Abstract
Introduction
Administration of betamethasone to women at risk of preterm delivery is known to be associated with reduced fetal growth via alterations in placental function and possibly direct effects on the fetus. The placental glucocorticoid receptor (GR) is central to this response and recent evidence suggests there are numerous isoforms for GR in term placentae. In this study we have questioned whether GR isoform expression varies in preterm placentae in relation to betamethasone exposure, fetal sex and birthweight.
Methods
Preterm (24–36 completed weeks of gestation, n = 55) and term placentae (>37 completed weeks of gestation, n = 56) were collected at delivery. Placental GR expression was examined using Western Blot and analysed in relation to gestational age at delivery, fetal sex, birthweight and beta-methasone exposure. Data was analysed using non-parametric tests.
Results
Eight known isoforms of the GR were detected in the preterm placenta and include GRα (94 kDa), GRβ (91 kDa), GRα C (81 kDa) GR P (74 kDa) GR A (65 kDa), GRα D1–3 (50–55 kDa). Expression varied between preterm and term placentae with a greater expression of GRα C in preterm placentae relative to term placentae. The only sex differences in preterm placentae was that GRα D2 expression was higher in males than females. There were no alterations in preterm placental GR expression in association with betamethasone exposure.
Discussion
GRα C is the isoform involved in glucocorticoid induced apoptosis and suggests that its predominance in preterm placentae may contribute to the pathophysiology of preterm birth.
Keywords: Placenta, Sex, Pregnancy, Fetal growth, Preterm, Glucocorticoid receptor
1. Introduction
Excess exposure to maternal glucocorticoids during pregnancy can significantly impact lifelong health [1]. At least 80% of infants born less than 34 weeks gestation are exposed to exogenous glucocorticoids for the purposes of lung maturation [2]. The benefits of reducing respiratory distress syndrome are well documented however adverse consequences for preterm neonates exposed to antenatal glucocorticoids have been reported, and include an altered stress response [3], altered motor development and poor neuropsychological function in adolescence [4], higher blood pressure [5] and in animal models, altered kidney development [6]. In addition, excess exposure to glucocorticoids is not limited to preterm delivery. Maternal stress, mental illness or disease states that promote higher circulating concentrations of maternal cortisol during pregnancy can also perturb developmental programming of the fetal cardiovascular system, kidney, brain and immune system. This, in turn, may contribute to an increased risk of cardio-respiratory, metabolic disorders and allergy in later life [7]. A number of human studies have also suggested the response to glucocorticoids may occur in a sex specific manner but the mechanisms are currently unclear [8–15]. Understanding the mechanisms by which exposure to exogenous and endogenous glucocorticoids impacts the development of the human fetus requires an investigation of the placental response to glucocorticoids and the glucocorticoid receptor (GR) signalling pathway.
A number of pathways act together to confer either sensitivity or resistance to glucocorticoids during pregnancy. These include the pre- and post-receptor pathways, involving 11β hydroxysteroid dehydrogenase (11βHSD2) [9,16], glycoprotein P (Pgp) [17] and cortisol binding globulin (CBG) [18] which modulate cortisol exposure to the placenta and fetus. However these mechanisms do not sufficiently account for sex specific differences in the fetal-placental response to cortisol [10,11,19] and have lead us to examine the GR in more detail in the fetal-placental unit. GR is a ubiquitously expressed nuclear receptor comprised of 9 exons spanning ~80 kB. Exon 1 of GR gene has a 5′ untranslated region which can be spliced into 9 different promoter variants [20] and function in a tissue specific manner to regulate GR protein expression. Exons 2–9 can generate various isoforms of GR either through alternative splicing [21–23] or via alternative initiation of translation [23,24]. This can result in the expression of GRα, GRβ, GRγ, GR A and GR P proteins. GRα A is the well characterised functional isoform involved in transcriptional activation and transcriptional repression of multiple targets. However eight different GRα translational isoforms can originate from GRα mRNA through multiple start codons encoded by exon 2 and include GRα A (94 kDa) and GRα B (91 kDa), GRα C1–C3 (82–84 kDa) and GRα D1–D3 (53–56 kDa). The various translational isoforms of GRα function in a tissue specific manner to differentially regulate transcriptional activities [23]. The splice variants such as GRβ, inhibit activation of GRα through a dominant negative mechanism. Splice variants GRγ, GR A and GR P have low transactivation activities [25,26]. Current evidence suggests there may also be multiple translational isoforms of the splice variants due to the multiple start codons encoded by exon 2. Exon 2 is common to all of the GR isoforms. The presence of multiple isoforms of GR may confer sensitivity or resistance to glucocorticoids and that this response is not limited to the well characterised GRα A (94 kDa) but a far more complex process of several GR isoforms being co-expressed and potentially interacting with one another.
Previous work by Lu and Cidlowski [23] demonstrated that GR translational isoform expression varies between different cell types and this may mediate the differential regulatory patterns of glucocorticoid-induced gene activation or repression. Very few adult tissues express multiple isoforms of GR except for the pancreas, liver, colon and stomach [23]. We have previously identified that the term, human placenta expresses mRNA for the splice variants GRβ, GRγ and GR P [27] and the placental trophoblast expresses 12 protein isoforms of GR [28] which may be the only human tissue to do so indicating the placenta is a very unique organ. Interestingly not all 12 isoforms are found in every individual which implies there is a significant level of complexity in the placental response to cortisol. Variation in GR translational isoform expression plays a major putative role in regulating cellular glucocorticoid specificity and sensitivity [29,30]. In the placenta these differences in GR expression may mediate the sex specific differences in the response to cortisol and ultimately determining a difference in fetal growth and development.
Extensive data [8–15] supports cortisol as the primary driver of sex differences in fetal and neonatal development and survival. Our studies of maternal asthma [8,9,31], pre-eclampsia [32,33] and preterm delivery [34,35] indicate male and female fetuses and neonates institute different mechanisms to cope with an adverse environment or event. Males appear to induce a state of glucocorticoid resistance in response to a rise in maternal cortisol while females remain sensitive to changes in cortisol concentration. Further we have shown that GR expression in the fetal-placental unit varies with cell type and cellular location as well as in relation to fetal sex, maternal asthma or growth restriction [28]. Specifically, males may be glucocorticoid resistant in a high cortisol environment due to the dominant negative effects of GRβ, and possibly through GR A and GR P localisation to the nucleus [28]. Females may be more sensitive to glucocorticoids through an interaction of GRα A with GRα C and GRα D3 [28]. In this current study we have examined whether GR isoform expression varies in preterm placentae in relation to gestational age at delivery, fetal sex, birthweight and exposure to betamethasone.
2. Methods
The current study was approved by Queen Elizabeth Hospital (QEH) and Lyell McEwin Hospital (LMH) Human Research Ethics Committee, Womens’ and Childrens’ Hospital (WCH) Human Research Ethics Committee and The University of Adelaide. Women presenting in threatened preterm labour were recruited prospectively and consented.
