Abstract
The goal of this fMRI study was to examine how well developmental improvements in reasoning ability can be explained by changes in functional connectivity between specific nodes in prefrontal and parietal cortices. To this end, we examined connectivity within the lateral fronto-parietal network (LFPN) and its relation to reasoning ability in 132 children and adolescents aged 6–18 years, 56 of whom were scanned twice over the course of 1.5 years. Developmental changes in strength of connections within the LFPN were most prominent in late childhood and early adolescence. Reasoning ability was related to functional connectivity between left rostrolateral prefrontal cortex (RLPFC) and inferior parietal lobule (IPL), but only among 12–18-year olds. For 9–11-year olds, reasoning ability was most strongly related to connectivity between left and right RLPFC; this relationship was mediated by working memory. For 6–8-year olds, significant relationships between connectivity and performance were not observed; in this group, processing speed was the primary mediator of improvement in reasoning ability. We conclude that different connections best support reasoning at different points in development and that RLPFC-IPL connectivity becomes an important predictor of reasoning during adolescence.
Keywords: adolescent, child, development, dorsolateral, functional connectivity, inferior parietal lobule, parietal cortex, prefrontal cortex, processing speed, reasoning, rostrolateral, working memory
Introduction
Reasoning is among the most complex of cognitive operations that the human brain performs. It is also late-developing, with improvements in reasoning ability seen well into late adolescence (McArdle et al. 2002; Richland et al. 2006; Ferrer et al. 2009). Thus far, research on the neural underpinnings of reasoning ability has focused largely on age-related differences in the activation of specific brain regions (Wright et al. 2008; Crone et al. 2009; Eslinger et al. 2009; Dumontheil et al. 2010; Wendelken et al. 2011). However, the pattern of interaction between these regions is also critical, and recent investigations have begun to probe these interactions (Shokri-Kojori et al. 2012; Cocchi et al. 2013; Ebisch et al. 2013; Bazargani et al. 2014). Given the large improvements in reasoning ability that occur over childhood, a key question is whether and how these improvements are related to changes in functional connectivity—i.e., temporal correlation of activity between distinct brain regions. Here, we examine the relationship between functional connectivity and reasoning ability in a large longitudinal dataset, and investigate how changes in the pattern of network interactions over development support an improving capacity for reasoning.
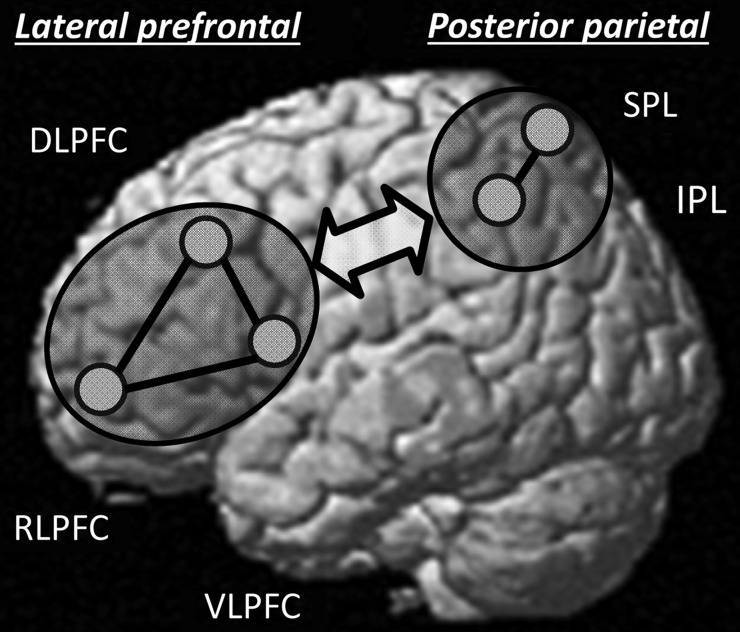
Two broad regions, lateral prefrontal cortex (PFC) and posterior parietal cortex (PPC), are consistently reported in fMRI studies of reasoning (for review, see Prado et al. 2011; Krawczyk 2012; see Fig. 1). While several subregions within PFC and PPC are engaged during reasoning, we have argued that 2 regions in particular—rostrolateral PFC (RLPFC) and the inferior parietal lobule (IPL)—play a central role in reasoning about relations between stimuli (Wendelken et al. 2008, 2012; Wendelken and Bunge 2010; Watson and Chatterjee 2012; Vendetti and Bunge 2014). This conclusion is based on studies of matrix reasoning (Christoff et al. 2001; Kroger et al. 2002; Baldo et al. 2010), analogical reasoning (Bunge et al. 2005; Green et al. 2006; Wendelken et al. 2008; Cho et al. 2010; Volle et al. 2011), transitive inference (Wendelken and Bunge 2010; Waechter et al. 2013), and relational matching (Christoff and Gabrielli 2002; Bunge et al. 2009; Wendelken et al. 2012).
Figure 1.
The fronto-parietal network of regions commonly associated with reasoning, including approximate locations of left hemisphere ROIs examined in this study. Right hemisphere ROIs were a mirror image of these.
Age differences in the patterns of RLPFC and IPL activation have been reported in several prior fMRI studies involving child and adult participants (Wright et al. 2008; Crone et al. 2009; Dumontheil et al. 2010). In recent work, we demonstrated a shift in processing related to higher order relational reasoning, from predominantly dorsolateral prefrontal cortex (DLPFC) in younger children to predominantly RLPFC in older children and adolescents (Wendelken et al. 2011), similar to what we have observed previously in adults with the same task (Bunge et al. 2009). In parallel with this shift in lateral PFC, and increasing specialization of RLPFC for higher order relational reasoning, we observed increasing specialization of the IPL in the vicinity of the intraparietal sulcus, and a negative relationship between IPL cortical thickness and RLPFC functional selectivity.
To our knowledge, only one other study has examined age differences in functional connectivity in relation to reasoning ability. In an fMRI study of 37 older children and adults, Bazargani et al. (2014) observed a positive relationship between short-range (fronto-insular) connectivity and reasoning task performance, but no relationship between reasoning and long-range (fronto-parietal) connectivity. Although the sample was not large enough to explore changing relationships across age groups, and the statistical analysis did not discriminate between specific region pairs, these results confirm the importance of inter-regional communication for reasoning performance.
While studies relating reasoning ability to functional connectivity are limited, a number of studies have examined the link between functional connectivity and IQ scores, which are based in part on measures of reasoning. One conclusion that emerges from this work is that better “global efficiency” of functional brain networks (i.e., a network with shorter paths for communication between nodes) is associated with better intellectual performance (Li et al. 2009; van den Heuvel et al. 2009; Langer et al. 2012). Consistent with this idea, but highlighting the role of one region in particular, Cole et al. (2012) demonstrated a relation between IQ and the “global brain connectivity” (i.e., average connectivity with the rest of the brain) of lateral PFC.
These functional connectivity studies suggest that improvements in reasoning ability might be best described by generalized increases in functional connectivity throughout the brain. In contrast, prior studies of relational reasoning in children and adults point toward a unique contribution of RLPFC and IPL. Thus, we hypothesized that it is the development of this RLPFC-IPL functional connection that best supports age-related improvements in reasoning ability. Moreover, we hypothesized that there might be age-related shift in the specific connections whose strength best accounts for individual variability in reasoning ability. Specifically, we reasoned that as reasoning-related activation shifts forward from DLPFC to RLPFC, so too might the importance of DLPFC connectivity give way to rising importance of RLPFC connectivity. In the current study, we set out to test these hypotheses by examining the relationships between age, functional connectivity, and reasoning ability across ages 6–18. Further, given that the development of reasoning ability has been linked to improvements in processing speed and working memory (Kail and Salthouse 1994), we also included measures of these lower level cognitive abilities to determine whether any observed relations between functional connectivity and reasoning ability were mediated by processing speed and/or working memory.
Materials and Methods
Participants
This study included 132 typically developing individuals (76 males; 117 right-handed) from the Neurodevelopment of Reasoning Ability (NORA) study, a longitudinal project designed to examine the behavioral and neural factors that underlie changes in reasoning ability across childhood and adolescence. Analyses of the development of functional specialization and white matter microstructure based on the NORA study have previously been published (Wendelken et al. 2011; Ferrer et al. 2013). Data were available from all 132 participants at the initial assessment (T1) and from 56 of these participants at the second assessment (T2). At T1, participants ranged in age from 6 years to 18.7 years old (11.1 ± 3.6 years [mean ± SD)], while at T2, participants ranged in age from 7.3 to 19.1 (11.9 ± 3.2 years). The interval between assessments ranged between 0.9 and 2.2 years (1.5 ± 0.3 years).
