Abstract
Background
Vocal cord dysfunction (VCD) is a respiratory disorder characterized by inappropriate vocal cord adduction during inspiration. The diagnosis of VCD is challenging, as expected flow volume loop abnormalities are uncommonly noted and laryngoscopy must be timed to coincide with symptoms.
Objective
To determine the potential role of Impulse Oscillometry (IOS) in the diagnosis of VCD. Methods: We conducted an analysis of 6 patients in which the diagnosis of VCD was being considered as well as 7 normal subjects and five subjects with asthma. All were evaluated with IOS, spirometry and patients underwent laryngoscopy. Two patients with suspected VCD who did not exhibit symptoms or abnormal pulmonary function at baseline underwent exercise challenge and repeat studies. One patient with suspected VCD underwent an additional irritant challenge.
Results
VCD was diagnosed by laryngoscopy in 3 of the 6 patients where the diagnosis of VCD was entertained. These three patients as a group all exhibited higher amplitude (mean, 9.3 cm H20/L/sec) and more variable spikes (SD, 4.8) on IOS impedance during inspiration, while the 3 patients where the diagnosis was not confirmed by endoscopy did not show these findings (mean, 2.0, P<0.0002; SD, 0.8, P<0.0001). This pattern was also not observed in the normal volunteers (mean, 1.8; SD, 0.7) and asthmatics at baseline (mean, 4.2; SD, 1.2) or following exercise challenge (mean, 1.5; SD, 0.5).
Conclusions
These findings support the conclusion that IOS displays a characteristic pattern in patients with VCD and thus may offer a rapid and non-invasive adjunct to the assessment and diagnosis of patients suspected to have this disorder.
Keywords: vocal cord dysfunction, impulse oscillometry, asthma, laryngoscopy, spirometry
Introduction
Vocal cord dysfunction (VCD) is a condition characterized by paradoxical adduction of the vocal cords during inspiration that manifests as respiratory distress. Diagnosis is challenging because of the transient nature and overlap of symptoms of VCD with asthma. VCD also may coexist with asthma or be misdiagnosed as asthma. 1–4 It is also reportedly more common in woman and in patients with gastroesophageal reflux disease (GERD). 5, 6 VCD may be misinterpreted as exercise-induced asthma and is one cause of upper airway obstruction during exercise in elite athletes. 7 Other triggers of VCD include inhalation of irritants, allergic rhinitis, and upper respiratory infections. 1, 8, 9 There may be a psychological component to VCD, as a relationship between VCD, anxiety and social stress has been suggested10 and may be considered a type of conversion disorder. Consistent with this observation, patients often respond to behavior therapy such as biofeedback. 1, 11 The diagnostic evaluation of VCD begins with history and physical exam, followed by spirometry and laryngoscopy. A characteristic finding indicative of extrathoracic upper airway obstruction is truncation of the inspiratory limb of the flow-volume loop (FVL). This finding, however, is not specific to VCD and often is not observed in spirometric evaluation of patients with VCD. In a study of 95 patients diagnosed with VCD, only 23% had this finding at baseline and several patients were unable to perform spirometry.12 Combining the findings from 288 VCD-related articles indicates FVL abnormalities were not detected in the majority of 1,587 patients with VCD. 1
The standard criteria to establish a diagnosis of VCD is direct observation with laryngoscopy of anterior vocal cord adduction during inspiration, or in both inspiration and expiration, accompanied by a residual posterior glottic chink.3, 13 These observations when accompanied by respiratory symptom establishes the diagnosis of VCD. Provocation with exercise challenge may be warranted, as these findings may not be observed at baseline. 14 Based on a comprehensive review of VCD studies mentioned above, 62% of 1,528 patients with VCD had these positive endoscopic finding.1 However, the need for additional non-invasive tests with efficacy that approximates laryngoscopy is warranted and has been highlighted in a recent investigation of new developments in the diagnosis and treatment of VCD over the past decade. 15
IOS is used as an adjunct in the evaluation of respiratory disease, particularly in young subjects and those who cannot perform effort dependent PFTs.16–18 IOS is based on the production of small pressure oscillations that are applied at the mouth and transmitted into the lungs, which in turn permits the measurement of the resistance and reactance to the impedance of the respiratory system during spontaneous quiet breathing and thus provides an indirect analysis of lung function. IOS has been applied in a number of clinical situations including detection of fixed airway obstruction, upper airway resistance in obstructive sleep apnea, tracheal stenosis, and significant inhalational exposures. 17–19
The observation of an unusual, acute spike in the impedance volume curve during inspiration in symptomatic patients with VCD was first suggested by Dr. Michael D. Goldman, one of the early developers of IOS, 20, 21 although to our knowledge there exists no reported evaluation of the impedance volume in VCD. 22, 23 This led us to examine the use of IOS to diagnose VCD in patients with a history suggestive of VCD. As will be shown, findings with IOS were consistent with the endoscopic assessment, thus supporting the concept that IOS appears to be a valuable adjunct in the diagnosis of VCD.
