Abstract
Paired-pulse transcranial magnetic stimulation (TMS) of the human motor cortex results in consecutive facilitatory motor-evoked potential (MEP) peaks in surface electromyography in intact humans. Here, we tested the effect of an incomplete cervical spinal cord injury (SCI) on early (first) and late (second and third) MEP peaks in a resting intrinsic finger muscle. We found that all peaks had decreased amplitude in SCI subjects compared with controls. The second and third peaks were delayed with the third peak also showing an increased duration. The delay of the third peak was smaller than that seen in controls at lower stimulation intensity, suggesting lesser influence of decreased corticospinal inputs. A mathematical model showed that after SCI the third peak aberrantly contributed to spinal motoneurone recruitment, regardless on the motor unit threshold tested. Temporal and spatial aspects of the late peaks correlated with MEP size and hand motor output. Thus, early and late TMS-induced MEP peaks undergo distinct modulation after SCI, with the third peak likely reflecting a decreased ability to summate descending volleys at the spinal level. We argue that the later corticospinal inputs on the spinal cord might be crucial for recruitment of motoneurones after human SCI.
Keywords: corticospinal volleys, primary motor cortex, spinal cord injury, transcranial magnetic stimulation, voluntary movement
Introduction
During the last decades, anatomical and electrophysiological studies demonstrated significant reorganization in the corticospinal tract after spinal cord injury (SCI) in animals and humans (Oudega and Perez 2012). Animal models of SCI showed that corticospinal axons undergo progressive fragmentation and Wallerian degeneration with sprouts formations rostral and caudal to the lesion (Bareyre et al. 2004; Rosenzweig et al. 2010). Anatomical studies in humans showed that surviving corticospinal axons may undergo demyelination and progressive atrophy extending at a distance from the injury epicenter (Bronson et al. 1978; Bunge et al. 1993; Buss et al. 2004). Corticospinal responses elicited by noninvasive cortical stimulation have delayed latencies and higher thresholds in individuals with SCI compared with controls (Ellaway et al. 2007; Perez 2012). However, limited information exists on cortico-cortical influences on corticospinal responses after SCI.
It is possible to obtain information about cortico-cortical inputs onto corticospinal neurons by recording the effects of cortical stimulation. Recordings from the epidural space in animals and humans showed that a single shock over the primary motor cortex evokes temporally synchronized descending waves in the corticospinal tract with frequencies of approximately 700 Hz (Patton and Amassian 1954; Di Lazzaro et al. 2012). The earliest wave is due to direct stimulation of the corticospinal neuron at or near the initial segment while later subsequent indirect (I) waves (termed I1, I2, and I3) may arise from transsynaptic activation of corticospinal neurons by intracortical circuits (Di Lazzaro et al. 2012). Studies using transcranial magnetic stimulation (TMS) have shown that it is possible to make inferences about the physiology of I-waves from surface electromyographic (EMG) recordings. Paired-TMS pulses can be precisely timed to increase the amplitude of motor-evoked potentials (MEPs) at interstimulus intervals of approximately 1.5 ms compatible with the I-waves recorded from the epidural space in control subjects (Tokimura et al. 1996; Ziemann, Tergau, Wassermann et al. 1998) and in individuals with motor disorders (Ho et al. 1999; Salerno and Georgesco 2001; Quartarone et al. 2002). The goal of our study was to examine the organization of early and late TMS-induced MEP peaks in an intrinsic finger muscle in individuals with chronic incomplete cervical SCI.
In uninjured subjects, MEP peaks measured by paired-pulse TMS are influenced by GABAergic inhibitory mechanisms at the level of the primary motor cortex (Tokimura et al. 1996; Ziemann, Tergau, Wassermann et al. 1998; Ziemann, Tergau, Wischer et al. 1998). However, early (first) and late (second and third) MEP peaks likely involve different mechanisms of action. The later peaks might be modulated by preferential activation of intracortical interneurons while the early peak likely involves depolarization of pyramidal neurons in deeper areas close to the axon initial segment (Di Lazzaro et al. 2012). After SCI, the resting excitability level of intracortical circuits is altered compared with controls (Shimizu et al. 2000), and retrograde degeneration in corticospinal axons is less likely to affect the axon initial segment (Kalil and Schneider 1975). Therefore, we hypothesized that early and late peaks measured by paired-pulse TMS will be impaired to a different extent in subjects with SCI.
To test our hypothesis, we used TMS over the hand representation of the primary motor cortex to examine the first, second, and third TMS-induced MEP peaks in an intrinsic finger muscle in individuals with incomplete cervical SCI and in age-matched controls. Our results indicate that early and late MEP peaks undergo distinct modulation after SCI, with the third peak likely reflecting a decreased ability to summate descending volleys at the spinal level. Furthermore, temporal and spatial characteristics of late peaks relates to aspects of residual hand voluntary motor output.
Materials and Methods
Subjects
Sixteen individuals with SCI (mean age = 53.4 ± 14.2 years, 2 female; Table 1) and 15 age-matched (P = 0.28) right-handed healthy controls (mean age = 47.6 ± 15.1 years, 6 male) participated in the study. All subjects gave written informed consent prior to participation in the study, which was approved by the University of Pittsburgh Research Ethics Committee and conformed to the Declaration of Helsinki. Participants with SCI had an intact (score = 2) or impaired (score = 1), but not absent innervation in dermatome C6 during light touch and pin prick stimulus using the ASIA (American Spinal Cord Injury Association) sensory scores and residual hand motor function (Table 1). The Semmens–Weinstein monofilament test (SWMT) was used to assess cutaneous sensibility in dermatome C6 in participants with SCI (Kalsi-Ryan et al. 2012). Three out of the 16 subjects were categorized as ASIA A (complete injury) due to the lack of sacral sparing (Marino et al. 2003), despite being able to elicit voluntary force with hand muscles. The remaining 13 subjects were classified as incomplete ASIA C or D (Table 1). All participants were able to exert maximal voluntary contraction (MVC) isometric forces into index finger abduction (Controls = 21.2 ± 8.5 N, SCI = 14.0 ± 6.8 N, P = 0.02). EMG activity during index finger abduction MVC was greater in controls (0.67 ± 0.19 mV) than in SCI subjects (0.30 ± 0.19 mV; P < 0.001).
Table 1.
