Abstract
Background:
Bisdemethoxycurcumin (BDMC) is an active component of curcumin and a chemotherapeutic agent, which has been suggested to inhibit tumor growth, invasion and metastasis in multiple cancers. But its contribution and mechanism of action in invasion and metastasis of non-small cell lung cancer (NSCLC) are not very clear. Therefore, we tried to study the effects of BDMC on regulation of epithelial-to-mesenchymal transition (EMT), which is closely linked to tumor cell invasion and metastasis.
Methods:
In this study, we first induced transforming growth factor-β1 (TGF-β1) mediated EMT in highly metastatic lung cancer 95D cells. Thereafter, we studied the effects of BDMC on invasion and migration of 95D cells. In addition, EMT markers expressions were also analyzed by western blot and immunofluorescence assays. The contribution of Wnt inhibitory factor-1 (WIF-1) in regulating BDMC effects on TGF-β1 induced EMT were further analyzed by its overexpression and small interfering RNA knockdown studies.
Results:
It was observed that BDMC inhibited the TGF-β1 induced EMT in 95D cells. Furthermore, it also inhibited the Wnt signaling pathway by upregulating WIF-1 protein expression. In addition, WIF-1 manipulation studies further revealed that WIF-1 is a central molecule mediating BDMC response towards TGF-β1 induced EMT by regulating cell invasion and migration.
Conclusions:
Our study concluded that BDMC effects on TGF-β1 induced EMT in NSCLC are mediated through WIF-1 and elucidated a novel mechanism of EMT regulation by BDMC.
Keywords: Bisdemethoxycurcumin, Epithelial-to-mesenchymal Transition, Lung Cancer, Wnt Inhibitory Factor-1, Wnt Pathway
INTRODUCTION
Lung cancer is the leading cause of cancer deaths worldwide,[1] and non-small cell lung cancer (NSCLC) accounts for almost 80% of all lung cancers. Majority of the NSCLC patients die due to metastasis rather than their primary tumor. Despite the recent progress in this field of lung cancer metastasis, the precise underlying mechanisms remain elusive. Therefore, it is important to elucidate the molecular mechanisms of NSCLC metastasis to develop novel effective therapeutic strategies.
Among many hallmarks of cancer, epithelial-to-mesenchymal transition (EMT) is a cellular program that is involved in the conversion of polarized epithelial cancer cells to more motile mesenchymal cells. This phenotypic conversion is directly linked with metastasis and is regulated by many transcriptional factors, such as zinc finger E-box binding homeobox 1/2 (ZEB1/2), Twist, B-cell-specific Moloney murine leukemia virus insertion site 1 (BMI-1), Snail, and Slug. In this transition, there is a suppression of the epithelial marker, E-cadherin, induction of the mesenchymal genes, N-cadherin, and vimentin.[2] EMT is usually triggered by stimuli from the various extracellular matrix proteins or soluble factors like transforming growth factor-β1 (TGF-β1), Wnt proteins, tumor necrosis factor-α (TNF–α) and other cytokines. Among these, TGF-β1 and Wnt pathways are quite active in lung cancer cells and thus play an important role in the induction of EMT in NSCLC. TGF-β1 is a multifunctional cytokine that regulates a wide range of cellular functions including induction of EMT.[3] It exerts its effect through transcription factor Snail.[4]
In addition, the canonical Wnt/β-catenin signaling pathway plays an important role in many cancers including NSCLC.[5,6] The secreted Wnt antagonists frizzled-related protein, dickkopf proteins, and Wnt inhibitory factor-1 (WIF-1) are important regulators in the canonical Wnt signaling pathway.[7] Wnt/β-catenin signaling pathway has been demonstrated to play an important role in the development of EMT and cancer metastasis.[8] The activity of β-catenin, which also acts in linking E-cadherin to the cytoskeleton at the cell-cell adhesion junctions to form the epithelial phenotype,[9] is regulated by these secreted Wnt proteins.
Curcumin, a mixture of compounds extracted from turmeric, has been reported to inhibit growth, invasion and metastasis of various tumor cells.[10] In addition, it has also been reported to induce EMT in pancreatic cancer cells. Bisdemethoxycurcumin (BDMC) is one of the three major active chemical components of curcumin. However, the basic mechanism of its action is largely unknown. In order to better understand its mechanisms of actions in NSCLC, we focused on a process of EMT to explain BDMC regulation of tumor growth, invasion and metastasis.
Therefore, in this study, we analyzed the relationship between BDMC and Wnt signaling pathway in the process of EMT induction in NSCLC cells. We explored the status of Wnt signaling pathway and expression of WIF-1 in 95D cells. In addition, we also investigated the role of the Wnt/β-catenin signaling pathway and WIF-1 in TGF-β1-induced EMT in the presence of BDMC.
