Abstract
Objective:
The objective was to evaluate the protective effects of dexmedetomidine (DEX), a selective agonist of α2-adrenergic receptor, on sepsis-induced diaphragm injury and the underlying molecular mechanisms.
Data Sources:
The data used in this review were mainly from PubMed articles published in English from 1990 to 2015.
Study Selection:
Clinical or basic research articles were selected mainly according to their level of relevance to this topic.
Results:
Sepsis could induce severe diaphragm dysfunction and exacerbate respiratory weakness. The mechanism of sepsis-induced diaphragm injury includes the increased inflammatory cytokines and excessive oxidative stress and superfluous production of nitric oxide (NO). DEX can reduce inflammatory cytokines, inhibit nuclear factor-kappaB signaling pathways, suppress the activation of caspase-3, furthermore decrease oxidative stress and inhibit NO synthase. On the basis of these mechanisms, DEX may result in a shorter period of mechanical ventilation in septic patients in clinical practice.
Conclusions:
Based on this current available evidence, DEX may produce extra protective effects on sepsis-induced diaphragm injury. Further direct evidence and more specific studies are still required to confirm these beneficial effects.
Keywords: Dexmedetomidine, Diaphragm, Drug Effects, Sepsis
INTRODUCTION
Sepsis is a systemic inflammatory response to infection, with high morbidity and mortality in a clinical setup.[1] Patients with sepsis are hyper-stressed and often require drugs for sedation and analgesia to reduce anxiety and stress. Severe sepsis is also frequently associated with acute lung injury, which necessitates mechanical ventilation support.[2,3] Hence, a sedative agent is required in patients with sepsis.
Dexmedetomidine (DEX), a selective agonist of α2-adrenergic receptor, is widely used in intensive care units due to its sedative and analgesic effects. Based on available literature search, The Canadian Agency for Drugs and Technologies in Health stated that DEX was associated with a shorter period of mechanical ventilation and lower intensive care unit stay when compared with traditional sedative agents such as midazolam and propofol.[4] The latest meta-analysis done in 2014 covering 1624 participants found that DEX also could reduce breathing support time by approximately one-fifth and consequently the length of stay time in intensive care units by one-seventh in patients requiring long-term sedation (more than 24 h) under mechanical ventilation.[5] This can be explained by the anti-inflammatory, organ-protective, and anti-sympathetic properties of DEX.[5,6]
In septic animals, DEX was effective in suppressing the inflammatory response and thus decreasing mortality rates.[7,8,9,10,11] Similarly, in a clinical setup, DEX infusion decreased inflammatory cytokine production significantly compared with midazolam[12] and propofol[13] in septic patients. Considering these anti-inflammatory effects, DEX can improve 28-day survival rates compared with lorazepam in patients with sepsis.[6]
Sepsis can induce severe diaphragm dysfunction[14,15] and exacerbate respiratory weakness.[16] A recent study indicates that during sepsis, diaphragm is much more affected as compared with limb muscle.[17] Sepsis involves a network of over-expressed inflammatory cytokines which have been proved to be associated with diaphragm dysfunction.[18,19] Due to its significant anti-inflammatory and organ-protective properties, we think DEX may produce extra protective effects on sepsis-induced diaphragm injury when used in septic patients for sedation and analgesia. In this review, we summarize the potential molecular mechanisms.
MOLECULAR MECHANISMS OF SEPSIS-INDUCED DIAPHRAGM INJURY
In animals, sepsis can induce an increase in plasma inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α).[20,21] Not only in plasma, sepsis also can increase inflammatory cytokines in muscles,[22,23] and muscle cells apparently constitute an important source of cytokines during sepsis.[23] Similarly, in human volunteers, skeletal muscle is capable of producing essential inflammatory mediators after endotoxin stimulation.[24] Hence, sepsis can induce comprehensive inflammatory response.
