Abstract
Background:
The auditory brainstem implants (ABIs) have been used to treat deafness for patients with neurofibromatosis Type 2 and nontumor patients. The lack of an appropriate animal model has limited the study of improving hearing rehabilitation by the device. This study aimed to establish an animal model of ABI in adult rhesus macaque monkey (Macaca mulatta).
Methods:
Six adult rhesus macaque monkeys (M. mulatta) were included. Under general anesthesia, a multichannel ABI was implanted into the lateral recess of the fourth ventricle through the modified suboccipital-retrosigmoid (RS) approach. The electrical auditory brainstem response (EABR) waves were tested to ensure the optimal implant site. After the operation, the EABR and computed tomography (CT) were used to test and verify the effectiveness via electrophysiology and anatomy, respectively. The subjects underwent behavioral observation for 6 months, and the postoperative EABR was tested every two weeks from the 1st month after implant surgery.
Result:
The implant surgery lasted an average of 5.2 h, and no monkey died or sacrificed. The averaged latencies of peaks I, II and IV were 1.27, 2.34 and 3.98 ms, respectively in the ABR. One-peak EABR wave was elicited in the operation, and one- or two-peak waves were elicited during the postoperative period. The EABR wave latencies appeared to be constant under different stimulus intensities; however, the amplitudes increased as the stimulus increased within a certain scope.
Conclusions:
It is feasible and safe to implant ABIs in rhesus macaque monkeys (M. mulatta) through a modified suboccipital RS approach, and EABR and CT are valid tools for animal model establishment. In addition, this model should be an appropriate animal model for the electrophysiological and behavioral study of rhesus macaque monkey with ABI.
Keywords: Animal Model, Auditory Brain Stem Implants, Aural Rehabilitation, Feasibility Studies, Macaca mulatta
INTRODUCTION
As an auditory rehabilitation device, the auditory brainstem implant (ABI) has been used to treat deafness in individuals with neurofibromatosis Type 2[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15] since the first device was implanted in 1979.[16,17] In the following years, progressively more effective multichannel ABIs have been developed to palliate the deficits due to loss of integrity of the auditory nerves. Furthermore, the indications for ABI have also been expanded to other treatments, such as the treatments for nontumor patients,[4,11,18,19,20,21,22,23,24,25] ossification or major cochlear malformations, aplasia or avulsion of the cochlear nerves, vestibular schwannoma on the only hearing ears, and bilateral temporal bone fractures. Thus, an increasingly greater number of patients have acquired auditory perception again with the help of ABI, and their quality of life has improved. Despite the enormous potential of this increasingly applied treatment, the auditory performance of many implanted patients is limited, and the variability between cases hinders a complete understanding of the roles played by the multiple parameters related to the efficacy of the implant. Although several researchers have committed to improve the efficacy of the implant, the predicament has not been resolved. To solve this problem, we attempted to establish a large animal model for ABIs in a rhesus macaque monkey to explore the array of the cochlear nucleus (CN) complex, which would thus lay the foundation for the investigation of the mechanisms of auditory recovery after implant surgery.
METHODS
Preparation
The care and handling of animals were conducted in compliance with the Chinese Animal Welfare Act, the Guidance for Animal Experimentation of Capital Medical University, and the Beijing guidelines for the care and use of laboratory animals. This study was approved by the Animal Ethics Committee of Beijing Neurosurgical Institute, Capital Medical University (No. 20131114). All procedures were in accordance with the principles of asepsis and animal welfare. Experiments were performed on six rhesus monkeys (Macaca mulatta). Four monkeys were male, and two monkeys were female. The mean age was 8.2 years, and the average weight was 7.5 kg. The monkeys lived in a controlled environment in Beijing Neurosurgical Institute Laboratory Animal Center. The animals were in good medical condition at the time of testing and had no history of exposure to known ototoxic drugs. All monkeys were treated in the same way (e.g., diet and cage status). They were maintained in individual cages in a controlled environment of 120 cm (H) ×80 cm (W) ×75 cm (D). The front, roof, and walls were composed of organic glass, whereas the bottom of the cage was composed of metal net. Each cage was equipped with a food container and water access. The room temperature was maintained at approximately 20°C with a relative humidity of 50–55%. The room lighting was automatically controlled with lights on from 7:00 to 19:00 daily. The monkeys were routinely released into a semi-closed environment that had environmental enrichment objects, such as toys, branches and climbing materials, mirrors, and TVs, as well as other objects.
In the ABI surgical procedure, general anesthesia was induced via intramuscular injections of ketamine (15 mg/kg, Gutian Pharmaceutical Co., Ltd., China), midazolam (0.5 mg/kg, Enhua Pharmaceutical Co., Ltd., China) and atropine (0.02 mg/kg, Jinyao Amino Acid Co., Ltd., China), and was maintained with isoflurane (1–1.5%). Orotracheal intubation was performed after an intravenous (IV) injection of fentanyl (0.001 mg/kg, Astrazeneca Ltd., UK) and rocuronium (0.3 mg/kg, N.V. Organon, The Netherlands). Lumbar puncture and urethral catheterization were performed prior to surgery. During the surgery, the electrocardiogram, blood pressure, pulse rate, SpO2, end-tidal carbon dioxide partial pressure and temperature were monitored. For the magnetic resonance imaging (MRI), computed tomography (CT) scanning, auditory brainstem response (ABR), electrical ABR (EABR) or lumbar puncture, the monkeys were initially anesthetized with intramuscular ketamine (15 mg/kg), midazolam (0.5 mg/kg) and atropine (0.02 mg/kg). All of these experiments were performed under IV propofol (PropoFlo, Abbott Laboratories, USA) anesthesia with an initial bolus of 1.5 mg/kg and a continuous infusion of 0.5 mg·kg− 1·min− 1. The pulse rate, SpO2, respiratory rate and temperature were monitored.