2.1. Maternal and neonatal characteristics
Following delivery maternal case notes were reviewed for antenatal betamethasone exposure, pregnancy related complications and maternal demographics. Maternal characteristics including maternal height, weight, age, parity and gravidity were recorded. Women who delivered at term were healthy individuals that had no complications during pregnancy and we excluded those women with pre-eclampsia, hypertension, gestational diabetes or pre-existing disease that included asthma, essential hypertension and diabetes. Women who smoked or were obese were included in the study as they are representative of the population. All women who delivered preterm were included except those pregnant with a baby with a congenital malformation. Neonatal data collected at delivery includes gestational age and birth weight. Birth weight centile (BWC) was calculated using www.gestation.net. “Small for gestational age” (SGA) was defined as birthweight less than the 10th BWC and “appropriate for gestational age” was birthweight >10th and < 90th BWC.
Gestational age was determined by date of the last menstrual period and confirmed by 18 week ultrasound. Placental weight, mode of delivery, complications and fetal sex were obtained. Preterm delivery was defined as delivery before 37 weeks gestation. Preterm placentae were collected from a range of gestational ages (24–36 completed weeks of gestation). Term placentae ranged from 37 to 41 weeks of gestation. Some women at risk of preterm delivery received 2 × 11.4 mg bolus doses of Celestone Chronodose (Merck Sharpe & Dohne Australia) injection that were administered 24 h apart. Forty four percent of women did not receive beta-methasone because women who deliver babies at greater than 34 weeks gestation do not routinely receive betamethasone. Furthermore some women presented in active preterm labour and then delivered before betamethasone could be administered.
Chorioamnioitis was determined retrospectively by histopathology of the placenta after delivery.
2.2. Placenta collection
Placental tissue (Preterm n = 55, Term n = 56) was collected within 1 h of delivery. Villous tissue was sampled randomly from 6 sites on the placenta, washed in saline, the top layer of the maternal side was removed to remove as much decidua as possible and then all pieces mixed together, chopped and then aliquoted into separate tubes and snap frozen in liquid nitrogen and stored at −80 °C until analysis.
2.3. Western blot
2.3.1. Cellular fraction preparation from placental tissue
Placental tissue was homogenised in complete cytosolic fractionation buffer [36] containing complete protease inhibitor cocktail. Lysates were spun at 6200 g for 5 min. Supernatants were kept for the cytosolic fraction. Nuclear fraction buffer with complete protease inhibitor cocktail was added to the pellet. Nuclear lysates were rotated at 4C° for 30 min, sonicated twice for 10 s at 30% amplitude using ultrasonic processor VCX 130 (Sonics, USA) and spun at 17,000 g for 8 min to remove debris. Supernatants were stored at −80 °C. Protein concentrations for each fraction were measured using Bradford assay.
2.3.2. Visualisation of target proteins
Cytosolic and nuclear protein fractions (60 μg) were electrophoresed on 3–8% Tris-acetate precast gels (Invitrogen, Life technologies, Carlsbad, California, USA) as previously described [11]. Blots were incubated with affinity purified polyclonal rabbit anti-human GR total (1:1500) (Bethyl Laboratories, Montgomery, TX, USA, Cat no. A303-491A) antibodies targeted to residue 150–200 of the GR receptor. The appropriate secondary antibody (goat anti-rabbit; 1:2500) was applied for 1 h. Membranes were subsequently probed with anti-β actin (1:4000, Bethyl laboratories, USA Cat no. A300-491A) and anti-lamin A/C (1:1500, Santa Cruz Biotechnology, Santa Cruz, California, USA Cat no. Sc-6215) antibodies as loading controls for cytoplasmic and nuclear fractions, respectively. The densitometric analysis was carried out using G:BOX Chemi Gel Imaging Systems (SYNGENE) to quantify the expression levels of different GR isoforms relative to β actin. Peptide competition with anti GR total antibody (1 μg/1.5 ml) incubated with 1× (1 μg) and 2× (2 μg) concentration of the control peptide (Bethyl Laboratories, USA Cat no: BP303-491A) was performed as a specificity control.
2.4. Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS v 19). An a-priori decision was made to analyse the data separately by sex when comparing the effects of preterm birth and betamethasone, as past research indicates that placental cortisol metabolism differs between males and females [37,38]. GR data was not normally distributed so non-parametric tests were used which included Mann–Whitney tests. Frequency data was analysed using Chi Squared. Spearman’s correlations were performed to assess associations. The alpha level was set at 0.05. GR data is expressed as median and interquartile range.
3. Results
3.1. Subjects
There were no significant differences in maternal characteristics (Table 1). Neonatal characteristics between healthy term controls (n = 56) and preterm pregnancies (n = 55) differed in relation to, gestational age, birthweight, and placental weight (Table 1). In the preterm cohort, 47% of women received antenatal betamethasone therapy, while 23% of preterm births that received betamethasone were associated with chorioamnionitis. Twenty percent of women that delivered preterm without betamethasone had histopathological evidence of chorioamnioitis.
Table 1.
Maternal and neonatal characteristics.
| Preterm no betamethasone n = 29 | Preterm betamethasone n = 26 | Term n = 56 | Pa (preterm comparison) | Pb (preterm v term) | |
|---|---|---|---|---|---|
| Gestational age (days) | 240 (3) | 226 (5) | 276 (8) | 0.02 | <0.001 |
| Maternal age (yrs) | 26 (1) | 29 (1) | 32 (6) | 0.09 | 0.14 |
| BMI | 28 (3) | 31 (3) | 30 (7) | 0.55 | 0.96 |
| Parity | 1 (0) | 1 (0) | 2 (1) | 0.66 | 0.14 |
| Gravidity | 3 (1) | 3 (1) | 3 (1) | 0.45 | 0.37 |
| Smoker | 3 (10%) | 2 (8%) | 10 (18%) | 0.73 | 0.19 |
| Male | 19 (66%) | 11 (42%) | 25 (44%) | 0.04 | 0.10 |
| Birth weight (g) | 2284 (108) | 1839 (145) | 3478 (467) | 0.017 | <0.001 |
| Birth weight centile | 48 (6) | 28 (5) | 46 (4) | 0.04 | 0.10 |
| Placental weight (g) | 478 (23) | 501 (47) | 658 (118) | 0.67 | 0.021 |
| Steroid exposed | 0 | 26 (100%) | 0 | – | – |
| Chorioamnionitis | 6 (20.6%) | 6 (23%) | 0 | 0.88 | – |
| Sepsis | 2 (7%) | 1 (4%) | 0 | – | – |
| Died | 0 | 1 (4%) | 0 | – | – |
| MOD | |||||
| Vaginal delivery | 18 (78%) | 10 (40%) | 42 (75%) | 0.01 | 0.01 |
| Elective CS | 3 (13%) | 7 (28%) | 8 (14.3%) | ||
| Emergency CS | 2 (8.6%) | 8 (32%) | 6 (10.7%) | ||
Data expressed as mean (standard error of the mean) or n (%) unless otherwise stated.