In dogs, TNF-α infusion is associated with a significant decline in isotonic and quasi-isometric diaphragm contraction.[25] In rats, after endotoxin injection, gene expression and production of TNF-α are elevated in the diaphragm tissue, and anti-TNF-α antibody preinjection can prevent the deterioration of the diaphragm muscle contractile properties.[18] Hence, TNF-α is an important mediator of sepsis-related impairment in diaphragm contractility. Possible mechanisms include: Activating caspase-8 and -3 pathway,[26] increasing oxidant activity in muscle fibers,[27,28] and activating nuclear factor-kappaB (NF-κB) signaling pathways.[29] Moreover, plasma from patients with septic shock can induce in vitro loss of myosin in skeletal myotubes, which is significantly associated with elevated plasma levels of IL-6 in septic shock patients.[19] With elevated serum IL-6 concentration, the muscle of IL-6 transgenic mice suffers from atrophy, which can be completely blocked by treatment with the anti-mouse IL-6 receptor antibody.[30] Lipopolysaccharide (LPS) induced sarcolemmal damage in diaphragm myofibers is accompanied by a significant increase in IL-1beta expression in the tissues.[31] IL-1alpha and -1beta can reduce myofibrillar protein in differentiated myotubes,[32] and IL-1 receptor antagonist can maintain the synthesis of both myofibrillar and sarcoplasmic proteins during sepsis.[33] One possible mechanism of IL-6 and -1 in inducing diaphragmatic contractile dysfunction is to stimulate NF-κB signaling and increase expression of atrogin1/muscle atrophy F-box and muscle RING-finger protein-1.[19,32] Hence, sepsis induces increase in inflammatory cytokines such as TNF-α, IL-6 and -1, which can lead to diaphragmatic force loss and atrophy.
Besides, sepsis can enhance oxidative stress in the skeletal muscle,[34,35] with diaphragm being no exception.[36] Sepsis-induced oxidative stress in the diaphragm can induce protein carbonyl formation,[37] impair mitochondrial respiratory functioning,[38,39] thus leading to diaphragmatic contractile dysfunction. Sepsis also can increase the activity of inducible nitric oxide synthase (iNOS),[40,41] which increases the amount of NO in diaphragm. NO can inhibit antioxidant enzyme activity[42] and increase oxidative stress.[43] NO also can produce excessive peroxynitrite and stimulate protein nitration formation in the mitochondrial matrix,[44,45] resulting into diaphragmatic mitochondrial dysfunction and contractile failure. Hence, sepsis induces excessive oxidative stress and superfluous production of NO which leads to diaphragmatic contractile dysfunction.
MOLECULAR MECHANISMS OF DEXMEDETOMIDINE IN DECREASING SEPSIS-INDUCED DIAPHRAGM INJURY
Dexmedetomidine can inhibit the increase of serum levels of TNF-α, IL-6 in endotoxemic rats[10,11] and in cecal ligation and puncture (CLP)-induced septic rats[7] and mice.[8] DEX also can attenuate the LPS-induced increase of inflammatory factor IL-1beta[9] and -8.[46] This suppressing effect of DEX on inflammatory mediators during sepsis is proved to be dose-dependent,[11] and the clinical using dose of DEX is proved to have the suppressing effect of LPS-induced inflammatory mediator production.[46] In vitro, DEX takes effects via α2-adrenergic receptors.[46] In vivo, it functions through its sympatholytic effect that leads to the activation of the vagus nerve, which then stimulates cholinergic anti-inflammatory pathway.[9] Hence, DEX may attenuate diaphragm atrophy and weakness in sepsis due to the inhibition of inflammatory cytokines by its anti-inflammatory effects.