Auditory brainstem implant instrument
The ABI was manufactured by Nurotron Biotechnology Co., Ltd. (Hangzhou, China) according to the specifications indicated by the research group. The electrode pad was comprised by medical silicone, slightly elliptical, and measured 12 mm × 4 mm × 2 mm. The 24 surface electrodes were attached to one side, and the other side was covered with a 1-cm-diameter polyethylene terephthalate mesh pad for stabilization purposes. This mesh could be easily cut on demand prior to implantation. The electrodes were composed of platinum and were circular with a 0.2 mm diameter.
Surgical procedure
We performed MRI/CT scanning for all six monkeys prior to the surgery to evaluate the intracranial status and gasification degree of the mastoid, and to obtain a reference for surgical planning; ABR testing was conducted to assess auditory function. A lumbar puncture was performed, the cerebrospinal fluid (CSF) was tested, and the results were subsequently reserved as a baseline. General anesthesia was performed via endotracheal intubation, and a left bench-park position was subsequently placed. A postauricular inverted L-shaped skin incision was performed under aseptic technique, and a right modified suboccipital retrosigmoid (RS) craniotomy was performed from the level of the superior nuchal line down to the foramen magnum. After the bone flap was removed, the transverse sinus, sigmoid sinus, and their intersection were exposed, and the foramen magnum was also opened [Figure 1]. A line incision was then performed on the dura mater along the sigmoid sinus. Under surgical microscopic guidance, the nerves in the cerebellopontine angle (CPA) (XI, X, IX, VIII, and VII) were detected and distinguished from bottom to top [Figure 2]. The ABR was used again to verify the normality of neural function. The right cochlear nerve (VIII) was subsequently cut under intraoperative neuromonitoring. By the anatomic markers of the glossopharyngeal nerve (IX), choroid plexus of the fourth ventricle and brainstem stub of cochlear nerve, the right Luschka's foramen of the fourth ventricle was located through which the outflow of CSF could be observed by microscope. Once the anatomical location was confirmed, the electrode assays were implanted in the lateral recess through Luschka's foramen. EABR was used to determine the optimal implantation. The electrode assays were subsequently fixed with autologous muscle, followed by dural closure, bone replacement and suturing of muscles and skin. At the end of the surgery, EABR and head CT were used to evaluate the validity of the electrophysiological function and anatomical placement. During the operation, the prophylactic use of IV ceftriaxone sodium 10 mg/kg was administered to prevent a potential intracranial infection. When the animals awoke from the anesthesia, they were transported to the observation room for postoperative monitoring. All monkeys remained under observation for 6 months after the ABI procedure.
Figure 1.
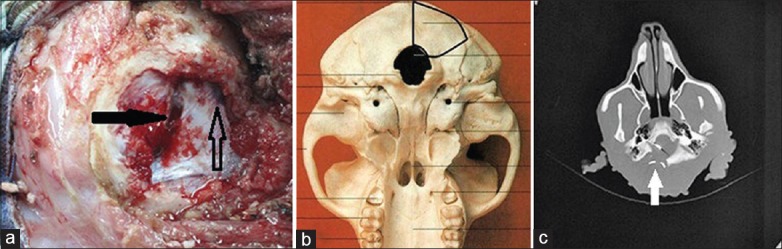
(a) An intra-operative bone window (marked with bold black line marked in Figure b), the solid arrow points to the transverse sinus, and the sigmoid sinus is marked with hollow arrow. The white arrow in Figure c points to the opened foramen magnum.
Figure 2.
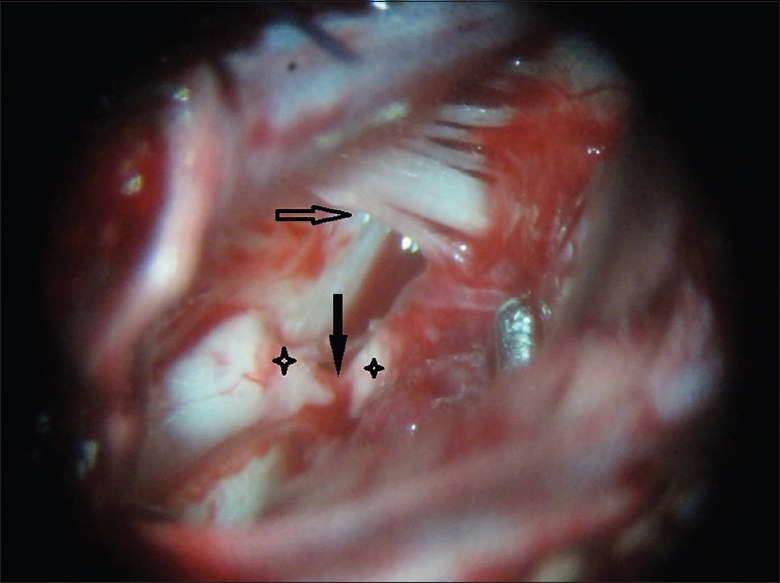
An intra-operative view of the cerebellopontine angle. The stars point to VIII cranial nerve, the solid arrow points to VII cranial nerve, and the hollow arrow points to XI, X and IX cranial nerves.
