Abstract
Cortical excitatory and inhibitory synapses are disrupted in schizophrenia, the symptoms of which often emerge during adolescence, when cortical excitatory synapses undergo pruning. In auditory cortex, a brain region implicated in schizophrenia, little is known about the development of excitatory and inhibitory synapses between early adolescence and young adulthood, and how these changes impact auditory cortex function. We used immunohistochemistry and quantitative fluorescence microscopy to quantify dendritic spines and GAD65-expressing inhibitory boutons in auditory cortex of early adolescent, late adolescent, and young adult mice. Numbers of spines decreased between early adolescence and young adulthood, during which time responses increased in an auditory cortex-dependent sensory task, silent gap-prepulse inhibition of the acoustic startle reflex (gap-PPI). Within-bouton GAD65 protein and GAD65-expressing bouton numbers decreased between late adolescence and young adulthood, a delay in onset relative to spine and gap-PPI changes. In mice lacking the spine protein kalirin, there were no significant changes in spine number, within-bouton GAD65 protein, or gap-PPI between adolescence and young adulthood. These results illustrate developmental changes in auditory cortex spines, inhibitory boutons, and auditory cortex function between adolescence and young adulthood, and provide insights into how disrupted adolescent neurodevelopment could contribute to auditory cortex synapse pathology and auditory impairments.
Keywords: dendritic spines, GAD65, kalirin, spinophilin, VGluT1
Introduction
During adolescence, the cerebral cortex undergoes changes including synapse refinement and increased myelination (Paus et al. 2008), and the symptoms of schizophrenia often emerge (Tandon et al. 2009). Reductions of dendritic spines, the postsynaptic targets of most cortical excitatory synapses, and reduced levels of the 65-kDa isoform of the GABA synthetic enzyme glutamate decarboxylase (GAD65) protein within inhibitory boutons, without reduction in inhibitory bouton number, are observed in the auditory cortex of individuals with schizophrenia (Sweet et al. 2009; Moyer et al. 2012; Shelton et al. 2015). Developmental studies in rodent, nonhuman primate, and human indicate that cortical dendritic spine and excitatory synapse densities peak prior to adolescence, and then decline during adolescence before reaching adult levels (Rakic et al. 1986, 1994; Zecevic et al. 1989; Huttenlocher and Dabholkar 1997; Zuo, Lin, et al. 2005; Glantz et al. 2007; Petanjek et al. 2011). Models of homeostatic plasticity would predict that the substantial reduction in excitatory synapse density during adolescence would be preceded, accompanied, or followed by a reduction in inhibitory synapses or synapse strength (Turrigiano and Nelson 2004). However, evidence from nonhuman primates suggests that cortical inhibitory synapses do not undergo extensive pruning during adolescence (Zecevic and Rakic 1991; Bourgeois and Rakic 1993; Erickson and Lewis 2002) [but see Anderson et al. (1995)], although evidence from rodent studies supports both stability (Micheva and Beaulieu 1996) and reductions (Blue and Parnavelas 1983; De Felipe et al. 1997) of cortical inhibitory synapses between adolescence and adulthood.
Changes occurring in auditory cortex excitatory and inhibitory synapses during adolescence, or in disease, may impact auditory cortex function. Both excitation and inhibition in auditory cortex shape cortical responses to auditory stimuli (Hefti and Smith 2000; Wang et al. 2000; Wang et al. 2002; Wehr and Zador 2003; Kaur et al. 2005; Liu et al. 2007; Wu et al. 2008). One aspect of auditory processing in which the auditory cortex plays a role is the detection of silent gaps embedded in noise (Eggermont 1999, 2000; Kirby and Middlebrooks 2012). Detection of gap-in-noise stimuli can be assessed in rodents using a modified prepulse inhibition (PPI) of the acoustic startle response paradigm (gap-PPI; Turner et al. 2006; Fitch et al. 2008; Truong et al. 2012), which relies on the integrity of the primary auditory cortex (Ison et al. 1991; Bowen et al. 2003). Manipulating inhibitory and excitatory neuron activity in auditory cortex can enhance or attenuate gap-PPI in adult mice (Weible et al. 2014), which suggests that both excitatory and inhibitory synapses in auditory cortex contribute to gap-PPI. In addition, gap-PPI responses have been shown to increase during adolescence in rats (Friedman et al. 2004; Sun et al. 2008; Sun et al. 2011). Therefore, gap-PPI can be used a read-out of adolescent maturation of excitatory and inhibitory synapses in the rodent auditory cortex.
Abnormal adolescent neurodevelopment may contribute to auditory cortex excitatory and inhibitory synapse pathology and auditory processing deficits in individuals with schizophrenia. Although the molecular mechanisms regulating cortical synapse pruning during adolescence are largely unknown, promising candidates include factors involved in regulating dendritic spine stability and elimination (Tada and Sheng 2006). One such protein is kalirin, a guanine nucleotide exchange factor that is localized to spines and dendrites in the brain, and promotes dendritic spine stability and spine growth (Yoshihara et al. 2009). Mice with a global knockout (KO) of kalirin have an adolescent-onset deficit in frontal cortex spine density (Cahill et al. 2009). Thus, kalirin KO mice provide a way to model the effects of spine disruption on the trajectories of auditory cortex inhibitory synapse structure and auditory cortex function during adolescence.
Here, we determined the developmental trajectories of excitatory and inhibitory synapse markers in auditory cortex between early adolescence and young adulthood in wild-type C57Bl/6NJ (WT) mice, and measured gap-PPI performance to evaluate auditory cortex function. We found that within-bouton GAD65 protein levels and numbers of GAD65-expressing boutons decreased between late adolescence and young adulthood, subsequent to the onset of dendritic spine pruning. Furthermore, we found that gap-PPI increased between early and late adolescence. However, in mice lacking the spine protein kalirin, there was no significant decrease in auditory cortex spine number between adolescence and young adulthood, and no significant changes in within-bouton GAD65 protein level and gap-PPI. These findings increase our understanding of adolescent developmental trajectories of excitatory and inhibitory synapse structure and function in the mouse auditory cortex, and illustrate how disrupted adolescent neurodevelopment could lead to excitatory and inhibitory synapse pathology and auditory processing deficits.
Materials and Methods
Experimental Animals
Experiments were carried out using WT and kalirin KO mice. KO mice were generated as described previously by inserting the neomycin resistance cassette in place of exons 27–28, the site of the GEF1 domain (Cahill et al. 2009). Mice were rederived on the C57Bl/6NJ background at The Jackson Laboratory (Bar Harbor, ME, USA). Heterozygous breeders were crossed to generate WT and KO experimental animals. Animals were identified with metal ear tags and tail snip DNA samples were obtained for genotyping prior to weaning at approximately postnatal day 24. Only male animals were included in experiments because of the effects of estrogen and estrous phase on PPI of acoustic startle (Koch 1998; Charitidi et al. 2012), the effects of estrogen on dendritic spine density and GAD protein (Murphy et al. 1998), and its interactions with kalirin (Ma et al. 2011). Animals were housed in standard microisolator caging (Allentown Caging Equipment, Allentown, NJ, USA) in groups of up to 4, maintained on a 12-h light/dark cycle (lights on at 7 AM), and were provided with food and water ad libitum. Cohorts of mice underwent behavioral testing beginning at postnatal day 28, 55, or 82 ± 4 days and then were sacrificed at postnatal day 31, 58, or 85 ± 4 days (hereafter referred to as 4, 8, and 12 weeks of age, respectively). These ages correspond in general to early adolescence, late adolescence, and young adulthood in rodents (Spear and Brake 1983; Spear 2000; Kilb 2012). Each mouse was used in behavioral experiments at only a single age. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Tissue Generation
Mice were weighed, deeply anesthetized with Nembutal (150 mg/kg), and transcardially perfused with ice-cold normal saline. Brains were rapidly extracted, bisected, and left hemispheres were immersed in 4% paraformaldehyde for 48 h at 4 °C. Hemispheres were allowed to sink in a 30% sucrose solution before being sectioned coronally at 40 µm on a cryostat. Sections were stored in cryoprotectant (30% ethylene glycol and 30% glycerol in phosphate buffer) at −30 °C until use. Brains from 6 animals per age of each genotype were analyzed.