Spinal cord injury participants
| SCI subject | Age (years) | Sex | Level | ASIA score | Etiology | Time since injury (years) | FDI MVC (N) | Maximum FDI EMG (mV) | SWMT (level) | Light touch | Pin prick | Spasm frequency score | Medication |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | M | C5 | D | T | 3 | 18.2 | 0.75 | 3.44 | 2 | 2 | 1 | None |
| 2 | 32 | M | C6 | A | T | 8 | 26.8 | 0.39 | 6.08 | 1 | 1 | 4 | None |
| 3 | 44 | F | C7 | A | T | 13 | 8.4 | 0.14 | 3.84 | 2 | 2 | 4 | Bac |
| 4 | 29 | M | C8 | A | T | 3 | 4.4 | 0.13 | n/k | 1 | 1 | 4 | Bac, Gbp, Dia, Tiz |
| 5 | 62 | M | C5 | D | NT | 6 | 12.6 | 0.51 | 2.93 | 2 | 2 | 0 | None |
| 6 | 61 | F | C7 | D | T | 15 | 7.3 | 0.23 | 2.93 | 1 | 1 | 2 | Bac, Gbp |
| 7 | 60 | M | C4 | C | NT | 5 | 10.9 | 0.29 | 3.91 | 1 | 1 | 2 | Gbp |
| 8 | 65 | M | C4 | D | T | 7 | 23.0 | 0.45 | 3.75 | 1 | 2 | 0 | None |
| 9 | 40 | M | C5 | D | T | 3 | 18.0 | 0.19 | 3.57 | 2 | 2 | 2 | Bac, Gpb |
| 10 | 68 | M | C4 | D | T | 4 | 15.6 | 0.53 | 4.39 | 1 | 1 | 3 | Lzp |
| 11 | 53 | M | C7 | C | T | 3 | 6.3 | 0.04 | 4.25 | 2 | 1 | 2 | Bac, Gbp, Tiz |
| 12 | 34 | M | C7 | D | T | 10 | 12.3 | 0.20 | 2.93 | 2 | 2 | 2 | Bac, Gbp |
| 13 | 59 | M | C5 | D | T | 6 | 17.6 | 0.25 | n/k | 1 | 1 | 1 | Bac |
| 14 | 61 | M | C4 | C | T | 1 | 5.6 | 0.08 | 3.32 | 2 | 2 | 1 | Gpb |
| 15 | 80 | M | C4 | D | T | 8 | 23.7 | 0.32 | 4.01 | 2 | 1 | 1 | None |
| 16 | 54 | M | C3 | C | T | 1 | 13.5 | 0.33 | n/k | 1 | 1 | 2 | Bac, Tiz |
Note: SCI, spinal cord injury; M, male; F, female; ASIA, American Spinal Injury Association; T, traumatic; NT, nontraumatic; FDI, first dorsal interosseous; MVC, maximum voluntary contraction; N, Newtons; EMG, electromyography; SWMT, Semmes–Weinstein monofilament test; range 1.65–6.60: high levels, more sensory impairment; n/k, not known; Light touch and Pin prick: 1 = impaired, 2 = intact; Spasm frequency score: 0 = no spasms, 1 = one or fewer spasms per day, 2 = 1–5 spasms per day, 3 = 5–10 spasms per day, and 4 = 10 or more spasms per day; Bac, baclofen; Gbp, gabapentin; Tiz, tizanidine; Dia, diazepam; Lzp, lorazepam.
EMG Recordings
Surface EMG was recorded from the first dorsal interosseous (FDI) muscle of the right (dominant) hand in healthy controls and from the less affected hand in individuals with SCI using Ag-AgCl electrodes (10 mm diameter). The signals were amplified, filtered (20–1000 Hz), and sampled at 2 kHz for off-line analysis (CED 1401 with Signal software, Cambridge Electronic Design, Cambridge, UK). During MVC, force exerted at the proximal interphalangeal joint of the index finger was measured by load cells (Honeywell, Ltd., range ± 498.1 N, voltage ±5 V, high-sensitivity transducer 0.045 V/N). Force was sampled at 200 Hz and stored on a computer for off-line analysis.
Experimental Setup
Subjects were tested at rest on different sessions. During testing, subjects were seated in an armchair with both arms flexed at the elbow by 90° with the forearm pronated and the wrist restrained by straps (Fig. 1A). At the start of the experiment, subjects performed 2–3 brief MVCs (3–5 s) with the index finger into abduction, separated by 30 s. EMG activity from the tested FDI muscle was displayed continuously on an oscilloscope, and verbal feedback was provided to the subjects to ensure that physiological measurements in the FDI were acquired at rest at all times. A total of 5.3 ± 2.0% trials in which mean rectified EMG activity exceeded ±2 SD of the mean average rectified EMG, measured 100 ms before the stimulus artifact, were excluded from further analysis (Bunday et al. 2014). A similar number of trials (5.1 ± 1.1%) were excluded when the criterion was set to have a root mean square EMG of <5 μV measured 100 ms before the stimulus artifact (Cuypers et al. 2014).
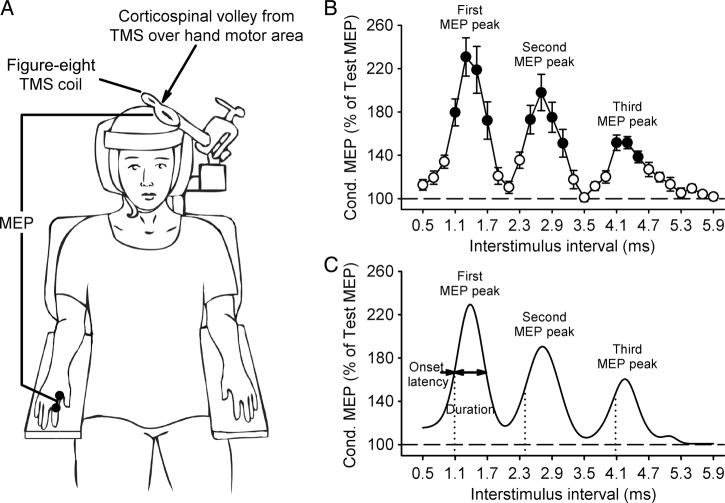
Figure 1.
Experimental setup. (A) Schematics of the experimental setup showing the posture of the hand and the coil position during testing. (B) Group data in control subjects (n = 15) showing MEP peaks tested by paired-pulse TMS in the resting FDI muscle. The abscissa shows ISI between paired pulses (0.5 and 5.9 ms), and the ordinate shows the size of the conditioned MEP (expressed as a % of the Test MEP). Note that the conditioned MEP was largely facilitated at stimulus intervals corresponding to the I1, I2, and I3 waves from epidural recordings (Ziemann and Rothwell 2000). Error bars indicate SEs. *P < 0.05. (C). Curve fitting analysis using a 3 Gaussian model for each MEP peak to estimate individual properties. The vertical dotted lines indicate the onset latency of each MEP peak localized by the fitting model and the horizontal dashed line represents the size of the Test MEP (baseline). The horizontal arrow indicates the duration of MEP peaks.
Transcranial Magnetic Stimulation
Transcranial magnetic stimuli were delivered from a Magstim 200 stimulator (Magstim Company) through a figure-eight coil (loop diameter, 7 cm; type number SP15560) with a monophasic current waveform. The coil was held tangentially to the skull with the handle pointing backwards and laterally at an angle of 45° to the sagittal plane. With this coil orientation, current flow was induced in a posterior–anterior direction (Sakai et al. 1997). The TMS coil was held to the head of the subject with a custom coil holder, while the head was firmly secured to a headrest by straps to limit head movements. TMS was delivered at 0.2 Hz. TMS measurements included MEPs, resting motor threshold (RMT), maximal MEP size (MEP-max), and MEP peaks (first, second, and third).
Motor-Evoked Potentials
RMT (Controls = 53.4 ± 12.4%, SCI = 61.1 ± 11.7%; P = 0.04) was determined as the minimum stimulus intensity required to elicit an MEP in the relaxed FDI of at least 50 µV in amplitude in 5 out of 10 consecutive trials (Rothwell et al. 1999). The MEP-max was defined in all participants at rest by increasing stimulus intensities in 5% steps of maximal device output until the MEP amplitude did not show additional increases (Controls = 4.66 ± 2.09 mV, SCI = 1.78 ± 2.42 mV; P < 0.01).