Postoperative observation
The animals were housed in a separate cage and monitored for food intake, skin wound healing condition, behavioral and neurological changes, and postoperative complications. The modified Tarlov classification[26] and the modified Canada disability scale[27] were used for neural defects and general status evaluation, respectively. Intramuscular ceftriaxone sodium 10 mg/kg was administered for the first 3 postoperative days. A lumbar puncture was again performed during the 1st week postoperation or as indicated by signs of intracranial infection. ABR and EABR testing were performed every 2 weeks from the 1st month after the implant surgery as the brain edema subsided. The CT scan was conducted whenever postoperative invalid EABR waves were encountered.
Auditory brainstem response test
Neural function in the monkeys was assessed using ABR before and 1-month after the ABI surgery. The anesthetized animal was placed in lateral recumbency with its head slightly elevated. The animal's ear canals were inspected and cleaned of debris if necessary, and the condition of the tympanic membrane was determined. Large debris or otitis media were not detected in any of the animals tested. Following sedation, the skin was wiped clean with alcohol wipes, and 0.22 gauge skin electrodes were placed subcutaneously behind each ear, on the forehead, and on the back of the neck[28,29,30,31] The electrode impedance was checked after placement into the skin and was below 2 k ohm for all recordings. The ABR recordings were obtained using a Bio-logic Auditory Evoked Potentials System (Version 6.2.0, Natus Medical Incorporated, USA) controlled by a laptop computer. The evoked responses were amplified 100 K times, and our physiological filters were set to pass at 100–500 Hz. For the ABR recordings, rarefaction clicks were delivered through insert earphones (ER-3A, Etymotic Research, Inc., IL, USA) at a rate of 13.3 stimuli/s and at a level of 80 dB nHL (peak sound pressure level). The ABR waveforms were averaged over a minimum of 1500 repetitions. Clicks and tone bursts were presented at 80 dB nHL and decreased in 10 dB nHL increments from 80 to 30 dB nHL and 5 dB nHL until the ABR response was no longer detectable. The latencies and amplitudes of peaks I, II, and IV were measured at 80 dB nHL. The monaural responses were recorded for each monkey and averaged (Biologic Traveler Express) in a 10 ms time window. The averages for 1000 sweeps were collected, and the responses were replicated to determine the waveform reliability.
Electrical auditory brainstem response recordings
The first EABR test was conducted every 2 weeks since the 1st month after the ABI procedure. The EABR was recorded using the Neurotron Scan software (Neurotron, China). The noninverting electrode was placed at the midline (CZ), and the inverting electrode was placed subcutaneously behind the ipsilateral ear. The ground electrode was placed on the forehead. The recording electrodes were passed through an external low pass filter (fc = 32 kHz) in an attempt to eliminate the frequency-modulated signal sent from the external to internal components of the ABI. Frequencies outside the 100–3000 Hz range were filtered out, and the response was sampled at 20,000 Hz, which provided data points at 0.05 ms intervals and interpolation between the points. The waveforms were marked using the Bio-logic Auditory Evoked Potentials System, which provided the latency values to the 0.01 ms. The latencies were reported to the second decimal point. Signal averaging was performed between −5 ms and 80 ms relative to the stimulus onset; however, a more limited time window of − 2–10 ms was used for the analyses. Sweeps that contained large signals between 2 ms and 80 ms latency were rejected from the average. At least 500 sweeps were typically averaged, and the responses were recorded at least twice. Monopolar pulses (MP 1 + 2) were delivered by the multichannel implants at the basal end of the array. The stimulation levels were increased in steps of 10–30 clinical units until the maximum levels were reached; in most cases, the EABRs recorded at least three increasing levels during this process.
Statistical analysis
All statistical procedures were performed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA). The measurement data were presented as the mean ± standard deviation (SD). For the analysis of the comparison of latencies and amplitudes of peaks I, II and IV between the bilateral ears in the ABR test, a dependent samples t-test was used. A paired samples t-test was applied for the pre- and post-operative CSF analysis. The P level was set to 0.05 for all analyses to determine the statistical significance.
RESULTS
Preoperative auditory brainstem response
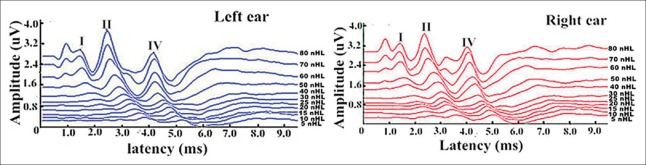
The average time for the ABI procedure was 5.2 h (range, 4.5–6 h), which included nearly half of the time for the EABR testing. The preoperative ABRs indicated that all animals possessed adequate auditory function, and the thresholds were 5–15 dB nHL [Figure 3]. The peaks I, II and IV in the ABR testing were distinguished at 80 dB nHL, and their latencies and amplitudes are shown in Table 1.
