Abstract
Background
The success of clinical dietary interventions depends on the motivation and willingness of study participants to adhere to the prescribed or provided diet. The aim of this study was to assess participants’ adherence to their provided diet over the 6-month duration of the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE).
Methods
Investigators assessed the dietary adherence of 46 men and women who completed the first phase of the CALERIE trial. Volunteers were randomized to 1 of 4 dietary intervention groups: control, calorie restriction, calorie restriction with exercise, and low-calorie diet. Participants were provided with foods during 2 weeks of baseline and during the first 12 weeks and the last 2 weeks of the intervention as outpatients, and they completed a daily self-report form to assess diet adherence. The data are expressed as mean ± standard deviation or standard error of the mean. Pearson’s correlation coefficient was determined to examine the relationship between assigned energy levels and total energy intake.
Results
Deviations reported were for eating nonstudy foods as well as not eating study foods. There were few deviations, and when converted to mean calories per day these did not affect total energy (weeks −3 to 2 = 10.25 ± 4.82, weeks 1–4 = 9.93 ± 12.52, weeks 5–11 = 8.38 ± 7.42, weeks 22–23 = 0.53 ± 3.97 kcal/d). The associations between assigned energy level and actual intake were high for all groups (P = .001), weeks −3 to −2 (r = 0.999), weeks 1–4 (r = 0.998), weeks 5–11 (r = 0.999), and weeks 22–23 (r = 0.998).
Conclusions
The data provide evidence that dietary adherence is good when all foods are provided and when participants are highly motivated.
Keywords: patient compliance, caloric restriction, nutrition assessment, adult, diet, reducing
Success in clinical dietary studies depends on the motivation and willingness of study participants to adhere to the dietary parameters set by the study protocol.(1) Each study presents unique challenges for study participants, thus potentially making dietary adherence difficult. Poor participant adherence to low-calorie diets in clinical studies has been implicated as one of several possible reasons for less than predicted weight loss. After exploring potential metabolic explanations for low efficacy of low-calorie diets in promoting weight loss, Heymsfield et al(2) concluded that low participant adherence to the prescribed diet was generally to be blamed. Low adherence to a long-term diet was also suspected to account for the gradual weight regain that was observed in long-term diet studies. By contrast, participant adherence to a diet has generally been good in studies where foods were prepared and provided at the clinical site(3,4) or were home-delivered.(5) Thus, in well-controlled dietary studies where all foods are prepared and provided to the participants, it may be assumed that adherence would be high, given the fact that the task of self-selecting the foods is eliminated. Similarly, ingestion of foods during self-selection periods may interfere with dietary compliance.(1) However, restrictions associated with dietary provision remove individual choice, and temptations in everyday living may make compliance difficult.
In the randomized clinical trial named the Comprehensive Assessment of the Long-term Effects of Reducing Intake of Energy (CALERIE), which examined calorie restriction and biomarkers of longevity,(6) participants were provided with all foods for the first 3 months (12 weeks) of intervention prior to an additional 2.5 months of self-selection of the dietary regimen, followed by a final 3 weeks with provided foods. The extent of the reduction in energy intake, the assignment to type of treatment, and the length of diet provisions were all factors challenging the participants’ perseverance faced with the dietary demands of the study. The aim of this investigation was to assess the adherence of participants to their provided diet over the 6-month duration of the CALERIE trial.
Methods
This study was part of the CALERIE randomized clinical trial previously described by Heilbronn et al.(6) The study protocol was approved by the Institutional Review Board of the Pennington Biomedical Research Center and an independent data safety monitoring board, and written consent was obtained from all participants.
Subjects
This randomized clinical study included 48 overweight patients (2 patients dropped out: 1 in the control group dropped out before month 3 and another in the very low-calorie diet group was lost to follow-up before month 3). Potential participants aged 18–50 years for men and 18–45 years for women completed 3 screening visits to ensure physical and psychological health; details of this were published elsewhere.(6) Participants were randomized into 1 of 4 groups: (1) control (total energy to meet weight maintenance needs); (2) calorie-restricted (CR: 25% energy deficit); (3) calorierestricted with exercise (CREX: 12.5% energy deficit plus increased physical activity equal to a 12.5% energy deficit), and (4) low-calorie liquid diet (LCD) until a 15% weight loss was achieved followed by a weight maintenance diet.
