant correlation of the object condition with the anterior MTL (r = 0.46, P < 0.001), but not posterior MTL (r = 0.00, P > 0.1). Alternatively, including the object condition as a covariate resulted in the conjunction condition significantly correlating with the posterior MTL (r = 0.31, P < 0.05), but not the anterior MTL (r = −0.15, P > 0.1). The same analysis across the entire cortical mantle in which one condition was covaried for the other, using a relatively liberal threshold (P < 0.01, uncorrected), produced similar evidence supporting the dissociation of anterior MTL regions associated with performance on the object condition and posterior MTL and cortical regions, including posterior cingulate/precuneus, with the conjunction condition (Supplementary Fig. 1).
Correlation of Task Performance with Thickness in a-MCI
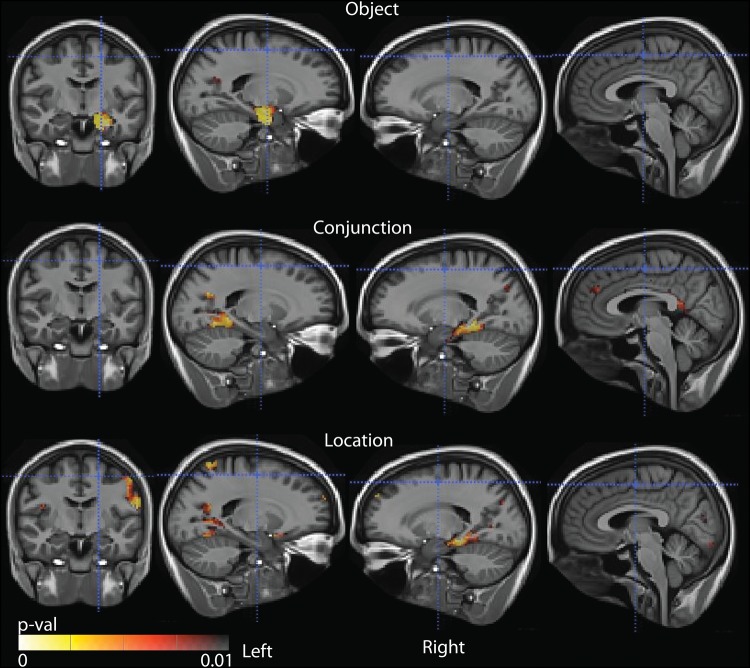
Given the likely variable degree of neurodegenerative changes in the a-MCI group, we also wanted to examine whether we saw similar dissociable correlations with the STM measures in this group alone. No voxels survived FWER correction for multiple comparisons when task performance was correlated with GM thickness only within the a-MCI group. Given the reduced power in this smaller sample, we also examined these relationships with a more liberal threshold of significance (P < 0.01, uncorrected), as the primary goal was to determine the degree to which these measures overlapped in their anatomic mappings. Figure 2 shows that the correlational trend exists in regions largely similar to those seen in the entire cohort. Importantly, the object and conjunction conditions show a similar spatial dissociation as in Figure 1, particularly in the MTL in which there is no overlap. Namely, the object condition is associated with thinning in the PRC/ERC and head of the hippocampus on the left whereas the conjunction (and location) condition is associated with thinning in the PHC and body/tail of the hippocampus. As performance in a-MCI was significantly worse in both conditions than CN, this indicates that the significant effects observed across all participants are unlikely to be driven by a purely group effect, which is also supported by the dissociation of neuroanatomic correlates across conditions.
Figure 2.
Statistical maps indicating P-value of correlation between cortical thickness and performance in respective cognitive tasks in MCI subjects. Only voxels with uncorrected P < 0.01 are shown.