In septic rats, DEX can significantly suppress CLP-induced NF-κB activation in lung tissue.[7] Moreover, after LPS stimulation, DEX also has an inhibitory effect on NF-κB activation in human whole blood.[46] Furthermore, in LPS-activated macrophages, DEX inhibits the translocation of NF-κB from the cytoplasm to the nucleus at clinically relevant dosages via α2-adrenergic receptors.[47] As inflammatory mediators can induce diaphragm atrophy by activating NF-κB signaling pathways,[19,29,32] DEX may produce protective effects in diaphragm muscle by inhibiting them.
In primary diaphragm muscle cell cultures, pharmacologic blockade of the NF-κB pathway and dominant-negative molecular inhibition of IκB kinase-beta strongly suppresses LPS-induced pro-inflammatory gene expression.[48] Moreover, in muscle-specific IκB-alpha super-repressor mice subjected to endotoxemia, the increase of pro-inflammatory cytokines in the diaphragm can be prevented.[49] These two experiments indicate that NF-κB signaling within skeletal muscle fibers is a key pathway leading to diaphragmatic weakness during sepsis, most likely via effects on multiple inflammatory mediators.[48,49] As inflammatory mediators can activate NF-κB signaling pathways,[19,29,32] inflammatory mediators and NF-κB can stimulate each other reciprocally. DEX may be able to break this vicious cycle by both suppressing inflammatory mediators and NF-κB signaling pathways.
Caspase-3 can break down actomyosin complexes, yielding proteins that are degraded by the ATP-ubiquitin-proteasome system. So in catabolic conditions, activation of caspase-3 is an initial step triggering accelerated muscle proteolysis.[50] Endotoxin administration can elicit significant diaphragm caspase-3 activation and caspase-mediated diaphragmatic weakness.[51] DEX can inhibit caspase-3 expression, attenuating isoflurane-induced injury in developing brain[52] or ischemia/reperfusion injury in the retina.[53] Thus, by inhibiting the activation of caspase-3, DEX may produce protective effects in diaphragm muscle during sepsis. In addition, both endotoxin and inflammatory cytokines can first activate p38[54] and Jun N-terminal kinase (JNK)[55] pathway, then triggering caspase-8 and -3 pathway[26] to induce diaphragm weakness. Luckily, DEX pretreatment can inhibit isoflurane-induced neuroapoptosis by inhibiting p38 and JNK pathways.[56] So by suppressing the upstream p38 and JNK pathways, DEX may further protect diaphragm muscle against sepsis-induced proteolysis.
It is proved that both antioxidant and NOS inhibitor can ameliorate diaphragmatic dysfunction.[57,58] Moreover, in acid-induced acute lung injury, preemptive use of DEX produces important protective effects on the liver against oxidative stress.[59] In pneumoperitoneum-related ischemia-reperfusion injury, DEX is proved to be effective in decreasing systematic[60] and local[61] oxidative stress. DEX can also markedly reduce the oxidative stress in skeletal muscle due to ischemia-reperfusion injury and exhibit more potent antioxidant activity than vitamin E.[62] Furthermore, short-term use of DEX can reduce iNOS in LPS-induced acute lung injury compared to thiopental sodium.[63] DEX can attenuate LPS-induced iNOS and NO accumulation in primary microglia[64,65] by inhibiting extracellular signal-regulated kinase activation.[65] Hence, DEX may preserve muscular force in sepsis by inhibiting excessive oxidative stress and the superfluous production of NO.
In clinical practice, septic patients treated with DEX have shorter time on the ventilator as compared with those treated with lorazepam, a benzodiazepine and this beneficial effect of DEX is more pronounced in septic patients than in nonseptic patients.[6] This outcome may be partly the result of DEX-induced reduction in pulmonary inflammatory mediators and lung tissue damage.[7] However, based on the molecular mechanisms discussed above, this outcome may be also the result of DEX-induced extra protective effects on sepsis-induced diaphragm injury.