Experimental Procedures
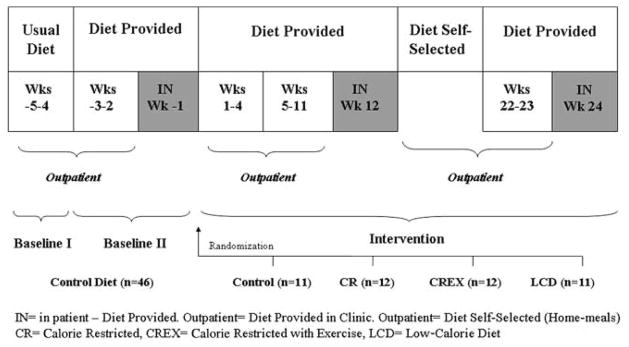
The study duration was 6 months, with a 5-week baseline period followed by a 24-week intervention period (Figure 1). Participants were provided with prepared foods during baseline II (outpatient period and diet-provided period: weeks −3 to −2) and during a 5-day inpatient stay (inpatient and diet-provided period: week −1). Participants were then randomized into 1 of 4 interventions described above. For the first 12 weeks of the intervention (outpatient period and diet-provided period: weeks 1–4 and weeks 5–11, and 5-day inpatient [week 12]), participants were provided with all foods according to their dietary assignment and received dietary training using the Health Management Resources Calorie System (HMR, Boston, MA). This method uses charts that list foods on a scale of 1–10, based on the number of calories they provide and typical portion sizes. During weeks 13–21 (outpatient period and diet-selected period), the subjects self-selected their diet, but not ad libitum. Selected foods should be in quantities to maintain the diet’s energy content prescribed on their intervention and individual energy target. Participants once again were provided with prepared foods during weeks 22 and 23 (outpatient period and diet-provided period) of the intervention. Additionally, two 5-day inpatient stays were required at weeks 12 and 24 (inpatient period and diet-provided period) of the intervention, and all foods were provided.
Figure 1.
Study design.
Diet and Adherence
All diets (except LCD) were based on the American Heart Association Step 1 dietary composition. LCD participants were placed on 890 kcal/d given as 4 nutrition shakes (Health One, provided by Health and Nutrition Technology, Carmel, CA) and 1 brownie (prepared with a packet of Health One, 10 g of corn oil, and 3 tablespoons of water) to ensure adequate gall-bladder emptying. When all foods were provided, a 5-day menu cycle was used, which was altered occasionally during the intervention. Participants ate 2 meals in the middle of each weekday with 1 meal plus a snack packaged for takeout. All weekend meals were packaged for takeout. Participants were allowed to consume a daily maximum of 2 alcoholic beverages away from the center and were required to adjust their energy intake accordingly. Alcohol was not allowed for the LCD participants. Every participant met with the study research dietitian once a week to discuss the diet.
During the period when foods were provided by the research center, each participant completed a daily self-report form to assess diet adherence. According to which diet was provided, participants recorded missed study foods and nonstudy foods eaten, including beverages containing caffeine and alcohol. Values were converted to grams per day and calories per day. When the participants deviated from the diet, the types of foods consumed and not consumed as well as the reasons for noncompliance were assessed. Alcoholic beverages consumed over the maximum allowance were counted as deviations. All diet deviations were analyzed to calculate total energy and recorded. Total daily energy intakes were determined by combining the energy provided with that of the diet deviations.
Statistical analyses were performed using SPSS for Windows (11.00 SPSS Inc, Chicago, IL). The data are expressed as mean ± standard deviation or standard error of the mean. Pearson’s correlation coefficient was determined to examine the relationship between assigned energy levels and total energy intake. P values less than 5% (P < .05) were considered statistically significant.