We tested for differences in attrition as a function of various measures, including age, gender, socioeconomic status (SES), and reasoning ability (see below), by regressing the number of time points on T1 values for each of these measures. There was no relationship between number of time points and age, gender, or reasoning score (all Ps > 0.2); however, there was a significant negative relationship between number of time points and SES (P = 0.015), indicating that lower SES participants were less likely than higher SES participants to return for T2 data collection.
Participants from the NORA project were included in the current study if they had usable fMRI data as well as data from 2 of 3 cognitive assessments of reasoning ability (at a given longitudinal time point). Scan data were considered usable if no more than 25% of total volumes in a given scan had volume-to-volume head motion in excess of 1 mm. All participants were screened for neurological impairment, psychiatric illness, history of learning disability, and developmental delay. All participants and their parents gave their informed assent (children under 12 years of age) or consent (adolescents aged 12 or more years and all parents) to participate in the study, which was approved by the Committee for Protection of Human Subjects at the University of California at Berkeley.
Behavioral Measures
Behavioral data were collected, at both T1 and T2, for a range of standardized cognitive tasks, including 3 standardized tests of reasoning ability: the “Block Design” and “Matrix Reasoning” subtests of the Wechsler Intelligence Scale for Children–Revised (WISC-R; Wechsler 1981), and the “Concept Formation” subtests of the Woodcock–Johnson Tests of Achievement (WJ-R; Woodcock and Johnson 1990). Block Design measures the ability to arrange a set of red-and-white blocks in such a way as to reproduce a 2D visual pattern shown on a set of cards. Matrix Reasoning measures the ability to select the geometric visual stimulus that accurately completes a series of stimuli that change along a particular dimension. Concept Formation measures the ability to identify and state the rules for concepts when shown illustrations of both instances and noninstances of the concept. All the tests are reported to have very high internal consistency and test–retest reliability, ranging from 0.94 to 0.95 (McGrew et al. 1991; McArdle et al. 2002).
In addition to our measures of reasoning ability, we also examined processing speed and working memory. Our measure of processing speed was the Cross Out subtest of the WJ-R, which measures how accurately one can identify geometric shapes that match a sample stimulus. Our measure of working memory was the Digit Span task (from WISC-R), which involves remembering sequences of numbers in forward or backward order over a short delay.
MRI Data Acquisition
Brain imaging data were collected on a Siemens 3T Trio system at the UC Berkeley Brain Imaging Center. Functional imaging data analyzed here were collected in four 4-min scans, during which participants were engaged in a propositional visual analogy task, described below. Importantly, as discussed below, the data used in the present study was filtered so as to remove activations time-locked to the task. Behavioral and fMRI data associated with this task are the subject of a separate manuscript (Vendetti et al. under review).
The analogy task was identical in design to that of a prior study from our laboratory (Wright et al. 2008), with updated stimuli. Each run consisted of 10 semantic and 10 analogy trials, presented in random order in a fast, event-related design. Participants had up to 10 s to answer each question. On semantic trials, participants saw a target stimulus (e.g., a picture of a notepad) and selected which of 4 probe objects was most closely associated with it (e.g., a pen). On analogy trials, participants saw 3 target stimuli, arrayed as an incomplete analogy of the form A:B::C:?, and were asked to indicate which of 4 probe stimuli best completed the array. Age-related improvements were observed for both semantic and analogy trials; adult-level performance was reached at around age 10 for semantic trials, and around age 14 for analogy trials.
Scan parameters were as follows: gradient-echo EPI sequence, TR = 2000 ms, TE = 25 ms, 33 axial slices, 2.0 × 1.8 × 3.0 mm voxels, no interslice gap, flip angle = 90°, field of view = 230 mm, 120 volumes per run). These 4 functional scans were preceded by collection of one or more high-resolution T1-weighted MPRAGE anatomical scans (TR = 2300 ms, TE = 2.98 ms, 1 × 1 × 1 mm voxels).
MRI Data Preprocessing
fMRI data preprocessing was carried out using SPM8 (Wellcome Trust Center for Neuroimaging, London). The first 3 volumes from each functional scanning run were discarded to allow for T1 equilibration. Functional images were corrected for differences in slice acquisition timing and were realigned to the first volume by means of 6-parameter rigid-body motion transformation. Motion parameters were extracted from this process and were used to inform a volume repair procedure (ArtRepair, Stanford Psychiatric Neuroimaging Laboratory). ArtRepair identified bad volumes on the basis of within-scan movement in excess of 1 mm and signal fluctuations in excess of 1.5 percent signal change, and then corrected bad volumes via interpolation. Following volume repair, the mean structural image was co-registered to the mean realigned functional image and then spatially normalized to SPM's T1 anatomical template. Normalization parameters obtained from this process were then applied to the un-normalized functional images to produce a set of functional images in SPM standard space (MNI152), with 3 × 3 × 3 mm voxels. As a final preprocessing step, functional images were smoothed with an 8-mm FWHM istotropic Gaussian kernel.
Regions of Interest
To obtain an unbiased set of regions of interest (ROIs) that would accurately represent the regions within lateral PFC and PPC that we aimed to examine, we extracted coordinates from a large set of ROIs that have been used previously to examine global connectivity properties (Power et al. 2011). In particular, we selected coordinates that were representative of the rostral, dorsal, and ventral subdivisions of lateral PFC (RLPFC, DLPFC, and VLPFC, respectively) as well as coordinates associated with the inferior and superior parietal lobules (IPL and SPL, respectively) in the left hemisphere. To obtain comparable ROIs on the left and right sides, right hemisphere ROIs were constructed as mirror images of those on the left. MNI coordinates of each ROI are listed in Table 1, and approximate locations are shown in Figure 1. Each ROI was constructed as a sphere with 5 mm diameter. We measured the strength of functional connectivity for all pairs of ROIs within a hemisphere and for all homologous pairs across hemispheres.
Table 1.
Regions of Interest (ROIs) were constructed as 5-mm spheres centered at the given MNI coordinates, with right hemisphere ROIs at positive X values and left hemisphere ROIs at negative X values
| ROI | Anatomical region | BA | X | Y | Z |
|---|---|---|---|---|---|
| RLPFC | Anterior middle frontal gyrus | 10 | ±34 | 55 | 4 |
| DLPFC | Middle frontal gyrus | 9 | ±42 | 25 | 30 |
| VLPFC | Inferior frontal gyrus | 45 | ±48 | 22 | 10 |
| IPL | Inferior parietal lobe | 40 | ±53 | −49 | 43 |
| SPL | Superior parietal lobe | 7 | ±22 | −65 | 48 |
BA, Brodmann's area. Coordinates are taken from Power et al. (2011).
Functional Connectivity Analysis
The purpose of the present study was to understand the relationship between reasoning ability and “intrinsic” patterns of functional connectivity—low-frequency correlations in regional activation that are relatively stable across task demands and that are thought to reflect the long-term history of coordination between regions (Seeley et al. 2007; Cole et al. 2014). Thus, we adopted the analytical methods of intrinsic functional connectivity analysis that are typically applied to resting-state data but that have also been successfully used with task data (e.g., Fair et al. 2007).
Intrinsic functional connectivity between regions was assessed by measuring low-frequency (f < 0.08) correlations between BOLD activation time series extracted for each ROI, for each participant. Several additional preprocessing steps were undertaken to reduce spurious variance unlikely to reflect neural activity, including high-pass filtering (f > 0.009), regression of the global white matter signal averaged from a white matter ROI, regression of ventricular signal averaged from a ventricular ROI, and correction for motion effects (see below). Because we were working with fMRI task data (collected while participants performed an analogical reasoning task), we also regressed out task-related activity from each ROI time series (cf. Fair et al. 2007).
To address potential issues associated with head motion during fMRI data collection (Power et al. 2012; Satterwaite et al. 2012; Van Dijk et al. 2012), we employed 2 primary techniques. First, motion vectors, consisting of volume-to-volume movement in 6 dimensions (3 translation directions and 3 rotation axes), were regressed out of each ROI time series. Second, data scrubbing was implemented as a final preprocessing step (Power et al. 2012). This procedure deleted time points corresponding to bad volumes (as identified by ArtRepair) and yielded a concatenated time series for each ROI.
Statistical Analysis
To characterize reasoning ability in each participant, we first carried out a confirmatory factor analysis, using T1 data from the Matrix Reasoning, Block Design, and Concept Formation tasks, across the entire sample of participants. Factor loadings obtained from this analysis were combined with task scores from T1 and, separately, T2, in order to produce a reasoning ability factor score for each participant at each available time point. These factor scores were converted to z-scores for subsequent analyses. Factor loadings were similar for each task, so each contributed similarly to calculation of the reasoning ability factor score.
