Abstract
Functionalized carbon nanotubes display unique properties that enable a variety of medicinal applications, including the diagnosis and treatment of cancer, infectious diseases and central nervous system disorders, and applications in tissue engineering. These potential applications are particularly encouraged by their ability to penetrate biological membranes and relatively low toxicity.
Nanomaterials possess fundamentally new properties and functions resulting from their small dimensions [1]. According to some estimates, nanotechnology could exceed the impact of the Industrial Revolution on society and is projected to become a $2.6 trillion business in 2014 [2]. Carbon nanotubes (CNTs) were described in 1991 by Iijima [3]. There are two classes of CNTs (Fig. 1): single-walled nanotubes (SWCNTs), which consist of a single graphite sheet seamlessly wrapped into a cylindrical tube with a diameter between 0.4 and 2.5 nm, and multiwalled carbon nanotubes (MWCNTs), which comprise more layers of graphite sheet with different diameters of up to 100 nm. The length of the tubes ranges from a few nanometers to a few micrometers. Their unique structure offers CNTs excellent physical and chemical properties [4] that enable wide industrial applications.
FIGURE 1.
Molecular structure of SWCNT and MWCNT.
Applications of nanotechnology in medicine have prompted the development of nanoparticles, nanostructured surfaces and nanoanalytical techniques for rapid and early diagnostics, smart drug delivery and real-time assessments of therapeutic and surgical efficacy. These technologies are highly promising for the mitigation of patient risk and disease progression and the realization of personalized medicine (theranostics) [5]. Until now, two families of therapeutic nanocarriers – liposomes and albumin nanoparticles – have already been used in clinical practice worldwide, and many other therapeutic agents are in preclinical phases of development and clinical trials.
Being able to readily penetrate plasma membrane [6], CNTs possess a large loading capability to carry various bioactive agents, such as drugs. Their intrinsic spectroscopic properties, including Raman scattering and photoluminescence, can provide valuable means for tracking, detecting and imaging diseases. They can also help monitor in vivo therapy status, pharmacodynamical behavior and drug delivery efficiency. In addition, their unique optical and thermodynamic properties can be used directly in medical diagnostics and therapy.
Poor dispersibility of CNTs has been the greatest obstacle to their use in nanomedicine. Many functionalization routes have been developed in recent years to solubilize CNTs and improve their biocompatibility [4,7–10].
In the past decade, we have witnessed the rapid development of nanotechnology in many fields. For example, the applications of CNTs in medicine have been highlighted in several review papers with a focus on cancer treatment. In this review, we summarize several medical applications in addition to drug delivery and in treatments of several diseases. Our focus is on the progress of the functionalizations of CNTs, which are the preconditions for CNT applications in medicine, the potential applications of CNTs in the treatment of intractable issues in medicine and the associated potential risks of CNT applications in nanomedicine.
Functionalizations of CNTs
Smooth surfaces without any hanging bonds make pristine CNTs chemically inert and incompatible with nearly all organic and inorganic solvents; thus, a solution-based CNT process is difficult to achieve, and this has formed a major drawback for CNTs’ applications in nanomedicine. Recently, researches have found that carbon atoms in both SWCNTs and MWCNTs can to some extent exhibit chemical reactivity toward many reagents and so both CNTs can be considered as new macromolecular form of carbon. For the knowledge of general chemistry of CNTs, readers can refer to Niyogi's and Tasis's reviews [4,11]. After modifications, CNTs show increased solubility [12].
Functionalizations of CNTs can increase their water miscibility and improve biocompatibility. Covalent functionalizations and non-covalent functionalizations are two main strategies to increase their water miscibility. Covalent functionalizations have been nicknamed ‘defect functionalizations’ because only defective carbon atoms on the sidewall or at the end of CNTs can be oxidized by strong oxidants to generate carboxylic acid groups or carboxylated fractions, which can be chemically modified via amidation or esterification (see Ref. [13] for a review on these two modifications). Various polymers [13], metals [14] and biological molecules [15] can be grafted to the surface of carboxylated CNTs. Addition reactions were also used in covalent modifications of CNTs, which derived from those traditionally for graphite surfaces or established for fullerenes [4,16]. By addition reactions, systematic and predictable chemistry on nanotube surface is achieved. For example, azomethine ylides generated by 1,3-dipolar cycloaddition [17] are very reactive in generating pyrrolidine rings on the sidewall of CNTs. The potential as a drug delivery system is an attractive aspect of CNTs’ applications. Because covalent functionalizations of CNTs are robust and easy to control, they also offer the possibility of introducing multiple functionalities, which are particularly crucial to enable the functionalized CNTs to be used as a drug delivery system. Covalent functionalizations can also diversify CNTs’ surface properties, from which favorable CNTs can be selected. With some informatics platforms (e.g. Pipeline Pilot), the properties of molecules (made from building blocks) such as similarities, hydrophobicity, solubility and topological and stereochemical properties can be calculated and those building blocks that can result in diverse properties are selected. This computation-guided diversity design and techniques for combinatorial library synthesis can effectively modify CNTs’ surfaces to modulate their biological properties. Our group applied this strategy to construct a MWCNT library containing 80 members. Through multiple biological screenings, functionalized MWCNTs with reduced cytotoxicity and immune responses were discovered. This study indicated that the covalent surface modification combined with biological screening was a promising approach to reducing the potential toxicity of CNTs [18].
Non-covalent functionalizations are also highlighted in medical applications of CNTs. The conjugated electronic structure of nanotubes will not be impaired and some intrinsic physical properties of CNTs, such as near-infrared fluorescence and Raman scattering, can be reserved [19,20]. Two approaches have been taken for non-covalent functionalizations of CNTs. One is to wrap polymers around the CNT sidewall [8] and the other is through π–π stacking interaction between aromatic rings of the loaded materials and π-electrons of graphite sheets on the surface of CNTs [21–23]. Such non-covalent coatings will disrupt the van der Waals interactions that cause SWCNTs to aggregate into bundles [24,25] and might also improve their water miscibility. CNTs have a high affinity for single-stranded DNA and RNA and form an electrostatic complex with the polynucleotide molecules, which can not only impart aqueous solubility but also deliver genes into cells [6,26,27].