Correlation with Estimates of Recollection and Familiarity
We also examined the relationship of performance of the current task with estimates of recollection and familiarity instituting a study-test delay more standard of putative episodic memory tasks. When we looked across all participants, we found that recollection correlated with all of the STM measures (object: r = 0.37, P < 0.01; location: r = 0.39, P < 0.01; conjunction: r = 0.39, P < 0.01). Alternatively, estimates of familiarity appeared to strongly correlate with object discrimination but only approached significance with the location and conjunction measure (object: r = 0.42, P < 0.001; location: r = 0.22, P = 0.06; conjunction: r = 0.20, P = 0.1). Further supporting the relative dissociation of the object, as opposed to conjunction, condition with familiarity, we found that this correlation was still significant even when including the conjunction condition as a covariate (r = 0.39, P = 0.001). In other words, the object condition explained the variance in familiarity significantly above and beyond that of the conjunction condition.
Discussion
We examined STM for objects, locations, and object-location conjunctions in a-MCI, a condition frequently associated with prodromal AD and the presence of significant MTL pathology. Consistent with the potential importance of the MTL for each of these types of representations in STM, a-MCI patients performed significantly worse than CN adults in all conditions. Additionally, we saw a dissociation in the locus of cortical thinning within the MTL and other isocortical regions in relationship to object memory versus conjunctions. Finally, performance on different conditions of the STM task appeared to map, to a degree, differentially on the LTM constructs of recollection and familiarity. Overall, this work supports the differential role of MTL structures for specific representations in STM and may have clinical implications on the nature of memory loss in prodromal AD.
Consistent with the notion that the MTL may support some aspects of STM, we found that patients likely to have pathology in this brain region display impairment on all 3 conditions examined here. Previous work has argued that only when STM requires the binding of information, such as object-location or object-object associations, is the MTL, particularly hippocampus, required (Hannula et al. 2006; Olson et al. 2006; Finke et al. 2008; Yee et al. 2014). Indeed, utilizing essentially the same experimental paradigm as the current study, Olson and colleagues found that hippocampal amnesics were selectively impaired on the conjunction condition after delays of 1 and 8 s, but performed normally on the ‘feature’ condition (collapsed performance on the object and location conditions). Notably, this was found when working memory load was equated across conditions, as with the current study, by not informing participants in the feature condition whether they would be tested on object or location information. Importantly, all of the patients in the Olson et al. study had hippocampal lesions whereas only half had lesions that extended into extrahippocampal MTL structures. Further, the majority of patients developed amnesia as a result of an anoxic event, which is generally associated with relatively selective involvement of the hippocampus proper without involving cortical MTL regions (Rempel-Clower et al. 1996; Yonelinas et al. 2002).
The current findings then contrast with this prior result because of the concomitant impairment in the single feature conditions, most saliently for objects. Several potential explanations may account for this disparity across the studies. Most compelling is the greater involvement of extrahippocampal MTL structures in a-MCI patients than that of the amnesic participants studied by Olson and colleagues. The first areas of NFT pathology in AD include the perirhinal and entorhinal cortices, or the anterior subhippocampal region, before direct involvement of the hippocampus (Arnold et al. 1991; Braak and Braak 1991). Consistent with this early involvement, several studies have demonstrated significant atrophy in both of these regions that can exceed the relative atrophy of whole hippocampal measurements (Killiany et al. 2002; Augustinack et al. 2013; Yushkevich et al. 2015). If these extrahippocampal MTL structures are important in supporting nonbound, item, or object-level information, then one would expect performance on such measures to be affected in a population enriched in prodromal AD patients (i.e., MCI), as was found in the current study.
Indeed, accuracy on the object condition correlated with cortical thickness of anterior MTL structures, including in the region of the PRC. That item or object-level representations in STM could be supported by extrahippocampal MTL structures has been suggested by several studies, particularly related to paradigms which used novel face stimuli. For example, in a carefully designed experimental paradigm in which the STM nature of the task was confirmed with the introduction of a distractor condition disrupting performance, Race and colleagues tested amnesic patients with both isolated hippocampal lesions and more extensive MTL injury (Race et al. 2013). They found that STM for faces was impaired only in those patients with the involvement of MTL regions outside of the hippocampus. Moreover, this difference could not be attributed to any difference in visuoperceptual integrity. Finally, these investigators also found that STM for faces was disrupted by a distractor task that required configural processing of faces (essentially treating the face as a unitized object) versus feature detection. This finding is consistent with the notion that extrahippocampal MTL regions, particularly PRC, may be particularly important for unitized or object-level representations (Norman and O'Reilly 2003; Giovanello et al. 2006; Diana et al. 2010; Norman 2010; Ranganath 2010).
