Abstract
Metronidazole is a very common antibacterial and antiprotozoal with wide usage across the globe, including the least developed countries. It is generally well-tolerated with a low incidence of serious side-effects. Neurological toxicity is fairly common with this drug, however majority of these are peripheral neuropathy with very few cases of central nervous toxicity reported. We report the imaging findings in two patients with cerebellar dysfunction after Metronidazole usage. Signal changes in the dentate and red nucleus were seen on magnetic resonance imaging in these patients. Most of the cases reported in literature reported similar findings, suggesting high predilection for the dentate nucleus in metronidazole induced encephalopathy.
Key words: Metronidazole, dentate, toxicity, magnetic resonance imaging
Introduction
Metronidazole is a synthetic 5-nitroimidazole antibiotic with wide usage as an anti-anaerobic and anti-protozoal drug. Metronidazole has been used for the treatment of infections for more than 50 years now and is still considered as the drug of choice for the treatment of anaerobic bacterial infections with low rate of resistance.1 Metronidazole is considered to be a cost-effective drug with favorable pharmacokinetic properties and minor adverse effects. Minor adverse reactions to metronidazole include nausea, mouth dryness, vomiting and diarrhea. Neurotoxicity is rare, seen predominantly as peripheral neuropathy, dizziness, vertigo and headache.2 Cerebellar toxicity is a rare but serious side effect of metronidazole toxicity with classic findings on magnetic resonance imaging (MRI).3 We discuss two cases of metronidazole-induced cerebellar dysfunction, presenting with signal changes of the dentate nucleus. Although the pathophysiology of metronidazole neurotoxicity remains unclear, most lesions secondary to metronidazole neurotoxicity are completely reversible. Moreover, the toxicity does not seem to be a dose- or duration-related phenomenon.
Case Report #1
Our first case is a young 22-year-old male with chronic sinus inflammatory disease who presented with seizures at an outside hospital. He underwent workup with an MRI scan, which showed a small peripherally enhancing lesion in the right frontal lobe with central contents showing restricted diffusion suggestive of brain abscess. He was evaluated by neurosurgery and no surgical intervention was recommended based on its small size and lack of surrounding edema. Ear, nose, and throat specialist was consulted and ethmoidectomy performed due to concern for contiguous spread from a sinus infection. Cultures from his sinuses were reportedly positive for grampositive cocci in clusters. Patient was started on antibiotics including intravenous (IV) ceftriaxone (2 gm, q12h) and IV metronidazole (500 mg q8h) as well as prophylactic anti-epileptic with levetiracetam (500 mg oral tablet). He was discharged on these medications after one week. Patient was on these medications for around four months when he started developing lethargy, weakness, gait abnormality and pain in lower extremities. His symptoms had progressively worsening over the past two weeks. Patient was admitted to our University hospital. Coordination tests including finger-to-nose exam revealed mild dysmetria in both arms and the patient was falling in all directions during gait assessment. Patient was diagnosed with drug-induced neuropathy. Repeat MRI of the brain was performed which showed near-complete resolution of the right frontal brain abscess. The scan also revealed symmetric areas of increased FLAIR signal with restricted diffusion involving the dentate, the facial nerve and the red nuclei, bilaterally (Figure 1). Given the clinical symptoms, medication history and unique imaging findings, the patient was diagnosed with metronidazole-induced cerebellar toxicity. Patient was discharged after two days with discontinuation of ceftriaxone and metronidazole. Patient was however advised to continue intake of levetiracetam. Patient had significant improvement in his symptoms over the next few weeks. Complete resolution of signal changes was noted on the follow-up MRI of the brain done after 5 weeks.
Figure 1.
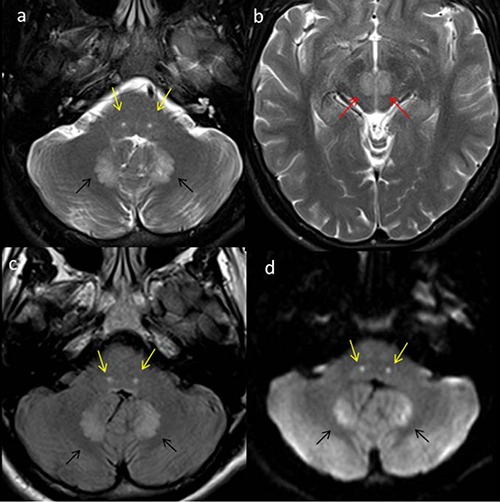
Multiple axial magnetic resonance imaging of the brain in a 22-year old male on metronidazole presenting with cerebellar symptoms. Axial T2 images (a,b) reveal symmetric areas of increased signal in the dentate (black arrows), the facial (yellow arrows) and the red nuclei (red arrows), bilaterally. Axial fluid attenuated inversion recovery images (c) showing similar changes with restricted diffusion noted on the diffusion-weighted image (d).