Auditory Cortex Mapping
Every sixth section was stained for Nissl substance using a 0.1% thionin solution in acetate buffer to identify primary auditory cortex and laminar boundaries. Animals were blocked into groups of six (one of each genotype and age group combination per block) and coded during mapping, so that the investigator was blinded to genotype and age group. Area Au1, which spans approximately 2.18–3.64 mm posterior to bregma and 4.5 mm lateral to the midline, was identified on the Nissl sections using a mouse brain atlas (Paxinos and Franklin 2004). An average of 5.47 Nissl sections per animal contained Au1, with a range of 5–7 sections per animal. Using Stereo Investigator software (MicroBrightField, Inc., Natick, MA, USA), a contour outline of cortical layers 2 through 4 was drawn, and the volume of these layers in left hemisphere Au1 was estimated for each animal. Three auditory cortex-containing sections per animal were randomly selected, and 3 sections adjacent to these were used for immunohistochemistry.
Immunohistochemistry
Free-floating immunohistochemistry to label pre- and postsynaptic components of excitatory and inhibitory synapses was carried out as previously described, with minor modifications (Sweet et al. 2009; Moyer et al. 2012, 2013; Kirkwood et al. 2013; see Supplementary Methods for specific details). To label components of excitatory and inhibitory synapses, we used an anti-spinophilin antibody raised in rabbit at 1 : 1000 (Millipore, Billerica, MA, USA; AB5669), an anti-vesicular glutamate transporter 1 (VGluT1) antibody raised in guinea pig at 1 : 250 (Millipore, AB5905), an anti-GAD65 antibody raised in mouse at 1 : 250 (Millipore, MAB351), and Alexa Fluor 568-conjugated phalloidin at 1 : 500 (Life Technologies, Grand Island, NY, USA; A12380).
Microscopy and Image Processing and Analysis
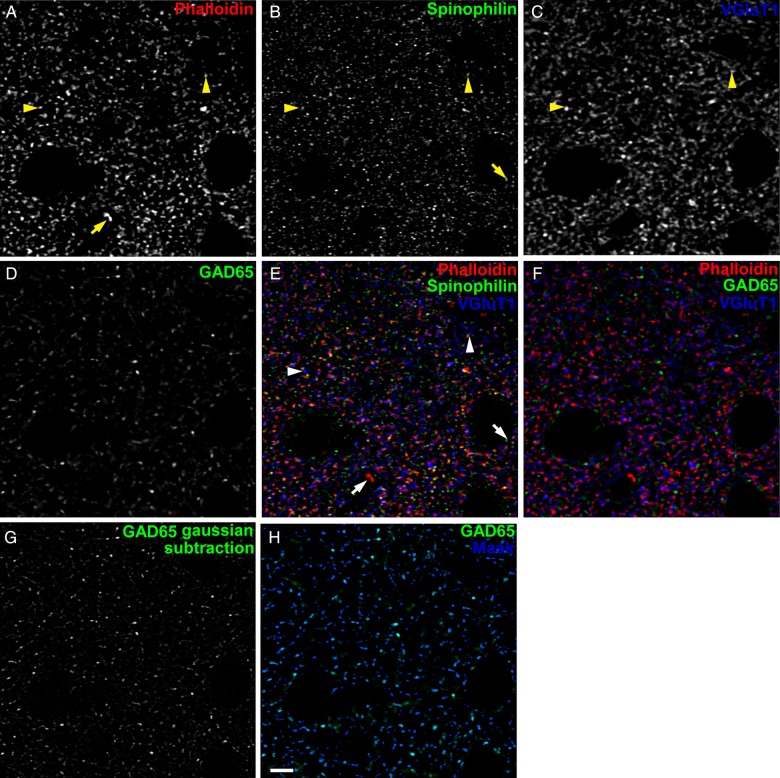
Details of spinning disk confocal microscopy and Z-stack sampling can be found in Supplementary Methods. Postprocessing of confocal image stacks included noise subtraction, deconvolution, a subtraction of Gaussian transformations for edge enhancement, and intensity- and morphology-based segmentation. Details on processing steps can be found in Supplementary Methods, and are variations on previously published methods (Fish et al. 2008; Moyer et al. 2012, 2013; Kirkwood et al. 2013). Examples of deconvolved and Gaussian-subtracted immunoreactive (IR) puncta as well as the IR puncta mask are shown in Figure 1.
Figure 1.
Examples of labeled puncta and masking process. (A) Phalloidin-labeled puncta. (B) Spinophilin-IR puncta. (C) VGluT1-IR puncta. Arrowheads in (A–C,E) indicate co-localized phalloidin and anti-spinophilin labels with close apposition to VGluT1-IR puncta. (D) GAD65-IR puncta. (E) Phalloidin-labeled puncta (red) and spinophilin-IR puncta (green) exhibit a large degree of overlap. Many phalloidin (red)- and spinophilin (green)-co-labeled puncta are apposed to VGluT-IR puncta (blue), such as the examples indicated by arrowheads, suggestive of pre- and postsynaptic components of excitatory synapses. Arrows in (A,B,E) indicate spinophilin-IR or phalloidin-labeled puncta without overlap, which were not quantified in this study. (F) GAD65-IR puncta (green) exhibit little overlap with phalloidin-labeled puncta (red) or VGluT1-IR puncta (blue). (G and H) Example of masking IR puncta. (G) GAD65-IR puncta after Gaussian smoothing to enhance object edge detection and facilitate masking. (H) Original GAD65-IR puncta (green) from D, with mask overlaid (blue). IR puncta intensity data were extracted from original, non-Gaussian-subtracted images. Scale bar is 5 µm.
IR and Fluorescently-Labeled Puncta Quantification
The details of inclusion criteria for quantification of labeled puncta can be found in Supplementary Methods. As previously described (Shelton et al. 2015), quantification of phalloidin-labeled puncta was contingent upon a minimum of 1 voxel overlap with the IR spinophilin signal, to increase specificity for presumptive dendritic spines. For completeness, we also report the converse analysis of spinophilin-labeled puncta, where quantification was contingent upon a minimum of 1 voxel overlap with the phalloidin signal (see Supplementary Results). Puncta density was determined by taking the total number of mask objects for each animal, divided by the sum of the total volumes of each sampling site for the animal. The number of puncta for each animal was generated using the Nv × Vref approach by multiplying the density by the estimated volume of left hemisphere area Au1 layers 2–4.
Auditory Testing
All behavioral testing was conducted in the Rodent Behavior Analysis Core of the University of Pittsburgh Schools of Health Sciences. Details of acclimation and test days can be found in Supplementary Methods.
Acoustic Startle Reflex and Noise-PPI
The acoustic startle reflex was assessed by measuring the reflexive response of mice presented with 75, 85, 95, 105, and 115 dB white noise, startle eliciting stimuli, presented against a 65-dB white noise background. PPI is the attenuation of the acoustic startle response that occurs when a nonstartling stimulus precedes the startle eliciting stimulus [reviewed in Swerdlow et al. (2001)]. To assess noise-PPI, we measured startle responses to the 115-dB startle eliciting stimulus when preceded by 5, 10, 15, or 20 dB increases in the 65-dB white noise background, 100 ms prior to the startle eliciting stimulus. Details of the test session design and testing equipment can be found in Supplementary Methods.