Figure 3.
Bilateral auditory brainstem response waves of one monkey. Peaks I, II and IV have been marked.
Table 1.
The latency and amplitude of peaks I, II and IV in the ABR testing for six monkeys (mean ± SD)
| Items | Latency (ms) | Amplitude (µV) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bilateral ears | Left ear* | Right ear | t | P† | Bilateral ears | Left ear* | Right ear | t | P† | |
| Peak I | 1.23 ± 0.11 | 1.21 ± 0.12 | 1.25 ± 0.11 | –0.613 | 0.554 | 0.21 ± 0.13 | 0.19 ± 0.09 | 0.26 ± 0.22 | –0.571 | 0.618 |
| Peak II | 2.26 ± 0.12 | 2.26 ± 0.16 | 2.25 ± 0.09 | 0.112 | 0.913 | 0.68 ± 0.34 | 0.70 ± 0. | 0.46 ± 0.47 | 0.960 | 0.369 |
| Peak IV | 3.89 ± 0.19 | 3.97 ± 0.19 | 3.82 ± 0.17 | 1.494 | 0.166 | 0.19 ± 0.18 | 0.18 ± 0.17 | 0.22 ± 0.24 | –0.273 | 0.792 |
*The left ears were tested twice, and one for the right; †P: Comparison between left and right ears. ABR: Auditory brainstem response; SD: Standard deviation.
Electrical auditory brainstem response waves
Only one-peak EABR waves were induced during the ABI operation [Figure 4]. However, both one-peak and two-peak EABR waves were observed during the postoperative testing [Figure 5]. The intra- and post-operative EABR waves confirmed that the devices were accurately placed where the stimulus could excite the complete auditory pathway. For both one-peak and two-peak EABR waves, the latencies did not appear to change with the stimulus intensity; however, the amplitudes had a positive correlation with the intensity. The postoperative CT scans confirmed the anatomic site of the implant electrode arrays [Figure 6]. Throughout the EABR testing, all monkeys remained stable, and no abnormal changes in the pulse rate, SpO2, respiratory rate, or abnormal limb movements were identified.
Figure 4.
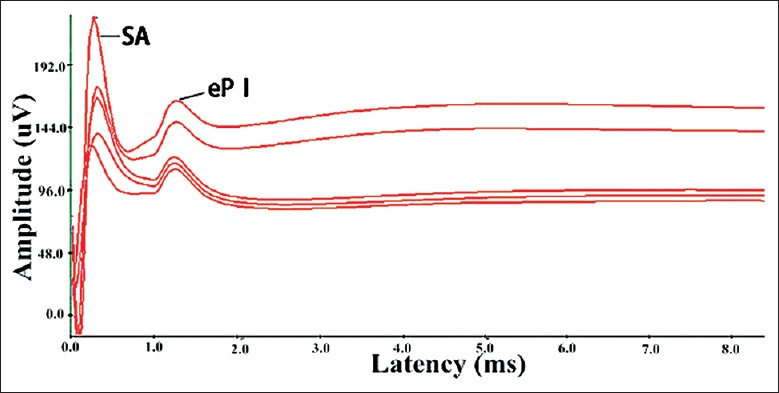
The one intra-operative electrical auditory brainstem response wave (eP I) followed a stimulus artifact (SA), and the stimulus intensity from top to bottom of the five lines were 200, 150, 100, 50 and 0 current levels.
Figure 5.
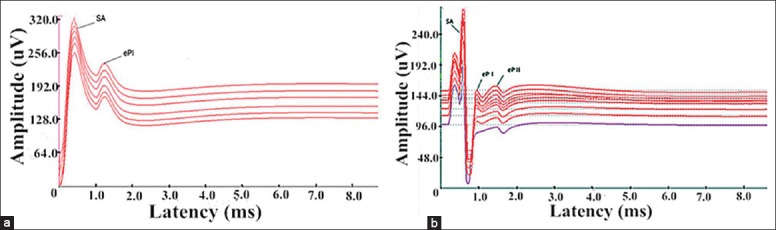
(a) One-peak postoperative electrical auditory brainstem response (EABR) wave (eP I), the stimulus intensities from top to bottom were 120, 100, 80, 50, 30 and 0 current levels (CLs); (b) Two distinguishable EABR waves (eP I and II), the stimulus intensities from top to bottom were 150, 150, 150, 120, 110, 100, 80, 50 and 30 CLs.
Figure 6.
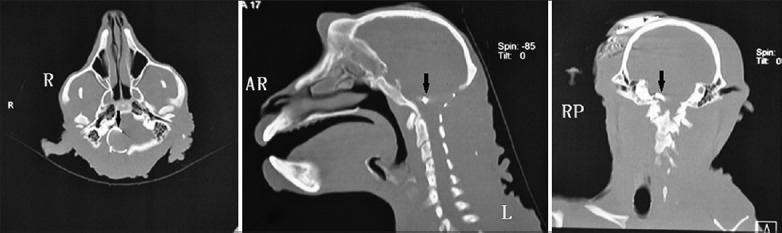
A group of bone windows in postoperative computed tomography scans showed auditory brainstem implants (arrows) in the lateral recess of the fourth ventricle, which was the target anatomic location.