Owing to its biocompatibility and good solubility under various physiological conditions, poly(ethylene glycol) (PEG) is heavily used for various purposes in biomedical applications [28–32]. PEGylated CNTs are able to extend the blood circulating time [33], reduce the uptake by the reticuloendothelial system and block the nonspecific binding of serum proteins in mice [29]. It has been reported that when the molecular weight of the PEG chain was less than 2000, the PEGylation did not prevent cell uptake of SWCNTs, whereas longer chains could reduce the nonspecific cell uptake in vivo [29,34]. PEG grafted to lipidophilic polymers has better biological biocompatibility. SWCNT functionalized with PEG grafted to poly(γ-glutamic acid) and poly(maleic anhydride-alt-octadecene) exhibited a long blood circulation (t1/2 = 22.1 hours) [35]. Oligothiophene-terminated PEG synthesized by Lee et al. [23] was another PEG-based amphiphilic molecule, which could effectively disperse CNTs in aqueous solution with less PEG.
The encapsulation of materials in CNTs has attracted interest. Some metals and compounds have been placed into CNTs via physical and chemical processes [36–40]. One application of the technique is to drive metal-embedded CNTs using magnetic fields to target cells more efficiently [41].
After suitable modifications, CNTs can exhibit increased water miscibility and reduced toxicity [42]. As different bioactive agents are conjugated to CNTs, multi-functionalized drug delivery systems and theranostics that integrate imaging, drug delivery and targeting functions can be realized.
Potential clinical applications of CNTs
Cancer therapy
Cancer is one of the deadliest diseases in the world. Efficacy and specificity are two basic requirements for cancer therapy. The application of nanomaterials as drug carriers can improve anticancer therapeutic efficacy by both passive and active targeting mechanisms. In this regard, CNTs might provide new directions for cancer treatment.
Antitumor chemotherapy
Chemotherapy is currently an indispensable method of treating advanced tumors, besides surgical intervention and radiation. Traditional chemotherapy has been hindered because of two main roadblocks: low specificity of chemotherapeutic drugs, which leads to severe systemic toxicity, and drug resistance (whether acquired or intrinsic to cancerous cells) leading to low efficacy. CNTs have been explored as novel drug delivery vehicles with low toxicity and immunogenicity. Different types of therapeutic molecules have been reported to be delivered by CNTs, and some better outcomes than with traditional vehicles have been achieved. Readers can refer to Refs. [43–45] for reviews on this subject. When used in antitumor chemotherapy, the high surface area of CNTs allows for efficient loading of chemotherapy drugs [46]. Because the enhanced permeability and retention effect are universal in solid tumors [47], CNTs loaded with drugs (which can be regarded as macromolecular agents) can extravasate in tumor tissues over time; the concentration in tumor will reach several folds higher than that of the plasma. An accumulation of 13% of the injected PEGylated SWCNTs in tumor was reported by Dai's group [29]. The same group also conjugated paclitaxel to SWCNTs and delivered SWCNT–paclitaxel conjugate in vivo to achieve higher efficacy in suppressing tumor growth and avoided obvious toxic effects to normal organs in a murine 4T1 breast cancer model [33]. The higher therapeutic efficacy and lower side-effects could be attributed to prolonged blood circulation, higher tumor uptake (tenfold higher than Taxol) and slower release of drug from SWCNTs. Another widely used anticancer drug, cisplatin, has also been processed with SWCNT as ‘longboat delivery system’ and achieves increased uptake in cancerous cells [48].
CNTs functionalized with antibodies of antigens overexpressed on the cancerous cell surface or ligands recognizing specific receptors on the cancerous cell can be directed to the cancerous cell surface (Fig. 2). By virtue of endocytosis, the CNTs can be taken up by the cell before the chemotherapeutic drugs are cleaved off CNTs; thus, targeting delivery is realized. Folic acid [48,49], integrin antagonist [29], epidermal growth factor [50] and Herceptin (which can recognize the HER2/neu receptor on some breast cancer cells) [51] have been reported to be used as such targeting molecules.
FIGURE 2.
One example of CNT used as a drug carrier. Cisplatin is covalently ligated to surficially oxidized CNTs as an effective anti-tumor agent, and a folic acid molecule is further coupled to the cisplatin as a targeting molecule. The large surface area of CNTs makes it possible to carry more cisplatins into tumor cells. Reproduced, with permission, from Ref. [48]. Copyright 2008 American Chemical Society.
Antitumor immunotherapy
As an adjuvant anti-tumor treatment, antitumor immunotherapy is usually highlighted for fewer adverse effects, better patient tolerance and the potential to improve prognosis notably [52]. Although several clinical trials of immunotherapy have achieved promising results in treating malignancies [53,54], low treatment efficacy of immunotherapy remains a pressing issue [55]. Properties such as lower immunogenicity than common protein carriers, the ability to translocate across the cell membrane without causing toxicity and enhanced immune response when attached to an antigen have validated the use of CNTs as vaccine delivery tools [56]. In a pilot research by Yang's group [57], the conjugate of MWCNTs and tumor lysate protein (tumor cell vaccine) can considerably and specifically enhance the efficacy of antitumor immunotherapy in a mouse model bearing the H22 liver tumor. In vitro, CNTs conjugated to tumor immunogens can play a similar part as professional antigen-presenting cells (such as mature dendritic cells) to efficiently bring tumor antigens to immune effector T cells, owing to the high avidity of antigen on the surface and the negative charge [58] (Fig. 3). Adjuvant effects and complement system activation effects of CNTs have also been assumed as possibilities [59,60]; however, the mechanism remains unknown.
FIGURE 3.
