Abstract
Background:
At present, China has listed the compound tablet containing a fixed dose of rosiglitazone and metformin, Avandamet, which may improve patient compliance. The aim of this study was to evaluate the efficacy and safety of Avandamet or uptitrated metformin treatment in patients with type 2 diabetes inadequately controlled with metformin alone.
Methods:
This study was a 48-week, multicenter, randomized, open-labeled, active-controlled trial. Patients with inadequate glycaemic control (glycated hemoglobin [HbA1c] 7.5–9.5%) receiving a stable dose of metformin (≥1500 mg) were recruited from 21 centers in China (from 19 November, 2009 to 15 March, 2011). The primary objective was to compare the proportion of patients who reached the target of HbA1c ≤7% between Avandamet and metformin treatment.
Results:
At week 48, 83.33% of patients reached the target of HbA1c ≤7% in Avandamet treatment and 70.00% in uptitrated metformin treatment, with significantly difference between groups. The target of HbA1c ≤6.5% was reached in 66.03% of patients in Avandamet treatment and 46.88% in uptitrated metformin treatment. The target of fasting plasma glucose (FPG) ≤6.1 mmol/L was reached in 26.97% of patients in Avandamet treatment and 19.33% in uptitrated metformin treatment. The target of FPG ≤7.0 mmol/L was reached in 63.16% of patients in Avandamet treatment and 43.33% in uptitrated metformin treatment. Fasting insulin decreased 3.24 ± 0.98 μU/ml from baseline in Avandamet treatment and 0.72 ± 1.10 μU/ml in uptitrated metformin treatment. Overall adverse event (AE) rates and serious AE rates were similar between groups. Hypoglycaemia occurred rarely in both groups.
Conclusions:
Compared with uptitrated metformin, Avandamet treatment provided significant improvements in key parameters of glycemic control and was generally well tolerated. Registration number: ChiCTR-TRC-13003776.
Keywords: Avandamet, Efficacy, Type 2 Diabetes
INTRODUCTION
Type 2 diabetes is characterized by chronic hyperglycemia resulting in microvascular and macrovascular complications which may contribute to an increased risk of early cardiovascular morbidity and early mortality. Now-a-days, the primary approach for type 2 diabetes management is steadily and continuously blood glucose control, with the aim of decreasing the risk of diabetic complications.[1,2] A Diabetes Outcome Progression Trial (ADOPT),[3] which compared three antidiabetic agents currently available for the treatment of type 2 diabetes, showed that in terms of glycemic control, rosiglitazone showed a long-term glycemic control. Since metformin and rosiglitazone act through different mechanisms, their combined use may be indicated in patients whose disease is poorly controlled with a maintenance dose of metformin. Studies[3,4] have shown that rosiglitazone in combination with metformin can decrease glycated hemoglobin (HbA1c) while increase insulin sensitivity and improve β-cell function.
At present, China has listed the compound tablet containing a fixed dose of rosiglitazone and metformin, Avandamet, which may improve patient compliance. Data showed that, due to the increased number of patients with medication, it was very difficult to long-term adherence to their drug regimen. A compound tablet containing two different fixed dose drugs due to a different mechanism can reduce the number of medication, maintaining a more strict glycemic control, improving the compliance of patients with general. The aim of this randomized open study was to compare the efficacy and safety of Avandamet and up titrated metformin in patients uncontrolled with metformin alone.
METHODS
Study population
Patients aged 18–65 years who have been diagnosed with type 2 diabetes (defined according to World Health Organization criteria) inadequately controlled with metformin were considered for this 48-week, multicenter, randomized, open-label, parallel group study. Participants were recruited from 21 centers in China. The study was conducted in accordance with Good Clinical Practice guidelines and the 1996 version of the Declaration of Helsinki. Written informed consent was obtained from all participants. The study protocol and informed consent were approved by the ethics committee of Peking University People's Hospital according to local requirements. Participants with body mass index (BMI) ≥24 kg/m2, 7.5% ≤HbA1c ≤9.5%, 7 mmol/L ≤ fasting plasma glucose (FPG) ≤15 mmol/L at screening, receiving 0.75–1.0 g metformin alone at constant dosage for at least 6 weeks prior to entry were included in this trial.