MEP Peaks
An established paired-pulse TMS paradigm was used to assess TMS-induced MEP peaks at rest (Tokimura et al. 1996; Ziemann, Tergau, Wassermann et al. 1998). Note that peaks registered by surface EMG electrodes are compatible with the peaks of the I-waves recorded from the epidural space. A conditioned stimulus (S2) was delivered at interstimulus intervals (ISIs) from 0.5 to 5.9 ms (tested in 0.2 ms steps, 28 intervals; Fig. 1B) after a test stimulus (S1). The S1 elicited a test MEP and the S2 elicited a conditioned MEP. The S1 intensity was adjusted to match the MEP size (∼1 mV) in both groups (Controls = 0.93 ± 0.32 mV, SCI = 0.87 ± 1.16 mV; P = 0.85). The S2 intensity was set at 90% of RMT. MEPs at each ISI were tested twice and each time 10 MEPs were collected. Because the size of the test MEP in SCI subjects was around 50% of their MEP-max in an additional control experiment, the S1 was also set to produce an MEP of 50% of the MEP-max in controls (∼3 mV; 2.65 ± 0.90, n = 10). Because the latency of the later MEP peaks is influenced by the intensity of the S1 (Ziemann, Tergau, Wassermann et al. 1998), testing was also conducted in controls by eliciting a small test MEP of approximately 0.05 mV (n = 7). MEP peaks were calculated by expressing the size of the conditioned MEP as a percentage of the size of the test MEP ([conditioned MEP × 100]/test MEP) response obtained in the same block.
MEP Peaks Analysis Using a 3 Gaussian Model
We fitted our previous data into a 3 Gaussian model. We used the sum of 3 Gaussian curves (Thickbroom 2011; Delvendahl et al. 2014), accounting for changes in background level, to estimate the onset latency and duration of each peak (Fig. 1C). For each peak i with a given latency ti, amplitude Ai, and width (Gaussian sigma) σi, and overall baseline y0, and small-ISI baseline y0,L, all peaks were modeled as
where y is the peak amplitude (see MEP peaks section for calculation) and t is the ISI. Data were fitted to the model for each subject using a 1000-iteration bootstrapping procedure (Efron and Tibshirani 1993; DiCiccio and Efron 1996) using the MATLAB bootci function. On each iteration, a dataset was created by sampling individual normalized MEPs with replacement. A curve fit was then performed using a trust region reflective least-squares fit algorithm (Coleman and Li 1996). Parameter estimates for each subject were chosen as the mean of the 1000 fits, and 95% confidence intervals (CIs) were computed across this sample. Peaks where amplitudes had CIs not inclusive of 0 were deemed significant. As a result of the curve fitting, 12/16 individuals with SCI and 14/15 controls demonstrated three MEP peaks and were included in the group analyses.
A previous model estimated the peaks summation and subsequent activation of spinal motoneurones (Thickbroom 2011). We modified this model to include the temporal dynamics of all peaks recruitment. Recruitment curves were modeled for the 3 peaks for data derived from each individual control and SCI subject. First, the peak amplitude at each time point (0–6 ms) was converted to a ratio (peak size of 150% has an amplitude of 0.5). Second, the magnitude of the spinal motoneurone excitatory postsynaptic potentials (EPSPs) was modeled as the time-dependent summation of all peaks. Third, total motor unit firing rates were determined by comparing the EPSP to a population of motor units with threshold modeled to follow a normal distribution with a minimum of 0 and maximum of 1 (defined as the maximum total cumulative facilitation in controls), a variable mean (mean motor unit threshold), and a fixed width (σ, 1/6th of the maximum activation). The integral of the motor unit threshold distribution from 0 to the EPSP magnitude at each time point was used to estimate the proportion of the spinal motoneurone pool activated. Intervals of 0–2 ms (first peak), 2–4 ms (second peak), and 4–6 ms (third peak) were used to assess the proportion of motoneurones recruited by each peak. We also assessed mean motor unit recruitment across a range of threshold values intervals from 0.2–0.4 (25%), 0.4–0.6 (50%), 0.6–0.8 (75%), and 0.8–1.0 (100%).
Motor Output
We examined the onset of voluntary contraction during brief, fast, isometric contractions with the index finger into abduction following a visual cue. Four blocks of 20 trials were recorded with a 2–3 min rest period between blocks. Reaction time (RT) was defined as the time when the mean rectified EMG signal exceeded 2SD of the mean resting EMG. We assessed the coefficient of variation (CV) of EMG (SD/mean EMG) signals in the FDI muscle during 20% of isometric MVC. EMG was rectified and averaged 100 ms before TMS for each trial.
Data Analysis
Normal distribution was tested by the Shapiro–Wilk's test and homogeneity of variances by the Levene's test of equality and Mauchly's test of sphericity. When normal distribution could not be assumed, data were log transformed. When sphericity could not be assumed, the Greenhouse–Geisser correction statistic was used. Repeated-measures ANOVAs were performed to determine the effect of GROUP (Controls and SCI) and ISI (0.5–5.9 ms) on the amplitude of each peak. Repeated-measures ANOVAs were also performed to determine the effect of GROUP and PEAKS (first, second, and third) on the onset latency, duration, and frequency of each peak. Additional ANOVA tests were conducted to compare the effect of motor unit THRESHOLD (25%, 50% 75%, and 100% interval) and GROUP on the proportion of motor unit recruitment. Bonferroni post hoc tests were used to test for significant comparisons. Independent t-tests were used to compare age, force, EMG, RMT, and MEP amplitude between groups. Additional repeated ANOVAs and t-tests were performed on each group separately. A Pearson correlation analysis was used as needed (Bonferroni corrected for multiple comparisons). The significance level was set at P < 0.05, and group data are presented as mean ± SD in the text.
Results
MEP Peaks
Figure 2A illustrates examples of test (black traces) and conditioned (gray traces) MEPs in the resting FDI muscle from a representative subject in each group. Note that the amplitude of the conditioned MEP increased to a lesser extent in the SCI compared with the control subject. Consistent with previous results, in controls, the first MEP peak included ISIs from 1.1 to 1.7 ms (P < 0.001), the second from 2.5 to 3.1 ms (P < 0.001), and the third from 4.1 to 4.5 ms (P < 0.01).
Figure 2.