SWNT bundles can present antibody with a higher local density to stimulate T cells to release Interleukin-2 (IL-2). Left: schematic representation of anti-CD3-adsorbed SWNT scaffolds inducing T-cell (B3Z cells) stimulation (not to scale). The SWNT scaffold is labeled as (SWNT), anti-CD3 as (Ab). Right: stimulating ability comparison of anti-CD3 absorbed by SWNT and soluble anti-CD3 to T cells. Nitric acid treatment followed by lithium borohydride reduction is to increase the suitable surface area for protein absorption. The amount of secreted IL-2 is used to quantify the response of T cells to anti-CD3. All groups were treated with the same amount of anti-CD3. Reproduced, with permission, from Ref. [58]. Copyright 2008 American Chemical Society.
Antitumor hyperthermia therapy
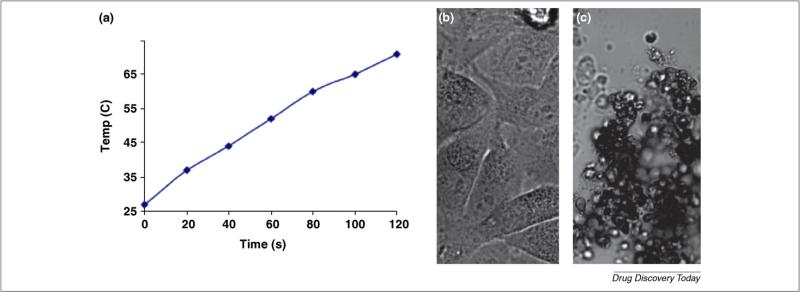
SWCNTs exhibit strong absorbance in the near-infrared region (NIR; 700–1100 nm) [61]. Coincidentally, biological systems are known to be highly transparent to the same spectral window. This can be used for optical imaging of nanotubes inside living cells, therefore, because of the low autofluorescence background of cells and tissues [51]. For example, it can be used to detect the pharmacokinetics of SWCNTs in vivo and is favorable compared with traditional fluorophores or radioactive labels for its simplicity and stability [62]. NIR radiation with laser pulse under controlled conditions could rupture the endosome and help the release of DNA carried by SWCNTs [49]. A related application of the NIR absorbance is the local thermal ablation of tumor cells by excessive heating of SWCNTs shackled in tumor cells (Fig. 4). Some progress in the technique has been achieved in recent years, and it has shown feasibility in clinical application [49,63,64].
FIGURE 4.
SWNT can absorb NIR radiation energy and cause local heating, which can lead to HeLa cell destruction. (a) Temperature evolution of a DNA-SWNT solution (approximately 25 mg/l) during continuous radiation by an 808 nm laser at 1.4 W/cm2 for two minutes. (b) Image of HeLa cells without internalized SWNTs after continuous 808 nm laser radiation at 3.5 W/cm2 for 5 min. No cell death was observed. (c) Image of dead and aggregated cells after internalization of DNA-SWNT and laser radiation at 1.4 W/cm2 for 2 min. The dead cells showed rounded and aggregated. Reproduced, with permission, from Ref. [49]. Copyright 2005 National Academy of Sciences, USA.
Other cancer treatment strategies
SWCNTs bind to the major groove of human telomeric i-motif, and the resultant electrostatic interactions between the positively charged C–C+ base pairs and the carboxyl groups on SWCNTs can increase i-motif stability [65].
SWCNTs can drive i-motif formation under cell-mimic crowding conditions and cause more water to be released, thereby driving i-motif formation [66]. In view of the significance of telomere, SWCNTs might have the potential to modulate the structure of human telomeric DNA in vivo, such as DNA B–A transitions and B–Z changes on SWCNTs in live cells, which could conceivably be used in cancer therapy in the future. Modulation of cellular signal transduction by nanoparticles can be achieved through interactions between nanoparticle and membrane-bound or cellular proteins. Our group found SWCNT–COOH was able to suppress the Smad-dependent bone morphogenetic protein signaling pathway and downregulate Id proteins via a non-apoptotic mechanism. This finding might have potential therapeutic applications in the treatment of cancers related to Id proteins or bone morphogenetic protein signaling, such as breast cancer [67].
Treatment of infectious diseases
Recent breakouts of SARS, avian flu and swine flu have indicated that infectious disease has become a critical public health issue with global concerns. Some infectious diseases, such as AIDS, have turned out to be deadly, and no effective therapies have been available to date. The medicinal application of nanotechnology has shed light on the quick diagnosis and effective therapy of infectious diseases. According to Kang et al. [68,69], pristine SWCNTs exhibited an antimicrobial effect in a size-dependent manner, indicating that they might be useful as building blocks for antimicrobial therapeutics. Organic modification on the surface of CNTs can generate sites for the attachment of bioactive molecules, the secondary structure of which can be preserved [70] and, hence, elicit specific anti-epitope antibodies [71,72]. The antibody recognition to the conjugates was facilitated by this coupling.
CNTs can also play a part in viral disease therapy by providing high-sensitive detecting devices. For example, a coordinated bio-sensor [73] made of Au nanoparticles and SWCNTs has been studied for detecting the nanomolar scale of HIV-1 PR, an aspartic protease responsible for virion assembly and maturation [74]. The realization of high-sensitive detection of this protease was promising to expedite development of effective HIV-1 PR inhibitors. Another example in viral disease diagnosis is the electrical detection of hepatitis C virus RNA [75]. A large surface-to-volume ratio and unique electronic properties made CNTs a welcome component for fabricating high-sensitive biodetectors, which were crucially needed in the diagnosis of viral diseases and the development of new anti-viral drugs. It was predictable, therefore, that CNTs might contribute considerably to the treatment of infectious diseases in the future.