Exclusion criteria included: (1) treatment with glucose-lowing agents other than metformin within 12 weeks before screening; (2) allergic to thiazolidinedione or their ingredients; (3) treatment with exogenous insulin or receiving insulin within 12 weeks before screening; (4) history of diabetic ketoacidosis; (5) hemoglobin disease or chronic anemia with the level of hemoglobin <11.0 g/dl (male) or <10.0 g/dl (female); (6) a history of kidney dysfunction with serum creatinine levels reaching 135 μmol/L (male) or 110 μmol/L (female); (7) alanine aminotransferase or aspartate aminotransferase >2.5 times the upper limit of normal, suffering from hepatic disease; (8) type 1 diabetes, gestational diabetes, or other type of diabetes (Mody, et al.); (9) a history of ischemic heart disease or heart failure according to the New York Heart Association Classification Class I–IV; (10) patients taking nitrates; (11) ongoing edema requiring pharmacological treatment; (12) systemic blood pressure >170 mmHg or diastolic blood pressure >100 mmHg while on anti-hypertensive treatment; (13) any chronic disease requiring continuous intermittent treatment with corticosteroids; (14) breastfeeding, pregnant or planning pregnancy women (women of childbearing age before interview one to use effective contraception for at least 1 month); (15) patients with abnormality during screening by the investigator judgement; (16) active drug or alcohol abuse within the last 6-month or any associated condition that could preclude completion of the study; (17) patients probably accept the iodinated contrast agent during study duration; (18) electrocardiogram with QTc >500 ms or unadjusted QT >600 ms; or patients with bundle branch block with QTc >530 ms.
Study design
This was a 48-week, multicenter, randomized, open-label, parallel group study, consisting of a screening visit and 48-week of follow-up after randomization. In an open regimen, patients were randomized into two groups receiving Avandamet 4 mg/1000 mg or metformin 1500 mg as the initial daily dose. Four weeks later, for each treatment group, drug dosage was increased to the maximum dosage (8 mg/2000 mg of Avandamet or 2500 mg of metformin daily) according to the protocol. If FPG level >7.0 mmol/L (126 mg/dl) and the maximum dosage was not reached, a dose increase was required at each visit; while a dose reduction was permitted if adverse events (AEs) occurred. The first patient was recruited on November 19, 2009 and the last patient completed follow-up visit on March 15, 2011. Registration number for this trial was ChiCTR-TRC-13003776 (http://www.chictr.org/cn/proj/search.aspx). The authors confirm that all ongoing and related trials for this drug/intervention are registered.
Study assessments
The primary efficacy outcome is the percentage of patients who reached the target HbA1c (HbA1c ≤7%) at week 48 in Avandamet treatment group and metformin treatment group. Hypothesis was made as that the between group difference more than 15% of the percentage of patients who reached HbA1c ≤7% at week 48 suggested that superiority was reached. The secondary outcomes included: (1) Changes in HbA1c from baseline; (2) changes in FPG and prandial plasma glucose (PPG) from baseline; (3) percentage of patients reached HbA1c ≤6.5% at week 48; (4) percentage of patients with the changes from baseline in HbA1c ≥0.7% in Avandamet group and metformin group; (5) changes in fasting insulin from baseline.
All AEs were recorded and judged by an investigator as the severity and possible relationship to study medication. Safety laboratory assessments (hematology and biochemistry) and vital signs were assessed at each study visit. Mild-to-moderate hypoglycemia was defined if the patient had symptoms or a self-measured capillary glucose level (CG) <63 mg/dl (3.5 mmol/L). Severe hypoglycemia was defined as any episode requiring assistance of another person with the CG below 2.8 mmol/L, unless the clinical situation prevented obtaining CG measurement.
Vital signs and anthropometric measurements were recorded at each visit. HbA1c, fasting insulin and lipids profile were measured centrally at randomization and every 3 months. HbA1c was measured by high-performance liquid chromatography (Ultra2 HbA1c Detector, PRIMUS Corporation, USA). FPG, PPG, levels of low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride were measured by immunonephelometry method (COBAS Integra 400 Plus System, Roche Diagnostics Ltd., Basel, Switzerland). Insulin level was measured by electrochemiluminescence immune assay (Elecsys 2010 System, Roche Diagnostics Ltd., Basel, Switzerland)). All study drugs were withheld on the morning of testing. An outline of the study assessments at clinic visits was shown in Table 1.
Table 1.
Outline of study assessments
| Assessment | Screening time (relative to baseline) | ||||||
|---|---|---|---|---|---|---|---|
| V0 (0–7 day) | V1 (week 0) | V2 (week 4) | V3 (week 12) | V4 (week 24) | V5 (week 36) | V6 (week 48) | |
| Vital sign | X | X | X | X | X | X | |
| Body weight | X | X | X | X | X | X | |
| Height | X | ||||||
| BMI | X | ||||||
| Waist and hippo circumference | X | X | X | ||||
| ECG | X | X | X | ||||
| Complete blood count | X | X | X | X | X | X | |
| Urine analysis | X | ||||||
| Β-HCG | X | ||||||
| FPG | X | X | X | X | X | X | |
| PPG | X | X | X | ||||
| HbA1c | X | X | X | X | X | ||
| Lipid (TC, LDL-C, TG, HDL-C) | X | X | X | ||||
| Hepatic function (ALT, AST, T-BIL, ALP) | X | X | X | ||||
| Renal function (BUN, CRE) | X | X | X | ||||
| FINS/CRP | X | X | X | ||||
| AEs | X | X | X | X | X | ||
AEs: Adverse Events; BMI: Body mass index; ECG: Electrocardiogram; Β-HCG: Beta-human chorionic gonadotropin; FPG: Fasting plasma glucose; PPG: Prandial plasma glucose; HbA1c: Glycated hemoglobin; TC: Total cholesterol; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; TG: Triglyceride; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; T-BIL: Total bilirubin; BUN: Blood urea nitrogen; CRE: Creatinine; CRP: C-reactive protein; FINS: Fasting insulin.