While much of the work linking PRC with object-level representations has come from the LTM literature, the current work and that of Race and colleagues add to the notion that a similar mapping may occur in STM. Indeed, performance on the object condition was associated relatively selectively with thinning of the anterior MTL, including the PRC. The fact that thinning of this region, likely reflecting early AD pathology, associates with performance enhances the argument for the necessary role of this region in certain STM tasks, expanding on findings in the functional imaging literature (Olsen et al. 2009; Schon et al. 2013). This finding in humans is also consistent with monkey studies of significant STM impairment following PRC lesions (Zola-Morgan et al. 1989; Eacott et al. 1994). Interestingly, performance on the object condition tended to best discriminate patients with MCI from controls, particularly as evidenced by the reaction time data, suggesting this condition may have been particularly difficult in MCI. This observation is quite consistent with recent work demonstrating early functional change in the lateral ERC/PRC and the potential that object level, nonspatial memory may be particularly vulnerable in the very early stages of AD frequently associated with MCI (Wolk et al. 2013; Khan et al. 2014; Yassa 2014). It is thought that medial ERC/parahippocampus and connected regions (posterior cingulate) then “catch up” in NFT pathology. Notably, cortical thinning in these regions overlapped to a greater extent in the conditions requiring spatial or bound information (i.e., location and conjunction condition).
The current findings are in keeping with notion that the subregional MTL contributions to STM overlap with those of LTM (Ranganath 2010). As noted earlier, the PRC has been argued to support object level, unitized information, which is often tightly linked conceptually to familiarity-based memory. Alternatively, PHC and the hippocampus proper, respectively, support contextual features and the linking of this context to item-level representations, which is thought to underlie recollection-based memory. Support for a similar dissociation in STM in the current study comes from 2 levels. One, we found that performance on the object condition in STM, but not location or conjunction, correlated strongly with an estimate of familiarity obtained in the same subjects in a LTM task. However, only a single dissociation was found, as all 3 conditions correlated similarly with recollection. Second, and more convincingly, we found a double dissociation in the relationship of cortical thinning, both across groups and within just MCI patients, between the object and the conjunction conditions. Specifically, we found more anterior MTL regions, including PRC, correlated with object memory performance whereas nonoverlapping posterior MTL regions, including PHC, correlated with the conjunction condition, as well as the location condition in the MCI only analysis.
However, it is worth noting that while the posterior MTL association with the conjunction condition included posterior hippocampus in the analysis of the MCI group alone utilizing a more liberal threshold, only the PHC was included in the significant cluster associated with the analysis across the entire cohort. The lack of hippocampal involvement appears to conflict with the notion from the LTM literature that this structure is specifically involved in the linking of items with contextual aspects of an episode. The PHC and PRC have significant connections, particularly projections from the PHC to PRC (Suzuki and Amaral 1994; Aggleton 2012). It is possible that in the STM domain, these connections support object-location conjunctions without requirement of the hippocampus whereas the more sustained representations of LTM do necessitate its involvement. That said, one should be cautious in over-interpretation given the potential that the lack of hippocampal association may be a threshold effect, as suggested by the MCI only analysis. Moreover, the PHC has strong reciprocal connectivity with the posterior hippocampus (Aggleton 2012) and, as described later, is part of a ‘posterior MTL network’ that together may support associative/contextual memory (Ranganath and Ritchey 2012). Thus, it is possible that the involvement of any of the nodes in this network, such as PHC, could have functional consequences for the posterior hippocampus.