Results
Forty-six overweight men and women completed the CALERIE I study. Twenty-one were male; 30 were Caucasian, 15 African American, and 1 other. The mean age was 37.7 ± 1 years and the mean body mass index (BMI) was 27.7 ± 0.2 kg/m2 (Table 1).
Table 1.
Baseline characteristics of Subjects
| Characteristics | All Subjects (n = 46) | Males (n = 21) | Females (n = 25) |
|---|---|---|---|
| Race, n Caucasian/African | |||
| American/other | 30/15/01 | 15/5/1 | 15/10/0 |
| Age, y | 37.7 ± 1 (26–48) | 38.0±2(26–48) | 37.4 ± 1 (26–44) |
| Weight, kg | 81.4 ± 1 (61.0–104.0) | 88.2 ± 2 (77.0–104.0) | 75.7 ± 1 (61.0–2.0) |
| Body mass index, kg/m2 | 27.7 ± 0.2 (24.7–31.3) | 27.7 ± 0.4 (25.1–31.3) | 27.7 ± 0.3 (24.7–30.5) |
Values are given as Mean ± standard error of the mean [range]
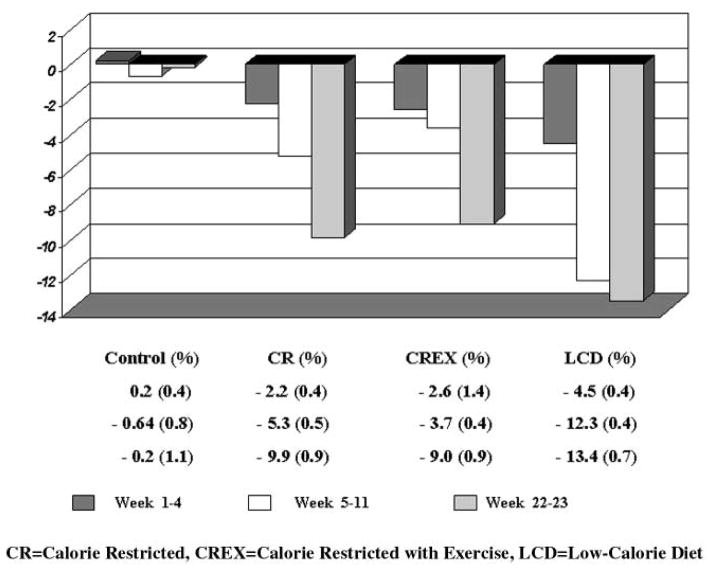
The mean weight loss, by group, from the outpatient baseline period (weeks −3 to −2) to the end of the outpatient intervention periods (weeks 22–23) was −0.2% ± 1.1% (control), −9.9% ± 0.9% (CR), −9.0% ± 0.9% (CREX), and −13.4% ± 0.7% (LCD) (Figure 2).
Figure 2.
Change in weight by periods and treatment
When meals were provided, 27 participants (59%) consumed all of the meals. Five participants reported that they deviated from their diets no more than 5 times during the entire study. As expected, episodes of nonadherence were few during the inpatient stays. Three participants did not consume entire portions of study foods during the first stay at the clinical unit because of reported dislike for the foods (2 participants did not eat mushrooms and 1 did not eat olives). There were no reported or observed deviations for the remaining inpatient stays.