Statistical analysis
SAS version 9.2 (SAS Institute Inc., USA) was used for all analyses. A sample size of 400 patients (200 Avandamet and 200 metformin-uptitrated) was required to ensure 85% power to detect a superiority difference of 15% in Avandamet treated patients who achieve the target HbA1c (≤7%) to metformin-uptitrated treated patients (i.e., assuming in metformin monotherapy group 50% of patients at the end of the study achieving HbA1c ≤7%, 65% of patients in Avandamet group achieving HbA1c ≤7% were needed) at the level of α = 0.05 (two-sided). Assuming 15% of loss rate and 15% of screening failure rate, totally there were 554 patients needed to be screened and 471 patients needed to be randomized, to ensure that ultimately 400 patients complete the study.
Demographic and other baseline characteristics were analyzed using descriptive statistics. The primary endpoint, the percentage of patients who achieved the target HbA1c (HbA1c ≤7%) at week 48, was compared between Avandamet treatment group and metformin treatment group by Chi-square analysis. Other efficacy analyses for continuous variables were compared by using a one-way analysis of variance test while frequency of dichotomous variables was performed by Chi-square analysis.
This analysis was performed on the full analysis set (FAS). This group comprised all randomized patients who were treated with at least one dose of study medication, had a baseline HbA1c measurement and had at least one on-treatment HbA1c measurement. Safety analyses conducted on the treated patients’ data set included all patients who received at least one dose of study medication. Chi-square test was used to compare the incidence of AEs between groups. Regression techniques were applied to assess the associations of variables with all continuous and categorical endpoints, respectively.
RESULTS
Study characteristics
Five hundred and eighty-eight patients who met the inclusion criteria were recruited for the study. At the end of run-in period, 398 patients who still met the inclusion criteria were randomly assigned into Avandamet treatment group and metformin treatment group, while 156 participants in Avandamet treatment group and 160 participants in metformin treatment group were included in the FAS [Figure 1]. Baseline characteristics of patients between Avandamet treatment group and metformin treatment group were comparable except that the average duration of diabetes in Avandamet group was significantly longer than that in metformin group (3.56 ± 3.49 years vs. 2.73 ± 3.10 years, P = 0.01) [Table 2].
Figure 1.
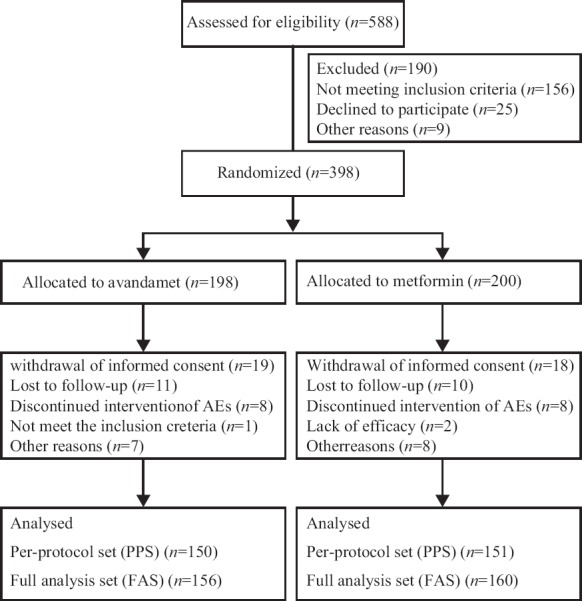
Flow diagram of this study.
Table 2.
Baseline characteristics of the patients*
| Variables | Avandamet (n = 198) | Metformin (n = 200) |
|---|---|---|
| Age (years) | 51.38 ± 9.57 | 51.25 ± 8.84 |
| Gender (male, %) | 57.7 | 59.8 |
| Diabetic duration (years) | 3.57 ± 3.49 | 2.73 ± 3.10 |
| Body weight (kg) | 74.82 ± 11.18 | 74.67 ± 10.74 |
| Height (cm) | 165.92 ± 8.75 | 164.94 ± 8.62 |
| BMI (kg/m2) | 27.10 ± 2.75 | 27.39 ± 3.06 |
| Waist circumference (cm) | 93.41 ± 8.15 | 93.48 ± 9.30 |
| SBP (mmHg) | 129.98 ± 13.62 | 129.61 ± 15.98 |
| DBP (mmHg) | 80.31 ± 9.72 | 80.97 ± 9.09 |
*Baseline characteristics were comparable between the two groups except for diabetic duration (P = 0.01). BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure.