Pa is the P value for the comparison between just the preterm groups that were either exposed or not exposed to betamethasone.
Pb is the P value for the comparison between the combined preterm groups and the term group.
MOD = mode of delivery.
3.2. GR isoforms expressed in the term and preterm placenta
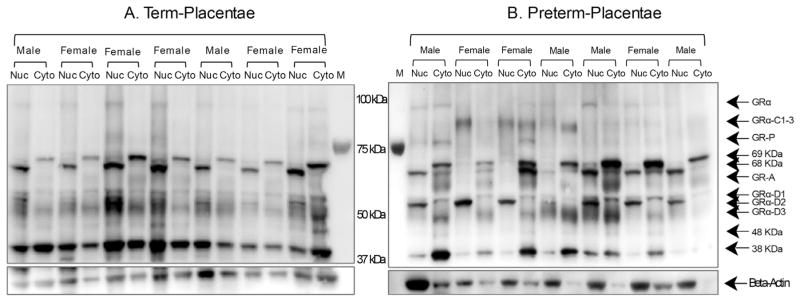
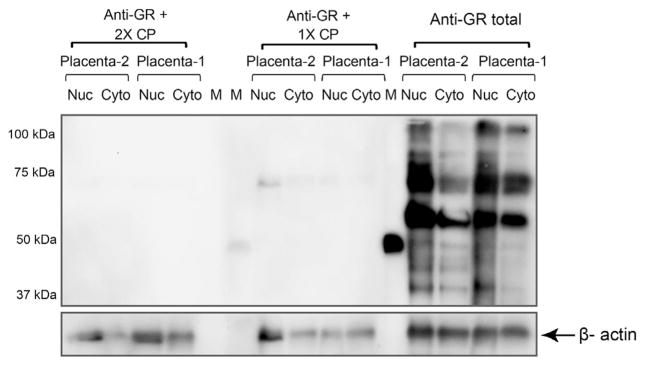
Total GR antibody identified 13 specific bands in protein extracts of whole placental tissue derived from term (n = 56) and preterm pregnancies (n = 55). Molecular weights (MW) of 94, 91, 81, 74, 69, 68, 65, 60, 55, 52, 50, 48 and 38 kDa were observed in both term and preterm placentae (Fig. 1). Some of these MWs are equivalent to known isoforms including GRα (94 kDa), GRβ (91 kDa), GRα C (81 kDa) GRP (74 kDa) GRA (65 kDa), GRα D1–3 (50–55 kDa). Some isoforms are unknown proteins including the 69, 68, 60, 48 and 38 kDa proteins. Not all isoforms were expressed in every individual with expression varying in relation to gestational age and fetal sex (Table 2). Following pre-absorption of the GR antibody with the control peptide all MW forms were not detectable on the Western blot (Fig. 2).
Fig. 1.
Representative Western blot of placental GR isoforms from term and preterm deliveries. Nuclear and cytoplasmic protein extracts of placentae from healthy pregnancies (Panel A) and pregnancies associated with a preterm delivery (Panel B) were exposed to either GR total antibodies. Twelve proteins were detected ranging from 38 to 94 kDa. Blots were probed with β-actin as a loading control.
Table 2.
Expression profiles of GR isoforms in preterm and term placenta, according to fetal sex. Data are presented as median (IQR) relative protein expression and the percentage of placenta that expressed each isoform.
| GR isoform | Preterm
|
Term
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Male n = 30 | % Detected | Female n = 25 | % Detected | Male n = 25 | % Detected | Female n = 31 | % Detected | ||
| GRα A | Cytosol | 0.08 (0–0.83) | 17 (57) | 0.06 (0–0.84) | 14 (56) | 0.07 (0–0.18) | 15 (60) | 0.06 (0–0.23) | 18 (58) |
| Nuclear | 0.07 (0–0.76) | 17 (57) | 0 (0–1) | 9 (36) | 0.12 (0–0.48) | 17 (68) | 0.09 (0.05–0.30) | 26 (84) | |
| GR β | Cytosol | 0 (0–0.51) | 9 (30) | 0 (0–0.22) | 3 (12) | 0 (0–0.70) | 8 (32) | 0 (0–0.03) | 8 (26) |
| Nuclear | 0 (0–0.27) | 3 (10) | 0 (0–0.42) | 1 (4) | 0 (0–0) | 4 (16) | 0 (0–0) | 7 (23) | |
| GRα C | Cytosol | 0.20 (0–2.01)* | 20 (67) | 0.14 (0–0.95) | 13 (52) | 0 (0–0.05) | 8 (32) | 0 (0–0.21) | 15 (48) |
| Nuclear | 0 (0–2.94) | 12 (40) | 0.19 (0–3.10)# | 14 (56) | 0 (0–0.07) | 7 (28) | 0 (0–0.07) | 11 (35) | |
| GR P | Cytosol | 0 (0–1.09) | 10 (33) | 0 (0–0.80) | 7 (28) | 0 (0–0.07) | 8 (32) | 0 (0–0.11) | 11 (35) |
| Nuclear | 0 (0–0.39) | 11 (37) | 0 (0–1.00) | 4 (16) | 0 (0–0.12) | 10 (40) | 0.04 (0–0.12) | 16 (52) | |
| 69 kDa | Cytosol | 0 (0–1.05) | 3 (10) | 0 (0–7.01) | 2 (8) | 0 (0–0) | 1 (4) | 0 (0–0) | 5 (16) |
| Nuclear | 0 (0–0) | 1 (3) | 0 (0.13) | 2 (8) | 0 (0–0) | 3 (12) | 0 (0–0) | 5 (16) | |
| 68 kDa | Cytosol | 0 (0–4.19) | 12 (40) | 0 (0–3.41) | 8 (32) | 0 (0–0.37) | 9 (36) | 0 (0–0.24) | 10 (32) |
| Nuclear | 0 (0–8.42) | 8 (27) | 0 (0–2.56) | 7 (28) | 0 (0–0) | 5 (20) | 0 (0–0.25) | 13 (42) | |
| GRA | Cytosol | 0.79 (0–12.64)* | 20 (67) | 0.93 (0.18–9.18) | 16 (64) | 0 (0–0) | 11 (44) | 0.09 (0–1.58) | 19 (61) |
| Nuclear | 0.67 (0.1–8.15) | 25 (83) | 0.46 (0.06–5.71) | 15 (60) | 0.31 (0–1.40) | 14 (56) | 0.37 (0–0.99) | 22 (71) | |
| 60 kDa | Cytosol | 0.16 (0–5.11) | 16 (53) | 0.03 (0–2.26) | 10 (40) | 0.06 (0–0.40) | 13 (52) | 0.10 (0–0.62) | 16 (52) |
| Nuclear | 0.2 (0–2.91) | 13 (43) | 0 (0–2.04) | 6 (24) | 0 (0–0.36) | 10 (40) | 0 (0–0.21) | 10 (32) | |
| GRα D3 | Cytosol | 0.68 (0–9.67) | 22 (73) | 0.61 (0.08–6.06) | 17 (68) | 0.15 (0–0.64) | 14 (56) | 0.24 (0–0.51) | 20 (65) |
| Nuclear | 0 (0–30.16) | 14 (47) | 0.19 (0–16.51) | 12 (48) | 0.20 (0–0.89) | 14 (56) | 0.20 (0–1.25) | 22 (71) | |
| GRα D2 | Cytosol | 0 (0–8.58) | 8 (27) | 0 (0–3.54) | 3 (12) | 0 (0–0) | 2 (8) | 0 (0–0) | 2 (6) |
| Nuclear | 0 (0–13.10)§ | 14 (47) | 0 (0–5.25)¥ | 4 (16) | 0 (0–0) | 3 (12) | 0 (0–0) | 2 (6) | |
| GRα D1 | Cytosol | 1.94 (0.27–16.07) | 24 (80) | 2.89 (0.70–10.71) | 18 (72) | 1.18 (0.22–2.51) | 20 (80) | 1.22 (0.12–3.25) | 24 (77) |
| Nuclear | 1.39 (0–11.07) | 19 (63) | 0.75 (0–13.17) | 12 (48) | 1.37 (0–3.38) | 18 (72) | 1.61 (0–2.77) | 23 (74) | |
| 48 kDa | Cytosol | 0 (0–2.01)* | 14 (47) | 0.20 (0–2.55)# | 11 (44) | 0 (0–0) | 5 (20) | 0 (0–0) | 7 (23) |
| Nuclear | 0 (0–1.78)§ | 9 (30) | 0 (0–2.83) | 7 (28) | 0 (0–0.15) | 7(28) | 0 (0–0.03) | 8 (26) | |