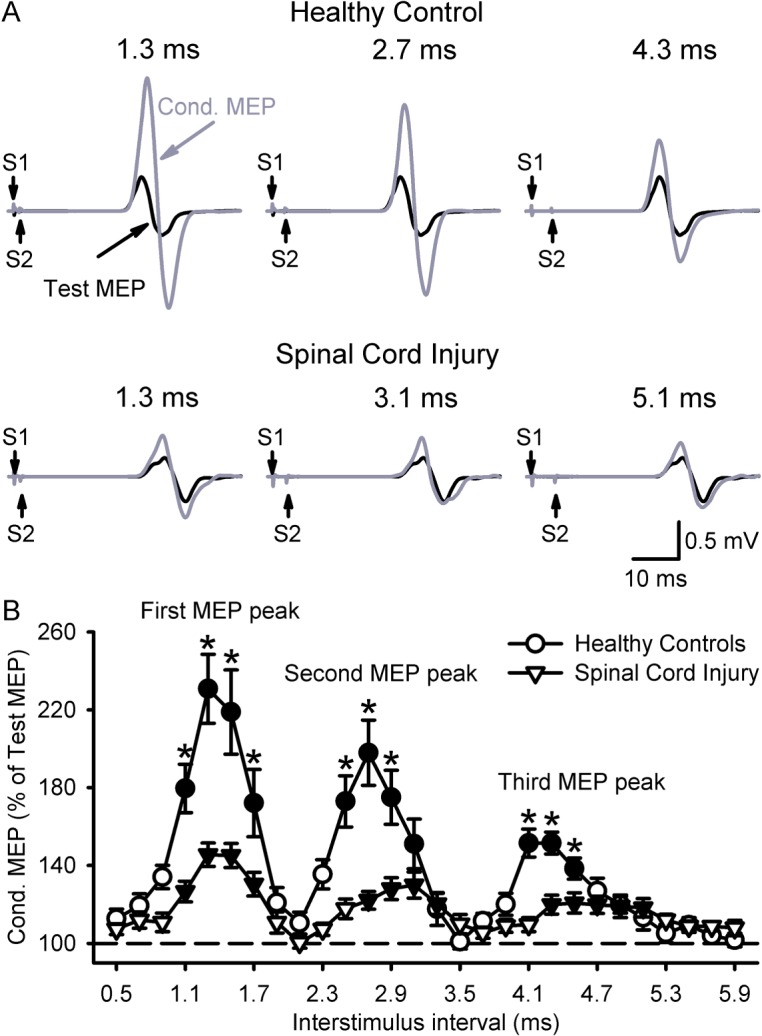
MEP peaks. (A) MEPs tested in the resting FDI muscle in a control (upper panel) and SCI subject (lower panel) at the peak amplitude interval for the first (left trace: Control, 1.3 ms; SCI, 1.3 ms), second (middle trace: Control, 2.7 ms; SCI, 3.1 ms), and third (right trace: Control, 4.3 ms; SCI, 5.1 ms) MEP peaks. Traces show the average of 20 test (black) and conditioned (gray) MEPs. Arrows indicate the test (S1) and conditioning (S2) stimulus. (B) Group data in controls (n = 15, circles) and subjects with SCI (n = 16, triangles). The abscissa shows the ISIs tested (0.5–5.9 ms, in 0.2-ms steps). The ordinate shows the size of the conditioned MEP (expressed as a % of the Test MEP, horizontal dashed line). Note that the amplitude of all peaks was decreased in subjects with SCI compared with controls. Also, note that the onset of the second and third MEP peaks was delayed in SCI subjects compared with controls and that the third MEP peak showed increased duration after SCI. Error bars indicate SEs. Filled symbol denotes statistical significance compared with baseline (P < 0.05). *P < 0.05 compared with SCI.
Our overall analysis showed a significant effect of GROUP (F1,29 = 12.5, P < 0.01), ISI (F8,231 = 27.1, P < 0.001), and a GROUP × ISI interaction (F8,231 = 7.5, P < 0.001; Fig. 2B) on peaks amplitude. Post hoc testing showed that the amplitude of all peaks was decreased in SCI subjects compared with controls (P < 0.01). Note that 16/16 showed a decrease in the first and second peaks, and 14/16 showed a decrease in the third peak. Mean background rectified EMG activity in the FDI remained similar across conditions (F7,191 = 0.8, P = 0.57) and groups (F1,29 = 1.7, P = 0.19). Figure 3A shows the group curve fitting analysis to estimate the onset latency and duration of each MEP peak. Here, we found a significant effect of GROUP (F1,24 = 14.2, P < 0.001), PEAKS (F2,37 = 1952.4, P < 0.001), and a GROUP × PEAKS interaction (F2,37 = 5.2, P = 0.01; Fig. 3B) on peaks onset latency. Post hoc testing showed that the onset latency of the first peak was similar across groups but delayed for the second and third peaks after SCI (Table 2). Note that a decrease in the S1 intensity (∼0.05 mV) in controls delayed the onset latency of the second and third peaks compared with higher S1 intensities. Also, the delay of the third but not the second peak seen after SCI was less than controls at lower S1 intensity (Table 2).
Figure 3.
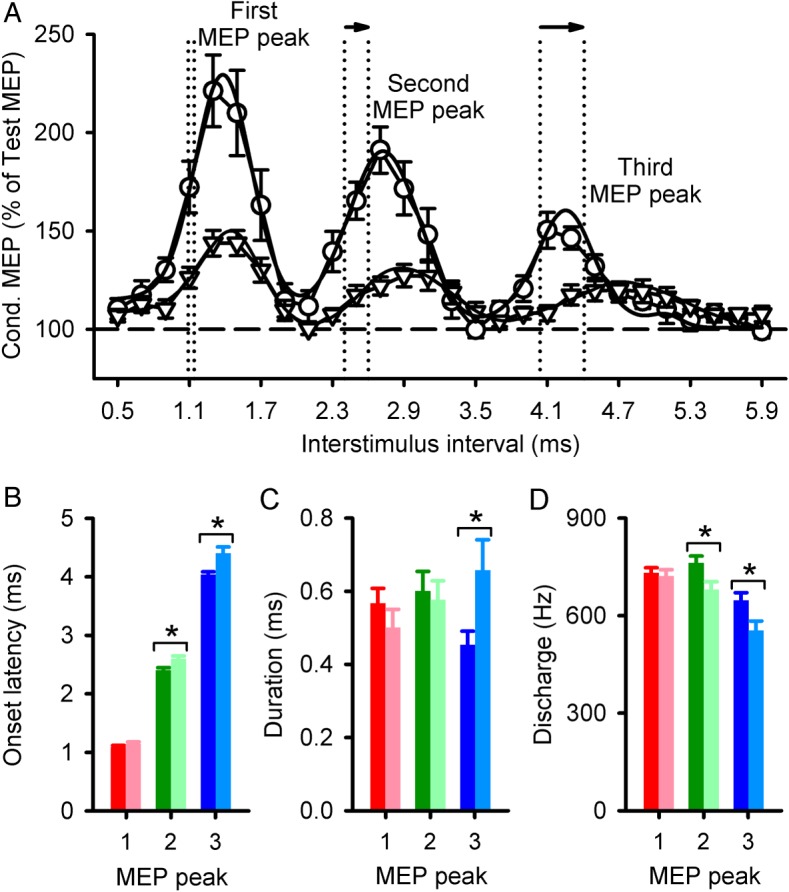
MEP peaks onset latency and duration estimated by a Gaussian model. (A) Data estimated by a Gaussian model for each peak in controls (circles) and subjects with SCI (triangles). Each symbol shows the group mean of conditioned MEPs (expressed as a % of the Test MEP), with the solid line representing the respective curve fit. The vertical dotted lines indicate the onset localized by the fitting model. Note the delayed onset, as shown by the arrows, in SCI subjects compared with controls for the second and third MEP peak. The horizontal dashed line represents the size of the Test MEP. Group data (Controls, n = 14; SCI, n = 12) showing onset latency (B), duration (C), and discharge frequency (D) of each peak. The abscissa shows each MEP peak (1, 2, and 3). The ordinate shows the onset and duration (milliseconds), and discharge frequency (Hz) of each peak in SCI subjects (light bars) and controls (dark bars). Error bars indicate SEs. *P < 0.05.
Table 2.
Onset latency of MEP peaks
| MEP peak | Onset latency (ms) |
P-value | |||
|---|---|---|---|---|---|
| Controls, 0.05 mV | Controls, 1.0 mV | Controls, 3.0 mV | SCI, 1.0 mV | ||
| First | 0.90 ± 0.13 | 1.09 ± 0.11 | 1.11 ± 0.11 | 1.14 ± 0.12 | <0.001 |
| Second | 2.66 ± 0.18 | 2.40 ± 0.17 | 2.37 ± 0.22 | 2.60 ± 0.17 | <0.01 |
| Third | 4.97 ± 0.44 | 4.08 ± 0.29 | 4.06 ± 0.26 | 4.41 ± 0.37 | <0.001 |
Note: Values are mean ± SD onset latency of MEP peaks for test stimulus (S1) intensities of 0.05, 1.0, and 3.0 mV in controls and 1.0 mV in SCI subjects. P-values represent ANOVA tests performed across S1 intensities on each MEP peak. Note that a decrease in the S1 intensity (∼0.05 mV) in controls delayed the onset latency of the second and third peaks compared with higher S1 intensities (1.0 and 3.0 mV), whereas the delay of the third but not the second peak seen after SCI was less than controls at lower S1 intensity (∼0.05 mV).