In conclusion, when used in patients with sepsis for sedation and analgesia, DEX may produce protective effects on diaphragm against sepsis-induced injury. The mechanisms include reducing inflammatory cytokines, inhibiting NF-κB signaling pathways, suppressing the activation of caspase-3, decreasing oxidative stress, and inhibiting iNOS [Figure 1]. As most of the mechanisms come from the studies of the protective effects of DEX on other organs in sepsis or other organ injury models, further direct evidence is still needed to confirm these beneficial effects.
Figure 1.
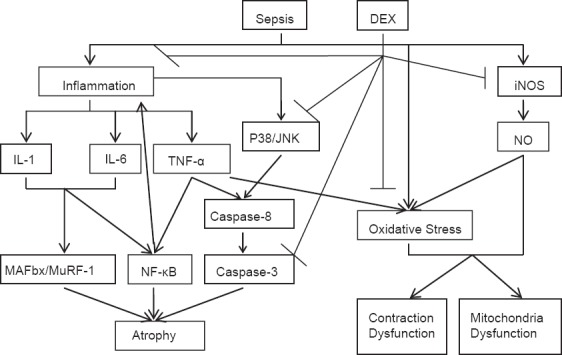
DEX: Dexmedetomidine; IL-1: Interleukin-1; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-alpha; JNK: Jun N-terminal kinase; MAFbx: Muscle atrophy F-box; MuRF-1: Muscle RING-finger protein-1; iNOS: Inducible nitric oxide synthase; NO: Nitric oxide; ↓activation; ⊥inhibition.
Footnotes
Edited by: Jian Gao and Yuan-Yuan Ji
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine 1992. Chest. 2009;136:e28. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Azevedo LC, Park M, Salluh JI, Rea-Neto A, Souza-Dantas VC, Varaschin P, et al. Clinical outcomes of patients requiring ventilatory support in Brazilian intensive care units: A multicenter, prospective, cohort study. Crit Care. 2013;17:R63. doi: 10.1186/cc12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schädler D, Elke G, Engel C, Bogatsch H, Frerichs I, Kuhlen R, et al. Ventilatory strategies in septic patients. Results from a nationwide observational trial. Anaesthesist. 2013;62:27–33. doi: 10.1007/s00101-012-2121-2. [DOI] [PubMed] [Google Scholar]
- 4.CADTH Rapid Response Reports. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2014. Canadian Agency for Drugs and Technologies in Health. Dexmedetomidine for sedation of Patients in the ICU or PICU: Review of Clinical Effectiveness and Safety. [PubMed] [Google Scholar]
- 5.Chen K, Lu Z, Xin YC, Cai Y, Chen Y, Pan SM. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev. 2015;1:CD010269. doi: 10.1002/14651858.CD010269.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandharipande PP, Sanders RD, Girard TD, McGrane S, Thompson JL, Shintani AK, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14:R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Liu Y, Huang H, Zhu Y, Zhang Y, Lu F, et al. Dexmedetomidine inhibits inflammatory reaction in lung tissues of septic rats by suppressing TLR4/NF-κB pathway. Mediators Inflamm 2013. 2013:562154. doi: 10.1155/2013/562154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L, Bao H, Si Y, Wang X. Effects of dexmedetomidine on early and late cytokines during polymicrobial sepsis in mice. Inflamm Res. 2013;62:507–14. doi: 10.1007/s00011-013-0604-5. [DOI] [PubMed] [Google Scholar]
- 9.Xiang H, Hu B, Li Z, Li J. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation. 2014;37:1763–70. doi: 10.1007/s10753-014-9906-1. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004;32:1322–6. doi: 10.1097/01.ccm.0000128579.84228.2a. [DOI] [PubMed] [Google Scholar]
- 11.Taniguchi T, Kurita A, Kobayashi K, Yamamoto K, Inaba H. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J Anesth. 2008;22:221–8. doi: 10.1007/s00540-008-0611-9. [DOI] [PubMed] [Google Scholar]
- 12.Memis D, Hekimoglu S, Vatan I, Yandim T, Yüksel M, Süt N. Effects of midazolam and dexmedetomidine on inflammatory responses and gastric intramucosal pH to sepsis, in critically ill patients. Br J Anaesth. 2007;98:550–2. doi: 10.1093/bja/aem017. [DOI] [PubMed] [Google Scholar]