| 38 kDa | Cytosol | 1.74 (0.56–7.29) | 27 (90) | 2.88 (0.85–12.19) | 19 (76) | 0.84 (0–3.35) | 16 (64) | 0.88 (0.13–2.01) | 26 (84) |
| Nuclear | 0.92 (0.27–10.57) | 27 (90) | 1.85 (0.28–8.60)§ | 18 (72) | 0.82 (0–3.98) | 16 (64) | 0.85 (0.09–1.53) | 26 (84) | |
P < 0.05 compared to term male; P < 0.05 compared to term female; P < 0.05 compared to cytosol expression within the same group; P < 0.05 compared to preterm male.
Fig. 2.
Pre-absorption of GR antibody with control peptide. Nuclear and cytoplasmic protein extracts of placentae from healthy pregnancies (n = 2) were exposed to either GR total antibodies alone or in the presence of 1 times or 2 times the concentration of control peptide. There were no non-specific bands identified with antibody pre-absorption. Blots were probed with β-actin as a loading control.
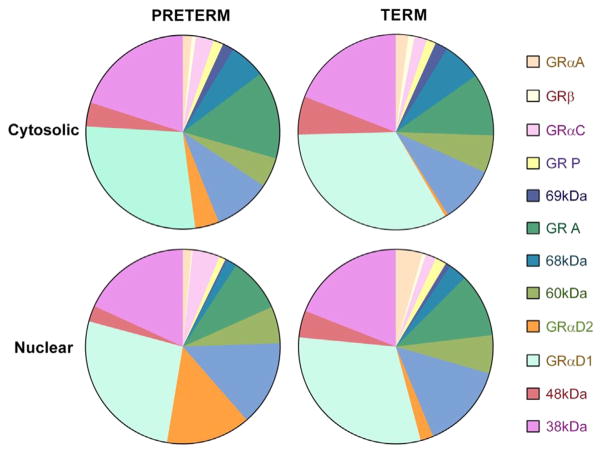
The relative expression of different GR isoforms is summarised in Fig. 3 comparing preterm placentae to term placentae. The most predominant isoforms were cytosolic and nuclear GRα D1 (cytosolic term 31% vs preterm 28%, nuclear term 29% vs preterm 26%, Chi-squared P > 0.05) and 38 kDa protein (cytosolic term 18% vs preterm 20%, nuclear term 18% vs preterm 18%, Chi-squared P > 0.05) expressed equally in term and preterm placentae. There were significant differences in the relative expression of nuclear GRα A (term 4.2% vs preterm 1.4% Chi-squared, P = 0.025), nuclear GRα C (term 1.7% vs preterm 4.5% Chi-squared, P = 0.03) and cytosolic and nuclear GRα D2 (cytosolic term 0.38% vs preterm 3.8% Chi-squared P = 0.02, nuclear term 2% vs preterm 13.9% Chi-squared, P = 0.001) (Fig. 3).
Fig. 3.
Percentage expression of GR isoforms in term and preterm placentae. Percentage was calculated as the individual GR isoform expression divided by total expression which was calculated by the sum of each densitometric measure of each GR isoform relative to β-actin. Values are a mean of all individuals either in the term group (n = 56) or preterm group (n = 55) separated by cellular location in the nucleus or cytoplasm.
3.3. Relative expression of all GR isoforms in term versus preterm placentae in relation to fetal sex
Some GR isoforms varied between term and preterm placentae in relation to the sex of the fetus. Male preterm placentae had higher cytoplasmic levels of GRα C (P = 0.006, Mann Whitney U test, Table 2) and GR A (P = 0.01, Mann Whitney U test, Table 2) relative to term male placentae (Table 2). Female preterm placentae had higher levels of nuclear GRα C relative to term placentae (P = 0.006, Mann Whitney U test, Table 2). Male preterm placentae had decreased expression of cytoplasmic 48 kDa protein than placentae of term males (P = 0.04 Mann Whitney U test, Table 2) while expression of this protein increased in female preterm placentae relative to term placentae (P = 0.05, Mann Whitney U test, Table 2). The cytoplasmic 38 kDa protein was increased in female preterm placentae compared to term placentae (P = 0.03 Mann Whitney U test, Table 2). All other isoforms were detectable in both term and preterm placentae though the levels were variable as demonstrated by the percentage of individuals with detectable expression (Table 2).
3.4. Effect of sex on GR isoform expression in the nucleus or cytoplasm of preterm placentae
There were no sex specific differences in the expression of GR between male and female preterm placentae except for the expression of nuclear GRα D2 which was increased in males relative to females (Table 2).
When examining the location of the GR isoforms in preterm placentae of males and females, GRα D2 was expressed at greater levels in the nucleus relative to cytoplasm in preterm placentae of males compare to females (P = 0.04, Wilcoxon ranked test). The unknown protein at 48 kDa was higher in the cytoplasm versus the nucleus of male preterm placentae (Wilcoxon ranked test, P = 0.04). The 38 kDa protein was higher in the cytoplasm than the nucleus of female preterm placentae (Wilcoxon ranked test, P = 0.05). All other isoforms did not vary significantly in relation to cellular location in the nucleus or cytoplasm.
3.5. Effect of betamethasone exposure and fetal sex on GR isoform expression
Betamethasone exposure did not affect the expression of any GR isoforms in the male preterm placentae. In female preterm placentae the unknown protein at 48 kDa was significantly increased in the nucleus in the presence of betamethasone exposure (P = 0.04, Mann Whitney U test). No other isoforms were affected by the administration of betamethasone.