The duration of the first (Controls = 0.57 ± 0.15 ms, SCI = 0.51 ± 0.16 ms, P = 0.73) and second (Controls = 0.60 ± 0.20 ms, SCI = 0.58 ± 0.14 ms, P = 0.98) peaks was similar between groups but increased for the third peak (Controls = 0.45 ± 0.14 ms, SCI = 0.67 ± 0.27 ms, P = 0.04; Fig. 3C) in SCI compared with controls. The overall frequency discharge of peaks was decreased in SCI individuals (637 ± 50 Hz) compared with controls (704 ± 22 Hz, P < 0.001; Fig. 3D). Specifically, the second (Controls = 763 ± 79 Hz, SCI = 680 ± 85 Hz, P = 0.04) and third (Controls = 648 ± 85 Hz, SCI = 554 ± 101 Hz, P = 0.04) but not the first (Controls = 731 ± 61 Hz, SCI = 722 ± 66 Hz, P = 0.97) peak discharge frequencies were decreased, in agreement with the delay in latency after SCI.
Figure 4 illustrates differences across MEP peaks. We found that differences in onset latency and frequency discharge were greater for the third (onset latency: P = 0.01, frequency discharge: P = 0.02) and second (onset latency: P = 0.04, frequency discharge: P = 0.03) compared with the first (Fig. 4A,B). Also, the third peak had a longer duration compared with the first (P < 0.01) and second (P = 0.04; Fig. 4C).
Figure 4.
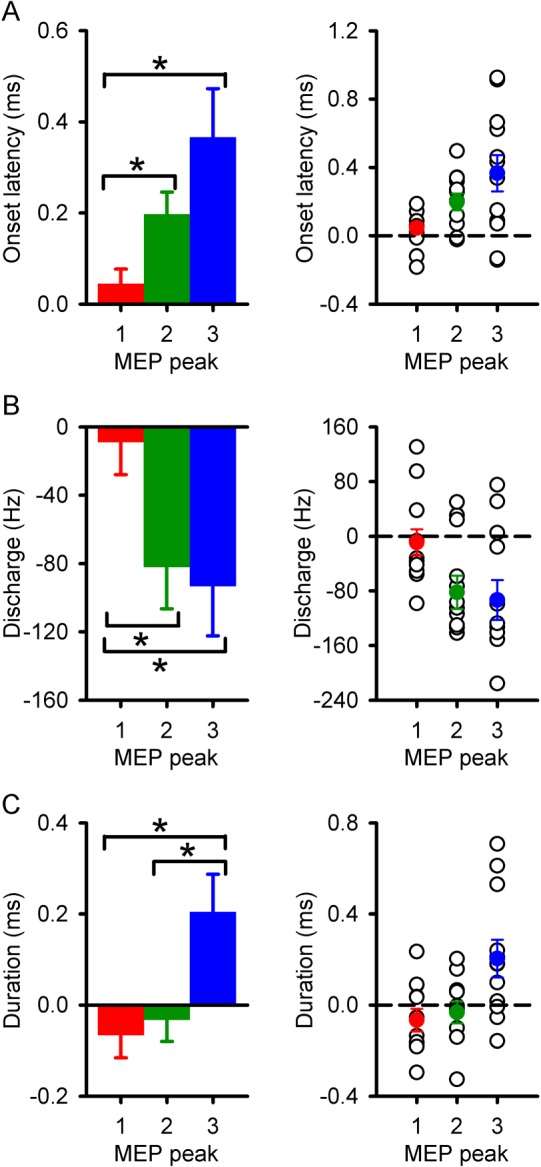
Differences across MEP peaks between groups. Left panel graphs show the difference in onset latency (A), discharge frequency (B), and duration (C) for the first (red), second (green), and third (blue) MEP peaks between SCI subjects and controls. Right panel graphs show individual data in each of the variables tested. The abscissa shows each MEP peak (1, 2, and 3). The ordinate shows the onset and duration (milliseconds), and discharge frequency (Hz) of each peak. Note that overall subjects with SCI showed more pronounced changes in duration, frequency discharge, and onset latency in the third compared with the second and first MEP peaks. Error bars indicate SEs. *P < 0.05.
When peaks were compared between groups by adjusting the size of the test MEP to be approximately 50% of the MEP-max, we found no effect of GROUP (F1,24 = 1.5, P = 0.23) but a significant effect of ISI (F9,208 = 10.9, P < 0.001) and a GROUP × ISI interaction (F9,208 = 2.1, P = 0.03; see Supplementary Fig. S1) on peaks amplitude. Post hoc analysis showed that the amplitude of all peaks was similar across groups. However, similar to our previous results, the onset latency was delayed for the second (by 0.24 ± 0.17 ms, P = 0.03) and third (by 0.34 ± 0.37 ms, P = 0.04) but not the first (P = 0.63) peak in individuals with SCI compared with controls. Also, SCI subjects had an increased duration of the third peak (P = 0.01). Mean background rectified EMG activity in the FDI remained similar across conditions (F6,153 = 1.0, P = 0.46) and groups (F1,24 = 0.1, P = 0.75).
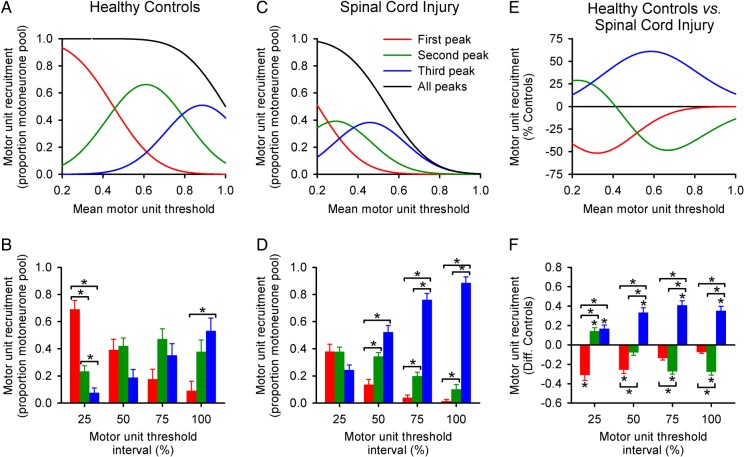
Proportion of Spinal Motoneurones Recruited by MEP Peaks
Figure 5A–F shows the results from a 3 Gaussian model on the proportion of motoneurones recruited by all peaks. In controls, the proportion of motor units recruited by the first MEP peak (69 ± 24%) was higher than the second (23 ± 15%, P < 0.001) and third (8 ± 13%, P < 0.001; Fig. 5B) at the 25% interval. Whereas the third (53 ± 36%, P = 0.03) peak made a higher contribution than the first (9 ± 26%) at the 100% interval. At 50% (P = 0.17) and 75% (P = 0.11) intervals, a similar contribution was found from all peaks.
Figure 5.
Proportion of spinal motoneurones recruited by MEP peaks. Graphs on the top row show the proportion of the spinal motoneurone pool recruited by the first (red), second (green), third (blue), and all MEP peaks together (black) as a function of the mean motor unit threshold in controls (A, n = 14) and SCI subjects (C, n = 12), and the difference between groups (E). Graphs on the bottom row show the quantified proportion of the spinal motoneurone pool recruited from the same subjects (Controls [B], SCI [D], and the difference between groups [F]). Note that the model revealed a pronounced contribution of the third peak to the recruitment of spinal motoneurones in subjects with SCI, regardless of the motor unit threshold tested. Error bars indicate SEs. *P < 0.05.
