Abstract
Obstructive sleep apnea (OSA) is a common public health issue. If left untreated, OSA may cause a large health economic burden from cardiovascular complications particularly stroke. The diagnosis of OSA can be made by polysomnography, but its availability is limited in the developing countries in Asia. STOP-BANG questionnaire is a good screening tool but may need some adjustment for Asian population. STOP-BANG stands for: Snoring history, Tired during the day, Observed stop breathing while sleep, High blood pressure, body mass index (BMI) more than 35 kg/m2, Age more than 50 years, Neck circumference more than 40 cm and male Gender. We compared clinical features in STOP-BANG questionnaire between 42 OSA induced hypertension patients and 82 healthy control subjects in the Faculty of Medicine, Khon Kaen University, Thailand. The best cutoff point for the BMI and the neck circumference were 24.5 kg/m2 and 36 cm, respectively. The sensitivity and specificity of the BMI cutoff point were 97.2% and 91.40, while those of the neck circumference were 94.7% and 82.9%. In conclusion, the appropriate cutoff points of BMI and neck circumference for Thai STOP-BANG questionnaire were 25 kg/m2 and 36 cm.
Key words: Obstructive sleep apnea, body mass index, neck circumference, Thais, STOP-BANG, screening tool, hypertension
Introduction
Obstructive sleep apnea (OSA) is a common public health problem worldwide and it has been shown to be associated with an increased incidence of cardiovascular complications such as death, stroke, or myocardial infarction.1 The 2014 American Heart Association/American Stroke Association guideline recommends that patients with stroke/transient ischemic disorder (TIA) showed be worked up for OSA.2 In this study, patients who had OSA and stroke had an improved outcome with continuous positive airway pressure.2
Extrapolating data from Western population and implementing in the Asian populations may not be possible as there are differences in genetic and anatomical factors.3 For example, the cutoff point for obesity based on body mass index (BMI) criterion for Western population is 30 kg/m2 meanwhile the BMI for Asian population is 25 kg/m2.4 It is also evident that there are differences in anatomical factors between Asian and Caucasian population such as retrognathia and oropharyngeal width which are main risk factors for OSA.3
Polysomnography is gold standard for diagnosis of OSA. However, the availability of this study is limited in some developing countries. Alternatively, the STOP-BANG questionnaire developed by Chung et al. has been widely used as a sensitive screening tool for OSA.5 It is composed of both subjective and objective questions. The STOP-BANG acronym stands for: Snoring history, Tired during the day, Observed stop breathing while sleep, High blood pressure, BMI more than 35 kg/m2, Age more than 50 years, Neck circumference more than 40 cm and male Gender. The authors recommended that if a patient had 3 or more criteria mentioned above, it is strongly suggestive for OSA. However, the cut-off values of this questionnaire are based on the data from Caucasians, therefore we hypothesized that to use it in Asian population, some adjustments are needed.
Materials and Methods
We compared the clinical features in the STOP-BANG questionnaire between OSA-induced hypertension patients and healthy control subjects. The study was conducted in the Faculty of Medicine, Khon Kaen University (Thailand). The study protocol was approved by the Ethics Committee for Human Research, Khon Kaen University. OSA induced-hypertension patients were diagnosed by: i) met the criteria of hypertension; ii) having average apnea-hypopnea index (AHI) by polysomnography more than or equal 5 times/hours and iii) no evidence of other secondary hypertension. The details of these patients are described elsewhere.6 Healthy control subjects were randomly selected from the database of medical students. The control subjects filled up the modified Berlin questionnaire and Epworth sleepiness scale less than 10 and defined as low risk for OSA with low scores.7-9 Subjects were excluded if having incomplete data to evaluate the OSA risk.
Demographic data and clinical features of both OSA-induced hypertension patients and healthy subjects were recorded and compared. The clinical features included age, gender, BMI, neck circumference, Mallampati classification, torus palatinus, and torus mandibularis. The neck circumference was measured across the cricothyroid membrane in an upright position. The Mallampati classification was defined by asking the subjects to protrude their tongue as much as possible and classified as class 1 to 4.10,11
The sample size of the study population was calculated by using the proportion comparison between OSA and healthy subjects using WinPepi program. The proportion of OSA was 0.35 and the healthy control was 0.07 with deviation of 5%, power of 90%, and missing data of 10%.12 The sample size was calculated to be 102 subjects (OSA 34 subjects and healthy control 68 subjects). Due to the incompleteness of the database of medical students, 120 healthy controls were selected by systematic sampling from the database (total of 1174 medical students).