Indeed, recent functional imaging and animal work has suggested that dissociable MTL networks can be defined based on nodes along the long axis of this region (Kahn et al. 2008; Aggleton 2012; Libby et al. 2012; Das et al. 2015). An “anterior MTL network” includes PRC, lateral ERC, anterior hippocampus (head), ventral temporopolar cortex, and orbitofrontal cortex. Alternatively, the “posterior MTL network” includes parahippocampus, medial ERC, posterior hippocampus (body/tail), midline parietal regions (retrosplenial cortex, posterior cingulate, and precuneus), and angular gyrus. While these networks likely support multiple cognitive functions, the anterior network has been linked to object perception/memory and the posterior network to associative/contextual memory. Consonant with the pathology of AD, we recently demonstrated the evidence for functional and structural disruption of both of these networks in MCI (Das et al. 2015). Notably, the regional relationship of cortical thinning to the object versus the conjunction/location conditions in the current study overlapped considerably with both MTL and cortical nodes of the anterior and posterior networks, respectively. The anatomic relationships described here also are similar to findings in a recent episodic memory study of mild AD in which encoding instructions varied between forming a unitized association (i.e., object level) versus a contextual association (i.e., relational) (Bastin et al. 2014). Performance on these conditions was related to a measure of cerebral metabolism, FDG-PET, using a partial least square analysis and revealed a similar dissociation of MTL and cortical regions as in the current STM task.
Interestingly, there was an apparent asymmetry in the relationship of the object and conjunction conditions with left and right hemisphere MTL clusters, respectively. It is possible that these findings reflect material-specific effects that have been described in the LTM literature for decades (Milner 1971). The object condition involved nameable objects, which may have resulted in a stronger left hemisphere representation. Alternatively, the conjunction condition involved spatial contextual information, which may favor the right hemisphere. Indeed, contextual memory involving allocentric spatial information (location of objects relative to environment), as tested here, may be particularly dependent on right MTL in contrast to egocentric spatial information (location of objects relative to self [Burgess et al. 2002; Feigenbaum and Morris 2004]). Nonetheless, it is worth noting that the conjunction asymmetry may be driven, in part, by threshold effects, as in the analysis of the MCI group alone using a more liberal threshold, greater symmetry of the posterior MTL effect was observed, but not for the object condition which remained strongly significant only on the left. Indeed, when we examined mean cortical thickness in the homologous cluster to the significant one on the right in the conjunction condition, we found both correlated with performance across participants (right: r = 0.52, P < 0.001, left: r = 0.41, P < 0.01). Alternatively, the object condition revealed greater asymmetry using the same approach, as the homologous right anterior MTL cluster was not significantly correlated with performance (right: r = 0.21, P > 0.1; left: r = 0.50, P < 0.001). Nonetheless, the degree to which any hemispheric mappings are more general phenomena of STM representations for items or conjunctions versus material-specific effects cannot be addressed with these data, but certainly merits future work varying stimulus materials and perhaps relative lesion involvement of right and left MTL.
The current findings should also be put into the context of a series of studies that have examined STM in preclinical and early symptomatic AD (Parra et al. 2009, 2010, 2011; Della Sala et al. 2012). These investigators employed an experimental paradigm that requires maintenance of either single features (e.g., color or shape) versus binding of these features. In general, they have reported that throughout the spectrum of severity, from asymptomatic carriers of an autosomal dominant gene mutation associated with familial AD to patients with mild clinical AD, there is disproportionate impairment on conditions requiring binding of features, but relative sparing when single features were required to be maintained in STM. Superficially, these results may appear to conflict with the findings related to the object condition of the current study. However, it is notable that their binding condition required the conjunction of features within an object representation, as opposed to interobject relationships. Thus, this condition may more closely parallel the object condition of the paradigm applied here rather than the object-location conjunction condition. Indeed, these investigators and others have argued that the within-object binding of their task is likely dependent on anterior extrahippocampal MTL structures, possibly PRC and ERC, which is in distinction from the associative interitem or context-rich relational representations, which are posited to be rely on hippocampal function (Didic et al. 2011; Parra 2014; Parra et al. 2015). Alternatively, single features may occupy earlier aspects of visual processing, more likely to be spared in early clinical stages of AD.
There are several limitations to this study. As with all studies of MCI, it must be acknowledged that this is a heterogeneous diagnostic category and that only a proportion of individuals will truly have prodromal AD. In light of a modest sample size, there is risk in this or any study of a disproportionate fraction of nonAD patients with other sources of memory decline. However, it should be noted that the regions of cortical thinning linked with task performance are in regions that are largely expected to be associated with pathology in early AD, and it is likely that a significant proportion of our MCI patients do have prodromal AD.