To examine age-related differences in reasoning ability, we fit a nonlinear model relating reasoning ability to age, utilizing the cumulative distribution function of the normal distribution (“pnorm” in R) as our nonlinear function. This function assumes that changes are monotonic with age, and also that, across the population, the age of maximal change is normally distributed around a population mean. There are 2 parameters in this model: age of maximal change (mean, µ) and spread (standard deviation, σ). To select optimal parameters for each modeled relation, we first considered only the T1 data and examined all possible values for µ between 6 and 19 years, in half-year increments, and all possible values for σ between half and 13 years, also in half-year increments. We then selected the parameters that yielded the maximum F-statistic and entered these optimal parameters into a nonlinear mixed model that included the full dataset (cross-sectional plus longitudinal), with age as a random factor conditioned on participant. These mixed-model analyses allowed us to account for within-individual changes while modeling age-related differences with all the available T1 and T2 data. Mixed-model analyses were conducted using the “nlme” package in R (R Development Core Team 2011).
To examine age-related differences in functional connectivity, we followed the same approach as with reasoning ability. First, we computed correlations between functional connectivity and age for each ROI pair, for the cross-sectional sample. Next, for each connection that demonstrated a significant correlation, we conducted a nonlinear mixed-model analysis with the full dataset.
To examine developing patterns of influence of connectivity on reasoning ability, we split our sample roughly evenly into 3 age groups based on age at T1, producing a younger third (N = 44, 6–8 years, mean ± SD: 7.2 ± 0.8 years), a middle third (N = 42, 9–11 years, 10.4 ± 0.9 years), and an older third (N = 46, 12–18 years, 15.4 ± 1.9 years). Specific age cutoffs were selected in order to roughly balance the group sizes. T2 data were available for 21 participants in the younger age group, 17 participants in the middle age group, and 18 participants in the older age group. Because participants were assigned to age groups on the basis of their age at T1, age ranges for each group were larger when T2 data were included: ages 6–10 for the younger group, 9–13 for the middle group, and 12–19 for the older group. For each age group, as well as for the full age range, and for each ROI pair, we conducted a multiple linear regression of reasoning ability on connectivity and age using T1 data, and a linear mixed-model regression of reasoning ability on connectivity and age using data from both longitudinal time points. Where effects were present, we included age group as indicator variable to test for an interaction between age group and connectivity in their effect on reasoning ability. In a follow-up analysis, in addition to examining connectivity of individual ROI pairs, we also computed average network connectivity across all ROI pairs and tested, as above, for effects of average network connectivity on reasoning ability.
To test whether or not processing speed or working memory mediate observed effects of functional connectivity on reasoning ability, we used structured equation modeling to relate age, functional connectivity, and reasoning ability with either processing speed or working memory. We limited our examination of this question to analysis of cross-sectional data and to those functional connections that demonstrated a relation with reasoning ability. For each connection, we used a χ2 test to compare a full model that included a path from functional connectivity to reasoning ability with a reduced model that did not. The specific models are described in the Results section. Structured equation modeling was conducted with the “lavaan” package in R (Rosseel 2012).
Results
Development of Reasoning Ability
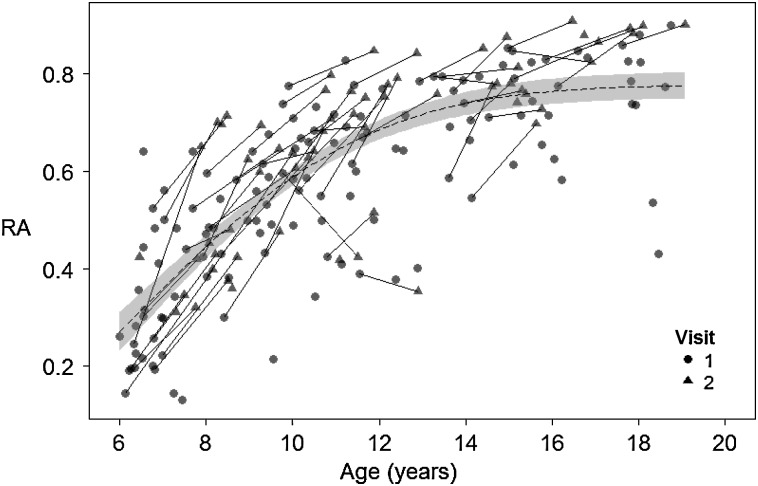
First, we examined age-related changes in reasoning ability. Fitting a nonlinear model to the cross-sectional data, we obtained an age of maximum change (μ) of 6 years with a spread (σ) of 4 years. Applying these parameters in a mixed-model regression to the full longitudinal dataset, we observed that this nonlinear relationship was highly significant (b = 5.3, t = 17.2, P < 0.001). Thus, these analyses indicate a rapid increase in reasoning ability during childhood, particularly before age 10, followed by a slower increase through adolescence (see Fig. 2).
Figure 2.
Age-related differences and longitudinal changes in reasoning ability, across the entire sample of participants. Age is on the x-axis, with reasoning ability factor score (RA) on the y-axis. Lines connecting data points indicate within-person longitudinal changes, while shape indicates the longitudinal time point (visit). The dotted line indicates the best-fitting cumulative normal distribution (with μ = 6, σ = 4), and shading shows the standard error of this fit line.
Age-Related Differences in Functional Connectivity Within the Reasoning Network
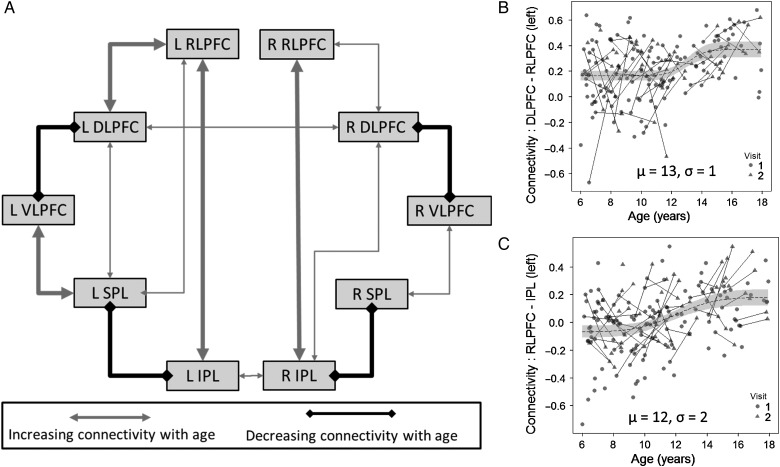
We next sought to characterize patterns of developmental change in functional connectivity. A graphical summary of significant age-related changes is given in Figure 3A. Complete results, including cross-sectional correlations between age and connectivity as well as longitudinal mixed-model results, are reported in Table 2. In consideration of the 25 pairwise connections that were examined here, we consider findings with P < 0.002 as statistically significant, in accordance with the Bonferroni correction.
Figure 3.
(A) Age-related differences in functional connectivity within the reasoning network. Regions are connected here if they demonstrated a significant pattern of age-related changes (calculated using nonlinear mixed modeling). Thin lines indicate P < 0.05 uncorrected for multiple comparisons, thick lines indicate P < 0.05 Bonferroni-corrected. (B) Scatter plot of the relationship between age left DLPFC-RLPFC connectivity. (C) Scatter plot of the relationship between age and left RLPFC-IPL connectivity.
Table 2.