Baseline and clinical characteristics of the participants in both groups were compared using descriptive statistics. Univariate logistic regression analyses were applied to calculate the crude odds ratios of individual variables for having OSA. All clinically significant variables or P<0.20 by the univariate analyses were included in subsequent multivariate logistic regression analyses. Analytical results were presented as crude odds ratios (OR), adjusted OR, and 95% confidence intervals (CI). Significant risk factors were calculated for the best cut-off points by the receiver operator characteristic curve (ROC curve). Data analyses were performed with STATA software (College Station, TX, USA) and SPSS software (Chicago, IL, USA).
Results
In this study, 42 OSA-induced hypertension patients and 82 control subjects who had a complete set of clinical data were included. All clinical features of both groups were significantly different (Table 1). The OSA-induced hypertension patients were significantly older (59.5 vs 21.0 years), with higher proportion of males (64.3 vs 59.8%), more obese (78.6 vs 6.1%), higher incidence of Mallampati class 3 or more (54.8 vs 24.4%), larger neck circumference (41.3 vs 32.0 cm), higher incidence of torus palatinus (26.6 vs 0%) and of torus mandibularis (9.5 vs 0%).
Table 1.
Clinical features of obstructive sleep apnea patients and healthy control.
| Factors | OSA (n=42) | Controls (n=82) | P |
|---|---|---|---|
| Age, years (SD) | 59.48 (9.82) | 21.00 (3.00) | <0.001 |
| Male, n (%) | 27 (64.28) | 49 (59.76) | 0.698 |
| Body mass index >25, n (%) | 33 (78.57) | 5 (6.10) | 0.001 |
| Mallampatic 3+, n (%) | 23 (54.76) | 20 (24.39) | <0.001 |
| Neck diameter, cm (SD) | 41.33 (3.67) | 32.00 (4.60) | <0.001 |
| Torus palatinus, n (%) | 11 (26.62) | 0 | <0.001 |
| Torus mandibularis, n (%) | 4 (9.5) | 0 | 0.012 |
OSA, obstructive sleep apnea; SD standard deviation.
By multiple logistic regression analysis, only two factors BMI and neck circumference, were associated significantly with OSA-induced hypertension (Table 2). The adjusted odds ratios for both factors were 1.49 (95% CI: 1.06, 2.09) and 1.67 (95% CI: 1.11, 2.51), respectively. By the ROC curve analyses, the best cut-off points for the BMI and the neck circumference were 24.5 kg/m2 (Figure 1A) and 36 cm (Figure 1B). The sensitivity and the specificity for BMI cut-off point were 97.2% and 91.4%, whereas those for the neck circumference were 94.7% and 82.9%.
Table 2.
Factors associated with having obstructive sleep apnea-induced hypertension by multiple logistic regression analysis.
| Variables | Univariate OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Neck circumference, cm | 2.17 (1.60, 2.95) | 1.67 (1.11, 2.51) |
| Body mass index, kg/m2 | 2.42 (1.69, 3.46) | 1.49 (1.06, 2.09) |
OR, odds ratio; CI, confidence interval. Data are adjusted for gender, body mass index, neck circumference, Mallampati classification and toruses.
Figure 1.
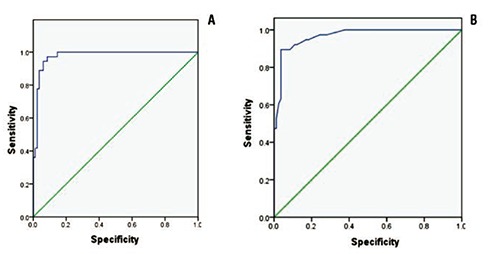
A receiver operator characteristic curve (ROC curve) of body mass index (A) and neck circumference (B) having obstructive sleep apnea induced hypertension.