3.6. Effect of birthweight, placental weight and labour on preterm placental GR isoform expression
When examining the effect of growth by comparing appropriate for gestational age (AGA) fetus to a small for gestation age (SGA) fetus there were no significant differences in GR isoform expression in placentae of preterm males. SGA preterm female placentae had decreased expression of nuclear GR A (Mann Whitney U test, P = 0.005), GRα D2 (Mann Whitney U test, P = 0.05) and cytoplasmic GRα D1 (Mann Whitney U test, P = 0.03) and unknown 48 kDa protein (Mann Whitney U test, P = 0.05) relative to AGA preterm placentae. Chorioamnioitis had no effect on placental GR isoform expression or % of isoforms expressed.
4. Correlations
A number of isoforms were negatively correlated with placental weight in female preterm placentae which include cytoplasmic GRα A (R = −0.553, P = 0.03, Spearman’s Correlation) GRα C (R = −0.612, P = 0.01, Spearman’s Correlation), GR P (R = −0.53, P = 0.03, Spearman’s Correlation) and 48 kDa (R = −0.613, P = 0.01 Spearman’s Correlation). Nuclear 38 kDa protein was negatively correlated with preterm female birthweight (R = −0.60, P = 0.01, Spearman’s Correlation). There were no correlations in male preterm placentae between placental weight or birthweight or birthweight centile and GR isoform expression.
5. Discussion
This study has demonstrated that there are 8 known isoforms of the glucocorticoid receptor present in the preterm placenta which varied in expression in relation to gestational age at delivery, mode of delivery, fetal sex, betamethasone exposure, fetal size and receptor location in the cytoplasm or nucleus. There were 5 unknown proteins that may be GR isoforms. Of particular interest was the predominance of translational isoforms; GRα C and D2, splice variant; GR A and the unknown 48 kDa protein identified in pre-term placentae relative to term placentae. Furthermore fetal sex was a key contributor to differences in GR expression where female preterm placental GR expression was dependent on birth weight, placental weight and betamethasone exposure but male preterm placental GR expression was unaltered by any of these factors. Previous work would suggest the placenta responds to glucocorticoids in a sex specific manner [8,9,19]. Specifically, placentae from females born preterm [16,39,40] adjust glucocorticoid metabolic capacity in response to excess maternal cortisol or exogenous glucocorticoids remaining sensitive to intracellular cortisol concentrations as reflected by changes in inflammatory cytokine gene expression [19]. Conversely male placentae appear to not alter glucocorticoid metabolic capacity or cytokine gene expression under the same conditions [9,19]. The current data suggests the sex specific placental response to maternal cortisol or betamethasone may be dependent on the expression of a combination of GR isoforms and indicates a greater level of complexity than previously considered when only GRα A was measured and studied. Furthermore we observed that each individual appears to have their own particular GR isoform profile which may be significant in understanding the future health of offspring exposed to excess glucocorticoid.
Interestingly GRα A did not change significantly in the analyses conducted on preterm placentae. Its expression was detectable in 57–70% of all preterm placentae which was surprising as it was expected to be found in 100% of placentae. Relative to the other isoforms its expression was minor constituting less than 2% of all isoforms expressed. The only observation associated with GRα A was that its expression was negatively correlated with preterm female placental weight suggesting cortisol may regulate placental growth via GRα A. Increased exposure of the placenta to cortisol in the absence of glucocorticoid metabolism results in decreased placental growth [41] and exposure to betamethasone for threatened preterm labour can reduce placental size slightly by 6% [42] which could be mediated by GRα A However other isoforms were also associated with placental weight including GRα C, GR P and 48 kDa suggesting an interaction between isoforms may be important in regulating placental size and growth.
GRα C expression was increased in male preterm placentae relative to term placentae and decreased in female preterm placentae. GRα C is the most potent activator of glucocorticoid induced apoptosis while GRα D isoforms are the least potent at inducing apoptosis [43]. Apoptosis induced by glucocorticoids is an intrinsic mechanism involving activation of the mitochondrial BIM pathway leading to caspase 9 and 3 activation and cell death [44]. Recent work by Whitehead et al., 2013 [45] reported increased markers of intrinsic apoptosis including BIM and BAD in the maternal circulation and the placenta of growth restricted preterm pregnancies. Similar studies report overexpression of some apoptotic genes in preterm placenta [46] and with premature rupture of membranes [47]. This may be significant in understanding the pathophysiology of male preterm delivery where an increase in glucocorticoid-induced cell death mediated by GRα C may be initiated prior to preterm labour. Given there was no association of GRα C with betamethasone administration or SGA in male placentae it would suggest GRα C is not up regulated under these conditions but further research will need to be conducted.
Several unknown isoforms were identified in both term and preterm placentae. The GR gene has multiple start codons present in exon 2 at residues 1, 27, 86, 90, 98, 316, 330 and 336 which allows ribosomes to initiate translation at different sites along the gene which results in the formation of the GRα isoforms; A, B, C1–3 and D1–3 [23]. However exon 2 is also present in the splice variants of GR and it is possible that translational isoforms for GR β, GR γ, GR A and GR P may exist and account for the presence of GR proteins at 69, 68, 60, 48 and 38 kDa. Given some of these unknown isoforms were altered in relation to fetal sex and gestational age it suggests they have some physiological activity. Furthermore, we have previously reported that when total GR gene translation is silenced, the protein expression of these unknown isoforms along with the known isoforms, are reduced supporting the hypothesis that all protein bands identified by Western are GR proteins [28] but confirmation is required as these unknown bands could also be degraded forms of GR.
Increased exposure to maternal glucocorticoids is known to be involved in alterations of fetal growth and development with long term impacts on later health [48,49]. There are many mechanisms involved in the regulation of fetal exposure to glucocorticoids which are altered in the presence of SGA or growth restricted fetus. In the maternal circulation corticosteroid binding globulin (CBG) regulates the circulating concentrations of free cortisol modulating the concentration of cortisol available to the placenta. In human pregnancies complicated by SGA, maternal CBG concentrations were significantly reduced [18]. Placental glucocorticoid metabolism by 11β hydroxysteroid dehydrogenase type 2 (11β HSD 2) has been shown to be reduced in the presence of a growth restricted fetus [50]. Wyrwoll et al. [41] examined the impact of knocking out placental 11β HSD 2 in the mouse placenta and reported that homozygous knock out mice had normal fetal growth due to compensatory increases in amino acid transport but reduced placental growth at embryonic day 15. However by day 18, fetal and placental growth were reduced in the homozygous mice due to reduced glucose transport, no change in amino acid transport and reduced placental vascular development. Similar findings have been reported in a number of animal models using excess glucocorticoid administration as an exposure [51]. Sex specific differences in the fetal response to betamethasone exposure have been demonstrated in the sheep with female birth weight reduced more than males with glucocorticoid exposure in both normal and growth restricted pregnancies [52]. We have identified that glycoprotein P, a glucocorticoid efflux pump present on the trophoblast apical membrane, was decreased in preterm placentae of pregnancies complicated by SGA [53]. These data suggest that many key mechanisms involved in protecting the fetus from glucocorticoids are compromised in SGA pregnancies resulting in the placenta and fetus being exposed to higher concentrations of steroid. However there have been limited studies examining the role of placental GR in pregnancies complicated by SGA.