Case Report #2
Our second patient is an 80 year old lady who presented with gradually worsening ataxia and gait abnormalities. Her past medical history includes hypertension, chronic renal disease, hyperlipidemia and coronary artery disease. The patient had periprosthetic femur fracture a month prior and underwent a successful revision in the operating room. In addition, she also developed diarrhea during her hospital stay with positive Clostridium difficile assay and was started on metronidazole (500 mg 1 tab every 8 hours). On admission, the patient was evaluated by neurology and MRI and MR angiogram of the brain was requested to rule out posterior fossa stroke. Her neurological examination revealed horizontal nystagmus to the right and positive cerebellar signs. MRI of the brain showed symmetrical areas of increased T2/FLAIR signal involving the dentate nuclei (Figure 2). There was no restricted diffusion, intracranial hemorrhage or abnormal parenchymal enhancement. Based on the clinical history of prolonged metronidazole intake, we considered the possibility of metronidazole induced cerebellar toxicity. The clinical team discontinued Metronidazole, based on the recommendations of the neurologist and the MR imaging findings. The patient was discharged to physiotherapy and rehabilitation services. There was near complete resolution of cerebellar signs and symptoms on follow-up office visit after 3 weeks. Follow-up imaging was deemed unnecessary and was not requested.
Figure 2.
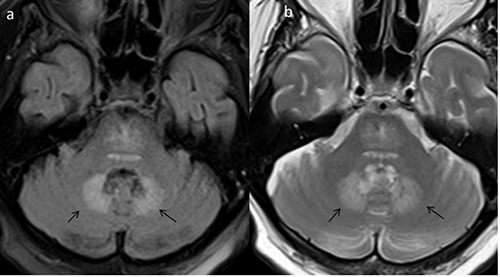
Axial fluid attenuated inversion recovery (a) and T2-weighted images (b) in an 80 year old lady on Metronidazole, presenting with ataxia and gait abnormalities. Symmetric areas of increased T2/fluid attenuated inversion recovery signal noted in the dentate nuclei, bilaterally (black arrows). Follow-up imaging was not performed as there was complete resolution of clinical signs and symptoms.
Discussion
Metronidazole is an antimicrobial and antiprotozoal with broad usage in medical and surgical patients. It is a synthetic 5-nitroimidazole which is in inactive state on administration and turns to metabolically active form on reaching the target. It is a well-tolerated drug with very low incidence of severe side effects. The most common side-effects include nausea, headaches and metallic taste in mouth. Neurological side-effects are uncommon with the vast majority being mild to moderate peripheral neuropathy.4 As the drug penetrates cerebrospinal fluid (CSF), it can cause central neurological (CNS) side effects. This can range from seizures to diffuse encephalopathy or acute/chronic cerebellar symptoms. Imaging is usually not indicated in this group of patients and is usually done to rule out other intracranial pathologies. Moreover, imaging is unrevealing in most of the patients presenting with seizures or diffuse encephalopathy.5 However, recently there have been many cases of acute/ chronic cerebellar toxicity reported in literature. Interestingly, most of the patients in this group have findings on MR imaging which includes signal changes in the dentate nucleus. Recognizing the pattern of imaging abnormality is important as terminating the usage of Metronidazole can result in rapid clinical improvement and resolution of signal changes. The exact mechanism of cerebellar toxicity is unclear. Ahmed et al.6 first described the clinical and neuroimaging findings of metronidazole induced cerebellar toxicity in 1995. Multiple prior studies done in animal (rats) subjects have shown axonal degeneration after treatment with Metronidazole, with symmetric lesions in the cerebellar and cochlear nuclei. It was postulated that Metronidazole and its metabolites binds to neuronal RNA and inhibit protein synthesis resulting in reversible axonal swelling.7 Metronidazole crosses blood-brain barrier and can result in imaging and histological findings similar to Wernicke’s encephalopathy. Metronidazole neurotoxicity is not dependent upon the dosage and duration of usage with no significant difference between the oral and intravenous routes of administration. Metronidazole-induced cerebellar toxicity typically involves the dentate nuclei, splenium of corpus callosum and the dorsal brainstem. Lesions are bilateral and symmetric in almost all the patients. Cerebellar dentate nuclei and the inferior colliculus are the most characteristic location in metronidazole-induced central nervous system toxicity. These lesions appear as T2 and FLAIR hyperintense areas with no contrast enhancement or mass effect. Many