Age-related differences in functional connectivity
| ROIs | Cross-sectional correlation |
Longitudinal nonlinear mixed model |
||||||
|---|---|---|---|---|---|---|---|---|
| r | t | P | µ | σ | b | t | P | |
| Prefrontal–prefrontal | ||||||||
| RLPFC-RLPFC (Bi) | −0.11 | −1.3 | 0.18 | |||||
| RLPFC-DLPFC (L) | 0.30 | 3.6 | 0.0004 | 13 | 1 | 0.18 | 5.2 | <0.0001 |
| RLPFC-DLFCP (R) | 0.17 | 2.0 | 0.05 | 19 | 1 | 0.25 | 1.0 | 0.32 |
| RLPFC-VLPFC (L) | −0.04 | −0.50 | 0.61 | |||||
| RLPFC-VLPFC (R) | −0.11 | −1.3 | 0.19 | |||||
| DLPFC-DLPFC (Bi) | 0.23 | 2.7 | 0.008 | 14.5 | 1.5 | 0.17 | 4.1 | 0.0001 |
| DLPFC-VLPFC (L)† | −0.26 | −3.1 | 0.002 | 11 | 2.5 | −0.14 | −3.4 | <0.0001 |
| DLPFC-VLPFC (R) | −0.34 | −4.1 | <0.0001 | 13.5 | 2.5 | −0.25 | −6.1 | <0.0001 |
| VLPFC-VLPFC (Bi) | −0.03 | −0.38 | 0.70 | |||||
| Parietal–parietal | ||||||||
| IPL-IPL (Bi) | 0.15 | 1.8 | 0.07 | |||||
| IPL-SPL (L) | −0.47 | −6.1 | <0.0001 | 11 | 2 | −0.32 | −7.8 | <0.0001 |
| IPL-SPL (R)† | −0.38 | −4.6 | <0.0001 | 13.5 | 2.5 | −0.30 | −5.0 | <0.0001 |
| SPL-SPL (Bi) | −0.08 | −1.0 | 0.33 | |||||
| Prefrontal–parietal | ||||||||
| RLPFC-IPL (L) | 0.39 | 4.8 | <0.0001 | 12 | 2 | 0.25 | 6.1 | <0.0001 |
| RLPFC-IPL (R) | 0.37 | 4.5 | <0.0001 | 12.5 | 1 | 0.25 | 6.2 | <0.0001 |
| RLPFC-SPL (L) | 0.19 | 2.1 | 0.03 | 11.5 | 1 | 0.11 | 2.8 | 0.008 |
| RLPFC-SPL (R) | 0.06 | 0.74 | 0.45 | |||||
| DLPFC-IPL (L) | 0.09 | 1.1 | 0.29 | |||||
| DLPFC-IPL (R) | 0.21 | 2.5 | 0.01 | 15.5 | 1 | 0.15 | 3.3 | 0.002 |
| DLPFC-SPL (L) | 0.27 | 3.1 | 0.002 | 11.5 | 1 | 0.10 | 2.8 | 0.006 |
| DLPFC-SPL (R) | 0.02 | 0.21 | 0.83 | |||||
| VLPFC-IPL (L) | 0.06 | 0.71 | 0.48 | |||||
| VLPFC-IPL (R) | 0.13 | 1.5 | 0.12 | |||||
| VLPFC-SPL (L) | 0.27 | 3.1 | 0.002 | 12.5 | 1 | 0.15 | 4.6 | <0.0001 |
| VLPFC-SPL (R) | 0.25 | 3.0 | 0.003 | 8.5 | 1 | 0.18 | 4.1 | <0.0001 |
For the cross-sectional analysis, reported statistics include the correlation r-value, the t-value, and the associated P-value. For the nonlinear mixed-model analysis of longitudinal data, which used the cumulative distribution function of the normal distribution, 5 values are reported: µ, the best-fit age of maximal change; σ, the best-fit standard deviation or spread; b, the parameter estimate for the best-fitting model; the associated t-value; and the associated P-value. Reported P-values are uncorrected for multiple comparisons. Given the 25 comparisons reported here, we consider P < 0.002 to be significant, in accordance with the Bonferroni correction. Mixed-model analyses were performed only in cases where the cross-sectional correlation survived P < 0.05 uncorrected.
†Mixed model did not converge; reported values are from cross-sectional nonlinear regression.
Within lateral PFC, a strong age-related increase in functional connectivity was observed between left DLPFC and left RLPFC (Fig. 3B), with best-fit parameters indicating maximal change at around age 13. In contrast, an age-related decrease in connectivity was observed between DLPFC and VLPFC in both hemispheres. Using Fisher r-to-z transformation, we confirmed that the age-related patterns for DLPFC-RLPFC and DLPFC-VLPFC differed significantly from one another, on the left and on the right (Ps < 0.001). Connectivity between left and right DLPFC also demonstrated an overall increase with age. Within PPC, we observed a large decrease in functional connectivity between IPL and SPL in both hemispheres.
Regarding fronto-parietal connectivity, we observed 2 notable age-related increases. For RLPFC, there was a strong age-related increase in connectivity with IPL, on the left (Fig. 3C) and on the right. This increase in IPL connectivity with RLPFC was significantly greater than changes in connectivity between IPL and either DLPFC (left: P = 0.006; right: P = 0.08) or VLPFC (left: P = 0.003; right: P = 0.02). For VLPFC, in contrast, there was an age-related increase in connectivity with SPL in both hemispheres. DLPFC demonstrated different patterns in the left and right hemispheres—specifically, increasing connectivity with SPL on the left and increasing connectivity with IPL on the right.
Examining the parameters of the best-fitting nonlinear models, we noted that age-related differences in functional connectivity were generally largest between the ages of 10 and 14, an age range that corresponds to pre- and early adolescence. This finding held for prefrontal–prefrontal differences (increases and decreases with age), for IPL-SPL age-related decreases, and for RLPFC-IPL age-related increases. There was greater variability in the timing of maximal change for other prefrontal–parietal connections, with right VLPFC-SPL increases peaking at around age 8.5 and right DLPFC-IPL increases peaking at around age 15.5.
Functional Connectivity and Reasoning Ability
Our primary aim was to understand how changing patterns of functional connectivity support developmental improvements in reasoning. We consider here the relation between reasoning ability and functional connectivity of different ROI pairs, across the entire age range and within each of the 3 age groups. Analyses are presented in the following order, according to our prior hypotheses: 1) fronto-parietal connections involving RLPFC, 2) fronto-frontal connections involving RLPFC, 3) connections involving DLPFC that are not included above. We report results from both cross-sectional regressions of reasoning ability on connectivity and age (denoted “cross”) and corresponding longitudinal mixed-model regressions (denoted “long”), including parameter estimates (b), t-values, and uncorrected P-values. We report all effects with uncorrected P-values <0.1. We consider a result to be significant if it survives Bonferroni correction for multiple comparisons (across pairs of regions and age groups) as follows: 1) P < 0.004, for the 12 tests relevant to our primary hypothesis, involving RLPFC-parietal connectivity; 2) P < 0.003, for the 15 tests relevant to our secondary hypothesis, involving RLPFC-frontal connections; and 3) P < 0.002, for the 24 additional tests involving connections with DLPFC.
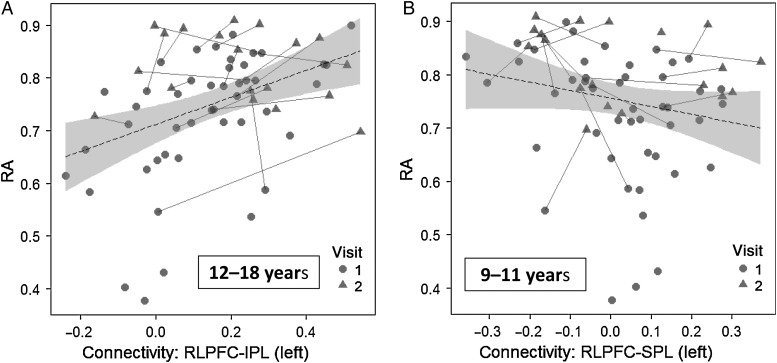
Based on prior findings demonstrating the involvement of left RLPFC and IPL in reasoning tasks, our main prediction was that reasoning ability would be supported by left RLPFC-IPL connectivity. Across the entire age range, this effect was not significant (cross: b = 0.47, t = 1.7, P = 0.09; long: P > 0.1). However, when this analysis was limited to 12–18-year olds, the effect of left RLPFC-IPL connectivity on reasoning ability was highly significant (cross: b = 1.7, t = 3.5, P = 0.001; long: b = 0.93, t = 3.5, P = 0.003; Fig. 4A). Moreover, there was an interaction between connectivity and membership in the older age group in their effect on reasoning ability (cross: t = 2.4, P = 0.02; long: t = 2.3, P = 0.02). The correlation between left RLPFC-IPL connectivity and reasoning ability was not significant for either the younger or middle age groups, and the correlation between right RLPFC-IPL connectivity and reasoning ability was not significant for any age group (all Ps > 0.1).
Figure 4.
Graphs depicting the relationship between functional connectivity and reasoning ability (RA) in older children and adolescents. (A) A scatter plot of the positive relation between left RLPFC-IPL connectivity and reasoning ability. (B) A scatter plot of the negative relation between left RLPFC-SPL connectivity and reasoning ability.
Although we predicted that RLPFC-IPL connectivity would be most strongly related to reasoning ability, we also examined other fronto-parietal connections. Across the full age range, there was no effect of either left or right RLPFC-SPL connectivity on reasoning ability (all Ps > 0.1). But notably, for the older group, in stark contrast to the positive relationship between reasoning ability and left RLPFC-IPL connectivity, there was a weak negative relationship between reasoning ability and left RLPFC-SPL connectivity (cross: b = −1.0, t = −1.7, P = 0.08; long: b = −0.83, t = −2.5, P = 0.02; Fig. 4B). With regard to fronto-parietal connections involving DLPFC or VLPFC, we observed no significant relationship between connectivity and reasoning after accounting for age, either across the entire age range or within each age group, either cross-sectionally or longitudinally (all Ps > 0.1).