A recent examination of human placentae from SGA term pregnancies identified increased expression of GRβ, GRα D1, 38 and 48 kDa unknown GR proteins [28]. Conversely, in preterm placentae of SGA pregnancies, female placentae had decreased expression of GR A, GRα D2, GRα D1 and unknown 48 kDa isoform with no differences in GR expression for placentae of males with SGA. All of the known GR isoforms altered in SGA placentae of both term and preterm pregnancies have been reported to either inhibit or mediate a hyposensitive response to glucocorticoid in other organs. For example, GRβ is well characterised as an antagonist of GRα A involved in mediating glucocorticoid resistance [54,55]. GR A is a splice variant first identified in myeloma cells that is missing exons 5 to 7 which encodes the ligand binding domain and therefore has low transactivational activity [56]. GRα D translational isoforms are associated with a hyposensitive response to glucocorticoids, but can function in the absence of glucocorticoid to activate GR regulated pathways [55]. The data suggest that the SGA placentae reduce sensitivity to glucocorticoids as gestation progresses and part of the pathophysiology of SGA may depend on the level of expression and function of GR isoforms associated with low transactivational activities.
Betamethasone exposure was not associated with a significant number of alterations in GR expression with the 48 kDa protein being the only protein to change in female preterm placentae. This is in line with other human studies that indicated betamethasone had no effect on GRα or GRβ in preterm placentae [57]. Other glucocorticoid-regulated placental mechanisms are different following the timing of exposure to betamethasone and numerous in vitro studies confirm the placenta is responsive to glucocorticoids [58]. We have identified that timing of exposure to betamethasone is associated with greater oxidative stress in male preterm placentae [39], increased markers of vasoconstriction including normetanephrine and endothelin-1 in female preterm placentae [40] and increased 11β HSD 2 activity in female placentae [16]. In animal models betamethasone exposure during pregnancy results in altered placental vascular development [59] and altered GR expression fetal organs such as the hippocampus [60]. A weakness in the current study is that we did not have data on the length of time between the last betamethasone dose and delivery which may influence GR expression. Previously we have shown that when betamethasone is still bioactive at less than 72 h after the last dose of betamethasone there are alterations in markers of fetal adrenal function and placental glucocorticoid metabolism [16]. It is possible that sex specific differences in the placental response to glucocorticoid are not conferred by a change in the expression of one single GR isoform but regulated by the combination of GR isoforms expressed. Thus the response to betamethasone may be dependent on the expression pattern of all GR isoforms rather than one single isoform.
Understanding the clinical relevance of the GR isoform expression will provide an understanding of how changes in the GR proteomic profile within individuals or within groups can drive differences in the response to cortisol and affect perinatal outcome and potentially, long term health outcomes. In this current study we have demonstrated that GR isoform expression varies in pre-term placentae in relation to gestational age at delivery, fetal sex and birthweight but are unaffected by exposure to betamethasone.
Acknowledgments
Funding support
VC salary is funded by the National Health and Medical Research Council Senior Research Fellowship (APP1041918) and NH salary is funded by the National Health and Medical Research Council Peter Doherty Training Fellowship (ID 1016379). This research work was funded by the University of Adelaide.
Footnotes
Disclosure statement
The authors have nothing to disclose.
References
- 1.Gluckman PD, Cutfield W, Hofman P, Hanson MA. The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum Dev. 2005;81(1):51–9. doi: 10.1016/j.earlhumdev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Moss TJ. Respiratory consequences of preterm birth. Clin Exp Pharmacol Physiol. 2006;33(3):280–4. doi: 10.1111/j.1440-1681.2006.04359.x. [DOI] [PubMed] [Google Scholar]
- 3.Erni K, Shaqiri-Emini L, La Marca R, Zimmermann R, Ehlert U. Psychobiological effects of prenatal glucocorticoid exposure in 10-year-old-children. Front Psychiatry. 2012;3:104. doi: 10.3389/fpsyt.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ter Wolbeek M, de Sonneville LM, de Vries WB, Kavelaars A, Veen S, Kornelisse RF, et al. Early life intervention with glucocorticoids has negative effects on motor development and neuropsychological function in 14–17 year-old adolescents. Psychoneuroendocrinology. 2012;38(7):975–86. doi: 10.1016/j.psyneuen.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Huh SY, Andrew R, Rich-Edwards JW, Kleinman KP, Seckl JR, Gillman MW. Association between umbilical cord glucocorticoids and blood pressure at age 3 years. BMC Med. 2008;6:25. doi: 10.1186/1741-7015-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moritz KM, Johnson K, Douglas-Denton R, Wintour EM, Dodic M. Maternal glucocorticoid treatment programs alterations in the renin-angiotensin system of the ovine fetal kidney. Endocrinology. 2002;143(11):4455–63. doi: 10.1210/en.2002-220534. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93(446):26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 8.Scott NM, Hodyl NA, Murphy VE, Osei-Kumah A, Wyper H, Hodgson DM, et al. Placental cytokine expression covaries with maternal asthma severity and fetal sex. J Immunol. 2009;182(3):1411–20. doi: 10.4049/jimmunol.182.3.1411. [DOI] [PubMed] [Google Scholar]
- 9.Murphy VE, Gibson PG, Giles WB, Zakar T, Smith R, Bisits AM, et al. Maternal asthma is associated with reduced female fetal growth. Am J Respir Crit Care Med. 2003;168(11):1317–23. doi: 10.1164/rccm.200303-374OC. [DOI] [PubMed] [Google Scholar]
- 10.Hodyl NA, Stark MJ, Osei-Kumah A, Bowman M, Gibson P, Clifton VL. Fetal glucocorticoid-regulated pathways are not affected by inhaled corticosteroid use for asthma during pregnancy. Am J Respir Crit Care Med. 2011;183(6):716–22. doi: 10.1164/rccm.201007-1188OC. [DOI] [PubMed] [Google Scholar]