patients also show restricted diffusion with varying apparent diffusion coefficient (ADC) values. Lower ADC values may suggest more acute nature of cell damage.1,8 Lesions are generally reversible with complete resolution of signal changes on follow-up imaging. Both of our patients had symmetric involvement of the dentate nuclei with FLAIR signal changes. One of these patients also showed restricted diffusion within the dentate. Cerebrospinal fluid analysis is usually unrevealing.9 It is important to rule out other pathologies which tend to involve the brainstem and cerebellum including demyelination pathologies, toxic and metabolic encephalopathies. Wernicke’s encephalopathy is the biggest mimicker of metronidazoleinduced cerebellar toxicity, primarily because it also has high propensity for the dentate nuclei. Clinical findings and history of alcoholism is usually evident in these patients. Other diseases such as osmotic demyelination tend to involve the basis pontis and spare the dentate nuclei.1,10 Many diseases can result in increased T1-signal within the dentate, which is usually a non-specific finding and represents mineralization or calcification. However, there are very few conditions which result in increased T2/FLAIR signal in the dentate. Extensive search of literature revealed very few conditions which result in such an imaging appearance. This includes Wernicke’s encephalopathy and metronidazole toxicity. Despite being uncommon, metronidazoleinduced neurotoxicity should always be considered as the main differential for symmetric increased T2 signal of the dentate nuclei. Other extremely rare causes for dentate T2-signal changes include methyl-bromide toxicity, maple-syrup urine disease and enteroviral encephalomyelitis.11,12 Complete reversal of symptoms and imaging findings is seen in most of the cases. Follow-up imaging is usually unnecessary once the clinical signs and symptoms have resolved.
Conclusions
To summarize, MR imaging findings of bilateral increased T2/FLAIR signal of the dentate nuclei are a very characteristic feature of metronidazole-induced neurotoxicity. Imaging is usually requested by the clinician expecting some structural cause for cerebellar symptoms. This imaging appearance should however prompt the neuroradiologist to search the clinical notes for metronidazole usage and alert the clinician. With increased awareness about this entity, many more such cased will be diagnosed in future.
References
- 1.Kim E, Na DG, Kim EY, et al. Imaging of metronidazole induced encephalopathy: Lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol 2007;28:1652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel K, Green-Hopkins Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis 2008;12:e111-4. [DOI] [PubMed] [Google Scholar]
- 3.Woodruff BK, Wijdicks EF, Marshall WF. Reversible metronidazole-induced lesions of the cerebellar dentate nuclei. N Engl J Med 2002;346:68-9. [DOI] [PubMed] [Google Scholar]
- 4.Graves TD, Condon M, Loucaidou M, Perry RJ. Reversible metronidazole-induced cerebellar toxicity in a multiple transplant recipient. J Neurol Sci 2009;285:238-40. [DOI] [PubMed] [Google Scholar]
- 5.Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011;72:381-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed A, Loes DJ, Bressler EL. Reversible magnetic resonance imaging findings in metronidazole induced encephalopahty. Neurology 1995;45:588-9. [DOI] [PubMed] [Google Scholar]
- 7.Bradley WG, Karlsson IJ, Rassol CG. Metronidazole neuropathy. Br Med J 1977;2:610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chacko J, Pramod K, Sinha S, et al. Clinical, neuroimaging and pathological features of 5-nitroimidazole-induced encephalo-neuropathy in two patients: Insights into possible pathogenesis. Neurol India 2011;59:743-7. [DOI] [PubMed] [Google Scholar]
- 9.Iyer RS, Chaturvedi A, Pruthi S, et al. Medication neurotoxicity in children. Pediatr Radiol 2011;41:1455-64. [DOI] [PubMed] [Google Scholar]
- 10.Zuccoli G1, Pipitone N. Neuroimaging findings in acute Wernicke’s encephalopathy: review of the literature. AJR Am J Roentgenol 2009;192:501-8. [DOI] [PubMed] [Google Scholar]
- 11.Tan CH, Chen YF, Chen CC, et al. Painful neuropathy due to skin denervation after metronidazole-induced neurotoxicity. J Neurol Neurosurg Psychiatry 2011;82:462-5. [DOI] [PubMed] [Google Scholar]
- 12.Groothoff MV, Hofmeijer J, Sikma MA, Meulenbelt J. Irreversible encephalopathy after treatment with high-dose intravenous metronidazole. Clin Ther 2010;32:60-4. [DOI] [PubMed] [Google Scholar]