A more theoretical limitation is whether performance on the current task truly resides completely within the STM domain or whether there is some contamination from LTM. While the delay interval for this task (8 s) is within the temporal frame generally considered within the realm of STM, as well as the number of studied items being within a typical STM span, it is possible that LTM processes may also be recruited for task performance and drive the group differences observed. Indeed, it has been proposed that another criterion to determine whether an experimental paradigm depends “purely” on STM is to require that performance be affected by an intervening distraction task during the retention period. As this was not done here, we cannot be certain that LTM did not contribute to accuracy. However, even if this is the case, the current data would still support the notion that memory even after very brief delays, on the order of seconds, is impaired in a-MCI with the material-specific anatomic mappings described.
Finally, it is worth noting that short-term and working memory may be more generally affected in prodromal AD (Gagnon and Belleville 2011; Saunders and Summers 2011; Wilson et al. 2011), particularly considering we included multi-domain MCI in the cohort, and that this more general impairment produced the uniform reduced performance observed across conditions. Indeed, fronto-parietal networks more traditionally thought to support this domain (e.g., [Xu and Chun 2006; Majerus 2013]) are regions that do display evidence of atrophy in early stages of disease (Dickerson et al. 2009). That involvement of these cortical regions may have contributed to impairment on the experimental task is, perhaps, supported by the overall poorer performance of md-MCI relative to sd-MCI group, consonant with the evidence of more extensive cortical neurodegeneration in the former (Bell-McGinty et al. 2005; Whitwell et al. 2007). However, it is also possible that this differential degree of impairment could be due to more extensive MTL involvement in md-MCI if this group is enriched in somewhat further progressed prodromal AD patients relative to sd-MCI, as commonly conceptualized. In fact, the somewhat disproportionate impairment in the object condition for the sd group relative to the other conditions, when compared with the md group, may reflect the fact that object STM maps onto anterior MTL regions (e.g., PRC) that are affected earlier in the AD disease process. Of course, the limited power of the MCI subgroup analysis should militate against over-interpretation of these findings. In terms of the degree of a more general STM impairment in the MCI group, it is also worth considering that digit span, a standard psychometric measure of STM, did not statistically differ between the MCI and CN group, but that this may be a reflection of the power and sensitivity of the measure. Nonetheless, the dissociable anatomic correlates and the specific relationship with MTL regions rather than traditional loci of short-term and working memory seems to argue against a more general STM dysfunction solely accounting for impaired performance on the task.
In conclusion, we found that patients with MCI displayed impairment not only on object-location conjunctions, but also single features, objects, and locations, in a putative STM task. Moreover, performance was linked to dissociable MTL regions and related nodes of their cortical networks. Importantly, object memory appeared particularly difficult for this group and was linked to anterior MTL structures, which are the earliest regions of NFT pathology in AD, and, therefore, a potentially useful cognitive measure for early disease detection and monitoring. Future work will need to examine the relative value of cognitive tasks that more clearly map onto these different MTL regions and networks to determine their relative value as screening measures and biomarkers in AD beyond standard psychometric tests.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by grants from NIH (AG028018, AG010124 and R03 EB016923), and the Alzheimer's Association (NIRG-12-242765).