METHODS
Cell lines and cell culture
Two invasive lung cancer cell lines 95C (less invasive) and 95D (highly invasive) were purchased from Cell Bank (CAS, Shanghai, China). Cells were cultured in RPMI-1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum (GIBCO, USA), 100 U/ml streptomycin, and 100 U/ml penicillin in a humidified incubator at 37°C with 5% CO2 level. BDMC, purchased from Sigma-Aldrich (MO, USA), was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 100 mmol/L BDMC and stored at − 20°C as the stock solution. The stock solution of BDMC was diluted with RPMI-1640 medium to the required concentration. Cells treated with DMSO only served as a vehicle control. The final concentration of DMSO was < 0.01%.
Immunofluorescence
Highly invasive 95D cells were seeded on sterile coverslips and cultured in a humidified 5% CO2 incubator at 37°C for 24 h, Thereafter, cells were treated with 5 ng/ml TGF-β1 alone or 5 μmol/L BDMC for 48 h. Finally, the cells were first fixed with 4% paraformaldehyde for 20 min at room temperature, permeabilized with 0.2% Triton X-100 for 15 min, and blocked with 2% bovine serum albumin in phosphate-buffered saline (PBS) for 60 min at room temperature. Subsequently, cells were incubated with specific primary antibodies (E-cadherin 1:300, and vimentin 1:400) at 4°C overnight. After being washed with PBS three times, the cells were stained with fluorescein isothiocyanate-conjugated secondary antibodies (Zhongshan Golden Bridge Biotechnology, China) for 1 h at room temperature. Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (Invitrogen, USA). The stained cells were visualized, and images were taken with a fluorescence microscope.
Western blot analysis
Whole cell, nuclear and cytosolic protein extracts of 95D cells were prepared using RIPA lysis buffer (Beyotime, China) containing phenylmethanesulfonyl fluoride. Equal amount of proteins were loaded onto 10% polyacrylamide gels for separation, and then transferred to the polyvinylidene fluoride membranes. These membranes were incubated with anti-WIF-1, anti-E-cadherin, anti-vimentin, anti-c-myc, anti-fibronectin, anti-β-catenin, anti-Snail (Cell Signaling Technology, USA), anti-GAPDH (Santa Cruz Biotechnology, USA) at 4°C overnight. The next day, membranes were incubated with horse reddish peroxidase-conjugated secondary antibodies (Zhongshan Golden Bridge Biotechnology, China) for 1 h at room temperature. Finally, protein bands were visualized using enhanced chemiluminescence (Beyotime, China) according to the manufacturer's protocol. GAPDH signal was used as the loading control.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from 95D cells using the Trizol reagent (Invitrogen, USA) and reversely transcribed into cDNA using a PrimeScript RT reagent Kit (Takara, China) according to the manufacturer's protocol. The following primer sequences were used in this study: 5’-CAACCGTCAATGTCCCTCTGC-3’ (forward) and 5’-TCCCTGGTAACCTTTGGAACAC-3’ (reverse) for WIF-1, 5’-CGGGAAATCGTGCGTGAC-3’ (forward) and 5’-TGGAAGGTGGACAGCGAGG-3’ (reverse) for β-actin. Polymerase chain reaction (PCR) was performed using the following conditions: 95°C for 30 s, 35 cycles of 94°C for 45 s, 55°C (WIF-1), or 54°C (β-actin) for 40 s, and 72°C for 1 min followed by 5 min extension at 72°C. All the PCR reactions were run in triplicate. β-actin amplification was used as an internal control. The intensity of each band amplified by RT-PCR.
Matrigel invasion and wound healing assays
For Matrigel invasion assays, 8-μm pore size Transwell migration chambers (Costar, Cambridge, MA, USA) coated with Matrigel (BD Bioscience, San Jose, CA, USA) were used to assess the cell invasion ability. Briefly, 5.0 × 104 95D cells, serum-starved for 24 h, were seeded into a Matrigel-coated chamber in a 24-well plate. The cells were treated with or without TGF-β1 plus or not plus BDMC. After 48 h, cells from the top surface of the insert were removed with cotton swabs. Invaded cells were then fixed with 100% methanol for 10 min and stained with 1% crystal violet (Sigma-Aldrich, MO, USA) for 10 min. The number of invaded cells was manually counted under an inverted microscope (×200, five random fields/well).