We turned next to consideration of RLPFC connectivity with other prefrontal regions. After accounting for age, there was no significant relationship between RLPFC-VLPFC or RLPFC-DLPFC connectivity and reasoning ability, left or right, across the entire age range or within any of the 3 age groups, cross-sectionally or longitudinally (all Ps > 0.1). However, there was a significant positive effect of cross-hemispheric connectivity between left and right RLPFC in the middle age group (cross: b = 1.1, t = 3.1, P = 0.003; long: b = 0.85, t = 3.0, P = 0.008). This effect was limited to the middle age group, as indicated by a significant interaction between group membership and connectivity (cross: t = 3.2, P = 0.002; long: t = 2.7, P = 0.009).
In contrast to RLPFC, cross-hemispheric connectivity was not associated with reasoning ability for DLPFC, VLPFC, IPL, or SPL (all Ps > 0.1). There were no other significant relationships between connectivity and reasoning ability, for any intraprefrontal, intraparietal, or fronto-parietal connections, for any age group (Ps > 0.07) [There was a weak negative relationship between left DLPFC-VLPFC connectivity and reasoning ability in the youngest group (cross: b = −0.68, t = −1.8, P = 0.07; long: did not converge). Although this was the strongest effect of connectivity on reasoning ability observed in the younger age group, it was not significant. This effect was not present in the other two age groups or across the entire age range (all Ps > 0.1)] . In addition, there was no effect of average network connectivity on reasoning ability in any group (all Ps > 0.1).
Mediation Analysis: Processing Speed and Working Memory
Given that the development of reasoning ability has been linked to the development of both processing speed and working memory (Fry and Hale 1996; Nettelback and Burns 2010), we sought to determine whether the observed relations between functional connectivity and reasoning ability were mediated by processing speed and/or working memory. We examined structured equation models that related reasoning ability, functional connectivity, and age, and which also included either processing speed or processing speed and working memory as potential mediators of the path from functional connectivity to reasoning ability. We compared each full model to a reduced model that eliminated the path from functional connectivity to reasoning ability. We report the χ2 test for this model comparison along with key parameters from the full model here, and present more detailed results for the full models in Table 3. Summary results, depicting significant relationships for each group, are shown in Figure 5. We note that only a subset of participants completed the working memory task, so models involving working memory were tested with a reduced sample (numbers are shown in Table 3). It is for this reason that we considered processing speed separately, as well as in combination with working memory.
Table 3.
Regression path parameters associated with each optimal model from the mediation analyses
| Regression path | Estimate | Std. Err. | Z-value | P-value |
|---|---|---|---|---|
| A. PS model: RLPFC-IPL connectivity, ages 12–18 (N = 46) Full model | ||||
| RA ∼ Age | 0.08 | 0.18 | 0.45 | 0.66 |
| RA ∼ PS | 0.20 | 0.19 | 1.04 | 0.30 |
| RA ∼ FC | 0.28 | 0.08 | 3.52 | <0.001 |
| PS ∼ Age | 0.58 | 0.11 | 5.19 | <0.001 |
| PS ∼ FC | 0.05 | 0.06 | 0.74 | 0.46 |
| FC ∼ Age | 0.38 | 0.26 | 1.43 | 0.15 |
| B. PS and WM model: RLPFC-IPL connectivity, ages 12–18 (N = 20) Full model | ||||
| RA ∼ Age | 0.76 | 0.23 | 3.32 | 0.001 |
| RA ∼ PS | 0.10 | 0.21 | 0.47 | 0.64 |
| RA ∼ WM | −0.12 | 0.10 | −1.28 | 0.20 |
| RA ∼ FC | 0.43 | 0.11 | 3.86 | <0.001 |
| WM ∼ Age | 0.37 | 0.53 | 0.70 | 0.48 |
| WM ∼ PS | 0.57 | 0.48 | 1.20 | 0.23 |
| WM ∼ FC | 0.46 | 0.24 | 1.89 | 0.06 |
| FC ∼ Age | −0.46 | 0.39 | −1.18 | 0.24 |
| C. PS model: RLPFC-RLPFC connectivity, ages 9–11 (N = 42) Full model | ||||
| RA ∼ Age | 0.37 | 0.39 | 0.94 | 0.34 |
| RA ∼ PS | 0.4 | 0.14 | 1.74 | 0.08 |
| RA ∼ FC | 0.25 | 0.09 | 2.91 | 0.004 |
| PS ∼ Age | 1.07 | 0.39 | 2.75 | 0.006 |
| PS ∼ FC | 0.12 | 0.09 | 1.35 | 0.18 |
| FC ∼ Age | −0.38 | 0.65 | −0.58 | 0.56 |
| D. PS and WM model: RLPFC-RLPFC connectivity, ages 9–11 (N = 31) Reduced model: RA ∼ FC removed | ||||
| RA ∼ Age | −0.05 | 0.36 | −0.13 | 0.90 |
| RA ∼ PS | 0.42 | 0.13 | 3.29 | 0.001 |
| RA ∼ WM | 0.26 | 0.12 | 2.30 | 0.02 |
| WM ∼ Age | 0.39 | 0.53 | 0.73 | 0.46 |
| WM ∼ PS | −0.10 | 0.19 | −0.56 | 0.58 |
| WM ∼ FC | 0.36 | 0.13 | 2.74 | 0.006 |
| FC ∼ Age | −1.0 | 0.71 | −1.41 | 0.16 |
| E. PS model: no connectivity, ages 6–8 (N = 44) Reduced model: RA ∼ Age removed | ||||
| RA ∼ PS | 0.68 | 0.18 | 3.7 | <0.001 |
| PS ∼ Age | 1.4 | 0.32 | 4.3 | <0.001 |
| F. PS and WM model: no connectivity, ages 6–8 (N = 19) Reduced model: RA ∼ Age removed | ||||
| RA ∼ PS | 0.61 | 0.26 | 2.38 | 0.02 |
| RA ∼ WM | 0.19 | 0.16 | 1.22 | 0.22 |
| WM ∼ Age | −0.36 | 0.99 | −0.37 | 0.72 |
| WM ∼ PS | 1.04 | 0.42 | 2.50 | 0.01 |
| PS ∼ Age | 1.64 | 0.40 | 4.14 | <0.001 |
We tested for mediating effects of either processing speed (PS) alone, or processing speed and working memory (WM). (A and B) For older children and adolescents (ages 12–18), we examined mediating effects on the relationship between left RLPFC-IPL functional connectivity (FC) and reasoning ability (RA). (C and D) For the middle group (ages 9–11), we examined mediating effects on the relationship between bilateral RLPFC connectivity and reasoning ability. (E and F) For younger children (ages 6–8), we examined mediating effects on the relationship between age and reasoning ability. Only a subset of participants completed the working memory task; numbers of participants are listed for each model. Relationships between age, processing speed, and functional connectivity are not shown for the models that include working memory, as these relationships are likely to be more accurately estimated in the PS-only models.
Figure 5.
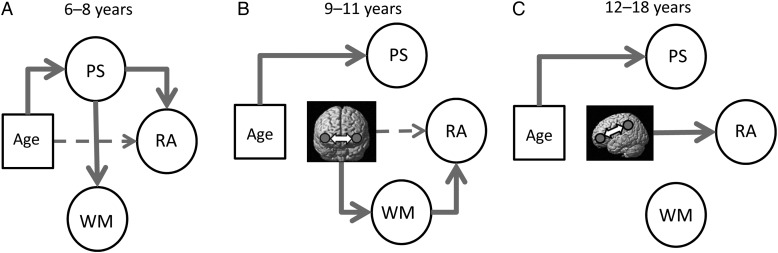
Summary of mediation analysis results, for models examining the relationships between age, functional connectivity, processing speed (PS), working memory (WM), and reasoning ability (RA). Dashed lines indicated relationships that do not hold in the presence of PS- and/or WM-mediating variables. (A) In younger children (6–8 years), processing speed mediated the relationship between age and reasoning ability. (B) In middle children (9–11), working memory mediated the relationship between functional connectivity (RLPFC bilateral connectivity) and reasoning ability. (C) In older children and adolescents (12–18 years), neither processing speed nor working memory mediated the relationship between functional connectivity (left RLPFC-IPL connectivity) and reasoning ability.