Gap-PPI
To assess detection of silent gaps embedded in background noise, we used a modified PPI of the acoustic startle reflex paradigm (Turner et al. 2006; Fitch et al. 2008). In this paradigm, the silent gap takes the place of the prepulse stimulus. If the animal detects the gap, the startle response is inhibited, and longer duration gaps are more salient and elicit greater inhibition of the startle response. Startle responses to silent gaps (1, 2, 4, 7, 10, 20, 40, or 100 ms in duration) embedded in a 70-dB white noise background were determined. Details of test session design and testing equipment can be found in Supplementary Methods.
Statistical Analyses
Details of ANOVA and ANCOVA models used can be found in Supplementary Methods. Statistical tests were conducted at the 0.05 significance level, and were done using SPSS software (IBM Corporation, Armonk, NY, USA). When appropriate, degrees of freedom were corrected for deviations from sphericity using the Greenhouse–Geisser adjustment.
Results
Spine Number Decreases in Auditory Cortex Between Early Adolescence and Young Adulthood
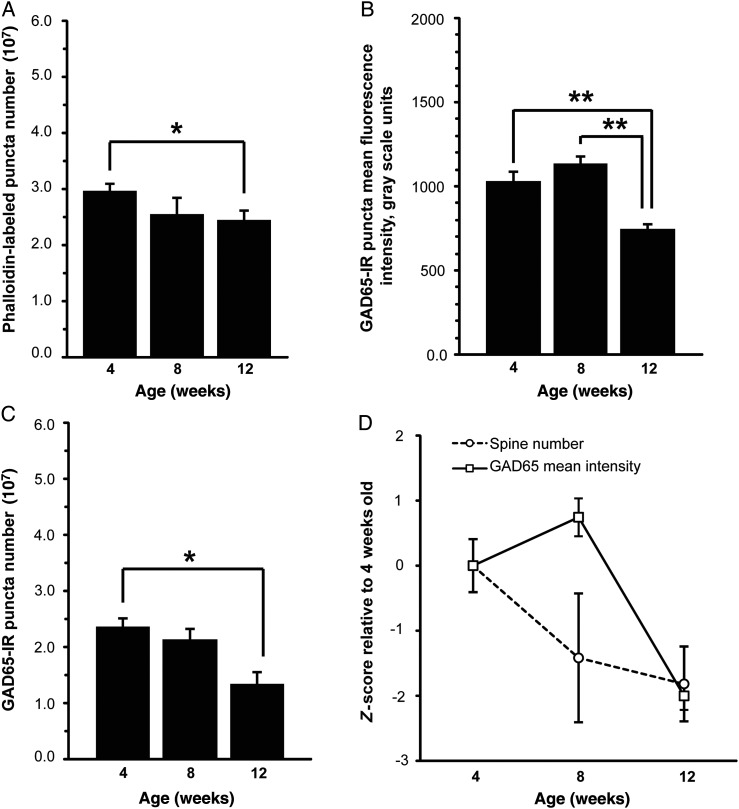
To determine whether adolescent pruning of dendritic spines occurs in the mouse auditory cortex, we quantified phalloidin-labeled puncta that exhibit overlap with spinophilin-IR puncta (hereafter referred to as phalloidin-labeled puncta). We found that the number of phalloidin-labeled puncta in auditory cortex decreased with increasing age in WT mice (P = 0.019; Fig. 2A). The number of phalloidin-labeled puncta was significantly decreased at 12 weeks compared with 4 weeks (an 18% decrease; P = 0.017), but differences between 4 and 8 weeks (a 14% decrease) and between 8 and 12 weeks (a 5% decrease) were not significant. The same age effect was observed when we examined the number of spinophilin-IR puncta that exhibit overlap with phalloidin-labeled puncta (hereafter referred to as spinophilin-IR puncta; P = 0.035; see Supplementary Fig. 1A). There was a nonsignificant reduction in the number of VGluT1-IR puncta between adolescence and young adulthood (18% reduction between 4 and 12 weeks; see Supplementary Fig. 2 and Supplementary Results). For additional analyses of fluorescence intensities and volume of phalloidin-labeled, spinophilin-IR, and VGluT1-IR puncta during this developmental time period, see Supplementary Results and Supplementary Figures 1 and 2.
Figure 2.
Changes in dendritic spines and GAD65-expressing boutons between early adolescence and young adulthood in layers 2–4 of primary auditory cortex of WT mice. (A) Mean number of phalloidin-labeled puncta that overlap with spinophilin-IR puncta decreases between 4 and 12 weeks of age in superficial auditory cortex of WT mice. (B) Mean GAD65 fluorescence intensity within GAD65-IR puncta significantly decreases between 8 and 12 weeks in WT mice. (C) Mean number of GAD65-IR puncta decreases between 4 and 12 weeks in WT mice. (D) Z-scored developmental trajectories of phalloidin-labeled puncta number and GAD65-IR puncta fluorescence intensity. There is a trend toward a significant interaction between age and synapse marker (P = 0.092). *P < 0.05; **P < 0.01. N = 6 per age group. Error bars are ±SEM.
Changes in Auditory Cortex GAD65-IR Boutons Occur Between Late Adolescence and Early Adulthood
GAD65-IR puncta mean fluorescence intensity (P = 0.001) and GAD65-IR puncta number (P = 0.015; Fig. 2B) decreased significantly with age in mouse auditory cortex. There was a 28% decrease in GAD65-IR puncta mean fluorescence intensity between 4 and 12 weeks of age (P = 0.004). Between 4 and 8 weeks of age, there was a nonsignificant 10% increase in puncta mean fluorescence intensity, and a significant 35% decrease between 8 and 12 weeks of age (P = 0.001). Similarly, GAD65-IR puncta number decreased significantly by 43% between 4 and 12 weeks of age (P = 0.015). There was a 10% decrease in GAD65-IR puncta number between 4 and 8 weeks of age that was not significant, and a trend level 37% reduction between 8 and 12 weeks of age (P = 0.093; Fig. 2C). GAD65-IR puncta volume also decreased significantly between 8 and 12 weeks of age (see Supplementary Fig. 3 and Supplementary Results). There was a trend toward a significant interaction between age and synapse marker for phalloidin-labeled puncta number and GAD65-IR puncta mean intensity (P = 0.092; Fig. 2D).