Wound healing assay was used to evaluate cell motility. Briefly, tumor cells were plated to grow as a monolayer in 24-well plates. A scratch was made in the cell monolayer using a 200 μl sterile pipette tip. The floating cells and debris were removed by washing twice with PBS. Cells were then treated with 5.0 ng/ml of TGF-β1 and 5.0 μmol/L BDMC while growing at 37°C. Thereafter, pictures of the scratch were taken at regular intervals from 0 h and 48 h. The percentage of the wound healing (the width of wound at 0 h – the width of wound at 48 h/the width of wound) was used to evaluate cell migration ability according to the previous published study.[11] Wound distance was evaluated using Image J Software (National Institutes of Health, USA). Each experiment was repeated three times.
Plasmid construction and transfection
To amplify WIF-1, WIF-1 cDNA was amplified by PCR using following primer sequences: WIF-1: 5’-CTAGCTAGCGCCACC ATGGCCCGGAGGAGCGCCTT-3(forward) and 5’-GAAGATCTTCACCAGATGTAATTGGAT-3’ (reverse). The amplified fragment was cloned into the NheI and BglII digested pIRES2-ZsGreen vector for overexpression of WIF-1. 95D cells were then transfected with pIRES2-ZsGreen-WIF-1 or pIRES2-ZsGreen vectors using lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol, The WIF-1 positive cells were selected with G418 (400 μg/ml) (Sigma). After 2 weeks of selection, 95D cells stably expressing WIF-1 (95D-WIF-1) and control vector pIRES2-ZsGreen (95D-pIRES2-ZsGreen) were collected and used for subsequent experiments. pIRES2-ZsGreen vector was obtained from Clontech (USA).
Small interfering-RNA knockdown of Wnt inhibitory factor-1
95D cells were seeded in 24-well plates (1.5 × 105 cells/well) and incubated for 24 h. Negative control small interfering-RNA (NC-siRNA) or WIF-1-siRNA (purchased from Santa Cruz Biotechnology) was transfected along with lipofectamine 2000 according to the manufacturer's instructions. Cells were later analyzed after 48–72 h for WIF knockdown by western blot.
Statistical analysis
Data collected from at least three independent experiments were presented as mean ± standard deviation (SD). Differences between groups were analyzed using Student's t-test. All the data were analyzed using statistical software SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 considered to be statistically significance.
RESULTS
Bisdemethoxycurcumin suppressed transforming growth factor-β1-induced epithelial-to-mesenchymal transition in 95D cells
To induce EMT, we treated 95D cells with TGF-β1 (5 ng/mL) for 48 h. As expected, 95D cells in the TGF-β1-treated group exhibited typical mesenchymal-like morphology. Furthermore, the expression of specific epithelial and mesenchymal markers was assessed by western blot. In accordance with EMT morphology changes, E-cadherin (epithelial cell marker) expression was downregulated by 60% upon TGF-β1 treatment. Similarly, TGF-β1 treatment caused about 3-, 2-, and 2-fold increase of the protein expression of vimentin, fibronectin, and Snail, respectively (P < 0.05). DMOS served as the control group.
To test the effect of BDMC on TGF-β1-induced-EMT, we treated 95D cells with either BDMC (5 μmol/L) or TGF-β1 alone or in combination with each other for 48 h. As observed earlier, 95D cells underwent EMT after being exposed to TGF-β1 for 48 h, but TGF-β1 treatment along with BDMC did not induce EMT as observed in cellular morphological phenotype (Figure 1a). Furthermore, consistent with the morphological phenotype, the expression profiles of EMT markers, E-cadherin, vimentin, fibronectin, and Snail also did not show expected shift (Figure 1b and 1c). Collectively, these in vitro results suggested that BDMC can repress TGF-β1-induced EMT in 95D cells.
Figure 1.
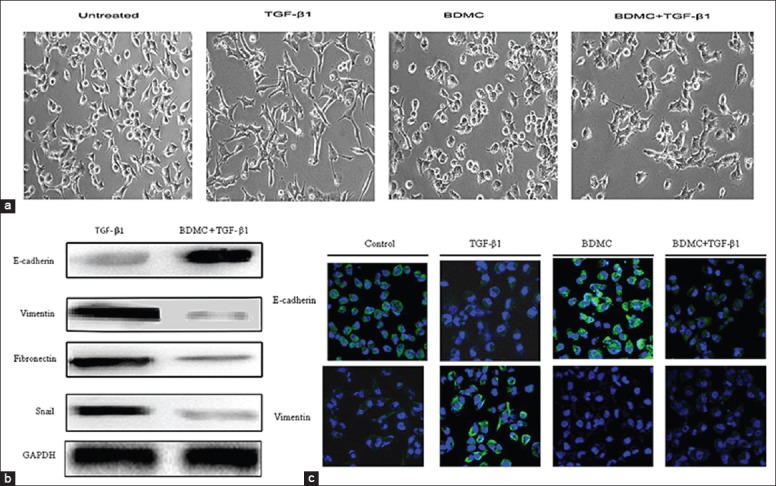
Bisdemethoxycurcumin (BDMC) inhibited transforming growth factor-β1 (TGF-β1)-induced epithelial-to-mesenchymal transition. (a) Phase contrast images of 95D cells untreated or treated with TGF-β1 (5 ng/mL), BDMC (5 μmol/L) or both after 48 h; (b) The protein expression of E-cadherin, vimentin, fibronectin, and Snail in 95D cells treated or not treated with TGF-β1 and BDMC together, GAPDH was a loading control; (c) The expression of E-cadherin and vimentin as detected by immunofluorescence in 95D cells untreated or treated with TGF-β1, BDMC or both. Blue signal represented DAPI nuclei staining, and green signal represented vimentin or E-cadherin expression (original magnification × 100).