We found a significant effect of GROUP (F1,24 = 4.9, P = 0.03), THRESHOLD (F3,72 = 3.3, P = 0.02), and a GROUP × THRESHOLD interaction (F3,72 = 4.7, P < 0.01; Fig. 5F) on the contribution of peaks to the recruitment of motoneurones. Post hoc testing showed that at the 25% interval, the first MEP peak contributed less to the recruitment after SCI compared with controls (Controls = 69 ± 24%, SCI = 38 ± 19; P < 0.01). Indeed, the first MEP peak contribution was 45 ± 28% less in SCI than in controls. Whereas at the 25% interval, the second (Controls = 23 ± 15%, SCI = 38 ± 12%; P = 0.03) and third (Controls = 8 ± 13%, SCI = 24 ± 13%; P = 0.01) peaks made a larger contribution in SCI than in controls. At the 100% interval, the first peak contribution did not differ from controls while the second peak showed a lesser contribution in SCI than in controls (Controls = 38 ± 32%, SCI = 10 ± 12%; P = 0.02). At the same interval, the third peak made a larger contribution to recruitment in SCI than in controls (Controls = 53 ± 36%, SCI = 88 ± 16%; P = 0.01). The third MEP peak contribution was larger in SCI than in controls, regardless of the motor unit threshold interval tested (P = 0.01).
Additional analysis revealed no differences in the recruitment of spinal motoneurones when changing the intensity of the S1 in control subjects (see Supplementary Fig. S2). We found a significant effect of THRESHOLD (F1,37 = 8.9, P < 0.01), but not for S1 INTENSITY (F2,29 = 1.9, P = 0.16) and S1 INTENSITY × THRESHOLD interaction (F2,33 = 0.6, P = 0.60) on the contribution of each peak to the recruitment of motoneurones. Post hoc testing showed that a similar contribution was found at each threshold interval (25%, 50%, 75%, and 100%) between different S1 intensities (∼0.05, ∼1, and ∼3 mV) for the first (P = 0.38), second (P = 0.18), and third (P = 0.56) MEP peaks in controls, suggesting that it is less likely that the aberrant pattern of motoneurone recruitment observed after SCI was related to changes in stimulus intensity.
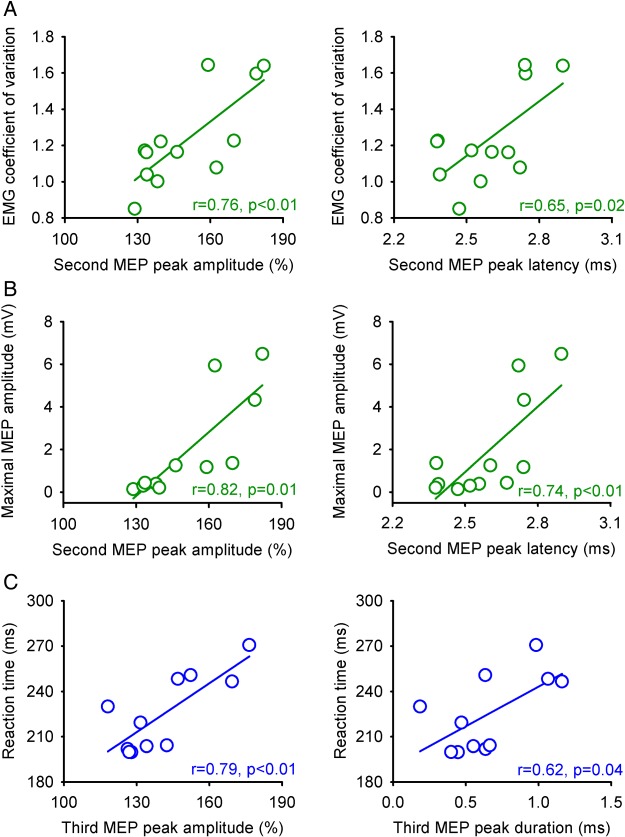
Correlations
We found that index finger RT was delayed (SCI = 227 ± 23 ms, Controls = 196 ± 33 ms, P = 0.02) and that the CV was increased (SCI = 1.25 ± 0.33, Controls = 1.02 ± 0.09, P = 0.01) in SCI subjects compared with controls. After SCI, the maximum amplitude (r = 0.76, P < 0.01) and onset latency (r = 0.65, P = 0.02) of the second MEP peak was correlated with the CV (Fig. 6A). Also, the second MEP peak maximum amplitude (r = 0.82, P = 0.01) and onset latency (r = 0.74, P < 0.01) was correlated with the MEP-max (Fig. 6B). The maximum amplitude (r = 0.79, P < 0.01) and duration (r = 0.62, P = 0.04) of the third peak correlated with SCI subjects RT (Fig. 6C). In contrast, no motor output parameter tested correlated with any characteristic of the first MEP peak (P > 0.22).
Figure 6.
Correlations in individuals with SCI. Correlation analysis between the maximum amplitude (upper left graph) and onset latency (upper right graph) of the second MEP peak with the coefficient of variation (A) and MEP maximum amplitude (B, see methods). Also, correlation analysis between the third MEP maximum amplitude (lower left graph) and duration (lower right graph) with index finger reaction time (C). In graphs showing amplitude, the abscissa shows the size of the conditioned MEP (expressed as a % of the Test MEP) and the ordinates show the coefficient of variation (CV) and reaction time (RT; milliseconds). In graphs showing onset latency and duration, the abscissa shows latency and duration values (milliseconds) and the ordinates show the CV and RT (milliseconds). Correlation analysis was Bonferroni corrected for multiple comparisons.
Discussion
We used a paired-pulse TMS protocol to study the organization of early and late TMS-induced MEP peaks in an intrinsic finger muscle after chronic incomplete cervical SCI. We found that all MEP peaks had decreased amplitude in SCI subjects compared with controls. The second and third peaks were delayed with the third peak also showing an increased duration. The delay of the third peak was smaller than that seen in controls at lower stimulation intensity, suggesting a lesser influence of decreased corticospinal inputs. A mathematical model showed that the third peak aberrantly contributed to recruit spinal motoneurones after SCI, regardless of the motor unit threshold tested. A relationship was found between temporal and spatial aspects of the late peaks and MEP size and hand voluntary motor output. We argue that later corticospinal inputs on the spinal cord might be crucial for recruitment of motoneurones after human SCI playing a critical role in the translation of motor commands after injury.