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Aggleton JP. 2012. Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function. Neurosci Biobehav Rev. 36(7):1579–1596. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. 1991. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1(1):103–116. [DOI] [PubMed] [Google Scholar]
- Augustinack JC, Huber KE, Stevens AA, Roy M, Frosch MP, van der Kouwe AJ, Wald LL, Van Leemput K, McKee AC, Fischl B et al. . 2013. Predicting the location of human perirhinal cortex, Brodmann's area 35, from MRI. Neuroimage. 64:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. 2008. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 12(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC. 2011. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics 9(4):381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Allen R, Vargha-Khadem F. 2010. Is the hippocampus necessary for visual and verbal binding in working memory? Neuropsychologia. 48(4):1089–1095. [DOI] [PubMed] [Google Scholar]
- Bastin C, Bahri MA, Mievis F, Lemaire C, Collette F, Genon S, Simon J, Guillaume B, Diana RA, Yonelinas AP et al. . 2014. Associative memory and its cerebral correlates in Alzheimers disease: Evidence for distinct deficits of relational and conjunctive memory. Neuropsychologia. 63:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-McGinty S, Lopez OL, Meltzer CC, Scanlon JM, Whyte EM, Dekosky ST, Becker JT. 2005. Differential cortical atrophy in subgroups of mild cognitive impairment. Arch Neurol. 62(9):1393–1397. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. 1991. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 82(4):239–259. [DOI] [PubMed] [Google Scholar]
- Brun A, Gustafson L. 1976. Distribution of cerebral degeneration in Alzheimer's disease. A clinico-pathological study. Arch Psychiatr Nervenkr. 223(1):15–33. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC et al. . 2005. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 25(34):7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. 2002. The human hippocampus and spatial and episodic memory. Neuron. 35(4):625–641. [DOI] [PubMed] [Google Scholar]
- Cave CB, Squire LR. 1992. Intact verbal and nonverbal short-term memory following damage to the human hippocampus. Hippocampus. 2(2):151–163. [DOI] [PubMed] [Google Scholar]
- Das SR, Avants BB, Grossman M, Gee JC. 2009. Registration based cortical thickness measurement. NeuroImage. 45(3):867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SR, Pluta J, Mancuso L, Kliot D, Yushkevich PA, Wolk DA. 2015. Anterior and Posterior MTL Networks in Aging and MCI. Neurobiol Aging. Suppl 1:S141–S150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PS, Anaki D, Saint-Cyr JA, Chow TW, Moscovitch M. 2006. Exploring the recognition memory deficit in Parkinson's disease: estimates of recollection versus familiarity. Brain. 129(Pt 7):1768–1779. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Parra MA, Fabi K, Luzzi S, Abrahams S. 2012. Short-term memory binding is impaired in AD but not in non-AD dementias. Neuropsychologia. 50(5):833–840. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. 2010. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. J Cogn Neurosci. 22(8):1808–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD et al. . 2009. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 19(3):497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didic M, Barbeau EJ, Felician O, Tramoni E, Guedj E, Poncet M, Ceccaldi M. 2011. Which memory system is impaired first in Alzheimer's disease? J Alzheimers Dis. 27(1):11–22. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G et al. . 2007. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6(8):734–746. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D, Murray EA. 1994. Preserved recognition memory for small sets, and impaired stimulus identification for large sets, following rhinal cortex ablations in monkeys. Eur J Neurosci. 6(9):1466–1478. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. 2007. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 30:123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenbaum JD, Morris RG. 2004. Allocentric versus egocentric spatial memory after unilateral temporal lobectomy in humans. Neuropsychology. 18(3):462–472. [DOI] [PubMed] [Google Scholar]
- Finke C, Braun M, Ostendorf F, Lehmann TN, Hoffmann KT, Kopp U, Ploner CJ. 2008. The human hippocampal formation mediates short-term memory of colour-location associations. Neuropsychologia. 46(2):614–623. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 1975. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 12(3):189–198. [DOI] [PubMed] [Google Scholar]