Bisdemethoxycurcumin inhibited transforming growth factor-β1 stimulated invasion and migration in 95D cells
To understand the role of BDMC in the metastatic ability of TGF-β1-stimulated 95D cells, we performed wound healing and transwell invasion assays. As shown in Figure 2a, the wound healing assay revealed that TGF-β1 treatment caused about 5-fold increase of migration capacity of 95D cells (t=2.844, P < 0.05 vs. control), but BDMC indeed inhibited the TGF-β1-induced migration of 95D cells. The transwell invasion assay showed that the number of the TGF-β1-stimulated cells (280.76 ± 20.57 cells/field) that invading through Matrigel was significantly more than the control group (120.56 ± 10.57 cells/field, t=3.341, P < 0.05), but BDMC significantly inhibited the TGF-β1-induced invasion of 95D cells through Matrigel (t=4.303, P < 0.01) [Figure 2b]. These results together demonstrated that BDMC can inhibit TGF-β1-induced migration and invasion of 95D cells without any cytotoxicity at concentration of 5 μmol/L. But, it was important to mention here that BDMC treatment alone also has some inhibitory effect on cell migration and invasion.
Figure 2.
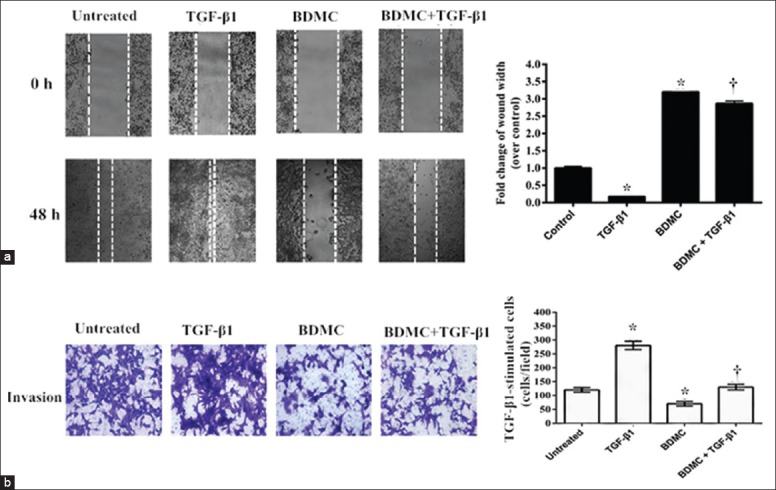
Bisdemethoxycurcumin (BDMC) attenuated transforming growth factor-β1 (TGF-β1)-induced migration and invasion. The effect of BDMC on the TGF-β1-induced migration and invasion of 95D cells was evaluated using: (a) Wound healing assay, where confluent cells were scratched to create a boundary and then cells were treated with TGF-β1 or BDMC alone or together. After 48 h, the wound widths were recorded, quantified, and plotted on right side panel (original magnification × 100). *P < 0.05 compared to controls; †P < 0.01, versus the group treated with TGF-β1 alone; (b) Similarly, 95D cells were plated on the Matrigel coated top chamber of 24-well plate. The cells were treated with TGF-β1or BDMC alone or together for 48 h and later, the number of cells that invaded through the Matrigel were counterstained and counted. The average of cell numbers/field calculated from multiple fields was plotted on the right-hand side panel. Cells in the control group were treated with DMSO in both the assays. *P < 0.05 compared to controls; †P < 0.01, versus the group treated with TGF-β1 alone (original magnification × 200).