Complication
All wounds healed well, and no CSF leakage, subcutaneous hydrops, wound infection, intracranial infection, or death occurred. According to the Tarlov classification, there were no serious neural defects in the six monkeys. On average, the animals recovered to preoperative health status in 5.7 days (range, 5–7 days), which was evaluated by the modified Canada disability scale. By the time of the last test, no monkeys had died or were sacrificed; they were all maintained for use in a subsequent study.
One animal presented with transient facial nerve paralysis postoperation, which was evidenced by a reduced ipsilateral less blink, a shallow forehead, and facial wrinkles. These signs gradually disappeared within 1-month postoperation.
Laboratory reports of cerebrospinal fluid
The CSF samples of the monkeys at different time points were colorless and clear, with a negative reaction in Pandy's test. The CSF items were not significant differents between the pre- and post-operative tests, including intracranial pressure (80.0 mmH2O vs. 81.7 mmH2O, P = 0.742), chloride (118.0 mmol/L vs. 120.0 mmol/L, P = 0.522), glucose (2.3 mmol/L vs. 2.4 mmol/L, P = 0.771), protein (17.2 mmol/L vs. 17.6 mmol/L, P = 0.946), and cells (1.7 cells/visual field vs. 0.3 cells/visual field, P = 0.184).
DISCUSSION
To the best of our knowledge, this is the first report regarding the rhesus macaque monkey (M. mulatta) implanted with ABI placement. The successful induction of an ABI model in the rhesus macaque monkey paves the way for future studies.
An appropriate surgical approach is vital for the exposure of the implant site and following ABI implantation. Two approaches have been used in clinical practice for ABIs: The translabyrinthine (TL) and RS approaches. The TL approach was originally advocated by Edgerton et al.[17] and is currently the only method approved by the US Food and Drug Administration (FDA) in ABI clinical trials. However, an anatomical study by Kuroki and Moller[32] on cadaver specimens has demonstrated that the TL approach to the CPA offers a limited view; thus, it is necessary to medically retract the sigmoid sinus to obtain a medial view of the root entry zone of the cochlear nerve. In another anatomical investigation, Friedland and Wackym[33] have demonstrated that the RS approach provides excellent visualization of the lateral recess of the fourth ventricle when a 30° endoscope is used, which provides a more direct appreciation of the implant site compared with the TL approach.
Previous studies[34,35,36] have proven that the RS approach has various advantages, such as the adequate control of bleeding, dissection of the entire tumor under direct view, easy identification of the facial and cochlear nerves at their root entry zone and the distal end in the internal auditory canal, and adequate exposure of the foramen of Luschka. In addition, the RS approach provides a unique opportunity for real-time intraoperative monitoring by direct recording of cochlear nerve action potentials,[37,38,39,40] which could prove useful in attempts at hearing preservation.
Compared with humans, the rhesus macaque monkey possesses a more flat, smaller (approximately 2 cm × 1.5 cm on one side) posterior fossa and relatively strong, thick head and neck muscles. The regular linear postauricular incision cannot provide satisfactory posterior fossa bone exposure, which, therefore, makes it difficult to distinguish nerves during the ABI procedure. After considering the advantages of the two approaches, the modified RS approach was adopted for this study. The inverted L-shaped flap and muscles, which had been fully freed, were turned outward, and the external occipital protuberance, middle line, superior nuchal line, part of superior border of the foramen magnum and mastoid process were completely exposed. During the nerve identification stage, the lower cranial nerves were recognized first, followed by the cochlear and facial nerves. The choroid plexus of the fourth ventricle and Luschka's foramen could then be located by anatomic landmarks. Prior to electrode array implantation, the cerebellum and brainstem were separated from the bottom to top, which proved to be easier than the operation on the locale of Luschka's foramen. The experiences with the six monkeys suggested that the modified approach is feasible for multichannel ABI implantation.
Several researchers have studied auditory evoked potentials in rhesus macaque monkeys.[30,41,42,43] They have concluded that peak I in rhesus macaque monkeys (which is analogous to peaks I and II in humans) was generated by eighth nerve fibers, whereas peak II (that is analogous to peak III in humans) was generated by the CN. The neural generator of peak III (which is analogous to peak IV in humans) was not determined by a specific near-field recording, although the researchers’ assumption was that this peak had its origin in the superior olivary complex. Finally, peak IV in monkeys (which is analogous to peak V in humans) was determined to originate from the contralateral lateral lemniscus. In our study, the latencies and amplitudes of peaks I, II and IV were accurately calculated. The latencies of the waves were similar to a previously published study;[28] however, the amplitudes appeared lower than the data from the same research. The comparisons between the bilateral ears were not significantly different in latency or amplitude.