The percentage of patients who did not complete the study did not differ significantly between Avandamet group and metformin group (23.23% vs. 24.00%, P > 0.05). Baseline characteristics of the lost-to-follow-up patients did not differ from those who completed the study. Baseline characteristics of the lost-to-follow-up patients between Avandamet group and metformin group did not differ either. Data was shown in supplement material.
Glycemic control
The primary efficacy outcome is the percentage of patients reached HbA1c ≤7% at week 48 in Avandamet group and metformin group. At the end of week 48, the percentage of patients who reached HbA1c ≤7% was 83.33% in Avandamet group and 70.00% in metformin group (P < 0.01). However, according to the protocol, the significance for the superiority of Avandamet treatment to metformin treatment was not reached (<15%). The percentage of patients who reached HbA1c ≤6.5% in the two groups was 66.03% and 46.88% respectively (P < 0.01) [Table 3]. At week 48, the average level of HbA1c in Avandamet group and metformin group was 6.78% ± 0.94% and 7.03% ± 0.89% respectively (P < 0.01), HbA1c change from baseline at week 48 was −1.49% ± 0.05% and −1.18% ± 0.09% respectively (P < 0.01). Changes of HbA1c during the 48-week were shown in Figure 2.
Table 3.
Comparisons of changes from baseline at week 48 between Avandamet and metformin
| Variables | Baseline | Week 48 | Adjusted changes from baseline | 95% CI | P |
|---|---|---|---|---|---|
| HbA1c (%) | |||||
| Avandamet | 8.26 ± 0.65 | 6.78 ± 0.94 | −1.49 ± 0.05 | −1.65, −1.32 | <0.01 |
| Metformin | 8.22 ± 0.60 | 7.03 ± 0.89 | −1.18 ± 0.09 | −1.33, −1.03 | |
| Patients reached HbA1c ≤6.5% (%) | |||||
| Avandamet | – | 66.03 | – | – | <0.01 |
| Metformin | – | 46.88 | – | – | |
| Patients reached HbA1c ≤7% (%) | |||||
| Avandamet | – | 83.33 | – | – | <0.01 |
| Metformin | – | 70.00 | – | – | |
| FPG (mmol/L) | |||||
| Avandamet | 9.03 ± 1.66 | 7.06 ± 2.18 | −1.98 ± 0.17 | −2.34, −1.62 | <0.01 |
| Metformin | 9.03 ± 1.90 | 7.66 ± 2.05 | −1.35 ± 0.11 | −1.72, −0.97 | |
| Patients reached FPG ≤6.1 mmol/L (%) | |||||
| Avandamet | – | 26.97 | – | – | >0.05 |
| Metformin | – | 19.33 | – | – | |
| Patients reached FPG ≤7.0 mmol/L (%) | |||||
| Avandamet | – | 63.16 | – | – | <0.01 |
| Metformin | – | 43.33 | – | – | |
| PPG (mmol/L) | |||||
| Avandamet | 13.69 ± 3.50 | 10.61 ± 3.17 | −3.0 ± 0.27 | −3.71, −2.48 | <0.01 |
| Metformin | 13.85 ± 3.38 | 11.86 ± 3.55 | −1.85 ± 0.27 | −2.44, −1.25 | |
| FINS (μU/ml) | |||||
| Avandamet | 13.75 ± 15.71 | 9.83 ± 12.97 | −3.24 ± 0.98 | −4.47, −2.01 | <0.01 |
| Metformin | 11.26 ± 8.88 | 10.79 ± 15.42 | −0.72 ± 1.10 | −3.25, 1.81 | |
| Body weight (kg) | |||||
| Avandamet | 75.20 ± 11.88 | 74.95 ± 12.89 | −0.09 ± 0.32 | −0.72, 0.55 | <0.01 |
| Metformin | 75.21 ± 10.74 | 72.66 ± 12.02 | −2.46 ± 1.03 | −3.55, −1.37 |
CI: Confidence interval; HbA1c: Glycated hemoglobin; FPG: Fasting plasma glucose; PPG: Prandial plasma glucose; FINS: Fasting insulin; “–” means not applicable.
Figure 2.
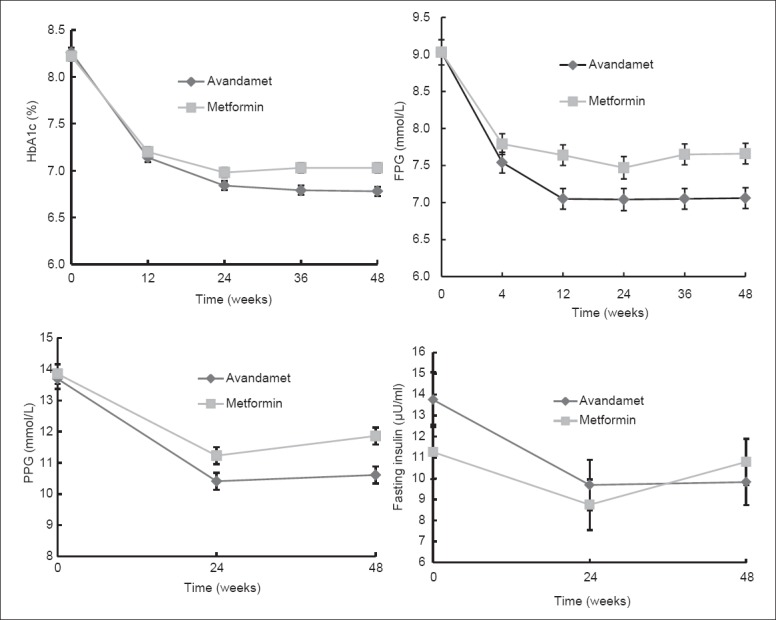
Changes of glycated hemoglobin, fasting plasma glucose, prandial plasma glucose and fasting insulin during 48 weeks in Avandamet group and metformin group.