Treatment of central nervous system disorders
Central nervous system (CNS) disorders consist of neurodegenerative diseases and brain tumors. Because of the unique and complicated environment and the restricted anatomical access (blood–brain barrier) of the CNS, it is more difficult to diagnose and treat CNS disorders than any other diseases. Nanotechnology is promising to revolutionize the status quo in this field. Because of their tiny dimensions and accessible external or exterior modifications, nanomaterials are able to cross the blood–brain barrier by various targeting mechanisms and, thus, they can act as effective delivery carriers for targeting brain. Many nanomaterials have been used successfully as suitable delivery systems for treating neurodegenerative diseases or brain tumors [76–79]. As a promising biomedical material, CNTs have been used in neurosciences [80–82]. The results of these studies have indicated that both pristine and chemically functionalized CNTs have positive effects on neuronal growth, although they also demonstrate cytotoxicity. One study has reported that the charge on the nanotubes could be manipulated to control neurite outgrowth [83], and it has been reported that CNTs functionalized with nerve growth factor or brain-derived neurotrophic factor could stimulate growth of neurons on the nanotube scaffold [84]. Keefer et al. [85] proved that conventional tungsten and stainless steel wire electrodes coated with CNTs could enhance both recording and electrical stimulation of neurons in culture, rats and monkeys. The benefits can be attributed to the capacity of CNTs to decrease electrode impedance and increase charge transfer. CNT-coated electrodes are expected to improve current electrophysiological techniques and to promote the development of long-lasting brain–machine interfacing devices, both of which will aid the diagnosis and treatment of CNS disorders.
Recent studies have also suggested that the nanoparticle-based formulations of some chemotherapeutic agents (e.g. doxorubicin [86]) have potential for the systemic chemotherapy of brain tumors with higher efficacy than the free agents. MWCNTs (p-MWCNTs or MWCNTs functionalized with DNA and siRNA) were found to be internalized by brain microglia (macrophage derived from migratory monocytes in brain that are believed to be an effective target for brain cancer treatment) in vitro and in vivo without inducing proliferative and cytokine changes [87,88]. These results indicated that MWCNTs could act as a safe nanovector delivery system for immunotherapies of brain cancers (e.g. gliomas). This field is still in its early infancy, and additional research is needed.
Applications in tissue engineering
The goal of tissue engineering is to replace diseased or damaged tissue with biologic substitutes that can restore and maintain normal functions [89,90]. Major advances in the knowledge of cell and organ transplantation and of chemistry of CNTs in recent years have aided in the sustained development of CNT-based tissue engineering and regenerative medicine. In this field, CNTs can be used as additives to reinforce the mechanical strength of tissue scaffolding [91] and conductivity by dispersing a small fraction of CNTs into a polymer [92] or to improve the benefits of native extracellular matrix (Fig. 5). For example, hybrid hyaluronic acid hydrogels with 2 wt% SWCNTs cross-linked by divinyl sulfone produced 300% enhancement in the storage modulus compared with hyaluronic acid [91]. To fully offer the mechanical and electrical properties compared with pure CNTs, CNT-based scaffolds have been developed [93,94]. These scaffolds can be used as molecular-level building blocks for the complex and miniaturized medical devices, which have enormous applications in biomedicine [95]. Research had shown that matrix containing CNTs had positive impacts on cell proliferation and differentiation [93,96–99]. In one such study, CNTs/chitosan scaffold absorbing recombinant human bone morphogenetic protein-2 could promote ectopic bone formation and most of the disassembled scaffold structures could, ultimately, be eliminated through the renal excretion route [98], which validated the safe application of CNTs in the artificial tissue. Until now, substrates prepared from CNTs (MWCNTs and SWCNTs) have been consistently reported to be biocompatible platforms for neuronal growth and differentiation [81,100,101] and directing bone formation [98,102].
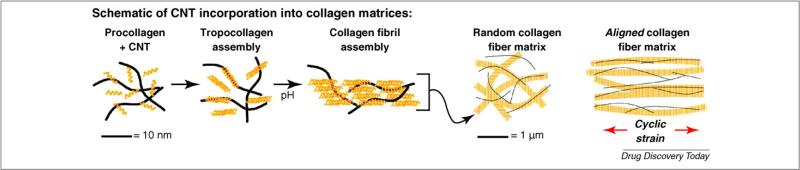
FIGURE 5.
Application of CNTs in tissue engineering. In this example, the self-assembly features of the structural protein collagen are combined with CNTs to produce a new class of mechanically robust and electrically conductive biomaterials. It harnesses the biochemical process of collagen fibrillogenesis and the biological process of cell-mediated collagen remodeling to induce alignment of CNTs in collagen matrices. Such CNT-collagen biomaterials can be used to develop nerve guidance materials for neural repair. Courtesy of J. Stegemann, University of Michigan.
Other applications of CNTs concerning tissue engineering include cell tracking and labeling, sensing cellular behavior, augmenting cellular behavior, and enhancing tissue matrices. For a panorama of the field, see Ref. [103].
Potential risks
The applications of nano-scale materials in nanomedicine are still in their infancy and facing some challenges [5]. Although intensive study of biodistribution and in vitro and in vivo toxicity of CNTs has been performed almost at the same time as their applications have been developed, our knowledge about their in vivo fates and potential health risks after exposure through various routes is still limited by the insufficient data and discrepancies of these results. Before the nature of CNTs is fully addressed, their promise in medicinal applications will be impaired by the issue of their potential risks. Most of the reported toxicological data are based on in vitro experiments with cell lines and under extraordinarily high doses, so we must be very cautious to extrapolate these results to human (see Refs. [45,104,105] for reviews). Recently, progresses have been made in the biodistribution, translocation and elimination of CNTs after systemic administration, making the first step toward safe medical applications of CNTs [106,107]. Research by us [18] and others [97] has demonstrated that toxicity and biocompatibility of CNTs can be controlled intentionally by chemical and material modifications. For an updated view of this area, please refer to several recent reviews on the topic [59,108–114].
Concluding remarks
In summary, chemically modifiable surfaces with a large surface area and tunable length, as well as unique physical properties, make CNTs welcome candidates in medicine. CNTs’ potential as drug carriers in targeted delivery has made them promising candidates for the diagnosis and treatment of refractory diseases such as cancer, CNS disorders and infectious diseases. Their unique mechanical properties also make them primary investigative topics in tissue engineering. Research on the potential toxicity of CNTs is still underway, but they have been widely accepted as a safer option than other nano-materials, such as quantum dots, or other drug carriers, such as virus carriers. Thus, the in vivo ADME profile of CNTs is still needed to realize their safe and efficient application in nanomedicine.