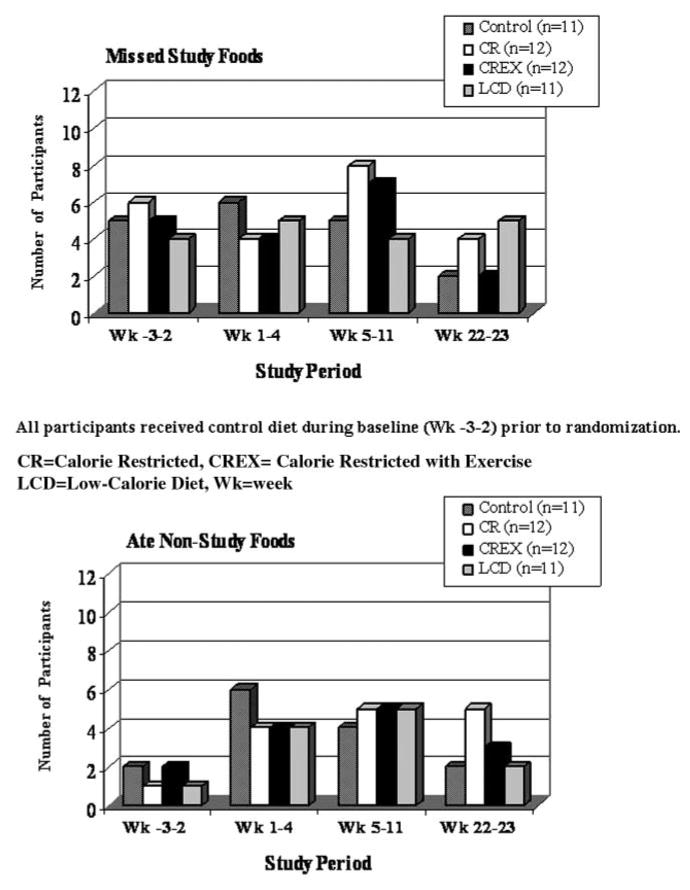
The numbers of participants in each treatment group who reported nonadherence to the diets provided during the outpatient time periods are shown in Figure 3. Time periods correspond to the outpatient baseline period (weeks −3 to −2) when all participants were given the control diet, the first month of the outpatient intervention period (weeks 1–4), the end of the outpatient intervention period (weeks 5–11), and the end of the study outpatient intervention period (weeks 22–23). During the initial outpatient baseline period when all participants received the control diet, 6 participants consumed nonstudy foods. Approximately 40% of all participants, 4–6 per group, said that they ate nonstudy foods during the 11 weeks of intervention. Participants belonging to the calorie-restricted groups, CR and CREX, reported lower adherence, with a higher number of episodes of missed study foods in weeks 5–11. Common reasons provided for the deviations included illness (2.7%), dislike for foods (2.1%), spilled beverages (3.5%), foods in social settings (2.7%), and temptations (89%). Dietary adherence generally improved when the participants were provided foods at the end of the study (weeks 22–23), possibly because of the anticipation of finishing a very long study.
Figure 3.
Number of participants in each intervention group who reported nonadherence to the diet by study period
Of the 15 participants who consumed alcohol during the study, 5 consumed more than the allotted amount or did not adjust their energy intake. Alcohol intake was higher in weeks 5–11, and the control group had the most prevalent intake, with 6 participants ingesting alcohol (54.5%). The intake of caffeinated beverages was similar in all groups. During the intervention, considering all periods of the study, 15 participants in the control group, 17 in the CR group, 16 in the CREX group, and 16 in the LCD group ingested caffeinated beverages.
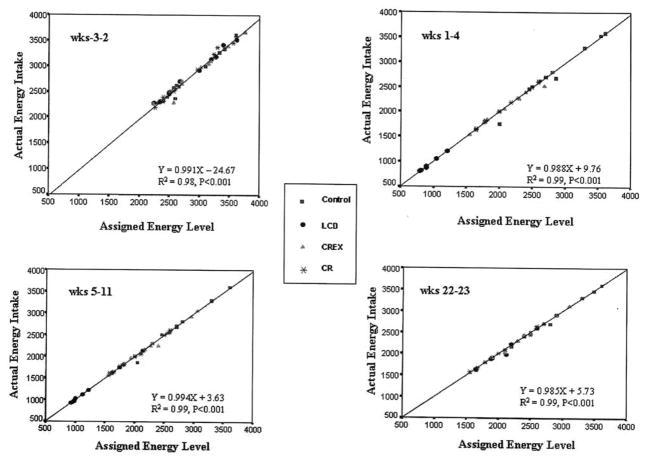
The energy values of the deviations were determined to estimate the extent of the nonadherence and the impact on total energy intake. When converted to mean calories per day, deviations for each diet group were considered small and did not affect total energy. The energy intake as mean calories per day was weeks −3 to −2 = 10.25 ± 4.82, weeks 1–4 = 9.93 ± 12.52, weeks 5–11 = 8.38 ± 7.42, and weeks 22–23 = 0.53 ± 3.97. For all participants as a group, <1% of assigned calories were not consumed, and nonstudy foods consumed represented <0.5% of total energy. Total actual energy intakes matched assigned energy levels for all groups (Figure 4). Pearson’s correlation coefficients were high in all groups for each time period studied (P = .001).