In terms of FPG, at week 48, the percentage of patients who reached FPG ≤6.1 mmol/L was 26.97% in Avandamet group and 19.33% in metformin group without significance. The percentage of patients who reached FPG ≤7.0 mmol/L was 63.16% and 43.33% respectively (P < 0.01). At week 48, the average FPG was 7.06 ± 2.18 mmol/L and 7.66 ± 2.05 mmol/L respectively (P < 0.01) in the two groups, FPG change from baseline at week 48 was −1.98 ± 0.17 mmol/L and −1.35 ± 0.11 mmol/L respectively (P < 0.01). At week 48, the average PPG level was 10.61 ± 3.17 mmol/L and 11.86 ± 3.55 mmol/L respectively (P < 0.01), PPG decreased from baseline at week 48 was 3.09 ± 0.27 mmol/L and 1.85 ± 0.27 mmol/L respectively (P < 0.01) [Table 3]. Changes of FPG and PPG during 48 weeks were shown in Figure 2.
Fasting insulin
At week 24 and week 48, changes of fasting insulin from baseline in Avandamet group was −3.89 ± 1.01 μU/ml and −3.24 ± 0.98 μU/ml respectively, while in metformin group, changes of fasting insulin from baseline was −2.40 ± 0.98 μU/ml and −0.72 ± 1.10 μU/ml respectively. Comparisons between the two treatment groups showed significant difference both at week 24 and week 48. Changes of fasting insulin during 48 weeks were shown in Figure 2.
Body weight
At week 48, the average body weight was 74.95 ± 12.89 kg in Avandamet group and 72.66 ± 12.02 kg in metformin group. Body weight decreased from baseline in the two groups was 0.09 ± 0.32 kg and 2.46 ± 1.03 kg respectively (P < 0.01).
Lipid profile
At week 48, changes of cholesterol from baseline in Avandamet group and metformin group were 0.33 ± 1.09 mmol/L and −0.23 ± 0.99 mmol/L respectively, with significant difference (P < 0.01). Changes of triglyceride from baseline in Avandamet group and metformin group were −0.05 ± 1.52 mmol/L and −0.32 ± 1.80 mmol/L respectively, with significant difference (P < 0.05). Changes of HDL-C from baseline in Avandamet group and metformin group were 0.09 ± 0.36 mmol/L and 0.02 ± 0.29 mmol/L respectively, with significant difference (P < 0.01). Changes of LDL-C from baseline in Avandamet group and metformin group were 0.27 ± 0.94 mmol/L and −0.05 ± 0.80 mmol/L respectively, with significant difference (P < 0.01).
C-reactive protein
At week 48, changes of hypersensitive C-reactive protein (CRP) from baseline in Avandamet group and metformin group were −1.06 ± 4.10 and −0.35 ± 6.50 respectively, with significant difference (P < 0.01).
Safety and tolerability
Over 48 weeks, both treatment regimens were generally well tolerated. The proportion of patients experiencing any AE (including hypoglycemia) was 24.24% (Avandamet-treated patients) and 31.50% (metformin-treated patients). AE frequency was comparable between groups. Serious AE (SAEs) were comparable between groups (1.52% in Avandamet-treated patients vs. 2.00% in metformin-treated patients, P > 0.05) [Table 4]. The most commonly reported AEs (occurring in >5% of Avandamet-treated patients vs. metformin-treated patients) were abdominal discomfort (7.1% vs. 9.5%, P > 0.05), dyslipidemia (7.6% vs. 8.5%, P > 0.05), upper respiratory tract infection (6.6% vs. 6.5%, P > 0.05), edema (6.6% vs. 0.5%, P < 0.01), diarrhea (3.5% vs. 11.5%, P < 0.01) and constipation (4.5% vs. 0.5%, P < 0.01). Hypoglycemic rate in Avandamet group was 0.51% while 2.00% in metformin group (P > 0.05). There were no clinically meaningful drug effects on any laboratory safety parameter. There were no cases of heart failure in either treatment group, but two occurrence of angina pectoris in Avandamet treated patients. One is a 61-year-old female patient who was incharged into hospital for the treatment of angina pectoris with the investigator judgement as probably correlated with study drug and discontinued Avandamet treatment. Another is a 51-year-old female patient who was incharged into hospital with the investigator judgement as probably not correlated with study drug and continued Avandamet treatment. Both patients recovered and discharged from hospital soon. No death in both groups.