Intraoperative electrophysiology during ABI implantation demonstrated that electrical stimulation from the implanted electrode elicits a response from the ascending auditory pathway, which is reflected in the recorded EABR. As the full surface of the CN is not visible, even an experienced neurosurgeon may have difficulty placing the electrode array into an optimal position every time. Distorted anatomy caused by the removal of a large tumor or “normal” anatomical variations makes this task even more difficult. Electrophysiologic guidance may help to reduce these problems,[44] even for less experienced surgeons. In our study, preoperative ABR in the rhesus macaque monkey contains three explicated waves [Figure 3]; however, only one-peak EABR waves can be observed during surgery [Figure 4], and one-peak or two-peak EABR waves can be observed postoperatively [Figure 5]. In humans, the EABR[45] elicited by the electrical stimulation of the CN is similar to the ABR; however, it typically does not contain components that correspond to peaks I or II in the ABR. The latencies of the peaks that are generated in the CN and the lateral lemniscus are shorter than the ABR.[46] Referred the relevant information of humans and analyzed the waves in the ABR and EABR of rhesus monkey, we concluded that the EABR waves in rhesus macaque monkeys do not contain components that correspond to peak I in ABR, and eP I and eP II [Figures 4 and 5] correspond to peaks II and IV [Figure 3], respectively. Furthermore, peaks II and IV were generated in the CN and contralateral lateral lemniscus. In addition, the latencies of eP I and eP II were less than peaks II and IV [Figures 4 and 5, and Table 1]. Of particular note was that the waveform of the recorded EABRs differed considerably among individuals. During testing, we could observe that as the stimulus intensity increased, the amplitude of the EABR wave in the same electrode also increased in a certain scope. However, no similar correlation was identified between the latency and stimulus intensity.
In the current study, we successfully established an ABI animal model in the rhesus macaque monkey, and short-term data supported its feasibility and safety. However, the long-term survival state and electrophysiology of monkeys with implants requires further investigation. Although EABR testing and postoperative CT could prove the effectiveness of this procedure in electrophysiology and anatomy, respectively, relevant behavioral studies should be considered in future implantations. Any disorder that affects the secretion, absorption or transportation will cause changes in the components of the CSF and/or neural activity. The short recovery time and stable CSF components of all animals may demonstrate the safety of the ABI.
We concluded that the rhesus macaque monkey is a valid ABI model, and the modified suboccipital RS approach can provide sufficient exposure for the implant surgery. During the intra- and post-operation of ABIs, the EABR test can guide us to the optimal implant sites, and the postoperative CT can confirm the implant location. eP I and eP II of EABR wave were generated in the CN and contralateral lateral lemniscus. Numerous studies have clearly established this species as an essential model for neurophysiologic investigations in conjunction with sophisticated behavior. We suggested that the demonstration of the feasibility of ABI procedures in the macaque monkey opens a promising field of research regarding the mechanisms of auditory recovery after ABI surgery.
Footnotes
Edited by: Xin Chen
Source of Support: This study was supported by a grant from the National Science and Technology Pillar Program during the 12th 5-year Plan Period (No. 2012BAI12B03).
Conflict of Interest: None declared.
REFERENCES
- 1.Kalamarides M, Grayeli AB, Bouccara D, Dahan EA, Sollmann WP, Sterkers O, et al. Hearing restoration with auditory brainstem implants after radiosurgery for neurofibromatosis type 2. J Neurosurg. 2001;95:1028–33. doi: 10.3171/jns.2001.95.6.1028. [DOI] [PubMed] [Google Scholar]
- 2.Kameswaran M, Vasudevan MC, Kumar RS, Nagasundaram J, Natarajan K, Raghunandhan S. Auditory brainstem implantation: The first Indian experience. Indian J Otolaryngol Head Neck Surg. 2005;57:58–63. doi: 10.1007/BF02907633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colletti V. Auditory outcomes in tumor vs. nontumor patients fitted with auditory brainstem implants. Adv Otorhinolaryngol. 2006;64:167–85. doi: 10.1159/000094651. [DOI] [PubMed] [Google Scholar]
- 4.Grayeli AB, Kalamarides M, Bouccara D, Ambert-Dahan E, Sterkers O. Auditory brainstem implant in neurofibromatosis type 2 and non-neurofibromatosis type 2 patients. Otol Neurotol. 2008;29:1140–6. doi: 10.1097/MAO.0b013e31818b6238. [DOI] [PubMed] [Google Scholar]