- 13.Tasdogan M, Memis D, Sut N, Yuksel M. Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. J Clin Anesth. 2009;21:394–400. doi: 10.1016/j.jclinane.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Supinski GS, Wang W, Callahan LA. Caspase and calpain activation both contribute to sepsis-induced diaphragmatic weakness. J Appl Physiol (1985) 2009;107:1389–96. doi: 10.1152/japplphysiol.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Supinski GS, Callahan LA. Calpain activation contributes to endotoxin-induced diaphragmatic dysfunction. Am J Respir Cell Mol Biol. 2010;42:80–7. doi: 10.1165/rcmb.2008-0275OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Jonghe B, Bastuji-Garin S, Durand MC, Malissin I, Rodrigues P, Cerf C, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35:2007–15. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 17.Jung B, Nougaret S, Conseil M, Coisel Y, Futier E, Chanques G, et al. Sepsis is associated with a preferential diaphragmatic atrophy: A critically ill patient study using tridimensional computed tomography. Anesthesiology. 2014;120:1182–91. doi: 10.1097/ALN.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 18.Shindoh C, Hida W, Ohkawara Y, Yamauchi K, Ohno I, Takishima T, et al. TNF-alpha mRNA expression in diaphragm muscle after endotoxin administration. Am J Respir Crit Care Med. 1995;152:1690–6. doi: 10.1164/ajrccm.152.5.7582314. [DOI] [PubMed] [Google Scholar]
- 19.van Hees HW, Schellekens WJ, Linkels M, Leenders F, Zoll J, Donders R, et al. Plasma from septic shock patients induces loss of muscle protein. Crit Care. 2011;15:R233. doi: 10.1186/cc10475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Léon K, Moisan C, Amérand A, Poupon G, L’Her E. Effect of induced mild hypothermia on two pro-inflammatory cytokines and oxidative parameters during experimental acute sepsis. Redox Rep. 2013;18:120–6. doi: 10.1179/1351000213Y.0000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebdrup L, Krog J, Granfeldt A, Larsen PØ, Vestergaard C, Hokland M, et al. Leukocyte, plasma, and organ-associated cytokine profiles in an animal model of acute inflammation. APMIS. 2008;116:352–60. doi: 10.1111/j.1600-0463.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 22.Crossland H, Constantin-Teodosiu D, Gardiner SM, Constantin D, Greenhaff PL. A potential role for Akt/FOXO signalling in both protein loss and the impairment of muscle carbohydrate oxidation during sepsis in rodent skeletal muscle. J Physiol. 2008;586:5589–600. doi: 10.1113/jphysiol.2008.160150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borge BA, Kalland KH, Olsen S, Bletsa A, Berggreen E, Wiig H. Cytokines are produced locally by myocytes in rat skeletal muscle during endotoxemia. Am J Physiol Heart Circ Physiol. 2009;296:H735–44. doi: 10.1152/ajpheart.01309.2008. [DOI] [PubMed] [Google Scholar]
- 24.Krogh-Madsen R, Plomgaard P, Akerstrom T, Møller K, Schmitz O, Pedersen BK. Effect of short-term intralipid infusion on the immune response during low-dose endotoxemia in humans. Am J Physiol Endocrinol Metab. 2008;294:E371–9. doi: 10.1152/ajpendo.00507.2007. [DOI] [PubMed] [Google Scholar]
- 25.Wilcox PG, Wakai Y, Walley KR, Cooper DJ, Road J. Tumor necrosis factor alpha decreases in vivo diaphragm contractility in dogs. Am J Respir Crit Care Med. 1994;150:1368–73. doi: 10.1164/ajrccm.150.5.7952566. [DOI] [PubMed] [Google Scholar]