Table 4.
AEs, hypoglycemia between the two groups (n)
| Variables | Avandamet (n=198) | Metformin (n=200) | P |
|---|---|---|---|
| AEs - number of patients | |||
| Total AEs | 48 | 63 | 0.09 |
| Cardiovascular disease | |||
| Palpitation | 3 | 1 | 0.13 |
| Dyslipidemia | 15 | 17 | 0.88 |
| Chest tightness | 2 | 2 | 0.98 |
| Gastrointestinal events | |||
| Constipation | 9 | 1 | 0.00 |
| Fatty liver | 0 | 3 | 0.22 |
| Liver dysfunction | 5 | 8 | 0.56 |
| Abdominal discomfort | 14 | 19 | 0.50 |
| Diarrhea | 7 | 23 | 0.002 |
| Blood system events | |||
| Thrombocytosis | 0 | 1 | 0.60 |
| Increase of white blood cell | 2 | 1 | 0.59 |
| Leukopenia | 6 | 0 | 0.05 |
| Anemia | 2 | 3 | 0.83 |
| Respiratory events | |||
| Upper-respiratory infection | 13 | 13 | 0.98 |
| Bronchitis | 0 | 1 | 0.60 |
| Urinary system diseases | |||
| Infection of urinary tract | 0 | 2 | 0.36 |
| Ureteral calculi | 0 | 1 | 0.60 |
| Renal dysfunction | 0 | 1 | 0.60 |
| Nervous system events | |||
| Dizzy | 5 | 1 | 0.00 |
| Insomnia | 0 | 2 | 0.36 |
| Headache | 0 | 1 | 0.60 |
| Numbness of limbs | 1 | 0 | 0.59 |
| Skin diseases | |||
| Itchy skin | 0 | 1 | 0.60 |
| Allergic | 1 | 0 | 0.59 |
| Orthopedics disease | |||
| Lumbago | 0 | 3 | 0.22 |
| Joint pain | 2 | 0 | 0.35 |
| Muscle soreness | 1 | 1 | 0.98 |
| Toe pain | 1 | 0 | 0.59 |
| Others | |||
| Weight gain | 2 | 0 | 0.35 |
| Edema | 13 | 1 | 0.002 |
| Fatigue | 0 | 3 | 0.22 |
| Mouth ulcer | 0 | 1 | 0.60 |
| Periodontitis | 1 | 2 | 0.77 |
| Gout | 0 | 1 | 0.60 |
| Glossitis | 0 | 1 | 0.60 |
| Blurre vision | 1 | 0 | 0.59 |
| Menstrual disorders | 1 | 0 | 0.59 |
| Eject dysfunction | 0 | 1 | 0.60 |
| Hypoglycemia | 1 | 4 | 0.32 |
| SAE - number of patients | |||
| Total SAE | 3 | 4 | 0.87 |
| The left ankle joint fracture of capitulum of fibula | 0 | 1 | 0.60 |
| Cerebral infarction | 0 | 1 | 0.60 |
| Acute appendicitis | 1 | 0 | 0.59 |
| Coronary heart disease (angina pectoris) | 2 | 0 | 0.35 |
| IgA nephropathy | 0 | 1 | 0.60 |
| Perianal abscess | 0 | 1 | 0.60 |
AEs: Adverse events; SAE: Serious adverse event.
DISCUSSION
The aim of this randomized open study was to compare the efficacy of Avandamet and up titrated metformin in patients uncontrolled with metformin alone in a Chinese population. Avandamet treatment for 48-week in patients poorly controlled with metformin alone led to statistically significant and clinically relevant reductions in HbA1c, FPG and PPG. Compared with metformin up titreated treatment, Avandamet treatment also led to statistically significant more reductions in HbA1c FPG and PPG. Treatment with Avandamet also showed trends toward a greater proportion of patients achieving HbA1c <7% as well as achieving HbA1c <6.5%. Results concluded from this study were comparable with that of the previous published studies in Caucasians. Fonseca et al.[4] reported in a study that after 26 weeks, compared with metformin-placebo treatment, rosiglitazone-metformin treatment led to a 1% in HbA1c decreased and 2.2 mmol/L in FPG decreased. Rosenstock et al.[5] reported from a randomized open-label trial that after 24 weeks of Avandamet treatment, the average HbA1c decreased 4.0% ± 2.2% and the average FPG decreased 7.7 ± 4.4 mmol/L, as well as 33% patients reached the target of HbA1c ≤6.5%. In another randomized double-blinded trial,[6] after 32 weeks of Avandamet treatment, the average HbA1c decreased 2.3% and the average FPG decreased 4.1 mmol/L with significance, and about 60% and 77% patients with Avandamet treatment reached the target of HbA1c ≤6.5% and HbA1c ≤7% respectively, which was higher than the proportion of patient receiving metformin treatment. Borges et al.[7] in an 80-week randomized double-blinded trial reported that, the Avandamet treatment was superior to metformin treatment both in HbA1c and FPG.