Bisdemethoxycurcumin attenuated the nuclear localization of-catenin and enhanced Wnt inhibitory factor-1 expression
To ascertain the involvement of Wnt signaling in attenuation of TGF-β1-mediated EMT phenotype in 95D cells by BDMC, we first analyzed the expression/activation of β-catenin protein. Treatment of 95D cells with TGF-β1 alone or in presence of two different concentrations (1 and 2.5 μmol/L) of BDMC, suggested that TGF-β1 treatment induced translocation of β-catenin (activity) into the nucleus whereas BDMC significantly inhibited this translocation in a concentration-dependent manner (Figure 3a). Similarly, consistent with nucleus translocation, the cytosolic fraction of β-catenin was reduced on TGF-β1 treatment and recovered back to the control levels by BDMC treatment. This result confirmed that there is indeed an involvement of Wnt signaling pathway in attenuation of TGF-β1-induced EMT by BDMC.
Figure 3.
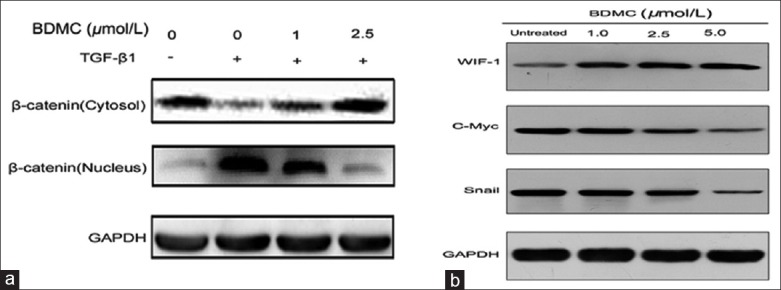
Analysis of bisdemethoxycurcumin (BDMC) effect on β-catenin and Wnt inhibitory factor-1 (WIF-1) proteins. (a) Western blot data of β-catenin, cytosolic and nuclear fraction from 95D cells treated with transforming growth factor-β1 alone and in combination with increasing doses of BDMC. GAPDH was a loading control; (b) Western blot data showed the expression of WIF-1, c-myc and Snail proteins in 95D cells treated with different doses of BDMC.
Furthermore, based on this observation that BDMC inhibited β-catenin translocation to the nucleus, we analyzed the expression β-catenin downstream target genes, c-myc and Snail. As expected [Figure 3b], their expressions were also reduced by BDMC in a dose-dependent manner. In contrast, protein expression analysis of WIF-1 (inhibitor of Wnt/β-catenin signaling pathway), revealed that BDMC enhanced its expression in a dose-dependent manner.
Wnt inhibitory factor-1 overexpression inhibits migration, invasion, and epithelial-to-mesenchymal transition
To further understand the contribution of WIF-1 in NSCLC cells migration and invasion, we first tested its mRNA (RT-PCR) and protein (western blot) expression in 95C and 95D cells with different invasive capacities [Figure 4a and 4b]. The 95C cells (less invasive) showed high expression at both mRNA and protein levels as compared to 95D cells (highly invasive). These data suggested that WIF-1 expression might associate negatively with the invasive property of NSCLC cells. Next, to confirm this possibility, we overexpressed WIF-1 in 95D cells by transfecting with pIRES2-ZsGreen-WIF-1 plasmid. The control cells were transfected with pIRES2-ZsGreen vector. The overexpression of WIF-1 was evaluated again by RT-PCR and western blot analysis as shown in Figure 4b. Further, when we analyzed overexpressing WIF-1 in migration and invasion of 95D cells, it was observed that the wound width of the 95D cells transfected with pIRES2-ZsGreen-WIF-1 was 2.7-fold wider compared with 95D cells transfected with vector group (Figure 4c, t=3.191, P < 0.05). Figure 4d showed the number of cells invading through the Matrigel in the WIF-1 overexpression group was significantly decreased (50.0 ± 14.0 cells/field) compared to the vector control (128.4 ± 10.0 cells/field). The invasion ability was decreased by 54% by WIF expression. And the result suggested that these cells had a significantly reduced migration and invasion through Matrigel in 95D cells transfected with WIF-1.
Figure 4.