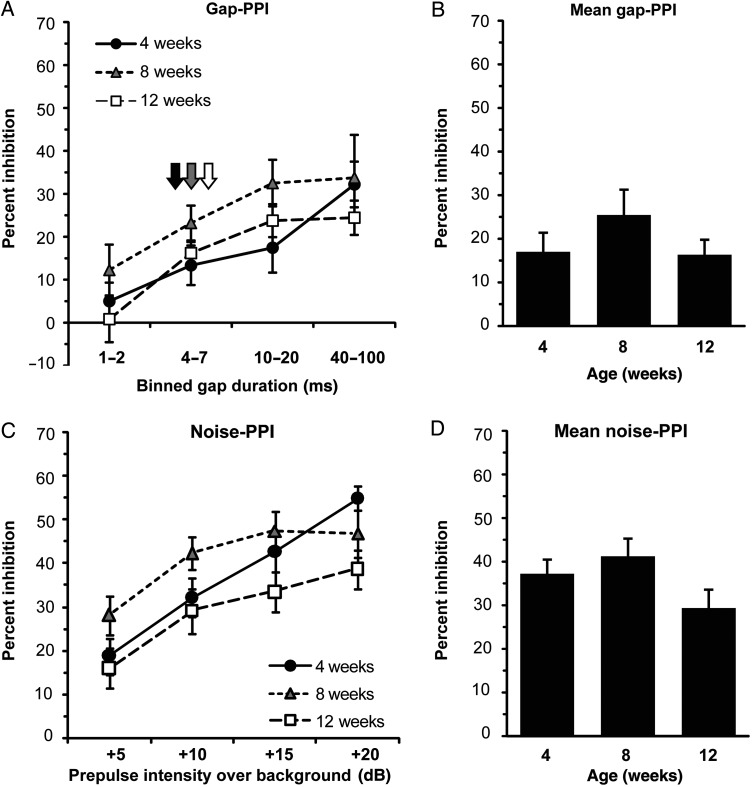
Gap-PPI Increases Between Early and Late Adolescence
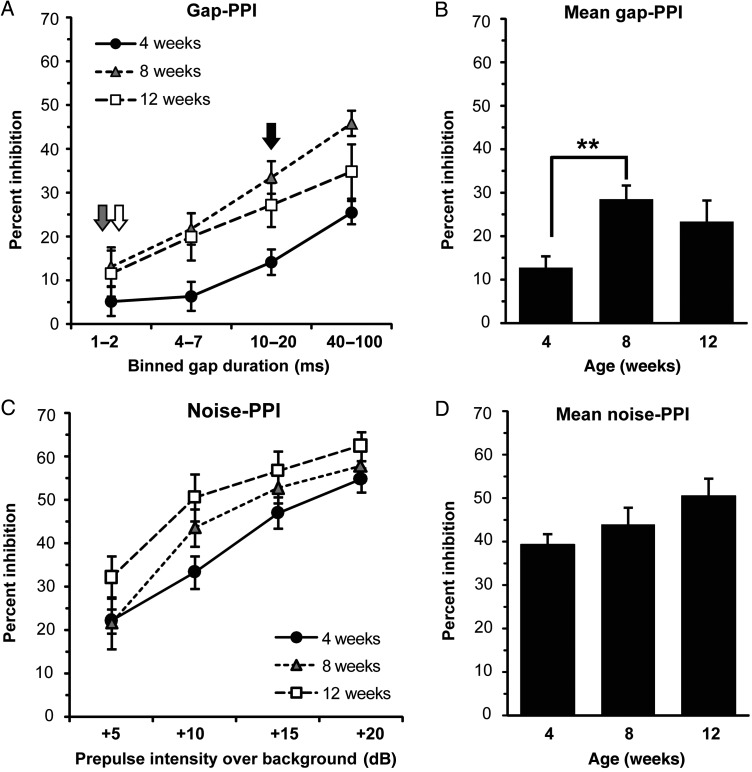
We found a significant effect of age on gap-PPI in WT mice (P < 0.001; Fig. 3A,B). Mean gap-PPI magnitude increased significantly (a 123% increase) in WT mice between 4 and 8 weeks of age (P = 0.003), with a trend toward being greater at 12 weeks compared with 4 weeks (an 82% increase; P = 0.064). The difference in gap-PPI (an 18% decrease) between 8- and 12-week-old mice was not significant. As a secondary measure of gap detection ability, we examined group-level thresholds for gap-PPI, defined as the lowest binned-gap durations to elicit significant PPI. The threshold for gap-PPI decreased from 10–20 to 1–2 ms gaps between 4 and 8 weeks of age in WT mice (Fig. 3A, arrows). Taken together, these results suggest that, compared with 4-week-old mice, 8-week-old mice exhibit significantly greater PPI responses to silent gap prepulses, and show increased sensitivity to shorter gap durations.
Figure 3.
Gap-PPI and noise-PPI between early adolescence and young adulthood in WT mice. (A) Magnitude of gap-PPI as a function of increasing binned-gap durations in WT mice. Arrows indicate gap detection threshold for 4 week (black), 8 week (gray), and 12 week (white) groups. (B) Gap-PPI data from (A) averaged across all gap durations in WT mice by age. Mean gap-PPI increases significantly between 4 and 8 weeks of age in WT mice. (C) Magnitude of noise-PPI as a function of increasing prepulse intensity in WT mice. (D) Noise-PPI data from (C) averaged across all prepulse intensities in WT mice by age. **P < 0.01. Animal numbers per group—age (N): 4 weeks (18), 8 weeks (17), 12 weeks (18). Error bars are ±SEM.
To determine whether the increase in gap-PPI during adolescence was specific for gap-PPI, we also examined noise-PPI of the acoustic startle reflex. There was a trend toward a significant effect of age on noise-PPI magnitude in WT mice (P = 0.089; Fig. 3C,D). However, in contrast to the marked developmental increase (123%) in gap-PPI responses that occurred between 4 and 8 weeks of age, the increase in noise-PPI measured between 4- and 8-week-old mice was 12% and not significant. Similarly, the change in noise-PPI between 8- and 12-week-old mice (a 15% increase) was also not significant.
Dendritic Spine Number, GAD65-Expressing Boutons, and Gap-PPI Responses Do Not Change Significantly Between Adolescence and Young Adulthood in Mice Lacking Kalirin
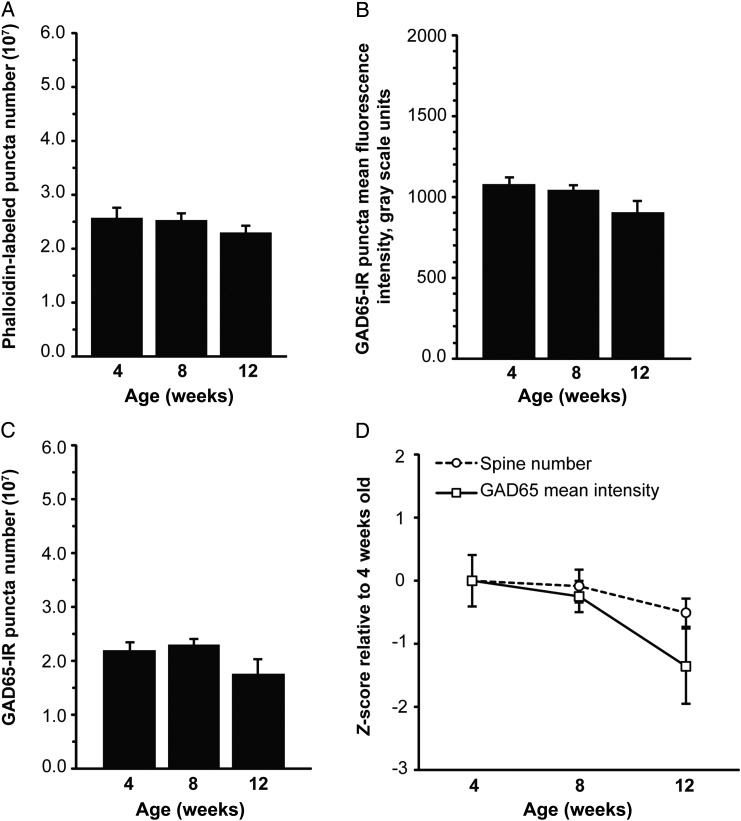
In kalirin KO mice, the decrease in the number of phalloidin-labeled puncta with age (a 10% decrease between 4 and 12 weeks) was not significant (P = 0.208; Fig. 4A), nor was a significant age effect observed for spinophilin-IR puncta (P = 0.255; see Supplementary Fig. 4A). There was a significant decrease in the number of VGluT1-IR puncta with age in kalirin KO mice (a 16% reduction between 4 and 12 weeks; see Supplementary Fig. 5 and Supplementary Results). For additional analyses of fluorescence intensity and volume of phalloidin-labeled, spinophilin-IR, and VGluT1-IR puncta between early adolescence and young adulthood in kalirin KO mice, see Supplementary Results and Supplementary Figures 4 and 5.
Figure 4.
Changes in dendritic spine number, and GAD65-expressing boutons between early adolescence and young adulthood in layers 2–4 of primary auditory cortex of kalirin KO mice. (A) The decrease in the number of phalloidin-labeled puncta that overlap with spinophilin-IR puncta with age is not significant in auditory cortex of kalirin KO mice. (B) The reduction in GAD65 fluorescence intensity within GAD65-IR puncta with age is not significant in kalirin KO mice. (C) Mean number of GAD65-IR puncta does not change significantly with age in kalirin KO mice. (D) Z-scored developmental trajectories of phalloidin-labeled puncta number and GAD65-IR puncta fluorescence intensity. There is no significant interaction effect between age and synapse marker. N = 6 per age group. Error bars are ±SEM.