First we examined, for the older group, the connection between left RLPFC and left IPL that demonstrated a significant relation with reasoning ability. For the processing speed model, removing the path from functional connectivity to reasoning ability led to significant reduction in model quality (Δχ2(1) = 10.9, P < 0.001). Indeed, this path was highly significant in the full model (b = 0.28, P < 0.001). In contrast, the paths from processing speed to reasoning ability, and from functional connectivity to processing speed, were not significant (Ps > 0.2). Thus, processing speed was unrelated to either reasoning ability or RLPFC-IPL connectivity in the older group. For the working memory model, removing the path from connectivity to reasoning ability again led to a significant worsening of the model fit (Δχ2(1) = 11.1, P < 0.001), and this path in the full model was highly significant (b = 0.43, P < 0.001). The path from working memory to reasoning ability showed a negative trend (b = −0.12, P = 0.2), while the path from connectivity to working memory was marginal and positive (b = 0.46, P = 0.06). Thus, while working memory was weakly related to both reasoning ability and RLPFC-IPL connectivity in 12–18-year olds, it did not mediate the effect of connectivity on reasoning in this group (Fig. 5C).
Next, we examined the connection between left and right RLPFC that demonstrated a positive relation with reasoning ability in the middle age group. Here again, for the processing speed model, removal of the path from connectivity to reasoning ability resulted in a significantly worse model fit (Δχ2(1) = 7.7, P < 0.01). This path was highly significant in the full model (b = 0.25, P = 0.004), while the path from processing speed to reasoning ability was marginally significant (b = 0.25, P = 0.08). The path from connectivity to processing speed was not significant (b = 0.12, P = 0.18). Thus, the impact of bilateral RLPFC connectivity on reasoning ability in the middle age group is not mediated by processing speed. For the working memory model, the results were different. In this case, removal of the path from connectivity to reasoning ability did not significantly affect the model fit (Δχ2(1) = 0.01, P > 0.2), and this path was not significant in the full model (b = 0.01, P > 0.2). However, the path from working memory to reasoning ability was significant (b = 0.26, P = 0.04), and there was a significant positive relationship between connectivity and working memory (b = 0.29, P = 0.008). Thus, working memory mediates the effects of bilateral RLPFC connectivity on reasoning ability in 9–11-year olds (Fig. 5B).
Finally, we sought to better understand the large age-related changes in reasoning ability in the youngest group, for whom changes in functional connectivity did not have a significant effect. Here, we sought to determine whether processing speed and/or working memory mediated the relationship between reasoning ability and age. We examined simple mediation models that included a direct path from age to reasoning ability as well as a mediating path through either processing speed or working memory, and compared these with reduced models that excluded the path from age to reasoning ability. In the processing speed model, the fit of the reduced model was marginally worse than that of the full model (Δχ2(1) = 3.2, P = 0.07). In the full model, the regression path from age to reasoning ability was similarly marginal (b = 0.82, P = 0.07) while the regression paths from age to processing speed (b = 1.4, P < 0.001) and from processing speed to reasoning ability (b = 0.68, P < 0.001) were highly significant. For the model that also included working memory, the reduced model was not significantly worse than the full model (Δχ2(1) = 1.3, P > 0.2). The regression path from age to reasoning ability in the full model was not significant (P > 0.2). There was a significant path connecting processing speed to working memory (b = 1.04, P = 0.01), but only positive trends in the relationships between age and working memory (b = 1.0, P = 0.17) and between working memory and reasoning ability (b = 0.21, P = 0.18). Thus, in children 6–8 years old, processing speed was the primary mediator of developmental improvements in reasoning ability (Fig. 5A).
Discussion
Age-Related Differences in Functional Connectivity
We observed marked changes in the pattern of functional connectivity in the network of regions that were examined, from childhood through adolescence. Within lateral PFC, the predominant pattern was one of differentiation of DLPFC (and to a lesser extent RLPFC) from VLPFC, suggesting that the functions of DLPFC and VLPFC may become increasingly disparate over development. In contrast, the present results suggest increased communication between RLPFC and DLPFC. This emerging pattern is consistent with a hierarchical model of lateral PFC, in which RLPFC depends on outputs of DLPFC (Koechlin et al. 2004; Badre and D'Esposito 2007).
A second prominent pattern of observed age-related changes were the selective increases in fronto-parietal connectivity. In particular, the observed increase in RLPFC-IPL connectivity was expected, as IPL is the region that in many adult studies demonstrates the strongest functional connectivity with RLPFC (Vincent et al. 2008; Boorman et al. 2009). The increase in VLPFC-SPL connectivity observed here is not as clearly linked to previously reported connectivity patterns in adults. Overall, the developmental changes in fronto-parietal connectivity suggest increasing communication between prefrontal regions and specific parietal targets. The logical outcome of these changes, a system in which different parietal subregions communicate preferentially with different prefrontal subregions, is consistent with reported parietal connectivity patterns in adults (Nelson et al. 2010; Mars et al. 2011).
Nonlinear modeling revealed that many of these age-related changes in connectivity peak between the ages of 10 and 14. This suggests that the transition to adolescence may be marked by widespread changes in patterns of functional connectivity. The extent to which this apparent transition in functional connectivity relates to other ongoing neurodevelopmental changes is an open question. Development of white matter tracts is a likely contributor to changes in functional connectivity. White matter volume demonstrates ongoing increase over middle to late childhood (Giedd et al. 1999); this is at least partly accounted for by myelination of axons, which continues throughout this period (Yakovlev and Lecours 1967). Developmental increases in white matter integrity (as measured by fractional anisotropy) vary as a function of tract; of particular note, the superior longitudinal fasciculus which serves as the major conduit of fronto-parietal communication, demonstrates ongoing increase in integrity through age 18. Thus, ongoing changes in white matter might enable the observed changes in functional connectivity.
In addition to changes in long-range fiber tracts, it is possible that synaptic changes could also affect developing patterns of functional connectivity. Large-scale synaptogenesis—the creation of new synaptic connections—is largely complete by early childhood (Rakic et al. 1994; Petanjek et al. 2008). Synaptic density plateaus during childhood and then begins a sharp decline with the onset of synaptic pruning. Notably, there are large regional differences in the timing of this transition, and PFC stands out for the late (mid-adolescent) onset and extended duration of large-scale pruning (Huttenlocher and Dabholkar 1997; Petanjek et al. 2011; Liu et al. 2012). Thus, overall changes in synaptic density are limited during middle to late childhood. However, synaptic reorganization and turnover continues throughout this period (Rakic et al. 1994; Selemon 2013), and could play a role in the changes in functional connectivity observed here.
Finally, there is evidence that hormonal changes that occur during puberty can affect both structural and functional connectivity (Peper et al. 2011). Hormonal changes, in particular, may help to explain the timing of the changes that we observed here.
Despite our efforts to mitigate the effects of motion on our results, findings related to age-related differences in functional connectivity must be interpreted with caution. The observed increases in fronto-parietal connectivity and decreases in intrafrontal and intraparietal connectivity fit into the pattern—well established in the literature (Fair et al. 2009; Supekar et al. 2009)—of age-related increases in long-range connectivity and decreases in short-range connectivity. But these also conform to the changes that one would expect due to decreases in head motion (Power et al. 2012; Satterthwaite et al. 2012; Van Dijk et al. 2012). Arguing against the idea that head motion could account for these results is the fact that we observe different patterns of developmental change among the shorter range connections that we examined and also among the longer range connections. These results highlight the importance of examining specific connections, instead of or in addition to looking at general classes of connections such as long range versus short range.
Also relevant to the interpretation of age-related changes is the fact that longitudinal attrition differed as a function of SES. Lower SES participants were less likely to return for a second scanning session, and so it is in theory possible that mixed-model results are biased toward changes that are seen in higher SES individuals. However, all of the effects that we obtained via mixed-model analysis with longitudinal data were also present when the analyses were restricted to cross-sectional data.
Fronto-parietal Connectivity and Reasoning Ability
In adults, RLPFC and IPL are 2 regions that are consistently engaged by higher order relational reasoning tasks. Thus, our main hypothesis concerned functional connectivity between RLPFC and IPL. Specifically, we hypothesized that connectivity between these regions should be an important predictor of reasoning ability. We considered that this might be the case regardless of maturity, or that it might only be true as the reasoning system approaches its adult state. In fact, we observed the latter pattern: left RLPFC-IPL connectivity was the strongest predictor of reasoning ability, but only in older children and adolescents. This result confirms the importance of RLPFC-IPL connectivity in the mature reasoning system, but also indicates that there are meaningful differences in the way that reasoning is performed in an immature neural system. It is an open question whether these differences reflect operation of different brain mechanisms in support of reasoning in younger versus older children, or if they reflect differential importance of the same set of mechanisms.