- Gagnon LG, Belleville S. 2011. Working memory in mild cognitive impairment and Alzheimer's disease: contribution of forgetting and predictive value of complex span tasks. Neuropsychology. 25(2):226–236. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Keane MM, Verfaellie M. 2006. The contribution of familiarity to associative memory in amnesia. Neuropsychologia. 44(10):1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. 2008. Medial temporal lobe activity predicts successful relational memory binding. J Neurosci. 28(1):116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. 2006. The long and the short of it: relational memory impairments in amnesia, even at short lags. J Neurosci. 26(32):8352–8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Wixted JT, Hopkins RO, Squire LR. 2012. Visual working memory capacity and the medial temporal lobe. J Neurosci. 32(10):3584–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. 2008. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 100(1):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. 1983. The Boston Naming Test. Philadelphia: Lea and Feibiger. [Google Scholar]
- Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA. 2014. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer's disease. Nat Neurosci. 17(2):304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, Tanzi R, Jones K, Albert MS. 2002. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 58(8):1188–1196. [DOI] [PubMed] [Google Scholar]
- Libby LA, Ekstrom AD, Ragland JD, Ranganath C. 2012. Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. J Neurosci. 32(19):6550–6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus S. 2013. Language repetition and short-term memory: an integrative framework. Front Hum Neurosci. 7:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R et al. . 2011. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. 1971. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull. 27(3):272–277. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D'Esposito M. 2000. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Brain Res Cogn Brain Res. 10(1–2):197–206. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, Mather M, D'Esposito M. 2000. Aging and reflective processes of working memory: binding and test load deficits. Psychol Aging. 15(3):527–541. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, D ME, Clark C, Fillenbaum G, Fillenbaum G, Fillenbaum G, Fillenbaum G, Fillenbaum G et al. . 1989. The consortium to establish a registry for Alzheimer's disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 39(9):1159–1165. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. 2002. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 15(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA. 2010. How hippocampus and cortex contribute to recognition memory: revisiting the complementary learning systems model. Hippocampus. 20(11):1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. 2003. Modeling hippocampal and neocortical contributions to recognition memory: a complementary learning systems approach. Psychol Rev. 110:611–646. [DOI] [PubMed] [Google Scholar]
- Olsen RK, Nichols EA, Chen J, Hunt JF, Glover GH, Gabrieli JD, Wagner AD et al. . 2009. Performance-related sustained and anticipatory activity in human medial temporal lobe during delayed match-to-sample. J Neurosci. 29(38):11880–11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Moore KS, Stark M, Chatterjee A. 2006. Visual working memory is impaired when the medial temporal lobe is damaged. J Cogn Neurosci. 18(7):1087–1097. [DOI] [PubMed] [Google Scholar]
- Parra MA. 2014. Overcoming barriers in cognitive assessment of Alzheimer's disease. Dement Neuropsychol. 8(2):95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Fabi K, Logie R, Luzzi S, Della Sala S. 2009. Short-term memory binding deficits in Alzheimer's disease. Brain. 132(Pt 4):1057–1066. [DOI] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Logie RH, Mendez LG, Lopera F, Della Sala S. 2010. Visual short-term memory binding deficits in familial Alzheimer's disease. Brain. 133(9):2702–2713. [DOI] [PubMed] [Google Scholar]
- Parra MA, Fabi K, Luzzi S, Cubelli R, Hernandez Valdez M, Della Sala S. 2015. Relational and conjunctive binding functions dissociate in short-term memory. Neurocase. 21(1):56–66. [DOI] [PubMed] [Google Scholar]
- Parra MA, Sala SD, Abrahams S, Logie RH, Mendez LG, Lopera F. 2011. Specific deficit of colour-colour short-term memory binding in sporadic and familial Alzheimer's disease. Neuropsychologia. 49(7):1943–1952. [DOI] [PubMed] [Google Scholar]
- Petersen RC. 2004. Mild cognitive impairment as a diagnostic entity. J Intern Med. 256:183–194. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR Jr., Jagust WJ, Shaw LM, Toga AW et al. . 2010. Alzheimer's disease neuroimaging initiative (ADNI): clinical characterization. Neurology. 74(3):201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, Jicha GA, Ivnik RJ, Smith GE, Tangalos EG et al. . 2006. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 63(5):665–672. [DOI] [PubMed] [Google Scholar]
- Pluta J, Yushkevich P, Das S, Wolk D. 2012. In vivo analysis of hippocampal subfield atrophy in mild cognitive impairment via semi-automatic segmentation of T2-weighted MRI. J Alzheimers Dis. 31(1):85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race E, LaRocque KF, Keane MM, Verfaellie M. 2013. Medial temporal lobe contributions to short-term memory for faces. J Exp Psychol Gen. 142(4):1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. 2010. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 20(11):1263–1290. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D'Esposito M. 2001. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 31(5):865–873. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. 2012. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 13(10):713–726. [DOI] [PubMed] [Google Scholar]