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program 2010CB933504), National Science Foundation of China (90913006), National Cancer Institute (P30CA021765) in the USA, the American Lebanese Syrian Associated Charities (ALSAC) and St. Jude Children's Research Hospital.
Biography
 YI ZHANG graduated in 2001 from the Hubei Polytechnic University with BS in Biological Engineering. He completed his Masters Degree at the School of Life Science, Shandong University in 2004. Since 2007, he has been a Ph.D. student in medicinal chemistry under the supervision of Prof. Bing Yan at Shandong University. His primary interests are in two areas, the first one is focused on the potential toxicology of carbon nanotubes, and the other is in the biomedical applications of carbon nanotubes and other nano-scale materials.
YI ZHANG graduated in 2001 from the Hubei Polytechnic University with BS in Biological Engineering. He completed his Masters Degree at the School of Life Science, Shandong University in 2004. Since 2007, he has been a Ph.D. student in medicinal chemistry under the supervision of Prof. Bing Yan at Shandong University. His primary interests are in two areas, the first one is focused on the potential toxicology of carbon nanotubes, and the other is in the biomedical applications of carbon nanotubes and other nano-scale materials.
 YUHONG BAI, born in 1983, received his B.S. in biology in 2005 from Shandong University in Jinan, China. Since 2006 he has been a graduate student pursuing M.S. and Ph.D. in drug toxicology under the supervision of Prof. Bing Yan at Shandong University. His research is on the reproductive toxicity of nanomaterials.
YUHONG BAI, born in 1983, received his B.S. in biology in 2005 from Shandong University in Jinan, China. Since 2006 he has been a graduate student pursuing M.S. and Ph.D. in drug toxicology under the supervision of Prof. Bing Yan at Shandong University. His research is on the reproductive toxicity of nanomaterials.
 BING YAN got his Ph.D. from Columbia University with Koji Nakanishi in 1990. He was a postdoctoral fellow at University of Cambridge from 1990 to 1991, and at University of Texas Medical School in Houston from 1991 to 1993. From 1993 to 2005, he worked at Novartis, Discovery Partners International, and Bristol-Myers Squibb. Now he is a faculty member at Department of Chemical Biology and Therapeutics, St. Jude Children's Research Hospital in Memphis, Tennessee and professor at Shandong University. He has published or edited 10 books and more than 120 peer reviewed papers. He is the editor of book series ‘Critical Reviews in Combinatorial Chemistry’ published by Francis & Taylor Group. He also serves as associate editor for ‘Journal of Combinatorial Chemistry’ published by American Chemical Society.
BING YAN got his Ph.D. from Columbia University with Koji Nakanishi in 1990. He was a postdoctoral fellow at University of Cambridge from 1990 to 1991, and at University of Texas Medical School in Houston from 1991 to 1993. From 1993 to 2005, he worked at Novartis, Discovery Partners International, and Bristol-Myers Squibb. Now he is a faculty member at Department of Chemical Biology and Therapeutics, St. Jude Children's Research Hospital in Memphis, Tennessee and professor at Shandong University. He has published or edited 10 books and more than 120 peer reviewed papers. He is the editor of book series ‘Critical Reviews in Combinatorial Chemistry’ published by Francis & Taylor Group. He also serves as associate editor for ‘Journal of Combinatorial Chemistry’ published by American Chemical Society.
References
- 1.Roco MC, et al. Nanotechnology Research Directions. Kluwer Academic Publishers; 2000. Biological, medical and health applications. [Google Scholar]
- 2.Lux Research Inc. The Nanotech Report - Investment Overview and Market Research for Nanotechnology. (4th Edn) 2006 [Google Scholar]
- 3.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- 4.Niyogi S, et al. Chemistry of single-walled carbon nanotubes. Acc. Chem. Res. 2002;35:1105–1113. doi: 10.1021/ar010155r. [DOI] [PubMed] [Google Scholar]
- 5.Sanhai WR, et al. Seven challenges for nanomedicine. Nat. Nanotechnol. 2008;3:242–244. doi: 10.1038/nnano.2008.114. [DOI] [PubMed] [Google Scholar]
- 6.Pantarotto D, et al. Functionalized carbon nanotubes for plasmid DNA gene delivery. Angew. Chem. Int. Ed. 2004;43:5242–5246. doi: 10.1002/anie.200460437. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y-T, et al. Noncovalent surface modification of carbon nanotubes for solubility in organic solvents. Carbon. 2006;44:1613–1616. [Google Scholar]
- 8.Star A, et al. Preparation and properties of polymer-wrapped single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2001;40:1721–1725. doi: 10.1002/1521-3773(20010504)40:9<1721::aid-anie17210>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Tasis D, et al. Soluble carbon nanotubes. Chem. Eur. J. 2003;9:4000–4008. doi: 10.1002/chem.200304800. [DOI] [PubMed] [Google Scholar]
- 10.Lee G-W, et al. Structural characterization of carboxylated multi-walled carbon nanotubes. Thin Solid Films. 2008;516:5781–5784. [Google Scholar]
- 11.Tasis D, et al. Chemistry of carbon nanotubes. Chem. Rev. 2006;106:1105–1136. doi: 10.1021/cr050569o. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, et al. Solution properties of single-walled carbon nanotubes. Science. 1998;282:95–98. doi: 10.1126/science.282.5386.95. [DOI] [PubMed] [Google Scholar]
- 13.Sun YP, et al. Functionalized carbon nanotubes: properties and applications. Acc. Chem. Res. 2002;35:1096–1104. doi: 10.1021/ar010160v. [DOI] [PubMed] [Google Scholar]
- 14.Lordi V, et al. Method for supporting platinum on single-walled carbon nanotubes for a selective hydrogenation catalyst. Chem. Mater. 2001;13:733–737. [Google Scholar]
- 15.Huang W, et al. Attaching proteins to carbon nanotubes via diimide-activated amidation. Nano Lett. 2002;2:311–314. [Google Scholar]