Figure 4.
Comparison of actual energy intake and assigned energy intake for each participant by study period. All participants received control diet during baseline (Wks −3–2) prior to randomization. CR= Calorie Restriction; CREX= Calorie Restriction and Exercise; LCD= Low-Calorie Diet
Discussion
A major obstacle in studies involving dietary modification is lack of participant adherence to the prescribed diet. In the CALERIE study, we explored the potential obstacles to participants’ adherence to the assigned diet. Obstacles considered were the extent of the reduction in energy intake, the type of treatment assigned, and the length of dietary provisions. As mentioned in the introduction, participant adherence to a diet has generally been high in studies where foods are prepared and provided at the clinical site(3,4) or home-delivered.(5) Yet, unique study features (such as patients going home for nights and weekends) may turn into obstacles that challenge a participant’s ability to adhere to study diets.(7) It is important to highlight that during weeks 13–21, patients were at home and self-selected their diets based on the intervention and individual energy target. Adherence was determined by the percentage of weight loss (Figure 2). Thereafter, participants once again were provided with prepared foods during weeks 22 and 23 (outpatient period and diet-provided period) of the intervention.
Nevertheless, we found that adherence to the diets prescribed in CALERIE did not suffer interference. There were fewer deviations during baseline and following the participants’ self-selected diets than during the intervention period. It may be postulated that at the beginning of the study participants were excited at being involved in the study and were motivated to follow the study protocol, willingly consuming the foods provided. Later, motivation may have waned, and participants may have become tired of the repetitive foods or may have given in to temptations to consume other foods. We attempted to alleviate boredom by introducing new menus during the study period. Adherence was best near the end of the study following the self-selected diet period.
In a survey of past participants of well-controlled diet studies, Hall and Most(4) examined possible challenges associated with study design features that may affect diet adherence. The length of studies, between 6 and 24 weeks, did not influence adherence, nor did the number of repeated days in a menu cycle. In general, when individuals deviate from a diet, eating all of the provided foods is more of a challenge than refraining from nonstudy foods. This was true in the present study, especially during the initial period of the intervention. In the survey by Hall and Most(4) participants who received an intervention diet exhibited better adherence than those who consumed a control diet during the entire study period. We did not observe differences in adherence between the diet intervention and control groups.
When our participants did not adhere to their provided diets, either the deviations were insignificant, negatively affecting total energy intake, or the energy level of nonstudy food consumed was equivalent to that of study food not consumed. Interestingly, when participants did deviate from their provided foods, the mean daily energy value of those nonstudy foods consumed was less at the end of the study following the self-selected diets than at the beginning of the study. This could suggest that the participants chose foods of lower energy content after they had completed training on selecting foods for their diets. The participants were good at making substitutions that allowed flexibility and individual choice and thus provided their own variety.