- Reitan R. 1958. Validity of the trail making test as an indicator of organic brain disease. Percept Motor Skills. 8:271–276. [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. 1996. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 16(16):5233–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. 2004. Revisiting Snodgrass and Vanderwart's object pictorial set: the role of surface detail in basic-level object recognition. Perception. 33(2):217–236. [DOI] [PubMed] [Google Scholar]
- Saunders NL, Summers MJ. 2011. Longitudinal deficits to attention, executive, and working memory in subtypes of mild cognitive impairment. Neuropsychology. 25(2):237–248. [DOI] [PubMed] [Google Scholar]
- Schon K, Ross RS, Hasselmo ME, Stern CE. 2013. Complementary roles of medial temporal lobes and mid-dorsolateral prefrontal cortex for working memory for novel and familiar trial-unique visual stimuli. Eur J Neurosci. 37(4):668–678. [DOI] [PubMed] [Google Scholar]
- Shrager Y, Levy DA, Hopkins RO, Squire LR. 2008. Working memory and the organization of brain systems. J Neurosci. 28(18):4818–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE et al. . 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. 2009. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 44(1):83–98. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. 1998. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentatory. 2nd ed. New York: Oxford University Press. [Google Scholar]
- Squire LR, Wixted JT. 2011. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 34:259–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. 1994. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J Neurosci. 14(3 Pt 2):1856–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. 2010. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 29(6):1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Cook PA, Klein A, Song G, Das SR, Duda JT, Kandel BM, van Strien N, Stone JR, Gee JC et al. . 2014. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage. 99:166–179. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Baddeley AD. 1974. Amnesia and memory for visual location. Neuropsychologia. 12(2):257–263. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1987. WMS-R Wechsler Memory Scale - Revised Manual. New York: The Psychological Corporation, Harcourt Brace Jovanovich, Inc. [Google Scholar]
- Whitwell JL, Petersen RC, Negash S, Weigand SD, Kantarci K, Ivnik RJ, Knopman DS, Boeve BF, Smith GE, Jack CR Jr.. 2007. Patterns of atrophy differ among specific subtypes of mild cognitive impairment. Arch Neurol. 64(8):1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickelgren WA. 1968. Sparing of short-term memory in an amnesic patient: implications for strength theory of memory. Neuropsychologia. 6:235–244. [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Bennett DA. 2011. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol. 68(3):351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O et al. . 2004. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 256(3):240–246. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Dunfee KL, Dickerson BC, Aizenstein HJ, DeKosky ST. 2011. A medial temporal lobe division of labor: insights from memory in aging and early Alzheimer disease. Hippocampus. 21(5):461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Mancuso L, Kliot D, Arnold SE, Dickerson BC. 2013. Familiarity-based memory as an early cognitive marker of preclinical and prodromal AD. Neuropsychologia. 51(6):1094–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Signoff ED, Dekosky ST. 2008. Recollection and familiarity in amnestic mild cognitive impairment: a global decline in recognition memory. Neuropsychologia. 46(7):1965–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Chun MM. 2006. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 440(7080):91–95. [DOI] [PubMed] [Google Scholar]
- Yassa MA. 2014. Ground zero in Alzheimer's disease. Nat Neurosci. 17(2):146–147. [DOI] [PubMed] [Google Scholar]
- Yee LT, Hannula DE, Tranel D, Cohen NJ. 2014. Short-term retention of relational memory in amnesia revisited: accurate performance depends on hippocampal integrity. Front Hum Neurosci. 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas A. 2002. The nature of recollection and familiarity: A review of 30 years of research. J Mem Lang. 46:441–517. [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. 2002. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 5(11):1236–1241. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Regehr G, Jacoby LL. 1995. Incorporating response bias in a dual-process theory of memory. J Mem Lang. 34(6):821–835. [Google Scholar]
- Yushkevich PA, Pluta JB, Wang H, Xie L, Ding SL, Gertje EC, Mancuso L, Kliot D, Das SR, Wolk DA. 2015. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum Brain Mapp. 36(1):258–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA. 1989. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci. 9(12):4355–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.