In terms of fasting insulin, results from this study indicated that Avandamet treatment led to a decrease of fasting insulin more than metformin treatment, which was also in adherence with previous reports. Kahn et al.[8] concluded from the results of ADOPT that compared with sulfonylurea, both rosiglitazone and metformin can decrease fasting insulin level, improving insulin sensitivity. Derosa et al. in a 1-year trial comparing metformin combinated with rosiglitazone or pioglitazone showed that,[9] except for glucose improving, metformin combinated with rosiglitazone decreased fasting and postprandial insulin level significantly. A 6-month small study in Chinese[10] also showed that metformin combination with rosiglitazone could decrease fasting and postprandial insulin level, which indicated that insulin resistance improving.
According to results from this study, compared with up titrated metformin treatment, Avandamet treatment was associated with less reduction in body weight as well as less decrease in lipid profile. Results were similar with those concluded from other studies. In Fonseca et al.'s study,[4] total cholesterol, HDL-C and LDL-C were significantly higher in rosiglitazone-metformin treatment and body mass was increased compared with metformin-placebo control group. Reasons for the increasing of lipid profile may be associated with the mechanism of glucotoxicity improvement or different percentage of usage of lipid lowering therapy between groups, or other reasons we still no know. The weight gain in Avandamet group might be associated with fluid retention or increased appetite which was explained in other studies previously.[4,5,6,11] The more reduction of CRP in Avandamet group might be associated with the efficacy in lowering free fatty acid or other inflammation factors.[4,12]
There was no significant difference of the number of AEs and SAEs between the two groups. The hypoglycemic rate between groups was comparable. The incidence rate of diarrhea in patients with Avandamet treatment was significantly lower than that of patients in metformin group, while the incidence rate of edema with Avandamet treatment was significantly higher. The types of AEs as well as the incidence rate of total AEs of this trial were similar with those results of other clinical trials.[3,4,5,6] In addition, no case of heart failure in either treatment group was reported during the 48-week of follow-up, but two occurrences of angina pectoris in Avandamet treated patients were reported. Both patients recovered and discharged from hospital soon, one continued the study drug while the other one discontinued. The incidence rate of angina pectoris between groups was comparable. Of course, longer duration and with the primary endpoint of study is needed to evaluate the cardiovascular outcome of Avandamet treatment in the future.
Although some of our study design was similar with that of previous studies carried out in Caucasians, there are some different points. First, this study was carried out in Chinese type 2 diabetes patients, which is a totally different ethnic population. Ethnicity difference might be associated with the incidence of type 2 diabetes as well as the treatment efficacy on glucose control or β-cell function or body weight changes,[13,14] therefore, results from this study will give us the answer whether the superior efficacy of Avandamet treatment to metformin treatment alone could be shown in a Chinese population. Secondly, baseline characteristics of patients were different from previous studies in Caucasian population such as BMI, HbA1c, FPG, duration of diabetes and so on. Results from different kinds of patients may result in a different conclusion, therefore, we just want to see whether the efficacy of rosiglitazone combination with metformin treatment was superior to that of up titrated metformin treatment. Third, different dosage forms between our study and other studies. In most previous study, they prescribed patients with metformin plus placebo or metformin plus rosiglitazone. In our study, we prescribed patients only one tablet with Avandamet or metformin. What's more, study duration in this trial was 48-week, which is a longer duration for us to evaluate the efficacy of Avandamet treatment.
According to the result concluded from this study in Chinese type 2 diabetes patients, Avandamet treatment led to a higher proportion of patients achieving HbA1c < 7% compared with metformin although the superiority was not reached. Clinical significance for this result might indicate that for poorly controlled Chinese type 2 diabetes patients with metformin alone, Avandamet treatment instead of up titrated metformin may be a good choice for glycemic control and insulin resistance improvement. Reasons for that might be the combination mechanisms of rosiglitazone and metformin, the possible better adherence of Avandamet treatment than up titrated metformin. Comparable results concluded from this study in Chinese type 2 diabetes patients also indicated that there may be no ethnicity difference in Avandamet treatment.
Of course, this study has some limitations. First, patients who lost follow-up in Avandamet group and metformin group were 23.23% and 24.00% respectively, the rate of which exceeded the expected lose rate (abscission rate up to not more than 15%), which may be the reasons for this study that failed to demonstrate the superiority of Avandamet treatment with respect to the higher dose of metformin monotherapy. But there was no significant difference in the abscission rate between groups with the P value of 0.9063, and the baseline characteristics of patients completed the trial as well as those of patients withdrawn did not show any significant difference between the Avandamet group and metformin group either (data was shown in supplement material), which might indicate that the higher loss rate was not resulted from different treatment group. Secondly, baseline characteristics of patients between Avandamet treatment group and metformin treatment group were comparable except that the average duration of diabetes in Avandamet group was significantly longer than that in metformin group (3.56 ± 3.49 years vs. 2.73 ± 3.10 years, P = 0.01). However, this difference at baseline did not seem to be associated with the efficacy difference between groups, just because patients in Avandamet group had a longer duration of diabetes which may indicate poorer insulin secretion and therefore poorer effect in hypoglycemic treatment. Controversely, at the end of the study, patients in Avandamet group had a higher decrease in HbA1c and a better control of glycemic treatment, which indicated that the baseline difference did not affect the final results.