- 11.Hodyl NA, Wyper H, Osei-Kumah A, Scott N, Murphy VE, Gibson P, et al. Sex-specific associations between cortisol and birth weight in pregnancies complicated by asthma are not due to differential glucocorticoid receptor expression. Thorax. 2010;65(8):677–83. doi: 10.1136/thx.2009.123091. [DOI] [PubMed] [Google Scholar]
- 12.Challis J, Newnham J, Petraglia F, Yeganegi M, Bocking A. Fetal sex and pre-term birth. Placenta. 2013;34(2):95–9. doi: 10.1016/j.placenta.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Morrison JL, Botting KJ, Soo PS, McGillick EV, Hiscock J, Zhang S, et al. Antenatal steroids and the IUGR fetus: are exposure and physiological effects on the lung and cardiovascular system the same as in normally grown fetuses? J Pregnancy. 2012;2012:839656. doi: 10.1155/2012/839656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waddell BJ, Hisheh S, Dharmarajan AM, Burton PJ. Apoptosis in rat placenta is zone-dependent and stimulated by glucocorticoids. Biol Reprod. 2000;63(6):1913–7. doi: 10.1095/biolreprod63.6.1913. [DOI] [PubMed] [Google Scholar]
- 15.Tare M, Miller SL, Wallace EM, Sutherland AE, Yawno T, Coleman HA, et al. Glucocorticoid treatment does not alter early cardiac adaptations to growth restriction in preterm sheep fetuses. BJOG. 2012;119(8):906–14. doi: 10.1111/j.1471-0528.2012.03309.x. [DOI] [PubMed] [Google Scholar]
- 16.Stark MJ, Wright IM, Clifton VL. Sex-specific alterations in placental 11beta-hydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. Am J Physiol Regul Integr Comp Physiol. 2009;297(2):R510–4. doi: 10.1152/ajpregu.00175.2009. [DOI] [PubMed] [Google Scholar]
- 17.Hodyl NA, Stark MJ, Butler M, Clifton VL. Placental P-glycoprotein is unaffected by the timing of antenatal glucocorticoid therapy but reduced in SGA preterm infants. Placenta. 2013;34(4):325–30. doi: 10.1016/j.placenta.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Ho JT, Lewis JG, O’Loughlin P, Bagley CJ, Romero R, Dekker GA, et al. Reduced maternal corticosteroid-binding globulin and cortisol levels in pre-eclampsia and gamete recipient pregnancies. Clin Endocrinol (Oxf) 2007;66(6):869–77. doi: 10.1111/j.1365-2265.2007.02826.x. [DOI] [PubMed] [Google Scholar]
- 19.Scott NM, Hodyl NA, Osei-Kumah A, Stark MJ, Smith R, Clifton VL. The presence of maternal asthma during pregnancy suppresses the placental pro-inflammatory response to an immune challenge in vitro. Placenta. 2011;32(6):454–61. doi: 10.1016/j.placenta.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Turner JD, Muller CP. Structure of the glucocorticoid receptor (NR3C1) gene 5′ untranslated region: identification, and tissue distribution of multiple new human exon 1. J Mol Endocrinol. 2005;35(2):283–92. doi: 10.1677/jme.1.01822. [DOI] [PubMed] [Google Scholar]
- 21.Turner JD, Schote AB, Keipes M, Muller CP. A new transcript splice variant of the human glucocorticoid receptor: identification and tissue distribution of hGR Delta 313–338, an alternative exon 2 transactivation domain isoform. Ann N Y Acad Sci. 2007;1095:334–41. doi: 10.1196/annals.1397.037. [DOI] [PubMed] [Google Scholar]
- 22.Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, et al. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318(6047):635–41. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell. 2005;18(3):331–42. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Lu NZ, Collins JB, Grissom SF, Cidlowski JA. Selective regulation of bone cell apoptosis by translational isoforms of the glucocorticoid receptor. Mol Cell Biol. 2007;27(20):7143–60. doi: 10.1128/MCB.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haarman EG, Kaspers GJ, Pieters R, Rottier MM, Veerman AJ. Glucocorticoid receptor alpha, beta and gamma expression vs in vitro glucocorticod resistance in childhood leukemia. Leukemia. 2004;18(3):530–7. doi: 10.1038/sj.leu.2403225. [DOI] [PubMed] [Google Scholar]
- 26.Beger C, Gerdes K, Lauten M, Tissing WJ, Fernandez-Munoz I, Schrappe M, et al. Expression and structural analysis of glucocorticoid receptor isoform gamma in human leukaemia cells using an isoform-specific real-time polymerase chain reaction approach. Br J Haematol. 2003;122(2):245–52. doi: 10.1046/j.1365-2141.2003.04426.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnson RF, Rennie N, Murphy V, Zakar T, Clifton V, Smith R. Expression of glucocorticoid receptor messenger ribonucleic acid transcripts in the human placenta at term. J Clin Endocrinol Metab. 2008;93(12):4887–93. doi: 10.1210/jc.2008-1077. [DOI] [PubMed] [Google Scholar]
- 28.Saif Z, Hodyl NA, Hobbs E, Tuck AR, Butler MS, Osei-Kumah A, et al. The human placenta expresses multiple glucocorticoid receptor isoforms that are altered by fetal sex, growth restriction and maternal asthma. Placenta. 2014;35(4):260–8. doi: 10.1016/j.placenta.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Wu I, Shin SC, Cao Y, Bender IK, Jafari N, Feng G, et al. Selective glucocorticoid receptor translational isoforms reveal glucocorticoid-induced apoptotic transcriptomes. Cell Death Dis. 2013;4:e453. doi: 10.1038/cddis.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Y, Bender IK, Konstantinidis AK, Shin SC, Jewell CM, Cidlowski JA, et al. Glucocorticoid receptor translational isoforms underlie maturational stage-specific glucocorticoid sensitivities of dendritic cells in mice and humans. Blood. 2013;121(9):155–62. doi: 10.1182/blood-2012-05-432336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy VE, Gibson P, Talbot PI, Clifton VL. Severe asthma exacerbations during pregnancy. Obstet Gynecol. 2005;106(5):1046–54. doi: 10.1097/01.AOG.0000185281.21716.02. [DOI] [PubMed] [Google Scholar]
- 32.Stark MJ, Dierkx L, Clifton VL, Wright IM. Alterations in the maternal peripheral microvascular response in pregnancies complicated by preeclampsia and the impact of fetal sex. J Soc Gynecol Investig. 2006;13(8):573–8. doi: 10.1016/j.jsgi.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Stark MJ, Clifton VL, Wright IM. Neonates born to mothers with preeclampsia exhibit sex-specific alterations in microvascular function. Pediatr Res. 2009;65(3):292–5. doi: 10.1203/pdr.0b013e318193edf1. [DOI] [PubMed] [Google Scholar]
- 34.Stark MJ, Clifton VL, Wright IM. Microvascular flow, clinical illness severity and cardiovascular function in the preterm infant. Arch Dis Child Fetal Neonatal Ed. 2008;93(4):F271–4. doi: 10.1136/adc.2007.123539. [DOI] [PubMed] [Google Scholar]