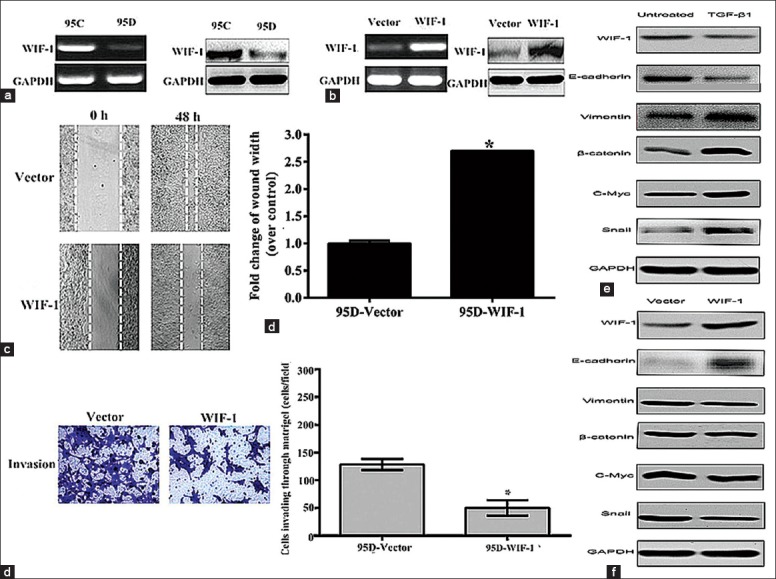
Wnt inhibitory factor-1 (WIF-1) overexpression inhibited cell migration and invasion, epithelial-to-mesenchymal transition. (a) Analysis of WIF-1 expression levels in 95C and 95D cells by reverse transcription-polymerase chain reaction (RT-PCR) (left) and western blot (right); (b) The analysis of overexpressed WIF-1 in 95D cells by RT-PCR (left) and by western blot (right); (c) Wound healing assay represented the migration of 95D cells transfected with pIRES2-ZsGreen-WIF-1 or pIRES2-ZsGreen vector (original magnification × 100); (d) Transwell invasion assay with 95D cells transfected with pIRES2-ZsGreen-WIF-1 or pIRES2-ZsGreen vector. Results were representative of three independent experiments. *P < 0.05 (t=3.819), versus controls; (e) Western blot data of nuclear β-catenin, WIF-1, E-cadherin, vimentin, c-myc, Snail from 95D cells treated with transforming growth factor-β1. GAPDH was a loading control; (f) Western blot data showed the expression of WIF-1, c-myc and Snail proteins in 95D cells transfected with pIRES2-ZsGreen-WIF-1 or pIRES2-ZsGreen vector (original magnification × 200).
In addition, we also evaluated the contribution of WIF-1 in TGF-β1-induced EMT. As shown in Figure 4e, TGF-β1 treatment itself inhibited the expression of WIF-1 and E-cadherin but upregulated the expression of vimentin, β-catenin, Wnt target gene c-myc, and the transcriptional factor Snail in 95D cells. However, overexpression of WIF-1 in these cells reversed these expression profiles [Figure 4f]. Thus, these results suggested that Wnt signaling might be involved in the inhibitory effects of WIF-1 on the TGF-β1-induced EMT.
Knockdown of Wnt inhibitory factor-1 by Wnt inhibitory factor-1 small interfering-RNA rescued 95D cells from suppressing transforming growth factor-β1-induced epithelial-to-mesenchymal transition by bisdemethoxycurcumin
Finally, to identify if WIF-1 contributes in BDMC regulation of TGF-β1-induced EMT, we first ablated WIF-1 by its specific siRNA. The 95D cells were transfected with either NC or WIF-1 siRNAs. WIF-1 siRNA significantly reduced the protein expression of WIF-1 compared to NC-siRNA group [Figure 5a]. Next, when we treated 95D cells with TGF-β1 and BDMC in a control and WIF-1 knockdown conditions, we observed that ablation of WIF-1 reversed the BDMC inhibitory effect on TGF-β1 induced EMT. The BDMC treatment cannot reverse the TGF-β1-induced changes in expression of E-cadherin, vimentin, fibronectin, and Snail proteins in WIF-1 ablated condition (Lane 4), whereas it did rescue the expression in lane 3 with control knockdown [Figure 5b]. These effects were more evident on E-cadherin and vimentin and fibronectin. Thus, these data indicated that BDMC effects on suppression of TGF-β1 induced EMT involve WIF-1 protein.
Figure 5.