- 26.Supinski GS, Ji X, Wang W, Callahan LA. The extrinsic caspase pathway modulates endotoxin-induced diaphragm contractile dysfunction. J Appl Physiol (1985) 2007;102:1649–57. doi: 10.1152/japplphysiol.00377.2006. [DOI] [PubMed] [Google Scholar]
- 27.Hardin BJ, Campbell KS, Smith JD, Arbogast S, Smith J, Moylan JS, et al. TNF-alpha acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol (1985) 2008;104:694–9. doi: 10.1152/japplphysiol.00898.2007. [DOI] [PubMed] [Google Scholar]
- 28.Danjo W, Fujimura N, Ujike Y. Effect of pentoxifylline on diaphragmatic contractility in septic rats. Acta Med Okayama. 2008;62:101–7. doi: 10.18926/AMO/30964. [DOI] [PubMed] [Google Scholar]
- 29.Bhatnagar S, Panguluri SK, Gupta SK, Dahiya S, Lundy RF, Kumar A. Tumor necrosis factor – α regulates distinct molecular pathways and gene networks in cultured skeletal muscle cells. PLoS One. 2010;5:e13262. doi: 10.1371/journal.pone.0013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsujinaka T, Fujita J, Ebisui C, Yano M, Kominami E, Suzuki K, et al. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Invest. 1996;97:244–9. doi: 10.1172/JCI118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin MC, Leung SY, Fang WF, Chin CH, Chung KF. Down-regulation of insulin-like growth factor I (IGF-I) in the mouse diaphragm during sepsis. Chang Gung Med J. 2010;33:501–8. [PubMed] [Google Scholar]
- 32.Li W, Moylan JS, Chambers MA, Smith J, Reid MB. Interleukin-1 stimulates catabolism in C2C12 myotubes. Am J Physiol Cell Physiol. 2009;297:C706–14. doi: 10.1152/ajpcell.00626.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vary TC, Owens EL, Beers JK, Verner K, Cooney RN. Sepsis inhibits synthesis of myofibrillar and sarcoplasmic proteins: Modulation by interleukin-1 receptor antagonist. Shock. 1996;6:13–8. doi: 10.1097/00024382-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Peralta JG, Llesuy S, Evelson P, Carreras MC, Flecha BG, Poderoso JJ. Oxidative stress in skeletal muscle during sepsis in rats. Circ Shock. 1993;39:153–9. [PubMed] [Google Scholar]
- 35.Llesuy S, Evelson P, González-Flecha B, Peralta J, Carreras MC, Poderoso JJ, et al. Oxidative stress in muscle and liver of rats with septic syndrome. Free Radic Biol Med. 1994;16:445–51. doi: 10.1016/0891-5849(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 36.Nethery D, DiMarco A, Stofan D, Supinski G. Sepsis increases contraction-related generation of reactive oxygen species in the diaphragm. J Appl Physiol (1985) 1999;87:1279–86. doi: 10.1152/jappl.1999.87.4.1279. [DOI] [PubMed] [Google Scholar]
- 37.Barreiro E, Gea J, Di Falco M, Kriazhev L, James S, Hussain SN. Protein carbonyl formation in the diaphragm. Am J Respir Cell Mol Biol. 2005;32:9–17. doi: 10.1165/rcmb.2004-0021OC. [DOI] [PubMed] [Google Scholar]
- 38.Peruchi BB, Petronilho F, Rojas HA, Constantino L, Mina F, Vuolo F, et al. Skeletal muscle electron transport chain dysfunction after sepsis in rats. J Surg Res. 2011;167:e333–8. doi: 10.1016/j.jss.2010.11.893. [DOI] [PubMed] [Google Scholar]
- 39.Vanasco V, Cimolai MC, Evelson P, Alvarez S. The oxidative stress and the mitochondrial dysfunction caused by endotoxemia are prevented by alpha-lipoic acid. Free Radic Res. 2008;42:815–23. doi: 10.1080/10715760802438709. [DOI] [PubMed] [Google Scholar]