- 16.Dyke CA, Tour JM. Overcoming the insolubility of carbon nanotubes through high degrees of sidewall functionalization. Chem. Eur. J. 2004;10:812–817. doi: 10.1002/chem.200305534. [DOI] [PubMed] [Google Scholar]
- 17.Hudson JL, et al. Water-soluble, exfoliated, nonroping single-wall carbon nanotubes. J. Am. Chem. Soc. 2004;126:11158–11159. doi: 10.1021/ja0467061. [DOI] [PubMed] [Google Scholar]
- 18.Zhou H, et al. A nano-combinatorial library strategy for the discovery of nanotubes with reduced protein-binding, cytotoxicity, and immune response. Nano Lett. 2008;8:859–865. doi: 10.1021/nl0730155. [DOI] [PubMed] [Google Scholar]
- 19.Yue G, et al. Generation of continuous and pulsed diagnostic imaging x-ray radiation using a carbon-nanotube-based field-emission cathode. Appl. Phys. Lett. 2002;81:355–358. [Google Scholar]
- 20.Choi JH, et al. Multimodal biomedical imaging with asymmetric single-walled carbon nanotube/iron oxide nanoparticle complexes. Nano Lett. 2007;7:861–867. doi: 10.1021/nl062306v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrov P, et al. Noncovalent functionalization of multi-walled carbon nanotubes by pyrene containing polymers. Chem. Commun. 2003;17:2094–2095. doi: 10.1039/b307751a. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, et al. Noncovalent engineering of carbon nanotube surfaces by rigid, functional conjugated polymers. J. Am. Chem. Soc. 2002;124:9034–9035. doi: 10.1021/ja026104m. [DOI] [PubMed] [Google Scholar]
- 23.Lee JU, et al. Aqueous suspension of carbon nanotubes via non-covalent functionalization with oligothiophene-terminated poly(ethylene glycol). Carbon. 2007;45:1051–1057. [Google Scholar]
- 24.Curran S, et al. Evolution and evaluation of the polymer/nanotube composite. Synth. Met. 1999;103:2559–2562. [Google Scholar]
- 25.Curran SA, et al. A composite from poly (m-phenylenevinyleneco-2,5-dioctoxy-p-phenylenevinylene) and carbon nanotubes: a novel material for molecular optoelectronics. Adv. Mater. 1998;10:1091–1093. [Google Scholar]
- 26.Singh R, et al. Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: toward the construction of nanotube-based gene delivery vectors. J. Am. Chem. Soc. 2005;127:4388–4396. doi: 10.1021/ja0441561. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, et al. Polyethylenimine-grafted multiwalled carbon nanotubes for secure noncovalent immobilization and efficient delivery of DNA. Angew. Chem. Int. Ed. 2005;44:4782–4785. doi: 10.1002/anie.200500042. [DOI] [PubMed] [Google Scholar]
- 28.Chen RJ, et al. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4984–4989. doi: 10.1073/pnas.0837064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat. Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 30.Fernando KAS, et al. High aqueous solubility of functionalized single-walled carbon nanotubes. Langmuir. 2004;20:4777–4778. doi: 10.1021/la036217z. [DOI] [PubMed] [Google Scholar]
- 31.Shim M, et al. Functionalization of carbon nanotubes for biocompatibility and biomolecular recognition. Nano Lett. 2002;2:285–288. [Google Scholar]
- 32.Zhao B, et al. Synthesis and characterization of water soluble single-walled carbon nanotube graft copolymers. J. Am. Chem. Soc. 2005;127:8197–8203. doi: 10.1021/ja042924i. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68:6652–6660. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeineldin R, et al. Role of polyethylene glycol integrity in specific receptor targeting of carbon nanotubes to cancer cells. Nano Lett. 2009;9:751–757. doi: 10.1021/nl8033174. [DOI] [PubMed] [Google Scholar]
- 35.Prencipe G, et al. PEG branched polymer for functionalization of nanomaterials with ultralong blood circulation. J. Am. Chem. Soc. 2009;131:4783–4787. doi: 10.1021/ja809086q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee RS, et al. Conductivity enhancement in single-walled carbon nanotube bundles doped with K and Br. Nature. 1997;388:255–257. [Google Scholar]
- 37.Smith BW, et al. Encapsulated C60 in carbon nanotubes. Nature. 1998;396:323–324. [Google Scholar]
- 38.Liu Z, et al. Encapsulation of polystyrene within carbon nanotubes with the aid of supercritical CO2. Carbon. 2004;42:458–460. [Google Scholar]
- 39.Hafner JH, et al. Structural and functional imaging with carbon nanotube AFM probes. Prog. Biophys. Mol. Biol. 2001;77:73–110. doi: 10.1016/s0079-6107(01)00011-6. [DOI] [PubMed] [Google Scholar]
- 40.Bao J, et al. Synthesis and magnetic behavior of an array of nickel-filled carbon nanotubes. Appl. Phys. Lett. 2002;81:4952–4954. [Google Scholar]
- 41.Cai D, et al. Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat. Methods. 2005;2:449–454. doi: 10.1038/nmeth761. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, et al. Biodistribution of carbon single-wall carbon nanotubes in mice. J. Nanosci. Nanotechnol. 2004;4:1019–1024. doi: 10.1166/jnn.2004.146. [DOI] [PubMed] [Google Scholar]
- 43.Klumpp C, et al. Functionalized carbon nanotubes as emerging nanovectors for the delivery of therapeutics. Biochim. Biophys. Acta Biomembr. 2006;1758:404–412. doi: 10.1016/j.bbamem.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Bianco A, et al. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005;9:674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Pastorin G. Crucial functionalizations of carbon nanotubes for improved drug delivery: a valuable option? Pharm. Res. 2009;26:746–749. doi: 10.1007/s11095-008-9811-0. [DOI] [PubMed] [Google Scholar]
- 46.Liu Z, et al. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano. 2007;1:50–56. doi: 10.1021/nn700040t. [DOI] [PubMed] [Google Scholar]
- 47.Maeda H, et al. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009;71:409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Dhar S, et al. Targeted single-wall carbon nanotube-mediated pt(IV) prodrug delivery using folate as a homing device. J. Am. Chem. Soc. 2008;130:11467–11476. doi: 10.1021/ja803036e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kam NWS, et al. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhirde AA, et al. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano. 2009;3:307–316. doi: 10.1021/nn800551s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welsher K, et al. Selective probing and imaging of cells with single walled carbon nanotubes as near-infrared fluorescent molecules. Nano Lett. 2008;8:586–590. doi: 10.1021/nl072949q. [DOI] [PubMed] [Google Scholar]