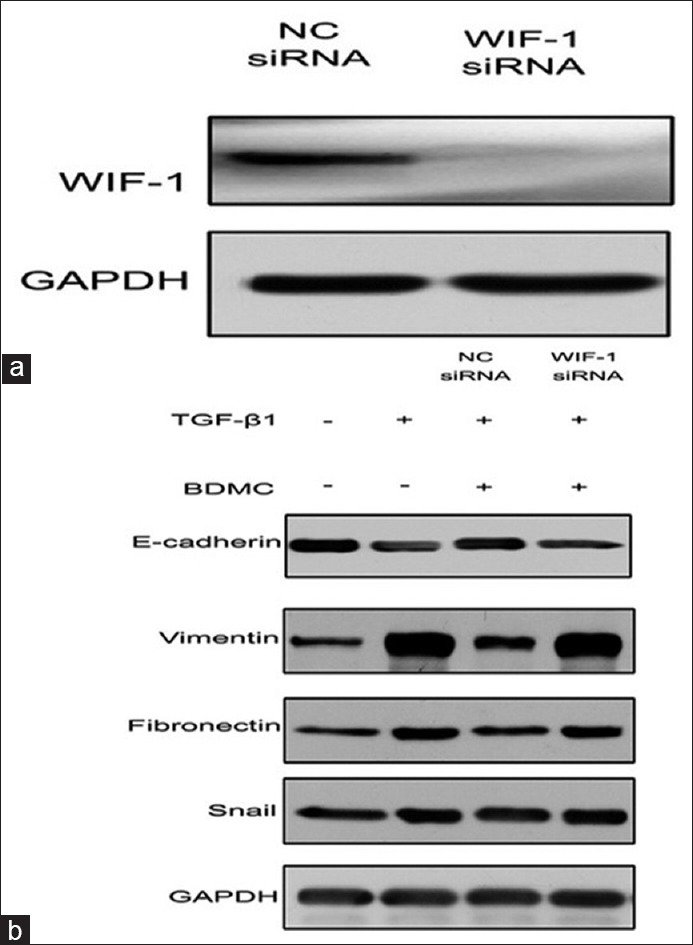
Wnt inhibitory factor-1 (WIF-1) ablation inhibited bisdemethoxycurcumin (BDMC) effect on transforming growth factor-β1 (TGF-β1)-induced epithelial-to-mesenchymal transition (EMT). (a) WIF-1 small interfering-RNA (siRNA) significantly inhibited its protein expression in 95D cells; (b) The 95D cells with no siRNA, control siRNA, and WIF-1 siRNA were treated with TGF-β1 and BDMC and analyzed for EMT marker proteins, E-cadherin, vimentin, fibronectin, and Snail. GAPDH was a loading control.
DISCUSSION
Despite curcumin being a promising chemotherapeutic agent with low toxicity, its actual mechanism of action is still not very clear. It consists of three forms including 77% pure curcumin (Cur), 17% DMC, and 3% BDMC.[12] BDMC was considered to be the most stable among three forms. Growing evidences indicate that curcumin can inhibit EMT in pancreatic cancer cells,[13] breast cancer,[14] and renal tubular epithelial cells[15] through multiple pathways, but exact mechanism is not very clear. Therefore, we focused our attention on studying the effect of BDMC on EMT pathway in NSCLC cells. It is well-known that EMT plays an important role in tumor invasion and metastasis. In this study, we used TGF-β1 induced EMT model in 95D cells to investigate the potential mechanism of anti-metastatic activity of commercially available BDMC (the purity was over 98%) on lung cancer.
Our data revealed that 95D cells treated with TGF-β1 underwent an EMT change in vitro. They displayed mesenchymal phenotype and expressed specific EMT markers. BDMC treatment inhibited this EMT phenotype. After reviewing the different molecular pathways that can regulate EMT, we focused our attention on Wnt/β-catenin pathway. The Wnt/β-catenin pathway, one of the major signaling pathways involved in EMT, can activate the EMT, which is linked to invasion and metastasis in tumor cells.[16] Our data indicated that BDMC inhibited the translocation of β-catenin to nucleus and also resulted in reduced expression of transcription factor, Snail. In cancer cells, Wnt/β-catenin pathway contributes to EMT by activating Snail, which in turn suppresses epithelial markers expression like E-cadherin.[17] Simultaneously, Snail also helps in crosstalk between β-catenin and TGF-β pathway.[18] Therefore, in 95D cells, TGF-β1-induced-EMT resulted in increased β-catenin nuclear translocation and higher Snail expression. When these cells were treated with BDMC, it reduced the translocation of β-catenin followed by reduced Snail protein, thus reversing the phenotype. In contrast, we also observed the inhibition of the WIF-1 protein expression by TGF-β treatment and its upregulation by BDMC treatment in a dose-dependent manner. Therefore, when trying to determine the possible mechanism of Wnt/β-catenin pathway inhibition by BDMC, we found that analysis of WIF-1 was the most plausible reason as it is reported to be a negative regulator of Wnt pathway. Overexpression of WIF-1 reversed TGF-β1 mediated effect on invasion, migration and EMT, whereas its ablation by SiRNA attenuated BDMC effects. Similarly, other group has also demonstrated that WIF-1 reduce tumor growth, cell migration and invasion, and a reversal of EMT in prostate cancer cells.[19] Furthermore, WIF-1 re-expression decreased tumorigenesis and metastasis in osteosarcoma.[20] Therefore, our finding along with published literatures suggested that WIF-1 plays a critical role in the invasive and/or metastatic potential of cancer cells. Our results also suggested that BDMC inhibits the invasive and/or metastatic potential of 95D cells, probably by directly regulating the WIF-1 gene expression.