- 35.Stark MJ, Clifton VL, Wright IM. Sex-specific differences in peripheral micro-vascular blood flow in preterm infants. Pediatr Res. 2008;63(4):415–9. doi: 10.1203/01.pdr.0000304937.38669.63. [DOI] [PubMed] [Google Scholar]
- 36.Wysocka J, Reilly PT, Herr W. Loss of HCF-1-chromatin association precedes temperature-induced growth arrest of tsBN67 cells. Mol Cell Biol. 2001;21(11):3820–9. doi: 10.1128/MCB.21.11.3820-3829.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy VE, Zakar T, Smith R, Giles WB, Gibson PG, Clifton VL. Reduced 11beta-hydroxysteroid dehydrogenase type 2 activity is associated with decreased birth weight centile in pregnancies complicated by asthma. J Clin Endocrinol Metab. 2002;87(4):1660–8. doi: 10.1210/jcem.87.4.8377. [DOI] [PubMed] [Google Scholar]
- 38.Clifton VL, Murphy VE. Maternal asthma as a model for examining fetal sex-specific effects on maternal physiology and placental mechanisms that regulate human fetal growth. Placenta. 2004;25(Suppl A):S45–52. doi: 10.1016/j.placenta.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Stark MJ, Hodyl NA, Wright IMR, Clifton VL. Influence of sex and glucocorticoid exposure on preterm placental pro-oxidant-antioxidant balance. Placenta. 2011;32(11):865–70. doi: 10.1016/j.placenta.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Stark MJ, Hodyl NA, Wright IM, Clifton V. The influence of sex and antenatal betamethasone exposure on vasoconstrictors and the preterm microvasculature. J Matern Fetal Neonatal Med. 2011;24(10):1215–20. doi: 10.3109/14767058.2011.569618. [DOI] [PubMed] [Google Scholar]
- 41.Wyrwoll CS, Seckl JR, Holmes MC. Altered placental function of 11beta-hydroxysteroid dehydrogenase 2 knockout mice. Endocrinology. 2009;150(3):1287–93. doi: 10.1210/en.2008-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braun T, Husar A, Challis JR, Dudenhausen JW, Henrich W, Plagemann A, et al. Growth restricting effects of a single course of antenatal betamethasone treatment and the role of human placental lactogen. Placenta. 2013;34(5):407–15. doi: 10.1016/j.placenta.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Gross KL, Lu NZ, Cidlowski JA. Molecular mechanisms regulating glucocorticoid sensitivity and resistance. Mol Cell Endocrinol. 2009;300(1–2):7–16. doi: 10.1016/j.mce.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gruver-Yates AL, Cidlowski JA. Tissue-specific actions of glucocorticoids on apoptosis: a double-edged sword. Cells. 2013;2(2):202–23. doi: 10.3390/cells2020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitehead CL, Walker SP, Lappas M, Tong S. Circulating RNA coding genes regulating apoptosis in maternal blood in severe early onset fetal growth restriction and pre-eclampsia. J Perinatol. 2013;33(8):600–4. doi: 10.1038/jp.2013.16. [DOI] [PubMed] [Google Scholar]
- 46.Demendi C, Borzsonyi B, Vegh V, Nagy ZB, Rigo J, Jr, Pajor A, et al. Gene expression patterns of the Bcl-2 and Bax genes in preterm birth. Acta Obstet Gynecol Scand. 2012;91(10):1212–7. doi: 10.1111/j.1600-0412.2012.01428.x. [DOI] [PubMed] [Google Scholar]
- 47.Saglam A, Ozgur C, Derwig I, Unlu BS, Gode F, Mungan T. The role of apoptosis in preterm premature rupture of the human fetal membranes. Arch Gynecol Obstet. 2013;288(3):501–5. doi: 10.1007/s00404-013-2774-3. [DOI] [PubMed] [Google Scholar]
- 48.Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 2: mechanisms. Nat Rev Endocrinol. 2014;10(7):403–11. doi: 10.1038/nrendo.2014.74. [DOI] [PubMed] [Google Scholar]
- 49.Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 1: outcomes. Nat Rev Endocrinol. 2014;10(7):391–402. doi: 10.1038/nrendo.2014.73. [DOI] [PubMed] [Google Scholar]
- 50.Shams M, Kilby MD, Somerset DA, Howie AJ, Gupta A, Wood PJ, et al. 11Beta-hydroxysteroid dehydrogenase type 2 in human pregnancy and reduced expression in intrauterine growth restriction. Hum Reprod. 1998;13(4):799–804. doi: 10.1093/humrep/13.4.799. [DOI] [PubMed] [Google Scholar]
- 51.Fowden AL, Forhead AJ, Coan PM, Burton GJ. The placenta and intrauterine programming. J Neuroendocrinol. 2008;20(4):439–50. doi: 10.1111/j.1365-2826.2008.01663.x. [DOI] [PubMed] [Google Scholar]
- 52.Miller SL, Sutherland AE, Supramaniam VG, Walker DW, Jenkin G, Wallace EM. Antenatal glucocorticoids reduce growth in appropriately grown and growth-restricted ovine fetuses in a sex-specific manner. Reprod Fertil Dev. 2012;24(5):753–8. doi: 10.1071/RD11143. [DOI] [PubMed] [Google Scholar]
- 53.Hodyl NA, Stark MJ, Butler M, Clifton VL. Placental P-glycoprotein is unaffected by timing of antenatal glucocorticoid therapy but reduced in SGA preterm infants. Placenta. 2013;34(4):325–30. doi: 10.1016/j.placenta.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Oakley RH, Jewell CM, Yudt MR, Bofetiado DM, Cidlowski JA. The dominant negative activity of the human glucocorticoid receptor beta isoform. Specificity and mechanisms of action. J Biol Chem. 1999;274(39):27857–66. doi: 10.1074/jbc.274.39.27857. [DOI] [PubMed] [Google Scholar]
- 55.Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132(5):1033–44. doi: 10.1016/j.jaci.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moalli PA, Pillay S, Krett NL, Rosen ST. Alternatively spliced glucocorticoid receptor messenger RNAs in glucocorticoid-resistant human multiple myeloma cells. Cancer Res. 1993;53(17):3877–9. [PubMed] [Google Scholar]
- 57.Johnstone JF, Bocking AD, Unlugedik E, Challis JR. The effects of chorioamnionitis and betamethasone on 11beta hydroxysteroid dehydrogenase types 1 and 2 and the glucocorticoid receptor in preterm human placenta. J Soc Gynecol Investig. 2005;12(4):238–45. doi: 10.1016/j.jsgi.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 58.Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev. 2006;27(2):141–69. doi: 10.1210/er.2005-0011. [DOI] [PubMed] [Google Scholar]
- 59.Hewitt DP, Mark PJ, Waddell BJ. Glucocorticoids prevent the normal increase in placental vascular endothelial growth factor expression and placental vascularity during late pregnancy in the rat. Endocrinology. 2006;147(12):75–81. doi: 10.1210/en.2006-0825. [DOI] [PubMed] [Google Scholar]
- 60.Sloboda DM, Moss TJ, Li S, Matthews SG, Challis JR, Newnham JP. Expression of glucocorticoid receptor, mineralocorticoid receptor, and 11beta-hydroxysteroid dehydrogenase 1 and 2 in the fetal and postnatal ovine hippocampus: ontogeny and effects of prenatal glucocorticoid exposure. J Endocrinol. 2008;197(2):213–20. doi: 10.1677/JOE-07-0375. [DOI] [PubMed] [Google Scholar]