There was no significant effect of age on GAD65-IR puncta fluorescence intensity in kalirin KO mice (a 16% reduction between 4 and 12 weeks; P = 0.194; Fig. 4B). Similarly, developmental changes in the number of GAD65-IR puncta (a 20% reduction between 4 and 12 weeks; P = 0.397; Fig. 4C) and the volume of GAD65-IR puncta (see Supplementary Fig. 6 and Supplementary Results) were not significant. There was no significant interaction between age and synapse marker for phalloidin-labeled puncta number and GAD65-IR puncta mean intensity (P = 0.292; Fig. 4D).
Changes in gap-PPI response magnitude between ages in the kalirin KO mice were not significant (P = 0.396) (a 3.5% decrease between 4 and 12 weeks, and a 50% increase between 4 and 8 weeks; Fig. 5A,B). The group-level thresholds for gap-PPI in the KO mice remained at 4–7 ms for all age groups (Fig. 5A, arrows). Taken together, these results suggest that kalirin KO mice do not exhibit significant improvement in gap-PPI during adolescence. We found that changes in noise-PPI with age in mice lacking kalirin were not significant (a 21% decrease between 4 and 12 weeks; P = 0.145; Fig. 5C,D).
Figure 5.
Gap-PPI and noise-PPI in kalirin KO mice at 4, 8, and 12 weeks of age. (A) Magnitude of gap-PPI as a function of increasing binned-gap durations in kalirin KO mice. Arrows indicate gap detection threshold for 4 week (black), 8 week (gray), and 12 week (white) groups. (B) Gap-PPI data from (A) averaged across all gap durations in KO mice by age. Mean gap-PPI magnitude is not significantly different between age groups. (C) Magnitude of noise-PPI as a function of increasing prepulse intensity in kalirin KO mice. (D) Noise-PPI data from (C) averaged across all prepulse intensities in KO mice by age. Mean noise-PPI magnitude is not significantly different between age groups. Animal numbers per group—age (N): 4 weeks (13), 8 weeks (15), 12 weeks (22). Error bars are ±SEM.
Discussion
Decrease in the Number of Dendritic Spines Between Early Adolescence and Young Adulthood in Superficial Auditory Cortex
We found that a reduction in the number of dendritic spines in superficial layers of the mouse auditory cortex occurs between early adolescence and young adulthood. The pattern of early postnatal overproduction and subsequent pruning of excitatory synapses has been extensively characterized in multiple cortical regions of the macaque, including motor, somatosensory, visual, and prefrontal cortex [O'Kusky and Colonnier 1982; Zecevic et al. 1989; Zecevic and Rakic 1991; Bourgeois and Rakic 1993; Bourgeois et al. 1994; reviewed in Rakic et al. (1994)], and in visual, auditory, and prefrontal cortex of human (Huttenlocher and Dabholkar 1997), and is primarily attributable to spine, rather than dendritic shaft, excitatory synapses (Bourgeois et al. 1994). Overproduction followed by pruning of excitatory synapses and spines has also been observed in studies of spine density and dynamics in the mouse (Alvarez and Sabatini 2007). In mouse somatosensory cortex, the density of asymmetric, presumably excitatory, synapses decreases by 37% between approximately 4 weeks of age [postnatal day (P) 32] and adulthood (P100–120; De Felipe et al. 1997). In mouse frontal cortex, a 20% loss of dendritic spines occurs between 4 and 8 weeks of age (Zuo, Lin, et al. 2005). In vivo spine imaging studies in mouse visual and frontal cortex have shown that this is attributable to spine elimination outpacing spine formation during the time between 4 and 8 weeks, followed by equalization of formation and elimination rates after 16 weeks of age (Grutzendler et al. 2002; Zuo, Lin, et al. 2005). In the present study, we found that the number of spines decreases by 18% between early adolescence and young adulthood in layers 2–4 of mouse auditory cortex. Although comparable with the reports of adolescent pruning in rodent cortex, this decrease in spines is smaller in magnitude compared with values reported in human and primate. For example, in human prefrontal cortex, spines are overproduced by as much as 3-fold (Petanjek et al. 2011). The number of synapses in monkey visual cortex is increased by 94% in 6-month juveniles compared with adult animals (O'Kusky and Colonnier 1982). Taken together, our findings in mouse cortex are consistent with the literature supporting pruning of spines and excitatory synapses between early postnatal development and adulthood across species and cortical regions; however, the magnitude of synapse overproduction and pruning in mouse cortex may not be as large as in human and nonhuman primates.
In the present study, we quantified spines located within layers 2–4 of mouse auditory cortex. However, the timing and magnitude of spine pruning may differ between layers 2, 3, and 4, as well as between layers 2 through 4 and other cortical layers. For example, developmental reductions between the peak in synapse density and adulthood in monkey cortex vary in magnitude between approximately 30% and 50%, depending on cortical layer and brain region (O'Kusky and Colonnier 1982; Zecevic et al. 1989; Zecevic and Rakic 1991). Evidence from monkey prefrontal and visual cortex suggests that the most dynamic changes in synapses during development occur in layers 1–3, and are less evident or absent in layers 4, 5, and 6 (O'Kusky and Colonnier 1982; Bourgeois et al. 1994). In monkey somatosensory cortex, the largest decrease in spine density (a 50% decrease) occurs in layer 3 (Zecevic and Rakic 1991). As synapse density is generally highest in the supragranular layers (Bourgeois and Rakic 1993; Bourgeois et al. 1994), changes in synapses in more superficial layers of auditory cortex may reflect the overall developmental trend for auditory cortex synapses, even though specific laminar trajectories likely differ. Differences in the timing, as well as the magnitude, of developmental changes may also exist between spines located on cells with somata in different cortical layers, and between different types of spiny neurons. Spine pruning begins earlier and is more pronounced on layer 3 neurons compared with layer 5 neurons of human prefrontal cortex (Petanjek et al. 2011). In monkey visual cortex, the early postnatal trajectories of spine development differ between the alpha and beta types of spiny stellate neurons in layer 4 (Lund and Holbach 1991). Differences in dendritic spine development between different types of spiny neurons may also exist in auditory cortex; however, in contrast to visual cortex, pyramidal rather than spiny stellate neurons serve as the primary thalamocortical recipient neurons in deep layer 3 and layer 4 of cat auditory cortex, although a small percentage of nonpyramidal, nonstellate cells identified in the middle layers also exhibit spines (Smith and Populin 2001). Given this evidence, it is possible that laminar and cell type-specific differences in the developmental trajectories of spines contribute to the overall spine reduction between adolescence and young adulthood in layers 2, 3, and 4 of mouse auditory cortex.
Decreases in the Number of GAD65-IR Boutons and Within-Bouton GAD65 Protein Levels Between Late Adolescence and Young Adulthood in Superficial Auditory Cortex
We identified a significant reduction in the number of GAD65-IR boutons in superficial auditory cortex between early adolescence and young adulthood, with the greatest decrement occurring after late adolescence. This finding extends the limited previous rodent data on adolescent development of inhibitory circuits in cortex. For example, inhibitory synapse number does not change significantly between early and late adolescence in rat somatosensory cortex (P30–P60, roughly equivalent to our 4- and 8-week age groups; Micheva and Beaulieu 1996), a period where we saw little change in the number of GAD65-IR boutons. However, when we extended our observations to early adulthood, we found a significant decrease in the number of GAD65-IR boutons. Other studies in rodent have evaluated type II or symmetric synapses, a population that includes, but is not restricted to, GABAergic synapses. Thus, in rat visual cortex, the density of type II synapses decreases by approximately 30% between early adolescence and young adulthood (P28–P90; Blue and Parnavelas 1983). In mouse somatosensory cortex, the density of symmetric synapses decreases by 8% between adolescence and adulthood (De Felipe et al. 1997).