In conclusion, our findings suggested that BDMC inhibits Wnt/β-catenin pathway by increasing WIF-1 expression, which subsequently leads to suppression of TGF-β1-induced EMT, migration, and invasion of 95D cells. In this study, we also showed the effect of WIF-1 on TGF-β1-induced EMT in NSCLC cells. In addition, this study helped us to explain the tentative molecular mechanisms of action for BDMC and its anti-cancer role in NSCLC.
Footnotes
Edited by: Xin Chen
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Dong P, Karaayvaz M, Jia N, Kaneuchi M, Hamada J, Watari H, et al. Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis. Oncogene. 2013;32:3286–95. doi: 10.1038/onc.2012.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Räsänen K, Vaheri A. TGF-beta1 causes epithelial-mesenchymal transition in HaCaT derivatives, but induces expression of COX-2 and migration only in benign, not in malignant keratinocytes. J Dermatol Sci. 2010;58:97–104. doi: 10.1016/j.jdermsci.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Cho HJ, Baek KE, Saika S, Jeong MJ, Yoo J. Snail is required for transforming growth factor-beta-induced epithelial-mesenchymal transition by activating PI3 kinase/Akt signal pathway. Biochem Biophys Res Commun. 2007;353:337–43. doi: 10.1016/j.bbrc.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 6.Wissmann C, Wild PJ, Kaiser S, Roepcke S, Stoehr R, Woenckhaus M, et al. WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol. 2003;201:204–12. doi: 10.1002/path.1449. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valenta T, Hausmann G, Basler K. The many faces and functions of ß-catenin. EMBO J. 2012;31:2714–36. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JS, Hur MW, Lee SK, Choi WI, Kwon YG, Yun CO. A novel sLRP6E1E2 inhibits canonical Wnt signaling, epithelial-to-mesenchymal transition, and induces mitochondria-dependent apoptosis in lung cancer. PLoS One. 2012;7:e36520. doi: 10.1371/journal.pone.0036520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen HW, Lee JY, Huang JY, Wang CC, Chen WJ, Su SF, et al. Curcumin inhibits lung cancer cell invasion and metastasis through the tumor suppressor HLJ1. Cancer Res. 2008;68:7428–38. doi: 10.1158/0008-5472.CAN-07-6734. [DOI] [PubMed] [Google Scholar]
- 11.Uygun K, Bilici A, Kaya S, Oven Ustaalioglu BB, Yildiz R, Temiz S, et al. XELIRI plus bevacizumab compared with FOLFIRI plus bevacizumab as first-line setting in patients with metastatic colorectal cancer: Experiences at two-institutions. Asian Pac J Cancer Prev. 2013;14:2283–8. doi: 10.7314/apjcp.2013.14.4.2283. [DOI] [PubMed] [Google Scholar]
- 12.Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–73. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 13.Sun XD, Liu XE, Huang DS. Curcumin reverses the epithelial-mesenchymal transition of pancreatic cancer cells by inhibiting the Hedgehog signaling pathway. Oncol Rep. 2013;29:2401–7. doi: 10.3892/or.2013.2385. [DOI] [PubMed] [Google Scholar]
- 14.Huang T, Chen Z, Fang L. Curcumin inhibits LPS-induced EMT through downregulation of NF-κB-Snail signaling in breast cancer cells. Oncol Rep. 2013;29:117–24. doi: 10.3892/or.2012.2080. [DOI] [PubMed] [Google Scholar]
- 15.Li R, Wang Y, Liu Y, Chen Q, Fu W, Wang H, et al. Curcumin inhibits transforming growth factor-ß1-induced EMT via PPARγ pathway, not Smad pathway in renal tubular epithelial cells. PLoS One. 2013;8:e58848. doi: 10.1371/journal.pone.0058848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SY, Jeon HM, Ju MK, Kim CH, Yoon G, Han SI, et al. Wnt/Snail signaling regulates cytochrome C oxidase and glucose metabolism. Cancer Res. 2012;72:3607–17. doi: 10.1158/0008-5472.CAN-12-0006. [DOI] [PubMed] [Google Scholar]
- 17.Stemmer V, de Craene B, Berx G, Behrens J. Snail promotes Wnt target gene expression and interacts with beta-catenin. Oncogene. 2008;27:5075–80. doi: 10.1038/onc.2008.140. [DOI] [PubMed] [Google Scholar]
- 18.Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell. 2008;19:4875–87. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yee DS, Tang Y, Li X, Liu Z, Guo Y, Ghaffar S, et al. The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition. Mol Cancer. 2010;9:162. doi: 10.1186/1476-4598-9-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin EM, Guo Y, Tu K, Xie J, Zi X, Hoang BH. Wnt inhibitory factor 1 decreases tumorigenesis and metastasis in osteosarcoma. Mol Cancer Ther. 2010;9:731–41. doi: 10.1158/1535-7163.MCT-09-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]