- 52.Schirrmacher V. Clinical trials of antitumor vaccination with an autologous tumor cell vaccine modified by virus infection: improvement of patient survival based on improved antitumor immune memory. Cancer Immunol. Immunother. 2005;54:587–598. doi: 10.1007/s00262-004-0602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu JS, et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 54.Berd D, et al. Immunopharmacologic analysis of an autologous, haptenmodified human melanoma vaccine. J. Clin. Oncol. 2004;22:403–415. doi: 10.1200/JCO.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 55.Figdor CG, et al. Dendritic cell immunotherapy: mapping the way. Nat. Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 56.Salvador-Morales C, et al. Complement activation and protein adsorption by carbon nanotubes. Mol. Immunol. 2006;43:193–201. doi: 10.1016/j.molimm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 57.Meng J, et al. Carbon nanotubes conjugated to tumor lysate protein enhance the efficacy of an antitumor immunotherapy. Small. 2008;4:1364–1370. doi: 10.1002/smll.200701059. [DOI] [PubMed] [Google Scholar]
- 58.Fadel TR, et al. Enhanced cellular activation with single walled carbon nanotube bundles presenting antibody stimuli. Nano Lett. 2008;8:2070–2076. doi: 10.1021/nl080332i. [DOI] [PubMed] [Google Scholar]
- 59.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 60.Bottini M, et al. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol. Lett. 2006;160:121–126. doi: 10.1016/j.toxlet.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 61.O'Connell MJ, et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science. 2002;297:593–596. doi: 10.1126/science.1072631. [DOI] [PubMed] [Google Scholar]
- 62.Cherukuri P, et al. Mammalian pharmacokinetics of carbon nanotubes using intrinsic near-infrared fluorescence. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18882–18886. doi: 10.1073/pnas.0609265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirsch LR, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chakravarty P, et al. Thermal ablation of tumor cells with antibody-functionalized single-walled carbon nanotubes. Proc. Natl. Acad. Sci. U. S. A. 2008;105:8697–8702. doi: 10.1073/pnas.0803557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, et al. Carboxyl-modified single-walled carbon nanotubes selectively induce human telomeric i-motif formation. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19658–19663. doi: 10.1073/pnas.0607245103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao C, et al. Single-walled carbon nanotubes binding to human telomeric i-motif DNA under molecular-crowding conditions: more water molecules released. Chem. Eur. J. 2008;14:5435–5439. doi: 10.1002/chem.200800280. [DOI] [PubMed] [Google Scholar]
- 67.Mu Q, et al. Suppression of human bone morphogenetic protein signaling by carboxylated single-walled carbon nanotubes. ACS Nano. 2009;3:1139–1144. doi: 10.1021/nn900252j. [DOI] [PubMed] [Google Scholar]
- 68.Kang S, et al. Antibacterial effects of carbon nanotubes: size does matter! Langmuir. 2008;24:6409–6413. doi: 10.1021/la800951v. [DOI] [PubMed] [Google Scholar]
- 69.Kang S, et al. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir. 2007;23:8670–8673. doi: 10.1021/la701067r. [DOI] [PubMed] [Google Scholar]
- 70.Yandar N, et al. Immunological profile of a Plasmodium vivax AMA-1 N-terminus peptide–carbon nanotube conjugate in an infected Plasmodium berghei mouse model. Vaccine. 2008;26:5864–5873. doi: 10.1016/j.vaccine.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 71.Pantarotto D, et al. Immunization with peptide-functionalized carbon nanotubes enhances virus-specific neutralizing antibody responses. Chem. Biol. 2003;10:961–966. doi: 10.1016/j.chembiol.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 72.Pantarotto D, et al. Synthesis, structural characterization, and immunological properties of carbon nanotubes functionalized with peptides. J. Am. Chem. Soc. 2003;125:6160–6164. doi: 10.1021/ja034342r. [DOI] [PubMed] [Google Scholar]
- 73.Shi H, et al. Coordinated biosensors –development of enhanced nanobiosensors for biological and medical applications. Nanomedicine. 2007;2:599–614. doi: 10.2217/17435889.2.5.599. [DOI] [PubMed] [Google Scholar]
- 74.Mahmoud KA, Luong JHT. Impedance method for detecting HIV-1 protease and screening for its inhibitors using ferrocene–peptide conjugate/Au nanoparticle/single-walled carbon nanotube modified electrode. Anal. Chem. 2008;80:7056–7062. doi: 10.1021/ac801174r. [DOI] [PubMed] [Google Scholar]
- 75.Dastagir T, et al. Electrical detection of hepatitis C virus RNA on single wall carbon nanotube-field effect transistors. Analyst. 2007;132:738–740. doi: 10.1039/b707025j. [DOI] [PubMed] [Google Scholar]
- 76.Abidian MR, et al. Conducting-polymer nanotubes for controlled drug release. Adv. Mater. 2006;18:405–409. doi: 10.1002/adma.200501726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aliabadi HM, Lavasanifar A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2006;3:139–162. doi: 10.1517/17425247.3.1.139. [DOI] [PubMed] [Google Scholar]
- 78.Richardson-Burns SM, et al. Polymerization of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) around living neural cells. Biomaterials. 2007;28:1539–1552. doi: 10.1016/j.biomaterials.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang F, et al. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–1900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 80.Gabay T, et al. Engineered self-organization of neural networks using carbon nanotube clusters. Physica A: Statist. Mech. Appl. 2005;350:611–621. [Google Scholar]