Discussion
The present results showed that the STOP-BANG questionnaire needed to adjust the cutoff values of the BMI and neck circumference suitable for Thai population. Subjects should referred for polysomnography if positive at least 3 questions: S, snoring history; T, tired during the day; O, observed stop breathing while sleep; H, high blood pressure; B, BMI more than 25 kg/m2; A, age more than 50 years; N, neck circumference more than 36 cm; G, male gender (modified from Chung et al.)5. For the criteria of obesity for Asians, the cut-off of the BMI should lower from 35 kg/m2 to 24.5 or round up to 25 kg/m2. The cut-off of the neck circumference also should be lowered from 40 cm to 36 cm for Asians. Our cut-off point settings improved the sensitivity and specificity over 80%. Similar to our study, Yagi et al. proposed the cutoff points of 24.1 kg/m2 for the BMI and 35.5 cm for the neck circumference for Japanese population.13
Using STOP-BANG questionnaire is very suitable for Thailand and other developing countries due to limited availability of polysomnography, the standard diagnostic tool for OSA. The STOP-BANG questionnaire has very high sensitivity particularly for severe OSA.14 This low cost tool can select appropriate patients for referral to sleep center for further polysomnography.
There are some limitations in the present study. Healthy control subjects were not performed polysomnography to exclude OSA. However, both the Berlin questionnaire and the Epworth sleepiness scale were used to exclude OSA, which has a sensitivity and specificity of 0.86, 0.95 and 0.49, 0.80,8,15 respectively. In addition, subjects were less likely to have OSA due to young age. Even though the age is a significant factor by univariate logistic regression, it was not included in the subsequent multivariate regression. This is because the difference of age group composition between the OSA and the healthy control subjects. Further studies, therefore, are needed to confirm the results of this study and to identify the appropriate cut-off point using the age- and sex-matched controls. Also, to increase their sensitivity and specificity, OSA patients with other complications than hypertension should be included and compared. Whether simple measurement of the neck circumference and the BMI calculation are sufficient to identify OSA patients in routine practice by primary health care personnel including physicians, nurses and volunteers should be examined in further study. OSA has been proved to be a contributing factor for major cardiovascular diseases; stroke, hypertension, sudden death, and also coronary artery disease. Treatment of OSA may reduce large economic burden from prevention of the morbidity and mortality from stroke and acute coronary syndrome.1 In conclusion, the appropriate cut-off points for the BMI and the neck circumference for STOP-BANG questionnaire were 25 kg/m2 and 36 cm for Asian people. All hypertensive patients should have their BMI and neck circumference measured to detect the risk factors for OSA. Public health campaign for OSA screening is also needed to reduce morbidity and mortality from OSA complications. Finally, proper referral to diagnose OSA is recommended.
References
- 1.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53. [DOI] [PubMed] [Google Scholar]
- 2.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160-236. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland K, Lee RW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology 2012;17:213-22. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157-63. [DOI] [PubMed] [Google Scholar]
- 5.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108:812-21. [DOI] [PubMed] [Google Scholar]
- 6.Sawanyawisuth K, Chindaprasirt J, Senthong V, et al. Lower BMI is a predictor of obstructive sleep apnea in elderly Thai hypertensive patients. Sleep Breath 2013;17:1215-9. [DOI] [PubMed] [Google Scholar]
- 7.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485-91. [DOI] [PubMed] [Google Scholar]
- 8.Sharma SK, Vasudev C, Sinha S, et al. Validation of the modified Berlin questionnaire to identify patients at risk for the obstructive sleep apnoea syndrome. Indian J Med Res 2006;124:281-90. [PubMed] [Google Scholar]
- 9.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5. [DOI] [PubMed] [Google Scholar]
- 10.Hiremath AS, Hillman DR, James AL, et al. Relationship between difficult tracheal intubation and obstructive sleep apnoea. Br J Anaesth 1998;80:606-11. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues MM, Dibbern RS, Goulart CW. Nasal obstruction and high Mallampati score as risk factors for obstructive sleep apnea. Braz J Otorhinolaryngol 2010;76:596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noknu S, Janthivas C, Thumaviriyakul H. Clinical predictors of obstructive sleep apnea hypopnea syndrome. Thai J Oto Head Neck Surg 2009;10:16-30. [Google Scholar]
- 13.Yagi H, Nakata S, Tsuge H, et al. Morphological examination of upper airway in obstructive sleep apnea. Auris Nasus Larynx 2009;36:444-9. [DOI] [PubMed] [Google Scholar]
- 14.Farney RJ, Walker BS, Farney RM, et al. The STOP-Bang equivalent model and prediction of severity of obstructive sleep apnea: relation to polysomnographic measurements of the apnea/hypopnea index. J Clin Sleep Med 2011;7:459-65B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drager LF, Genta PR, Pedrosa RP, et al. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol 2010;105:1135-9. [DOI] [PubMed] [Google Scholar]