Organization of Early and Late MEP Peaks After SCI
Our results in control subjects agree with previous findings showing TMS-induced MEP peaks at intervals of around 1.5 ms (Tokimura et al. 1996; Ziemann, Tergau, Wassermann et al. 1998). Note that the latency of these peaks is compatible with the latency of successive I-waves recorded from the epidural space (Ziemann and Rothwell 2000). In SCI individuals, we found that although all MEP peaks had decreased amplitudes, the second and third, but not the first MEP peak, showed delayed onset latencies. A critical question is which neuronal elements in the corticospinal pathway are involved in the changes in the MEP peaks observed in individuals with SCI. In uninjured subjects, later peaks likely reflect activation of GABAergic intracortical interneurons (Di Lazzaro et al. 2012). Modeling studies also proposed that later I-waves are influenced by the summation of EPSPs and IPSPs on distal synapses on corticospinal neurons (Rusu et al. 2014). Individuals with SCI show less GABAergic intracortical inhibition compared with controls, at least, in a resting condition (Shimizu et al. 2000). Therefore, because the I2- and I3-waves are affected by a TMS protocol testing intracortical inhibition (Nakamura et al. 1997; Di Lazzaro et al. 1998) one might expect that the second and third MEP peaks will be affected after SCI. However, intracortical inhibition has a similar effect on the magnitude of the second and third MEP peaks in control subjects (Wagle-Shukla et al. 2009), and we found that the amplitude, duration, and latency of the third MEP peak was more affected than that of the second MEP peak after SCI making this possibility less likely. This also agrees with previous studies proposing that early and late TMS-induced MEP peaks differ in their mechanisms of action (Di Lazzaro et al. 2012). Another possibility is that a decrease in corticospinal drive after the injury contributed to our results. Indeed, paired-pulse TMS protocols showed that in uninjured subjects the latency of the late MEP peaks is prolonged with decreasing intensities of the test MEP (Ziemann, Tergau, Wassermann et al. 1998). However, the effect of TMS intensity on the latency of the third MEP peak was less in people after SCI than in controls. We also found that the amplitude of all MEP peaks at lower TMS intensity increased in controls, which contrasted the results in individuals with SCI, suggesting that it is less likely that this was a main factor affecting our results. This is also supported by our control experiment showing that the amplitude of all peaks was similar between groups when higher test TMS intensity was used in controls. If higher TMS intensities result in more and larger I-waves as well as a D-wave (Di Lazzaro et al. 2010), temporal dispersion might be increased, which might explain why both groups showed similar peak amplitudes. Though, at higher intensity, SCI subjects continued to show delays in the second and third MEP peaks and increased duration of the third peak.
Our results indicate that a main contributing factor to the changes in MEP peaks after SCI is an impaired ability to summate descending volleys at the spinal level. EPSPs latencies on motoneurones show a direct relationship with the latencies of I-waves (Kernell and Chien-Ping 1967). This is consistent with results showing that individuals with SCI exhibited an impaired ability to summate EPSPs in motoneurones from activation of sensory afferents (Norton et al. 2008). This is also supported by our results showing that all MEP peaks had an altered contribution to the recruitment of spinal motoneurones in SCI subjects compared with controls. Particularly, the third peak showed an aberrant contribution to spinal motoneurone recruitment, regardless of the motor unit threshold tested. This contrasts results in controls, where the third peak recruited motoneurones to a lesser extent and at relatively higher motor unit thresholds (Thickbroom 2011). It is less likely that the amount of corticospinal drive affected the pattern of motor unit recruitment by the third MEP peak in SCI subjects, since in control subjects a similar pattern of recruitment was observed when large and small test MEP intensities were used. The most parsimonious explanation for our result is that after SCI, early volleys were not sufficiently strong to bring motoneurones to threshold. Therefore, more and later volleys were needed for motoneurones to reach threshold. Since subjects with SCI required higher stimulus intensities to elicit MEPs, it is also possible that in this group the threshold for the third peak was reached earlier than in controls. Indeed, evidence showed that in control subjects the third peak has a higher threshold than the first (Day et al. 1989; Sakai et al. 1997). Several of our SCI subjects were taking medication to reduce the symptoms of spasticity. Evidence showed that baclofen (Barry et al. 2013; Bunday et al. 2014), tizanidine (Chu et al. 2014), and diazepam (Greve et al. 1991) can affect the excitability of spinal cord circuits after SCI. However, a separate analysis revealed no differences in MEP peaks between SCI subjects taking and not taking these medications. This is also consistent with the lack of effect of baclofen on TMS-induced MEP peaks in control subjects (Ziemann, Tergau, Wischer et al. 1998). Because later peaks can reflect activity in cortico-cortical pathways transmitting information from other cortical areas (Amassian et al. 1987; Rothwell et al. 1991; Edgley et al. 1997), we cannot also exclude the possibility inputs arriving from other peripheral (Li et al. 2004) and/or central sources (Nishimura et al. 2007) contributed to our results.
Functional Significance
We found in SCI subjects that the maximum amplitude and onset latency of the second MEP peak were positively correlated with the variability of EMG signals during voluntary contraction. The discharge-rate variability across motor units in finger muscles influences the steadiness of a muscle contraction (Moritz et al. 2005). Thus, SCI subjects might have a decreased ability to activate regularly firing motor units over time and may therefore need more descending inputs to maintain the same level of activity (Thomas et al. 2014). This might explain why after SCI more facilitation of the second MEP peak was associated with more variability in EMG signals. This also agrees with our finding showing that individuals with larger facilitation of the second MEP peak were the ones showing larger corticospinal responses elicited by TMS. The maximum amplitude and duration of the third MEP peak were positively correlated with the speed to initiate finger voluntary contractions. This agrees with data from single motor unit recordings in controls showing that a preferred induced TMS current direction targeting the I3-wave resulted in longer latencies of corticospinal responses (Sakai et al. 1997), which has been associated with recruitment of the I3-wave (Day et al. 1989). A possible interpretation of our result is that longer reaction times might reflect a preferential activation of circuits mediating the third peak, because this peak makes the most pronounced contribution to motor unit recruitment after SCI. It is intriguing that these correlations were present only in SCI subjects. It is tempting to speculate that these associations reflect part of the reorganization taking place after SCI. Another possibility is that the relationship we found between the characteristics of the later peaks and hand voluntary motor output relates to the complexity of the task. Previous studies showed that during the preparation for grasp in control subjects, the modulation of a later peak predicted the muscle activity that was going to be used during grasp (Cattaneo et al. 2005). However, we want to notice that this relationship does not necessarily indicate a physiological role for the later peak in motor behaviors. Regardless of the factors contributing to these results and to the interpretation of our findings, our results indicate that mechanisms involved in later MEP peaks might play an important role in translating motor commands after SCI. Functionally, this may open new targets for inducing plasticity (Thickbroom et al. 2006; Cash et al. 2009), supporting the role of subcortical targets in motor recovery after SCI (Bunday and Perez 2012).
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke-National Institutes of Health (Grants R01 NS076589 and NS0900622 to M.A.P.), the Department of Veterans Affairs (Grant 3397626 to M.A.P.), and the Paralyzed Veterans of America (Grant 2968 to J.C.).