- 5.Vincenti V, Pasanisi E, Guida M, Di Trapani G, Sanna M. Hearing rehabilitation in neurofibromatosis type 2 patients: Cochlear versus auditory brainstem implantation. Audiol Neurootol. 2008;13:273–80. doi: 10.1159/000115437. [DOI] [PubMed] [Google Scholar]
- 6.Maini S, Cohen MA, Hollow R, Briggs R. Update on long-term results with auditory brainstem implants in NF2 patients. Cochlear Implants Int. 2009;10 Suppl 1:33–7. doi: 10.1179/cim.2009.10.Supplement-1.33. [DOI] [PubMed] [Google Scholar]
- 7.Colletti L, Shannon R, Colletti V. Auditory brainstem implants for neurofibromatosis type 2. Curr Opin Otolaryngol Head Neck Surg. 2012;20:353–7. doi: 10.1097/MOO.0b013e328357613d. [DOI] [PubMed] [Google Scholar]
- 8.Monteiro TA, Goffi-Gomez MV, Tsuji RK, Gomes MQ, Brito Neto RV, Bento RF. Neurofibromatosis 2: Hearing restoration options. Braz J Otorhinolaryngol. 2012;78:128–34. doi: 10.5935/1808-8694.20120020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanna M, Di Lella F, Guida M, Merkus P. Auditory brainstem implants in NF2 patients: results and review of the literature. Otol Neurotol. 2012;33:154–64. doi: 10.1097/MAO.0b013e318241bc71. [DOI] [PubMed] [Google Scholar]
- 10.Matthies C, Brill S, Kaga K, Morita A, Kumakawa K, Skarzynski H, et al. Auditory brainstem implantation improves speech recognition in neurofibromatosis type II patients. ORL J Otorhinolaryngol Relat Spec. 2013;75:282–95. doi: 10.1159/000350568. [DOI] [PubMed] [Google Scholar]
- 11.Merkus P, Di Lella F, Di Trapani G, Pasanisi E, Beltrame MA, Zanetti D, et al. Indications and contraindications of auditory brainstem implants: Systematic review and illustrative cases. Eur Arch Otorhinolaryngol. 2014;271:3–13. doi: 10.1007/s00405-013-2378-3. [DOI] [PubMed] [Google Scholar]
- 12.Otto SR, Shannon RV, Brackmann DE, Hitselberger WE, Staller S, Menapace C. The multichannel auditory brain stem implant: Performance in twenty patients. Otolaryngol Head Neck Surg. 1998;118:291–303. doi: 10.1016/S0194-59989870304-3. [DOI] [PubMed] [Google Scholar]
- 13.Otto S, Staller S. Multichannel auditory brain stem implant: Case studies comparing fitting strategies and results. Ann Otol Rhinol Laryngol Suppl. 1995;166:36–9. [PubMed] [Google Scholar]
- 14.Shannon RV, Fayad J, Moore J, Lo WW, Otto S, Nelson RA, et al. Auditory brainstem implant: II. Postsurgical issues and performance. Otolaryngol Head Neck Surg. 1993;108:634–42. doi: 10.1177/019459989310800603. [DOI] [PubMed] [Google Scholar]
- 15.Brackmann DE, Hitselberger WE, Nelson RA, Moore J, Waring MD, Portillo F, et al. Auditory brainstem implant: I. Issues in surgical implantation. Otolaryngol Head Neck Surg. 1993;108:624–33. doi: 10.1177/019459989310800602. [DOI] [PubMed] [Google Scholar]
- 16.Hitselberger WE, House WF, Edgerton BJ, Whitaker S. Cochlear nucleus implants. Otolaryngol Head Neck Surg. 1984;92:52–4. doi: 10.1177/019459988409200111. [DOI] [PubMed] [Google Scholar]
- 17.Edgerton BJ, House WF, Hitselberger W. Hearing by cochlear nucleus stimulation in humans. Ann Otol Rhinol Laryngol Suppl. 1982;91:117–24. [PubMed] [Google Scholar]
- 18.Colletti V, Carner M, Fiorino F, Sacchetto L, Miorelli V, Orsi A, et al. Hearing restoration with auditory brainstem implant in three children with cochlear nerve aplasia. Otol Neurotol. 2002;23:682–93. doi: 10.1097/00129492-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Grayeli AB, Bouccara D, Kalamarides M, Ambert-Dahan E, Coudert C, Cyna-Gorse F, et al. Auditory brainstem implant in bilateral and completely ossified cochleae. Otol Neurotol. 2003;24:79–82. doi: 10.1097/00129492-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Colletti V, Carner M, Miorelli V, Colletti L, Guida M, Fiorino F. Auditory brainstem implant in posttraumatic cochlear nerve avulsion. Audiol Neurootol. 2004;9:247–55. doi: 10.1159/000078394. [DOI] [PubMed] [Google Scholar]
- 21.Colletti V, Carner M, Miorelli V, Guida M, Colletti L, Fiorino F. Cochlear implant failure: Is an auditory brainstem implant the answer? Acta Otolaryngol. 2004;124:353–7. doi: 10.1080/00016480410016441. [DOI] [PubMed] [Google Scholar]
- 22.Colletti V, Fiorino FG, Carner M, Miorelli V, Guida M, Colletti L. Auditory brainstem implant as a salvage treatment after unsuccessful cochlear implantation. Otol Neurotol. 2004;25:485–96. doi: 10.1097/00129492-200407000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Cervera-Paz FJ, Manrique MJ. Traditional and emerging indications in cochlear and auditory brainstem implants. Rev Laryngol Otol Rhinol (Bord) 2005;126:287–92. [PubMed] [Google Scholar]
- 24.Ramsden R, Khwaja S, Green K, O’Driscoll M, Mawman D. Vestibular schwannoma in the only hearing ear: Cochlear implant or auditory brainstem implant? Otol Neurotol. 2005;26:261–4. doi: 10.1097/00129492-200503000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Colletti V, Shannon R, Carner M, Veronese S, Colletti L. Outcomes in nontumor adults fitted with the auditory brainstem implant: 10 years’ experience. Otol Neurotol. 2009;30:614–8. doi: 10.1097/MAO.0b013e3181a864f2. [DOI] [PubMed] [Google Scholar]