In conclusion, results from this randomized, open label, parallel group study indicated that compared with up titrated metformin treatment, the efficacy of Avandamet treatment in patients uncontrolled with metformin alone was better, the proportion of patients who reached the target HbA1c was higher in Chinese type 2 diabetes patients.
Footnotes
Edited by: Jian Gao and Li-Shao Guo
Source of Support: This study was supported by GlaxoSmithKline.
Conflict of Interest: None declared.
REFERENCES
- 1.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of Medical Care in Diabetes – 2011. Diabetes Care. 2011;34 Suppl 1:S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viberti G, Kahn SE, Greene DA, Herman WH, Zinman B, Holman RR, et al. A diabetes outcome progression trial (ADOPT): An international multicenter study of the comparative efficacy of rosiglitazone, glyburide, and metformin in recently diagnosed type 2 diabetes. Diabetes Care. 2002;25:1737–43. doi: 10.2337/diacare.25.10.1737. [DOI] [PubMed] [Google Scholar]
- 4.Fonseca V, Rosenstock J, Patwardhan R, Salzman A. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: A randomized controlled trial. JAMA. 2000;283:1695–702. doi: 10.1001/jama.283.13.1695. [DOI] [PubMed] [Google Scholar]
- 5.Rosenstock J, Rood J, Cobitz A, Huang C, Garber A. Improvement in glycaemic control with rosiglitazone/metformin fixed-dose combination therapy in patients with type 2 diabetes with very poor glycaemic control. Diabetes Obes Metab. 2006;8:643–9. doi: 10.1111/j.1463-1326.2006.00648.x. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstock J, Rood J, Cobitz A, Biswas N, Chou H, Garber A. Initial treatment with rosiglitazone/metformin fixed-dose combination therapy compared with monotherapy with either rosiglitazone or metformin in patients with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2006;8:650–60. doi: 10.1111/j.1463-1326.2006.00659.x. [DOI] [PubMed] [Google Scholar]
- 7.Borges JL, Bilezikian JP, Jones-Leone AR, Acusta AP, Ambery PD, Nino AJ, et al. A randomized, parallel group, double-blind, multicentre study comparing the efficacy and safety of Avandamet (rosiglitazone/metformin) and metformin on long-term glycaemic control and bone mineral density after 80 weeks of treatment in drug-naïve type 2 diabetes mellitus patients. Diabetes Obes Metab. 2011;13:1036–46. doi: 10.1111/j.1463-1326.2011.01461.x. [DOI] [PubMed] [Google Scholar]
- 8.Kahn SE, Lachin JM, Zinman B, Haffner SM, Aftring RP, Paul G, et al. Effects of rosiglitazone, glyburide, and metformin on ß-cell function and insulin sensitivity in ADOPT. Diabetes. 2011;60:1552–60. doi: 10.2337/db10-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derosa G, D’Angelo A, Ragonesi PD, Ciccarelli L, Piccinni MN, Pricolo F, et al. Metabolic effects of pioglitazone and rosiglitazone in patients with diabetes and metabolic syndrome treated with metformin. Intern Med J. 2007;37:79–86. doi: 10.1111/j.1445-5994.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Zhang N, Li Y, Shi Y, Li D, Xie Y, et al. Effects of metformin and rosiglitazone on peripheral insulin resistance and ß-cell function in obesity: A double-blind, randomized, controlled study. J Int Med Res. 2011;39:358–65. doi: 10.1177/147323001103900203. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu H, Tsuchiya T, Sato N, Shimomura Y, Kobayashi I, Mori M. Troglitazone reduces plasma leptin concentration but increases hunger in NIDDM patients. Diabetes Care. 1998;21:1470–4. doi: 10.2337/diacare.21.9.1470. [DOI] [PubMed] [Google Scholar]
- 12.Gada E, Owens AW, Gore MO, See R, Abdullah SM, Ayers CR, et al. Discordant effects of rosiglitazone on novel inflammatory biomarkers. Am Heart J. 2013;165:609–14. doi: 10.1016/j.ahj.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Gujral UP, Pradeepa R, Weber MB, Narayan KM, Mohan V. Type 2 diabetes in South Asians: Similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci. 2013;1281:51–63. doi: 10.1111/j.1749-6632.2012.06838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma RC, Chan JC. Type 2 diabetes in East Asians: Similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]