Other evidence emerges from studies of primate cortex, and suggests that GABAergic inhibitory synapses are not overproduced and pruned to the same extent as excitatory synapses during postnatal development (Zecevic and Rakic 1991; Bourgeois and Rakic 1993; Erickson and Lewis 2002). However, Anderson et al. (1995) found that the density of parvalbumin-IR terminals of the chandelier population of GABAergic interneurons decreases by nearly 90% in parallel with an approximate 50% decrease of layer 3 neuron spine density in monkey prefrontal cortex between adolescence and adulthood. Thus, the magnitude of reductions in cortical inhibitory boutons during adolescence may vary with GABAergic neuron subtype, and therefore, our finding of a 48% reduction in the number of GAD65-IR boutons between early adolescence and young adulthood using a single label for GAD65 that identifies boutons from multiple GABAergic subtypes (Fish et al. 2011) may obscure cell type-specific differences. Taken together, these reports suggest that adolescent developmental trajectories of inhibitory synapses may vary depending on species, GABAergic neuron population, and cortical region.
Even fewer studies have examined GAD65 protein levels in the cortex during adolescent development. A western blot analysis in human visual cortex demonstrated that GAD65 levels peak relatively late in human development, at approximately age 20 (Pinto et al. 2010). GAD65 immunoreactivity in the rat primary somatosensory cortex increases gradually between P6 and early adolescence (P33; Kiser et al. 1998). In rat auditory cortex, protein levels of GAD65 in rat auditory cortex measured by immunoblot increase over postnatal development through the end of adolescence (P56; Xu et al. 2010). However, our study extended observations into adulthood, and we identified a significant reduction in GAD65 levels between late adolescence and young adulthood. Our findings suggest that, in terms of GAD65 protein levels, inhibitory boutons in auditory cortex continue to mature after the end of adolescence. The implications of a developmental reduction in within-bouton GAD65 are not known; however, given that GAD65 mediates GABA synthesis in an activity-dependent manner (Martin et al. 1991), reduction in within-bouton GAD65 protein levels may translate into a reduction in GABA synthesis during periods of high inhibitory synaptic activity (Tian et al. 1999), for example, as occurs during gamma oscillations (Buzsaki and Wang 2012). Thus, within-bouton reduction in GAD65 between adolescence and young adulthood, if conserved across brain regions, could contribute to the decreased entrainment to gamma oscillations between early adolescence and young adulthood observed in medial prefrontal cortex neurons in mice (de Almeida et al. 2013).
Increase in Gap-PPI Between Early and Late Adolescence
We found an increase in gap-PPI magnitude and a decrease in gap-PPI threshold between 4 and 8 weeks of age in WT mice of the C57Bl/6NJ background, which suggests that gap-PPI matures during adolescence in mice of this strain. This is consistent with previous studies demonstrating that gap detection threshold decreases over the same developmental period in rats (Friedman et al. 2004; Sun et al. 2008, 2011). Gap-PPI is disrupted when cortical activity is silenced (Ison et al. 1991), and more specifically, when the auditory cortex is lesioned bilaterally (Bowen et al. 2003), suggesting that auditory cortex is required for gap-PPI. As the function of both inhibitory and excitatory neurons in auditory cortex is important for gap-PPI in young adult mice (Weible et al. 2014), the increase in gap-PPI between early and late adolescence may indicate functional maturation of auditory cortex inhibitory and/or excitatory synapses. We identified significant changes in inhibitory bouton features between late adolescence and young adulthood, but not between early adolescence and late adolescence when the significant developmental increase in gap-PPI was observed. These results suggest that the maturation of gap-PPI is not likely a functional consequence of changes in auditory cortex inhibitory boutons. However, it is possible that the developmental reductions in spine numbers between early adolescence and young adulthood might contribute to the improved gap-PPI observed between early and late adolescence.
While gap-PPI depends, at least in part, on the integrity of auditory cortex, noise-PPI can still be elicited even when the cortex is silenced, or auditory cortex is lesioned (Ison et al. 1991; Bowen et al. 2003). Although auditory cortex is not necessary for noise-PPI, the 2 paradigms likely share some subcortical circuitry. Therefore, the increase in gap-PPI with age could be attributable to changes occurring in auditory cortex, or within subcortical structures known to mediate noise-PPI (Koch 1999). We did not find a significant increase in noise-PPI between early and late adolescence. Thus, our findings suggest, but do not definitively demonstrate, that the increase in gap but not noise-PPI during adolescence is driven by changes in the auditory cortex.
Lack of Significant Adolescent to Adulthood Changes in Dendritic Spines and GAD65 Protein Levels in Superficial Auditory Cortex and Gap-PPI in Mice Lacking Kalirin
We found that auditory cortex dendritic spine number decreases and gap-PPI increases between early adolescence and young adulthood in auditory cortex in WT animals, whereas levels of GAD65 protein within inhibitory boutons decreased between late adolescence and young adulthood. These observations suggest that adolescent spine pruning is causally related to enhanced gap-PPI and to subsequent decreases in GAD65. In keeping with this hypothesis, when we examined kalirin KO mice, in which the decrease in auditory cortex spine number between early adolescence and young adulthood was not significant, within-bouton GAD65 levels and numbers of GAD65-IR puncta did not significantly differ between the end of adolescence and young adulthood. Additionally, we saw no significant improvement of gap-PPI over the course of adolescence in kalirin KO mice.
GAD65 expression is dependent on activity (Wei and Wu 2008), and several studies have reported that reducing neuronal activity or sensory input leads to decreased GAD expression (Hendry and Jones 1988; Welker et al. 1989; Patz et al. 2003). In contrast, after genetic deletion of GAD65, as well as inhibition of the GAD enzyme, dendritic spine density is increased (Murphy et al. 1998; Mataga et al. 2004), a sequence not compatible with our observations. Therefore, to the extent that a decrease in spine number between early adolescence and young adulthood in WT mice translates into a decrease in excitatory circuit activity, levels of GAD65 may decrease between late adolescence and young adulthood so as to maintain excitatory–inhibitory balance. Similarly, in kalirin KO mice, where decreases in spine number between adolescence and young adulthood were small in magnitude and not significant, parallel small and nonsignificant developmental changes in within-bouton GAD65 levels were also found. Alternatively, it is possible that the lack of a significant adolescent reduction in within-bouton GAD65 levels in kalirin KO mice results from a direct effect within GABAergic neurons, rather than from reductions in excitatory drive. Kalirin is expressed in GABAergic neurons (Ma et al. 2001, 2008), and in vitro transfection of hippocampal interneurons with exogenous kalirin-7 reduces GAD65 immunoreactivity (Ma et al. 2011).
Finally, we saw no significant improvement of gap-PPI over the course of adolescence in kalirin KO mice. A potential limitation of using the gap-PPI paradigm to assess auditory function in kalirin KO mice is that these mice exhibit impaired noise-mediated PPI (Cahill et al. 2009). However, we found that noise-intensity PPI is not impaired until young adulthood in mice lacking kalirin (see Supplementary Results). Therefore, the lack of a significant increase in gap-PPI between early and late adolescence in kalirin KO mice is likely not attributable to general PPI impairment (Cahill et al. 2009). The absence of a significant developmental increase in gap-PPI and of significant changes in auditory cortex dendritic spine numbers and inhibitory boutons during adolescence in mice lacking kalirin would be consistent with a dependence of gap-PPI functional maturation on the magnitude of developmental changes in auditory cortex synapses. For further discussion of the kalirin KO mouse findings and their relevance to studies of adolescent cortical synapse development and auditory processing in schizophrenia, see Supplementary Discussion.