- 26.Goldsmith HS, Steward E, Duckett S. Early application of pedicled omentum to the acutely traumatised spinal cord. Paraplegia. 1985;23:100–12. doi: 10.1038/sc.1985.18. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Mancilla B, Bédard PJ. Effect of nondopaminergic drugs on L-dopa-induced dyskinesias in MPTP-treated monkeys. Clin Neuropharmacol. 1993;16:418–27. doi: 10.1097/00002826-199310000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Torre P, 3rd, Fowler CG. Age-related changes in auditory function of rhesus monkeys (Macaca mulatta) Hear Res. 2000;142:131–40. doi: 10.1016/s0378-5955(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 29.Torre P, 3rd, Mattison JA, Fowler CG, Lane MA, Roth GS, Ingram DK. Assessment of auditory function in rhesus monkeys (Macaca mulatta): Effects of age and calorie restriction. Neurobiol Aging. 2004;25:945–54. doi: 10.1016/j.neurobiolaging.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Allen AR, Starr A. Auditory brain stem potentials in monkey (M. mulatta) and man. Electroencephalogr Clin Neurophysiol. 1978;45:53–63. doi: 10.1016/0013-4694(78)90341-3. [DOI] [PubMed] [Google Scholar]
- 31.Fowler CG, Chiasson KB, Leslie TH, Thomas D, Beasley TM, Kemnitz JW, et al. Auditory function in rhesus monkeys: Effects of aging and caloric restriction in the Wisconsin monkeys five years later. Hear Res. 2010;261:75–81. doi: 10.1016/j.heares.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuroki A, Møller AR. Microsurgical anatomy around the foramen of Luschka in relation to intraoperative recording of auditory evoked potentials from the cochlear nuclei. J Neurosurg. 1995;82:933–9. doi: 10.3171/jns.1995.82.6.0933. [DOI] [PubMed] [Google Scholar]
- 33.Friedland DR, Wackym PA. Evaluation of surgical approaches to endoscopic auditory brainstem implantation. Laryngoscope. 1999;109:175–80. doi: 10.1097/00005537-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Harner SG, Laws ER., Jr Posterior fossa approach for removal of acoustic neurinomas. Arch Otolaryngol. 1981;107:590–3. doi: 10.1001/archotol.1981.00790460002002. [DOI] [PubMed] [Google Scholar]
- 35.Jannetta PJ, Møller AR, Møller MB. Technique of hearing preservation in small acoustic neuromas. Ann Surg. 1984;200:513–23. doi: 10.1097/00000658-198410000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacker RK, Pitts LH. Acoustic neuroma. Neurosurgical Clin N Am. 1990;1:199–223. [PubMed] [Google Scholar]
- 37.Colletti V, Fiorino FG, Sacchetto L. Iatrogenic impairment of hearing during surgery for acoustic neuroma. Skull Base Surg. 1996;6:153–61. doi: 10.1055/s-2008-1058639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colletti V, Fiorino FG, Carner M, Tonoli G. Mechanisms of auditory impairment during acoustic neuroma surgery. Otolaryngol Head Neck Surg. 1997;117:596–605. doi: 10.1016/S0194-59989770039-1. [DOI] [PubMed] [Google Scholar]
- 39.Colletti V, Fiorino FG. Advances in monitoring of seventh and eighth cranial nerve function during posterior fossa surgery. Am J Otol. 1998;19:503–12. [PubMed] [Google Scholar]
- 40.Colletti V, Fiorino FG, Mocella S, Policante Z. ECochG, CNAP and ABR monitoring during vestibular Schwannoma surgery. Audiology. 1998;37:27–37. doi: 10.3109/00206099809072959. [DOI] [PubMed] [Google Scholar]
- 41.Doyle WJ, Saad MM, Fria TJ. Maturation of the auditory brain stem response in rhesus monkeys (Macaca mulatta) Electroencephalogr Clin Neurophysiol. 1983;56:210–23. doi: 10.1016/0013-4694(83)90075-5. [DOI] [PubMed] [Google Scholar]
- 42.Lasky RE, Maier MM, Snodgrass EB, Laughlin NK, Hecox KE. Auditory evoked brainstem and middle latency responses in Macaca mulatta and humans. Hear Res. 1995;89:212–25. doi: 10.1016/0378-5955(95)00140-7. [DOI] [PubMed] [Google Scholar]
- 43.Møller AR, Burgess J. Neural generators of the brain-stem auditory evoked potentials (BAEPs) in the rhesus monkey. Electroencephalogr Clin Neurophysiol. 1986;65:361–72. doi: 10.1016/0168-5597(86)90015-8. [DOI] [PubMed] [Google Scholar]
- 44.Waring MD. Intraoperative electrophysiologic monitoring to assist placement of auditory brain stem implant. Ann Otol Rhinol Laryngol Suppl. 1995;166:33–6. [PubMed] [Google Scholar]
- 45.Waring MD. Auditory brain-stem responses evoked by electrical stimulation of the cochlear nucleus in human subjects. Electroencephalogr Clin Neurophysiol. 1995;96:338–47. doi: 10.1016/0168-5597(95)00022-k. [DOI] [PubMed] [Google Scholar]
- 46.Nevison B. A guide to the positioning of brainstem implants using intraoperative electrical auditory brainstem responses. Adv Otorhinolaryngol. 2006;64:154–66. doi: 10.1159/000094650. [DOI] [PubMed] [Google Scholar]