- 40.Boczkowski J, Lanone S, Ungureanu-Longrois D, Danialou G, Fournier T, Aubier M. Induction of diaphragmatic nitric oxide synthase after endotoxin administration in rats: Role on diaphragmatic contractile dysfunction. J Clin Invest. 1996;98:1550–9. doi: 10.1172/JCI118948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambe A, Ungureanu-Longrois D, Danialou G, Lanone S, Benessiano J, Aubier M, et al. Role of nitric oxide on diaphragmatic contractile failure in Escherichia coli endotoxemic rats. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:167–75. doi: 10.1016/s1095-6433(97)00422-4. [DOI] [PubMed] [Google Scholar]
- 42.Lawler JM, Song W. Specificity of antioxidant enzyme inhibition in skeletal muscle to reactive nitrogen species donors. Biochem Biophys Res Commun. 2002;294:1093–100. doi: 10.1016/S0006-291X(02)00602-2. [DOI] [PubMed] [Google Scholar]
- 43.Arbogast S, Reid MB. Oxidant activity in skeletal muscle fibers is influenced by temperature, CO2 level, and muscle-derived nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2004;287:R698–705. doi: 10.1152/ajpregu.00072.2004. [DOI] [PubMed] [Google Scholar]
- 44.Boczkowski J, Lisdero CL, Lanone S, Samb A, Carreras MC, Boveris A, et al. Endogenous peroxynitrite mediates mitochondrial dysfunction in rat diaphragm during endotoxemia. FASEB J. 1999;13:1637–46. doi: 10.1096/fasebj.13.12.1637. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez S, Boveris A. Mitochondrial nitric oxide metabolism in rat muscle during endotoxemia. Free Radic Biol Med. 2004;37:1472–8. doi: 10.1016/j.freeradbiomed.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 46.Kawasaki T, Kawasaki C, Ueki M, Hamada K, Habe K, Sata T. Dexmedetomidine suppresses proinflammatory mediator production in human whole blood in vitro. J Trauma Acute Care Surg. 2013;74:1370–5. doi: 10.1097/TA.0b013e31828db978. [DOI] [PubMed] [Google Scholar]
- 47.Chang Y, Huang X, Liu Z, Han G, Huang L, Xiong YC, et al. Dexmedetomidine inhibits the secretion of high mobility group box 1 from lipopolysaccharide-activated macrophages in vitro. J Surg Res. 2013;181:308–14. doi: 10.1016/j.jss.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 48.Demoule A, Divangahi M, Yahiaoui L, Danialou G, Gvozdic D, Labbe K, et al. Endotoxin triggers nuclear factor-kappaB-dependent up-regulation of multiple proinflammatory genes in the diaphragm. Am J Respir Crit Care Med. 2006;174:646–53. doi: 10.1164/rccm.200509-1511OC. [DOI] [PubMed] [Google Scholar]
- 49.Okazaki T, Liang F, Li T, Lemaire C, Danialou G, Shoelson SE, et al. Muscle-specific inhibition of the classical nuclear factor-κB pathway is protective against diaphragmatic weakness in murine endotoxemia. Crit Care Med. 2014;42:e501–9. doi: 10.1097/CCM.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 50.Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113:115–23. doi: 10.1172/JCI200418330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Supinski GS, Callahan LA. Caspase activation contributes to endotoxin-induced diaphragm weakness. J Appl Physiol (1985) 2006;100:1770–7. doi: 10.1152/japplphysiol.01288.2005. [DOI] [PubMed] [Google Scholar]
- 52.Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, et al. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–85. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 53.Gencer B, Karaca T, Tufan HA, Kara S, Arikan S, Toman H, et al. The protective effects of dexmedetomidine against apoptosis in retinal ischemia/reperfusion injury in rats. Cutan Ocul Toxicol. 2014;33:283–8. doi: 10.3109/15569527.2013.857677. [DOI] [PubMed] [Google Scholar]