Consideration of Age-Dependent High-Frequency Hearing Loss
A potential confounding factor in our study is that the C57Bl/6 background on which the WT and kalirin KO mice were maintained is associated with young adult onset high-frequency hearing loss, beginning between 12 and 16 weeks of age (Willott et al. 1993; Kane et al. 2012). It is possible that this could have impacted our measures of synapse structure and function in the oldest age group tested. For example, sensory deprivation prevents dendritic spine loss, at least in somatosensory cortex (Zuo, Yang, et al. 2005), a process that might have reduced the magnitude of the observed dendritic spine reduction in our 12-week-old mice. Experimentally induced high-frequency hearing loss has been shown to both reduce and increase GAD expression in the auditory cortex (Sarro et al. 2008; Yang et al. 2011), leaving unsettled the question of any possible effects of hearing loss on GAD65 levels. However, it is worth noting that cortical synaptic measures in our study were not restricted to the region of auditory cortex corresponding to high frequencies, which suggests that the effects we observed were not solely attributable to high-frequency hearing loss.
Conclusions
Here, we report that dendritic spine numbers decrease in WT mouse auditory cortex between early adolescence and young adulthood, concurrent with increases in gap-PPI. Subsequent reductions in within-bouton levels of GAD65 protein, and numbers of inhibitory boutons, occur between late adolescence and young adulthood. The lack of a significant reduction in spine number or a significant increase in gap-PPI between early adolescence and young adulthood in kalirin KO mice suggests that subtle disruptions in the developmental trajectories of auditory cortex synapses in mice lacking kalirin lead to disrupted auditory cortex functional maturation. Taken together, these results increase our understanding of the relationship between structural changes of excitatory and inhibitory synapses during adolescence and functional changes in auditory cortex, and how disrupted adolescent development of auditory cortex synapses could contribute to the pathophysiology of schizophrenia.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org
Funding
This work was supported by the National Institute of Health (MH 096985 to K.N.F., DA 027679 to E.T., MH 071316 and MH 097216 to P.P., and MH 071533 to R.A.S.).
Supplementary Material
Notes
We thank Eloise Peet for technical support with the behavioral testing, and Nancy Petro and Dr Robert Ferrell for assistance with genotyping. Conflict of Interest: None declared.
References
- Alvarez VA, Sabatini BL. 2007. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 30:79–97. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Classey JD, Conde F, Lund JS, Lewis DA. 1995. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 67:7–22. [DOI] [PubMed] [Google Scholar]
- Blue ME, Parnavelas JG. 1983. The formation and maturation of synapses in the visual cortex of the rat. II. Quantitative analysis. J Neurocytol. 12:697–712. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. 1994. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 4:78–96. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. 1993. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 13:2801–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen GP, Lin D, Taylor MK, Ison JR. 2003. Auditory cortex lesions in the rat impair both temporal acuity and noise increment thresholds, revealing a common neural substrate. Cereb Cortex. 13:815–822. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Wang XJ. 2012. Mechanisms of gamma oscillations. Annu Rev Neurosci. 35:203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill ME, Xie Z, Day M, Photowala H, Barbolina MV, Miller CA, Weiss C, Radulovic J, Sweatt JD, Disterhoft JF et al. . 2009. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci USA. 106:13058–13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charitidi K, Meltser I, Canlon B. 2012. Estradiol treatment and hormonal fluctuations during the estrous cycle modulate the expression of estrogen receptors in the auditory system and the prepulse inhibition of acoustic startle response. Endocrinology. 153:4412–4421. [DOI] [PubMed] [Google Scholar]
- de Almeida J, Jourdan I, Murer MG, Belforte JE. 2013. Refinement of neuronal synchronization with gamma oscillations in the medial prefrontal cortex after adolescence. PLoS ONE. 8:e62978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felipe J, Marco P, Fairen A, Jones EG. 1997. Inhibitory synaptogenesis in mouse somatosensory cortex. Cereb Cortex. 7:619–634. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. 1999. Neural correlates of gap detection in three auditory cortical fields in the Cat. J Neurophysiol. 81:2570–2581. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. 2000. Neural responses in primary auditory cortex mimic psychophysical, across-frequency-channel, gap-detection thresholds. J Neurophysiol. 84:1453–1463. [DOI] [PubMed] [Google Scholar]
- Erickson SL, Lewis DA. 2002. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol. 448:186–202. [DOI] [PubMed] [Google Scholar]
- Fish KN, Sweet RA, Deo AJ, Lewis DA. 2008. An automated segmentation methodology for quantifying immunoreactive puncta number and fluorescence intensity in tissue sections. Brain Res. 1240:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish KN, Sweet RA, Lewis DA. 2011. Differential distribution of proteins regulating GABA synthesis and reuptake in axon boutons of subpopulations of cortical interneurons. Cereb Cortex. 21:2450–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch RH, Threlkeld SW, McClure MM, Peiffer AM. 2008. Use of a modified prepulse inhibition paradigm to assess complex auditory discrimination in rodents. Brain Res Bull. 76:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JT, Peiffer AM, Clark MG, Benasich AA, Fitch RH. 2004. Age and experience-related improvements in gap detection in the rat. Brain Res Dev Brain Res. 152:83–91. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. 2007. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 149:582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzendler J, Narayanan K, Gan W-B. 2002. Long-term dendritic spine stability in the adult cortex. Nature. 420:812–816. [DOI] [PubMed] [Google Scholar]
- Hefti BJ, Smith PH. 2000. Anatomy, physiology, and synaptic responses of rat layer V auditory cortical cells and effects of intracellular GABA(A) blockade. J Neurophysiol. 83:2626–2638. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG. 1988. Activity-dependent regulation of GABA expression in the visual cortex of adult monkeys. Neuron. 1:701–712. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. 1997. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 387:167–178. [DOI] [PubMed] [Google Scholar]
- Ison JR, O'Connor K, Bowen GP, Bocirnea A. 1991. Temporal resolution of gaps in noise by the rat is lost with functional decortication. Behav Neurosci. 105:33–40. [DOI] [PubMed] [Google Scholar]
- Kane KL, Longo-Guess CM, Gagnon LH, Ding D, Salvi RJ, Johnson KR. 2012. Genetic background effects on age-related hearing loss associated with Cdh23 variants in mice. Hear Res. 283:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Rose HJ, Lazar R, Liang K, Metherate R. 2005. Spectral integration in primary auditory cortex: laminar processing of afferent input, in vivo and in vitro. Neuroscience. 134:1033–1045. [DOI] [PubMed] [Google Scholar]
- Kilb W. 2012. Development of the GABAergic system from birth to adolescence. Neuroscientist. 18:613–630. [DOI] [PubMed] [Google Scholar]
- Kirby AE, Middlebrooks JC. 2012. Unanesthetized auditory cortex exhibits multiple codes for gaps in cochlear implant pulse trains. J Assoc Res Otolaryngol. 13:67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood CM, Ciuchta J, Ikonomovic M, Fish KN, Abrahamson EE, Murray PS, Klunk WE, Sweet RA. 2013. Dendritic spine density, morphology, and fibrillar actin content surrounding amyloid-B plaques in a mouse model of amyloid-B deposition. J Neuropathol Exp Neurol. 72:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser PJ, Cooper NG, Mower GD. 1998. Expression of two forms of glutamic acid decarboxylase (GAD67 and GAD65) during postnatal development of rat somatosensory barrel cortex. J Comp Neurol. 402:62–74. [PubMed] [Google Scholar]
- Koch M. 1998. Sensorimotor gating changes across the estrous cycle in female rats. Physiol Behav. 64:625–628. [DOI] [PubMed] [Google Scholar]
- Koch M. 1999. The neurobiology of startle. Prog Neurobiol. 59:107–128. [DOI] [PubMed] [Google Scholar]