- 81.Hu H, et al. Chemically functionalized carbon nanotubes as substrates for neuronal growth. Nano Lett. 2004;4:507–511. doi: 10.1021/nl035193d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Webster TJ, et al. Nano-biotechnology: carbon nanofibres as improved neural and orthopaedic implants. Nanotechnology. 2004;15:48–54. doi: 10.1088/0957-4484/15/1/009. [DOI] [PubMed] [Google Scholar]
- 83.Lovat V, et al. Carbon nanotube substrates boost neuronal electrical signaling. Nano Lett. 2005;5:1107–1110. doi: 10.1021/nl050637m. [DOI] [PubMed] [Google Scholar]
- 84.Sitharaman B, et al. Injectable in situ cross-linkable nanocomposites of biodegradable polymers and carbon nanostructures for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2007;18:655–671. doi: 10.1163/156856207781034133. [DOI] [PubMed] [Google Scholar]
- 85.Keefer EW, et al. Carbon nanotube coating improves neuronal recordings. Nat. Nanotechnol. 2008;3:434–439. doi: 10.1038/nnano.2008.174. [DOI] [PubMed] [Google Scholar]
- 86.Gulyaev AE, et al. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm. Res. 1999;16:1564–1569. doi: 10.1023/a:1018983904537. [DOI] [PubMed] [Google Scholar]
- 87.VanHandel M, et al. Selective uptake of multi-walled carbon nanotubes by tumor macrophages in a murine glioma model. J. Neuroimmunol. 2009;208:3–9. doi: 10.1016/j.jneuroim.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 88.Kateb B, et al. Internalization of MWCNTs by microglia: possible application in immunotherapy of brain tumors. Neuroimage. 2007;37(Suppl. 1):S9–S17. doi: 10.1016/j.neuroimage.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 89.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 90.Nerem RM, Sambanis A. Tissue engineering: from biology to biological substitutes. Tissue Eng. 1995;1:3–13. doi: 10.1089/ten.1995.1.3. [DOI] [PubMed] [Google Scholar]
- 91.Bhattacharyya S, et al. Carbon nanotubes as structural nanofibers for hyaluronic acid hydrogel scaffolds. Biomacromolecules. 2008;9:505–509. doi: 10.1021/bm7009976. [DOI] [PubMed] [Google Scholar]
- 92.Wang SF, et al. Preparation and mechanical properties of chitosan, carbon nanotubes composites. Biomacromolecules. 2005;6:3067–3072. doi: 10.1021/bm050378v. [DOI] [PubMed] [Google Scholar]
- 93.Edwards SL, et al. Tubular micro-scale multiwalled carbon nanotube-based scaffolds for tissue engineering. Biomaterials. 2009;30:1725–1731. doi: 10.1016/j.biomaterials.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 94.Firkowska I, et al. Highly ordered MWNT-based matrixes: topography at the nanoscale conceived for tissue engineering. Langmuir. 2006;22:5427–5434. doi: 10.1021/la053067e. [DOI] [PubMed] [Google Scholar]
- 95.Polizu S, et al. Applications of carbon nanotubes-based biomaterials in biomedical nanotechnology. J. Nanosci. Nanotechnol. 2006;6:1883–1904. doi: 10.1166/jnn.2006.197. [DOI] [PubMed] [Google Scholar]
- 96.Galvan-Garcia P, et al. Robust cell migration and neuronal growth on pristine carbon nanotube sheets and yarns. J. Biomater. Sci. Polym. Ed. 2007;18:1245–1261. doi: 10.1163/156856207782177891. [DOI] [PubMed] [Google Scholar]
- 97.Liopo AV, et al. Biocompatibility of native and functionalized single-walled carbon nanotubes for neuronal interface. J. Nanosci. Nanotechnol. 2006;6:1365–1374. doi: 10.1166/jnn.2006.155. [DOI] [PubMed] [Google Scholar]
- 98.Abarrategi A, et al. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials. 2008;29:94–102. doi: 10.1016/j.biomaterials.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 99.Sorkin R, et al. Compact self-wiring in cultured neural networks. J. Neural Eng. 2006;3:95–101. doi: 10.1088/1741-2560/3/2/003. [DOI] [PubMed] [Google Scholar]
- 100.Hu H, et al. Polyethyleneimine functionalized single-walled carbon nanotubes as a substrate for neuronal growth. J. Phys. Chem. B. 2005;109:4285–4289. doi: 10.1021/jp0441137. [DOI] [PubMed] [Google Scholar]
- 101.Mattson MP, et al. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J. Mol. Neurosci. 2000;14:175–182. doi: 10.1385/JMN:14:3:175. [DOI] [PubMed] [Google Scholar]
- 102.Zhao B, et al. A bone mimic based on the self-assembly of hydroxyapatite on chemically functionalized single-walled carbon nanotubes. Chem. Mater. 2005;17:3235–3241. [Google Scholar]
- 103.Harrison BS, Atala A. Carbon nanotube applications for tissue engineering. Biomaterials. 2007;28:344–353. doi: 10.1016/j.biomaterials.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 104.Constantine PF, Prabhakar RB. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomedicine. 2010;6:245–256. doi: 10.1016/j.nano.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 105.Oberdörster G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J. Intern. Med. 2010;267:89–105. doi: 10.1111/j.1365-2796.2009.02187.x. [DOI] [PubMed] [Google Scholar]
- 106.Qu G, et al. The effect of multiwalled carbon nanotube agglomeration on their accumulation in and damage to organs in mice. Carbon. 2009;47:2060–2069. [Google Scholar]
- 107.Schipper ML, et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat. Nanotechnol. 2008;3:216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 108.Colvin VL. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003;21:1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- 109.Brayner R. The toxicological impact of nanoparticles. Nano Today. 2008;3:48–55. [Google Scholar]
- 110.Gwinn MR, Vallyathan V. Nanoparticles: health effects—pros and cons. Environ. Health Perspect. 2006;114:1818–1825. doi: 10.1289/ehp.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nel A, et al. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 112.Oberdörster G, et al. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lewinski N, et al. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 114.Oberdorster G, et al. Toxicology of nanoparticles: a historical perspective. Nanotoxicology. 2007;1:2–25. [Google Scholar]