Prior investigations of the link between developing functional connectivity and higher cognition have also pointed to the importance of the fronto-parietal connection. For example, Ezekiel et al. (2013) demonstrated increased connectivity of lateral PFC to the anterior cingulate cortex (ACC) and to IPL during execution of the dimensional change card sorting task, in adults relative to children. Langeslag et al. (2012) demonstrated a relationship between nonverbal intelligence and increased functional connectivity between right parietal and frontal regions and between right parietal and dorsal ACC. Emerson and Cantlon (2012) reported a positive relationship between fronto-parietal connectivity and children's math ability. Complementing these developmental findings, Mackey et al. (2013) showed that intensive reasoning training in young adults is associated with increased RLPFC-parietal connectivity, with no change in DLPFC-parietal connectivity. Thus, our results add to the general finding that fronto-parietal connections can be critical for many higher cognitive tasks.
Notably, we did not observe a relation between average network connectivity and reasoning ability. Moreover, we did not observe a consistent relation between average fronto-parietal, or even RLPFC-parietal, connectivity and reasoning ability. Instead, we observed a very specific pattern: reasoning ability was positively related to RLPFC-IPL connectivity but negatively related to RLPFC-SPL connectivity. These results contrast with the results from prior studies of functional connectivity and general intelligence, which have linked IQ to generalized increases in functional connectivity (Li et al. 2009; van den Heuvel et al. 2009; Cole et al. 2012; Langer et al. 2012). The present results suggest that more specific links between IQ and functional connectivity might be found. On the other hand, just as reasoning ability is a specific component of IQ, connectivity changes associated with improvements in reasoning may be more specific than changes associated with more general intellectual improvement.
An Emerging Network for Reasoning
Beyond the hypothesized relationship between RLPFC-IPL connectivity and reasoning that was present in older children, we observed a compelling pattern of relations between functional connectivity and reasoning ability in the younger and middle child groups. We have demonstrated previously the occurrence of a developmental shift, from early childhood to adolescence, in the brain regions that are most engaged by higher order relational reasoning tasks (Wendelken et al. 2011). Specifically, while selective engagement for higher order reasoning is limited to DLPFC in younger children, it shifts to include RLPFC and IPL in adolescents and adults. Here, we observed a similar developmental shift in functional connectivity. In the youngest group of children, the factor that most related to reasoning ability was the connectivity of DLPFC and VLPFC. This was a negative relationship, with decreased connectivity associated (albeit weakly) with improved reasoning ability. Thus, at a relatively early stage in the development of the reasoning system, a key neurodevelopmental change may be the differentiation of DLPFC—the region that is most engaged by reasoning tasks in this age range—from nearby VLPFC. In the middle group of children, further differentiation of DLPFC from VLPFC appears less important for reasoning, perhaps because it is mainly complete or because of the shift toward a more prominent role for RLPFC. Instead, in this group, it was tighter coupling of left and right RLPFC that was most closely associated with reasoning ability. This pattern, along with the emerging importance of the RLPFC-IPL connection, reinforces the idea of an anterior shift within lateral PFC for higher order reasoning.
Processing Speed and Working Memory
Behavioral studies measuring concurrent and longitudinal relationships between cognitive abilities have pointed to processing speed and working memory as key mediators of reasoning development (e.g., Kail and Salthouse 1994; Li et al. 2004; Nettlebeck and Burns 2010; Demetriou et al. 2014). Large improvements in reasoning ability during the early school years, observed here as well as in prior studies (Goswami 1991; Ferrer and McArdle 2004), are complemented by large improvements in both processing speed and working memory (Fry and Hale 1996). In recent work, we demonstrated that processing speed mediates the concurrent relationship between global white matter integrity and reasoning across childhood (Ferrer et al. 2013). Thus, we reasoned that processing speed and/or working memory might similarly mediate the concurrent relationship between functional connectivity and reasoning ability.
As in prior behavioral work, processing speed was related to reasoning ability in our sample, particularly in the youngest group of children. However, processing speed did not mediate any of the observed effects of functional connectivity on reasoning ability. This finding reveals a difference between structural and functional connectivity. On one hand, as we have shown previously, the widespread increase in white matter coherence observed during development contributes to reasoning ability indirectly via its influence on processing speed. On the other hand, developmental changes (both increases and decreases) in functional connectivity between specific nodes in the lateral fronto-parietal network contribute to reasoning ability in a manner that cannot be explained by improved processing speed.
In older children and adolescents, the relationship between fronto-parietal connectivity and reasoning ability was not mediated by either processing speed or working memory. In middle children, in contrast, working memory did mediate the relationship between intraprefrontal connectivity and reasoning ability. In younger children, it was neither functional connectivity nor working memory, but rather processing speed, that had the strongest relation to reasoning ability. Thus, our results are consistent with a developmental cascade model of the relationship between processing speed, working memory, and reasoning ability (Fry and Hale 1996). Specifically, they indicate that development of reasoning ability is driven initially by improvements in processing speed and subsequently by improvements in working memory.
Summary
In previous work, we have argued that neurodevelopment of the reasoning system is characterized by shifting patterns of activation, particularly within lateral PFC. Here, we provide evidence that patterns of functional connectivity undergo a similar neurodevelopmental change. In particular, there is a shift in the importance of functional connections for reasoning toward connections that involve RLPFC, and ultimately toward specificity of RLPFC-parietal communication. This shift occurs alongside developmental changes in the relationships between reasoning, processing speed, and working memory. Much work remains to be done to understand how the specific brain regions and their interactions contribute to reasoning and its development. However, knowing which connections matter, and when, is a critical step toward a deeper mechanistic understanding.
Funding
Support was provided by the National Institutes of Health NINDS R01 NS057146 (S.B. and E.F.) and a James S. McDonnell Foundation Scholar Award (S.A.B.).
Notes
The authors thank Chloe Green, Elizabeth O'Hare, Brian Johnson, Mehdi Bouhaddou, Ori Elis, and Alexis Eils for assistance with data collection. Conflict of Interest: None declared.
References
- Badre D, D'Esposito M. 2007. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 19(12):2082–2099. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Bunge SA, Wilson SM, Dronkers NF. 2010. Is relational reasoning dependent on language? A voxel-based lesion symptom mapping study. Brain Lang. 113(2):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazargani N, Hillebrandt H, Christoff K, Dumontheil I. 2014. Developmental changes in effective connectivity associated with relational reasoning. Hum Brain Mapp. 35:3262–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman ED, Behrens TEJ, Woolrich MW, Rushworth MFS. 2009. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 62(5):733–743. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Helskog EH, Wendelken C. 2009. Left, but not right, rostrolateral prefrontal cortex meets a stringent test of the relational integration hypothesis. Neuroimage. 46(1):338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD. 2005. Analogical reasoning and prefrontal cortex: evidence for separable retrieval and integration mechanisms. Cereb Cortex. 15(3):239–249. [DOI] [PubMed] [Google Scholar]
- Cho S, Moody TD, Fernandino L, Mumford JA, Poldrack RA, Cannon TD, Knowlton BJ, Holyoak KJ. 2010. Common and dissociable prefrontal loci associated with component mechanisms of analogical reasoning. Cereb Cortex. 20(3):524–533. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JD. 2002. The frontopolar cortex and human cognition: evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 28(2):168–186. [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD. 2001. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 14(5):1136–1149. [DOI] [PubMed] [Google Scholar]
- Cocchi L, Halford GS, Zalesky A, Harding IH, Ramm BJ, Cutmore T, Shum DH, Mattingly JB. 2013. Complexity in relational processing predicts changes in functional brain network dynamics. Cereb Cortex. 24(9):2283–2296. [DOI] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. 2014. Intrinsic and task-evoked network architectures of the human brain. Neuron. 83(1):238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Yarkoni T, Repovs G, Anticevic A, Braver TS. 2012. Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J Neurosci. 32(26):8988–8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, van Leijenhorst L, Honomichl RD, Christoff K, Bunge SA. 2009. Neurocognitive development of relational reasoning. Dev Sci. 12(1):55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou A, Spanoudis G, Shayer M, van der Ven S, Brydges CR, Kroesbergen E, Podjarny G, Swanson HL. 2014. Relations between speed, working memory, and intelligence from preschool to adulthood: structural equation modeling of 14 studies. Intelligence. 46:107–121. [Google Scholar]
- Dumontheil I, Houlton R, Christoff K, Blakemore SJ. 2010. Development of relational reasoning during adolescence. Dev Sci. 13(6):F15–F24. [DOI] [PubMed] [Google Scholar]