- Liu BH, Wu GK, Arbuckle R, Tao HW, Zhang LI. 2007. Defining cortical frequency tuning with recurrent excitatory circuitry. Nat Neurosci. 10:1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JS, Holbach SM. 1991. Postnatal development of thalamic recipient neurons in the monkey striate cortex: I. Comparison of spine acquisition and dendritic growth of layer 4C alpha and beta spiny stellate neurons. J Comp Neurol. 309:115–128. [DOI] [PubMed] [Google Scholar]
- Ma XM, Huang JP, Kim EJ, Zhu Q, Kuchel GA, Mains RE, Eipper BA. 2011. Kalirin-7, an important component of excitatory synapses, is regulated by estradiol in hippocampal neurons. Hippocampus. 21:661–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Johnson RC, Mains RE, Eipper BA. 2001. Expression of kalirin, a neuronal GDP/GTP exchange factor of the trio family, in the central nervous system of the adult rat. J Comp Neurol. 429:388–402. [DOI] [PubMed] [Google Scholar]
- Ma XM, Wang Y, Ferraro F, Mains RE, Eipper BA. 2008. Kalirin-7 is an essential component of both shaft and spine excitatory synapses in hippocampal interneurons. J Neurosci. 28:711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DL, Martin SB, Wu SJ, Espina N. 1991. Regulatory properties of brain glutamate decarboxylase (GAD): the apoenzyme of GAD is present principally as the smaller of two molecular forms of GAD in brain. J Neurosci. 11:2725–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataga N, Mizuguchi Y, Hensch TK. 2004. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron. 44:1031–1041. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. 1996. Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. J Comp Neurol. 373:340–354. [DOI] [PubMed] [Google Scholar]
- Moyer CE, Delevich KM, Fish KN, Asafu-Adjei JK, Sampson AR, Dorph-Petersen KA, Lewis DA, Sweet RA. 2013. Intracortical excitatory and thalamocortical boutons are intact in primary auditory cortex in schizophrenia. Schizophr Res. 149:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer CE, Delevich KM, Fish KN, Asafu-Adjei JK, Sampson AR, Dorph-Petersen KA, Lewis DA, Sweet RA. 2012. Reduced glutamate decarboxylase 65 protein within primary auditory cortex inhibitory boutons in schizophrenia. Biol Psychiatry. 72:734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. 1998. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 18:2550–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kusky J, Colonnier M. 1982. Postnatal changes in the number of neurons and synapses in the visual cortex (area 17) of the macaque monkey: a stereological analysis in normal and monocularly deprived animals. J Comp Neurol. 210:291–306. [DOI] [PubMed] [Google Scholar]
- Patz S, Wirth MJ, Gorba T, Klostermann O, Wahle P. 2003. Neuronal activity and neurotrophic factors regulate GAD-65/67 mRNA and protein expression in organotypic cultures of rat visual cortex. Eur J Neurosci. 18:1–12. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. 2008. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 9:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. 2004. The Mouse Brain in Stereotaxic Coordinates: Compact Second Edition. San Diego: Elsevier Academic Press. [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. 2011. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto JG, Hornby KR, Jones DG, Murphy KM. 2010. Developmental changes in GABAergic mechanisms in human visual cortex across the lifespan. Front Cell Neurosci. 4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. 1986. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 232:232–235. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Goldman-Rakic PS. 1994. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res. 102:227–243. [DOI] [PubMed] [Google Scholar]
- Sarro EC, Kotak VC, Sanes DH, Aoki C. 2008. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABAA receptors in the auditory cortex. Cereb Cortex. 18:2855–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton MA, Newman JT, Gu H, Sampson AR, Fish KN, MacDonald ML, Moyer CE, DiBitetto JV, Dorph-Petersen K-A, Penzes P et al. . forthcoming. 2015. Loss of microtubule associated protein 2 immunoreactivity linked to dendritic spine loss in schizophrenia. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Populin LC. 2001. Fundamental differences between the thalamocortical recipient layers of the cat auditory and visual cortices. J Comp Neurol. 436:508–519. [DOI] [PubMed] [Google Scholar]
- Spear LP. 2000. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 24:417–463. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. 1983. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 16:83–109. [DOI] [PubMed] [Google Scholar]
- Sun W, Hansen A, Zhang L, Lu J, Stolzberg D, Kraus KS. 2008. Neonatal nicotine exposure impairs development of auditory temporal processing. Hear Res. 245:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Tang L, Allman BL. 2011. Environmental noise affects auditory temporal processing development and NMDA-2B receptor expression in auditory cortex. Behav Brain Res. 218:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. 2009. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 34:374–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. 2001. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 156:194–215. [DOI] [PubMed] [Google Scholar]
- Tada T, Sheng M. 2006. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 16:95–101. [DOI] [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS. 2009. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 110:1–23. [DOI] [PubMed] [Google Scholar]
- Tian N, Petersen C, Kash S, Baekkeskov S, Copenhagen D, Nicoll R. 1999. The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc Natl Acad Sci USA. 96:12911–12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong DT, Venna VR, McCullough LD, Fitch RH. 2012. Deficits in auditory, cognitive, and motor processing following reversible middle cerebral artery occlusion in mice. Exp Neurol. 238:114–121. [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. 2006. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 120:188–195. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. 2004. Homeostatic plasticity in the developing neocortex. Nat Rev Neurosci. 5:97–107. [DOI] [PubMed] [Google Scholar]
- Wang J, Caspary D, Salvi RJ. 2000. GABA-A antagonist causes dramatic expansion of tuning in primary auditory cortex. Neuroreport. 11:1137–1140. [DOI] [PubMed] [Google Scholar]
- Wang J, McFadden SL, Caspary D, Salvi R. 2002. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 944:219–231. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. 2003. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 426:442–446. [DOI] [PubMed] [Google Scholar]
- Wei J, Wu JY. 2008. Post-translational regulation of l-glutamic acid decarboxylase in the brain. Neurochem Res. 33:1459–1465. [DOI] [PubMed] [Google Scholar]
- Weible AP, Moore AK, Liu C, DeBlander L, Wu H, Kentros C, Wehr M. 2014. Perceptual gap detection is mediated by gap termination responses in auditory cortex. Curr Biol. 24:1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker E, Soriano E, Van der LH. 1989. Plasticity in the barrel cortex of the adult mouse: effects of peripheral deprivation on GAD-immunoreactivity. Exp Brain Res. 74:441–452. [DOI] [PubMed] [Google Scholar]
- Willott JF, Aitkin LM, McFadden SL. 1993. Plasticity of auditory cortex associated with sensorineural hearing loss in adult C57BL/6J mice. J Comp Neurol. 329:402–411. [DOI] [PubMed] [Google Scholar]
- Wu GK, Arbuckle R, Liu BH, Tao HW, Zhang LI. 2008. Lateral sharpening of cortical frequency tuning by approximately balanced inhibition. Neuron. 58:132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yu L, Zhang J, Cai R, Sun X. 2010. Early continuous white noise exposure alters l-alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunit glutamate receptor 2 and gamma-aminobutyric acid type a receptor subunit beta3 protein expression in rat auditory cortex. J Neurosci Res. 88:614–619. [DOI] [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. 2011. Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci USA. 108:14974–14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y, De Roo M, Muller D. 2009. Dendritic spine formation and stabilization. Curr Opin Neurobiol. 19:146–153. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Bourgeois JP, Rakic P. 1989. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Res Dev Brain Res. 50:11–32. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Rakic P. 1991. Synaptogenesis in monkey somatosensory cortex. Cereb Cortex. 1:510–523. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. 2005. Development of long-term spine stability in diverse regions of cerebral cortex. Neuron. 46:181–189. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan WB. 2005. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 436:261–265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.