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Amassian VE, Stewart M, Quirk GJ, Rosenthal JL. 1987. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 20:74–93. [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. 2004. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 7:269–277. [DOI] [PubMed] [Google Scholar]
- Barry MD, Bunday KL, Chen R, Perez MA. 2013. Selective effects of baclofen on use-dependent modulation of GABAB inibition after tetraplegia. J Neurosci. 33:12898–12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson R, Gilles FH, Hall J, Hedley-Whyte ET. 1978. Long term post-traumatic retrograde corticospinal degeneration in man. Hum Pathol. 9:602–607. [DOI] [PubMed] [Google Scholar]
- Bunday KL, Perez MA. 2012. Motor recovery after spinal cord injury enhanced by strengthening corticospinal synaptic transmission. Curr Biol. 22:2355–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Tazoe T, Rothwell JC, Perez MA. 2014. Subcortical control of precision grip after human spinal cord injury. J Neurosci. 34:7341–7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. 1993. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 59:75–89. [PubMed] [Google Scholar]
- Buss A, Brook GA, Kakulas B, Martin D, Franzen R, Schoenen J, Noth J, Schmitt AB. 2004. Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain. 127:34–44. [DOI] [PubMed] [Google Scholar]
- Cash RF, Benwell NM, Murray K, Mastaglia FL, Thickbroom GW. 2009. Neuromodulation by paired-pulse TMS at an I-wave interval facilitates multiple I-waves. Exp Brain Res. 193:1–7. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Voss M, Brochier T, Prabhu G, Wolpert DM, Lemon RN. 2005. A cortico-cortical mechanism mediating object-driven grasp in humans. Proc Natl Acad Sci USA. 102:898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu VW, Hornby TG, Schmit BD. 2014. Effect of antispastic drugs on motor reflexes and voluntary muscle contraction in incomplete spinal cord injury. Arch Phys Med Rehabil. 95:622–632. [DOI] [PubMed] [Google Scholar]
- Coleman TF, Li Y. 1996. An interior trust region approach for nonlinear minimization subject to bounds. SIAM J Optimiz. 6:418–445. [Google Scholar]
- Cuypers K, Thijs H, Meesen RLJ. 2014. Optimization of the transcranial magnetic stimulation protocol by defining a reliable estimate for corticospinal excitability. PLoS ONE. 9:e86380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. 1989. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 412:449–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvendahl I, Lindemann H, Jung NH, Pechmann A, Siebner HR, Mall V. 2014. Influence of waveform and current direction on short-interval intracortical facilitation: a paired-pulse TMS study. Brain Stimul. 7:49–58. [DOI] [PubMed] [Google Scholar]
- DiCiccio TJ, Efron B. 1996. Bootstrap confidence intervals. Statist Sci. 11:189–228. [Google Scholar]
- Di Lazzaro V, Profice P, Pilato F, Dileone M, Oliviero A, Ziemann U. 2010. The effects of motor cortex rTMS on corticospinal descending activity. Clin Neurophysiol. 121:464–473. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A, Pilato F. 2012. I-wave origin and modulation. Brain Stimul. 5:512–525. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. 1998. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol. 508:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. 1997. Comparison of activation of corticospinal neurons and spinal motor neurons by magnetic and electric transcranial stimulation in the lumbrosacral cord of the anaesthetized monkey. Brain. 120:839–853. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. 1993. An introduction to the bootstrap. New York: (NY: ): Chapman & Hall. [Google Scholar]
- Ellaway PH, Catley M, Davey NJ, Kuppuswamy A, Strutton P, Frankel HL, Jamous A, Savic G. 2007. Review of physiological motor outcome measures in spinal cord injury using transcranial magnetic stimulation and spinal reflexes. J Rehabil Res Dev. 44:69–76. [DOI] [PubMed] [Google Scholar]
- Greve JM, Ribeiro JB, Camargo AJ, Chiovato J, Battistella LR, Barros Filho TE. 1991. H-reflex in spastic patients due to spinal cord injury before and after the use of diazepam and baclofen. Rev Hosp Clin Fac Med Sao Paulo. 46:229–231. [PubMed] [Google Scholar]
- Ho KH, Lee M, Nithi K, Palace J, Mills K. 1999. Changes in motor evoked potentials to short-interval paired transcranial magnetic stimuli in multiple sclerosis. Clin Neurophysiol. 110:712–719. [DOI] [PubMed] [Google Scholar]
- Kalil K, Schneider GE. 1975. Retrograde cortical and axonal changes following lesions of the pyramidal tract. Brain Res. 89:15–27. [DOI] [PubMed] [Google Scholar]
- Kalsi-Ryan S, Beaton D, Curt A, Duff S, Popovic MR, Rudhe C, Fehlings MG, Verrier MC. 2012. The graded redefined assessment of strength sensibility and prehension: reliability and validity. J Neurotrauma. 29:905–914. [DOI] [PubMed] [Google Scholar]
- Kernell D, Chien-Ping W. 1967. Post-synaptic effects of cortical stimulation on forelimb motoneurones in the baboon. J Physiol. 191:673–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. 2004. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol. 91:767–783. [DOI] [PubMed] [Google Scholar]
- Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, Haak M, Hudson LM, Priebe MM, Committee ANS. 2003. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 26(Suppl. 1):S50–S56. [DOI] [PubMed] [Google Scholar]
- Moritz CT, Barry BK, Pascoe MA, Enoka RM. 2005. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol. 93:2449–2459. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. 1997. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 498:817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. 2007. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 318:1150–1155. [DOI] [PubMed] [Google Scholar]
- Norton JA, Bennett DJ, Knash ME, Murray KC, Gorassini MA. 2008. Changes in sensory-evoked synaptic activation of motoneurons after spinal cord injury in man. Brain. 131:1478–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega M, Perez MA. 2012. Corticospinal reorganization after spinal cord injury. J Physiol. 590:3647–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton HD, Amassian VE. 1954. Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 17:345–363. [DOI] [PubMed] [Google Scholar]
- Perez MA. 2012. Transcranial magnetic stimulation and spinal cord injury. In: Chen R, Rothwell J, editors. Cortical connectivity: brain stimulation for assessing and modulating cortical connectivity and function. New York (NY): Springer; p. 323–336. [Google Scholar]
- Quartarone A, Battaglia F, Majorana G, Rizzo V, Bagnato S, Messina C, Girlanda P. 2002. Different patterns of I-waves summation in ALS patients according to the central conduction time. Clin Neurophysiol. 113:1301–1307. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Courtine G, Jindrich DL, Brock JH, Ferguson AR, Strand SC, Nout YS, Roy RR, Miller DM, Beattie MS et al. . 2010. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci. 13:1505–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. 1999. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 52:97–103. [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. 1991. Stimulation of the human motor cortex through the scalp. Exp Physiol. 76:159–200. [DOI] [PubMed] [Google Scholar]
- Rusu CV, Murakami M, Ziemann U, Triesch J. 2014. A model of TMS-induced I-waves in motor cortex. Brain Stimul. 7:401–414. [DOI] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I. 1997. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res. 113:24–32. [DOI] [PubMed] [Google Scholar]
- Salerno A, Georgesco M. 2001. Short latency facilitation between pairs of threshold magnetic stimuli studied in amyotrophic lateral sclerosis. Neurophysiol Clin. 31:48–52. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Hino T, Komori T, Hirai S. 2000. Loss of the muscle silent period evoked by transcranial magnetic stimulation of the motor cortex in patients with cervical cord lesions. Neurosci Lett. 286:199–202. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW. 2011. A model of the contribution of late I-waves to alpha-motoneuronal activation: implications for paired-pulse TMS. Brain Stimul. 4:77–83. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Edwards DJ, Mastaglia FL. 2006. Repetitive paired-pulse TMS at I-wave periodicity markedly increases corticospinal excitability: a new technique for modulating synaptic plasticity. Clin Neurophysiol. 117:61–66. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Bakels R, Klein CS, Zijdewind I. 2014. Human spinal cord injury: motor unit properties and behaviour. Acta Physiol (Oxf). 210:5–19. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. 1996. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr Clin Neurophysiol. 101:263–272. [DOI] [PubMed] [Google Scholar]
- Wagle-Shukla A, Ni Z, Gunraj CA, Bahl N, Chen R. 2009. Effects of short interval intracortical inhibition and intracortical facilitation on short interval intracortical facilitation in human primary motor cortex. J Physiol. 587:5665–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC. 2000. I-waves in motor cortex. J Clin Neurophysiol. 17:397–405. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. 1998. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 511:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wischer S, Hildebrandt J, Paulus W. 1998. Pharmacological control of facilitatory I-wave interaction in the human motor cortex. A paired transcranial magnetic stimulation study. Electroencephalogr Clin Neurophysiol. 109:321–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.