- Ebisch SJH, Mantini D, Romanelli R, Tommasi M, Perrucci MG, Romani GL, Colom R, Saggino A. 2013. Long-range functional interactions of anterior insula and medial frontal cortex are differently modulated by visuospatial and inductive reasoning tasks. Neuroimage. 78:426–438. [DOI] [PubMed] [Google Scholar]
- Emerson RW, Cantlon JF. 2012. Early math achievement and functional connectivity in the fronto-parietal network. Dev Cogn Neurosci. 2(Suppl 1):S139–S151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Blair C, Wang J, Lipovsky B, Realmuto J, Baker D, Thorne S, Gamson D, Zimmerman E, Rohrer L et al. 2009. Developmental shifts in fMRI activations during visuospatial relational reasoning. Brain Cogn. 69(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekiel F, Bosma R, Morton JB. 2013. Dimensional change card sort performance associated with age-related differences in functional connectivity of lateral prefrontal cortex. Dev Cogn Neurosci. 5:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, Schlaggar BL, Petersen SE. 2009. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 5(5):e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. 2007. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 35(1):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer E, McArdle JJ. 2004. An experimental analysis of dynamic hypotheses about cognitive abilities and achievement from childhood to early adulthood. Dev Psychol. 40(6):935–952. [DOI] [PubMed] [Google Scholar]
- Ferrer E, O'Hare E, Bunge S. 2009. Fluid reasoning and the developing brain. Front Neurosci. 3(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer E, Whitaker KJ, Steele JS, Green CT, Wendelken C, Bunge SA. 2013. White matter maturation supports the development of reasoning ability through its influence on processing speed. Dev Sci. 16(6):941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AF, Hale S. 1996. Processing speed, working memory, and fluid intelligence: evidence for a developmental cascade. Psychol Sci. 7:237–241. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapaport JL. 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 2(10):861–863. [DOI] [PubMed] [Google Scholar]
- Goswami U. 1991. Analogical reasoning: what develops? A review of research and theory. Child Dev. 62:1–22. [Google Scholar]
- Green AE, Fugelsang JA, Kraemer DJ, Shamosh NA, Dunbar KN. 2006. Frontopolar cortex mediates abstract integration in analogy. Brain Res. 1096(1):125–137. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. 1997. Regional differences in synaptogenesis in human cerebral cortex. J Comput Neurol. 387:167–178. [DOI] [PubMed] [Google Scholar]
- Kail R, Salthouse TA. 1994. Processing speed as a mental capacity. Acta Psychol. 86(2–3):199–225. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. 2004. The architecture of cognitive control in the human prefrontal cortex. Science. 302:1181–1185. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC. 2012. The cognition and neuroscience of relational reasoning. Brain Res. 1428:13–23. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. 2002. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex. 12(5):477–485. [DOI] [PubMed] [Google Scholar]
- Langer N, Pedroni A, Gianotti LRR, Hänggi J, Knoch D, Jäncke L. 2012. Functional brain network efficiency predicts intelligence. Hum Brain Mapp. 33(6):1393–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeslag SJE, Schmidt M, Ghassabian A, Jaddoe VW, Hofman A, van der Lugt A, Verhulst FC, Tiemeier H, White TJH. 2012. Functional connectivity between parietal and frontal brain regions and intelligence in young children: the Generation R study. Hum Brain Mapp. 34(12):3299–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Lindenberger U, Hommel B, Aschersleben G, Prinz W, Baltes PB. 2004. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychol Sci. 15(3):155–163. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T. 2009. Brain anatomical network and intelligence. PLoS Comput Biol. 5(5):e1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Somel M, Tang L, Yan Z, Jian Xi, Guo S, Yuan Y, He L, Oleksiak A, Zhang Y et al. 2012. Extension of cortical synaptic development distinguishes humans from chimpanzees and macaques. Genome Res. 22(4):611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AP, Miller Singley AT, Bunge SA. 2013. Intensive reasoning training alters patterns of brain connectivity at rest. J Neurosci. 33(11):4796–4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Jbabdi S, Sallet J, O'Reilly JX, Croxson PL, Olivier E, Noonan MP, Bergman MC, Mitchell AS, Baxter MG et al. 2011. Diffusion-weighted imaging tractography-based parcellation of the human parietal cortex and comparison with human and macaque resting-state functional connectivity. J Neurosci. 31(11):4087–4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J, Ferrer-Caja E, Hamagami F, Woodcock R. 2002. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Dev Psychol. 38(1):115. [PubMed] [Google Scholar]
- McGrew KS, Werder JK, Woodcock RW. 1991. WJ-R technical manual. Allen (TX): DLM. [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, Velanova K, Donaldson DI, Phillips JS, Schlaggar BL et al. 2010. A parcellation scheme for human left parietal cortex. Neuron. 67(1):56–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettelbeck T, Burns NR. 2010. Processing speed, working memory, and reasoning ability from childhood to old age. Pers Indiv Diff. 48(4):379–384. [Google Scholar]
- Peper J, van den Heuvel MP, Mandl RCW, Hulshoff Pol HE, van Honk J. 2011. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology. 36(8):1101–1113. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Kostovix U, Uylings HB. 2008. Lifespan alterations of basal dendritic trees of pyramidal neurons in human prefrontal cortex: a layer-specific pattern. Cereb Cortex. 18(4):915–929. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simix G, Rasin MR, Uylings HB, Rakix P. 2011. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 108(32):13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL et al. 2011. Functional network organization of the human brain. Neuron. 72(4):665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado J, Chadha A, Booth JR. 2011. The brain network for deductive reasoning: a quantitative meta-analysis of 28 neuroimaging studies. J Cogn Neurosci. 23(11):3483–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Goldman-Rakic PS. 1994. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res. 102:227–243. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. 2011. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. ISBN 3-900051-07-0. Available from: URL http://www.R-project.org/.
- Richland L, Morrison R, Holyoak K. 2006. Children's development of analogical reasoning: insights from scene analogy problems. J Exp Child Psychol. 94(3):249–273. [DOI] [PubMed] [Google Scholar]
- Rosseel Y. 2012. lavaan: an R package for structural equation modeling. J Stat Softw. 48(2):1–36. [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. 2012. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 60(1):623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzburg AF, Keller J, Glover G, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27(9):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD. 2013. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry. 3:e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri-Kojori E, Motes MA, Rypma B, Krawczyk DC. 2012. The network architecture of cortical processing in visuo-spatial reasoning. Sci Rep. 2:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. 2009. Development of large-scale functional brain networks in children. PLoS Biol. 7(7):e1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. 2009. Efficiency of functional brain networks and intellectual performance. J Neurosci. 29(23):7619–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL. 2012. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 59(1):431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendetti MS, Bunge SA. 2014. Evolutionary and developmental changes in the lateral frontoparietal network: a little goes a long way for high-level cognition. Neuron. 84(5):906–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. 2008. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 100(6):3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volle E, Gonen-Yaacovi G, Costello ADL, Gilbert SJ, Burgess PW. 2011. The role of rostral prefrontal cortex in prospective memory: a voxel-based lesion study. Neuropsychologia. 49(8):2185–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter RL, Goel V, Raymont V, Kruger F, Grafman J. 2013. Transitive inference reasoning is impaired by focal lesions in parietal cortex rather than rostrolateral prefrontal cortex. Neuropsychologia. 51(3):464–471. [DOI] [PubMed] [Google Scholar]
- Watson CE, Chatterjee A. 2012. A bilateral frontoparietal network underlies visuospatial analogical reasoning. Neuroimage. 59(3):2831–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 1981. Wechsler intelligence scale for children—revised. New York: The Psychological Corporation. [Google Scholar]
- Wendelken C, Bunge SA. 2010. Transitive inference: distinct contributions of rostrolateral prefrontal cortex and the hippocampus. J Cogn Neurosci. 22(5):837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Chung D, Bunge SA. 2012. Rostrolateral prefrontal cortex: domain-general or domain-sensitive? Hum Brain Mapp. 33(8):1952–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Nakhabenko D, Donohue SE, Carter CS, Bunge SA. 2008. “Brain is to thought as stomach is to ??”: investigating the role of rostrolateral prefrontal cortex in relational reasoning. J Cogn Neurosci. 20(4):682–693. [DOI] [PubMed] [Google Scholar]
- Wendelken C, O'Hare ED, Whitaker KJ, Ferrer E, Bunge SA. 2011. Increased functional selectivity over development in rostrolateral prefrontal cortex. J Neurosci. 31(47):17260–17268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. 1990. Woodcock-Johnson psycho-educational battery—revised. Allen: (TX: ): DLM. [Google Scholar]
- Wright SB, Matlen BJ, Baym CL, Ferrer E, Bunge SA. 2008. Neural correlates of fluid reasoning in children and adults. Front Hum Neurosci. 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. 1967. The myelogenetic cycle of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford, UK: Blackwell Scientific; p. 3–70. [Google Scholar]