Although our adherence data are based on self-reports by the participants, energy intake data calculated from the reports reflect expected weight maintenance or loss for each intervention group. High adherence in the study may in part be explained by the participants’ motivation to follow a hypocaloric diet, the fact that the foods were provided for free, and the training received prior to the study on how to follow the diet. In addition, weekly meetings with a dietitian helped tremendously in terms of adherence. Furthermore, the participants were enrolled following a lengthy screening process and were provided with their meals and concurrent diet instruction; after this, we obtained success in the diet interventions leading to confidence in the interpretation of the endpoint measures, as can be observed in similar published papers on the matter(8–11)
Acknowledgments
We thank the remaining members of Pennington CALERIE Research Team, including Anthony Alfonso, Steven Anton, Catherine Champagne, Brenda Dahmer, Andy Deutsch, Paula Geiselman, Jana Ihrig, Michael Lefevre, Darlene Marquis, Connie Murla, Jennifer Rood, Aimee Stewart, and Vanessa Tarver. Our gratitude is extended to the excellent staffs of the Outpatient Clinic, Inpatient Clinic, Metabolic Kitchen, and Clinical Chemistry Laboratory. Our thanks also go to Health and Nutrition Technology, Carmel, CA, for providing us with all the Health One formula used in the study. Finally, our profound gratitude goes to all the volunteers, who spent so much time participating in this very demanding research study.
Footnotes
Financial disclosure: This work was supported by research grant U01 AG20478 from the National Institutes of Health (clinical trial registration: clinicaltrials.gov identifier: NCT00099151) and partially supported by a NORC Center Grant # P30DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” Emilia A. M. Moreira received a fellowship grant from Conselho Nacional de Desenvolvimento Cientifico e Tecnológico, CNPq, Brazil No. 20.1345/03-0.
References
- 1.Lichtentein AH, Prewitt E, Rasmussen H, et al. Managing participants and maximizing compliance. In: Dennis BH, Ershow A, Obarzanek E, Clevidence B, editors. Well Controlled Diet Studies in Humans: A Practical Guide to Design and Manegement. Chicago, IL: America Dietetic Association; 1999. pp. 96–108. [Google Scholar]
- 2.Heymsfield SB, Harp JB, Reitman ML, et al. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. Am J Clin Nutr. 2007;85:346–354. doi: 10.1093/ajcn/85.2.346. [DOI] [PubMed] [Google Scholar]
- 3.Windhauser MM, Evans MA, McCullough ML, et al. Dietary adherence in the Dietary Approaches to Stop Hypertension trial. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99(8):S76–S83. doi: 10.1016/s0002-8223(99)00420-4. [DOI] [PubMed] [Google Scholar]
- 4.Hall DM, Most MM. Dietary adherence in well-controlled feeding studies. J Am Diet Assoc. 2005;105:1285–1288. doi: 10.1016/j.jada.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Gleason JA, Bourdet KL, Koehn K, et al. Cardiovascular risk reduction and dietary compliance with a home-delivered diet and lifestyle modification program. J Am Diet Assoc. 2002;102:1445–1451. doi: 10.1016/s0002-8223(02)90320-2. [DOI] [PubMed] [Google Scholar]
- 6.Heilbronn LK, De Jonge L, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals. JAMA. 2006;295(13):1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Most MM, Fishell V, Binkoski A, et al. Clinical Nutrition Studies: Unique Applications. In: Berdanier CD, editor. CRC Handbook of Nutrition and Food. Boca Raton, FL: CRC Press; 2001. pp. 425–442. [Google Scholar]
- 8.Martin CK, Heilbronn LK, De Jonge L, et al. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 2007;15(12):2964–2973. doi: 10.1038/oby.2007.354. [DOI] [PubMed] [Google Scholar]
- 9.Redman LM, Rood J, Anton SD, et al. Calorie restriction and bone health in young, overweight individuals. Arch Intern Med. 2008;168(17):1859–1866. doi: 10.1001/archinte.168.17.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redman LM, Heilbronn LK, Martin CK, et al. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS ONE. 2009;4(2):1–9. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Jonge L, Moreira EAM, Martin CK, Ravussin E Pennington CALERIE Team. Impact of 6-month caloric restriction on autonomic nervous system activity in healthy, overweight, individuals. Obesity (Silver Spring) 2009;18(2):414–416. doi: 10.1038/oby.2009.408. [DOI] [PMC free article] [PubMed] [Google Scholar]