- 54.Supinski GS, Ji XY, Callahan LA. p38 Mitogen-activated protein kinase modulates endotoxin-induced diaphragm caspase activation. Am J Respir Cell Mol Biol. 2010;43:121–7. doi: 10.1165/rcmb.2008-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Supinski GS, Ji X, Callahan LA. The JNK MAP kinase pathway contributes to the development of endotoxin-induced diaphragm caspase activation. Am J Physiol Regul Integr Comp Physiol. 2009;297:R825–34. doi: 10.1152/ajpregu.90849.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao Z, Cao D, Han X, Liu C, Peng J, Zuo Z, et al. Both JNK and P38 MAPK pathways participate in the protection by dexmedetomidine against isoflurane-induced neuroapoptosis in the hippocampus of neonatal rats. Brain Res Bull. 2014;107:69–78. doi: 10.1016/j.brainresbull.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Nin N, Cassina A, Boggia J, Alfonso E, Botti H, Peluffo G, et al. Septic diaphragmatic dysfunction is prevented by Mn(III)porphyrin therapy and inducible nitric oxide synthase inhibition. Intensive Care Med. 2004;30:2271–8. doi: 10.1007/s00134-004-2427-x. [DOI] [PubMed] [Google Scholar]
- 58.Callahan LA, Nethery D, Stofan D, DiMarco A, Supinski G. Free radical-induced contractile protein dysfunction in endotoxin-induced sepsis. Am J Respir Cell Mol Biol. 2001;24:210–7. doi: 10.1165/ajrcmb.24.2.4075. [DOI] [PubMed] [Google Scholar]
- 59.Sen V, Güzel A, Sen HS, Ece A, Uluca U, Söker S, et al. Preventive effects of dexmedetomidine on the liver in a rat model of acid-induced acute lung injury. Biomed Res Int 2014. 2014 doi: 10.1155/2014/621827. 621827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cekic B, Geze S, Ozkan G, Besir A, Sonmez M, Karahan SC, et al. The effect of dexmedetomidine on oxidative stress during pneumoperitoneum. Biomed Res Int 2014. 2014 doi: 10.1155/2014/760323. 760323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cekic B, Besir A, Yulug E, Geze S, Alkanat M. Protective effects of dexmedetomidine in pneumoperitoneum-related ischaemia-reperfusion injury in rat ovarian tissue. Eur J Obstet Gynecol Reprod Biol. 2013;169:343–6. doi: 10.1016/j.ejogrb.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 62.Dong X, Xing Q, Li Y, Han X, Sun L. Dexmedetomidine protects against ischemia-reperfusion injury in rat skeletal muscle. J Surg Res. 2014;186:240–5. doi: 10.1016/j.jss.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 63.Cavalcanti V, Santos CL, Samary CS, Araújo MN, Heil LB, Morales MM, et al. Effects of short-term propofol and dexmedetomidine on pulmonary morphofunction and biological markers in experimental mild acute lung injury. Respir Physiol Neurobiol. 2014;203:45–50. doi: 10.1016/j.resp.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 64.Peng M, Wang YL, Wang CY, Chen C. Dexmedetomidine attenuates lipopolysaccharide-induced proinflammatory response in primary microglia. J Surg Res. 2013;179:e219–25. doi: 10.1016/j.jss.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 65.Zhang X, Wang J, Qian W, Zhao J, Sun L, Qian Y, et al. Dexmedetomidine inhibits inducible nitric oxide synthase in lipopolysaccharide-stimulated microglia by suppression of extracellular signal-regulated kinase. Neurol Res. 2015;37:238–45. doi: 10.1179/1743132814Y.0000000426. [DOI] [PubMed] [Google Scholar]