Methods
Subjects
Six patients 12–60 years of age with a clinical history suggestive of VCD were enrolled on an NIH IRB approved protocol following informed consent. All patients underwent a history, physical exam, spirometry, IOS and laryngoscopy using a standard protocol. 13 Two patients with suspected VCD with exercise that did not exhibit findings of VCD at baseline underwent a 15–20 min treadmill exercise challenge, which consisted of 5 minute intervals of increasing speed and incline, followed by IOS, spirometry and direct laryngoscopy when feasible. Exercise challenge intervals continued until there was profuse sweating and increased heart and respiratory rate. One patient was challenged with perfume, and other aromatic inhalations noted in the history to trigger symptoms. Patients’ test results from IOS were compared to 5 age matched asthmatics (FEV1 % post bronchodilator change > 15%) without a history of VCD; and 7 non-asthmatic (FEV1% mean, 110.4%, SD 11.8), non-atopic healthy controls (age 17–43) at baseline and following exercise challenge testing (FEV1% mean 107%, SD 11.2). For all subjects, the absolute impedance amplitude was determined from each Z5 tracing during each breath interval (highest peak to lowest trough) over 30 seconds of testing. The amplitudes in each group (controls, VCD, VCD ruled out, asthmatics) were compared in terms of severity (mean amplitude) and variability in impedance within each individual (standard deviation [SD]). To define and exclude testing artifact, 5 healthy controls underwent IOS while performing self-induced glottic closure.
Impulse Oscillometry
The IOS system (MasterScreen Impulse Oscillometry by CareFusion, Yorba Linda, CA) was calibrated as recommended by the manufacturer. Testing was performed and analyzed in accordance with ERS/ATS guidelines. 16, 18, 24, 25 Subjects were instructed to place their lips around the mouthpiece of the IOS pneumotachometer and to breathe normally. A nasal clip was used and the hands of the subject were placed on their cheeks to limit their expansion. Pulmonary impedance (Z,) which incorporates both lung resistance (R) and reactance (X) was measured at 5 Hz (Z5) and observed by the investigator in real-time t(s) as a function of flow volume (l) for a duration of 30–60 seconds. Patterns of rapid increased or decreased Z5 with inspiration were noted. Overall impedance was also measured during this time interval and reported as R, the energy required to propagate the pressure wave through the airways and X, which reflects the viscoelastic properties of the respiratory system. Mean values of reactance and resistance were calculated at frequencies from 5–20 Hz. For each trial the Z5 vs. flow volume curve was analyzed and stored. An average of 3 adequate trials of R and X values were also analyzed and graphically displayed. If there was an indication in the real-time impedance sampling that abnormal tracings were due to tongue or mouthpiece artifact, the trial was excluded. When challenge testing occurred, IOS was repeated following treadmill or inhalation challenge. Predicted values for R and X were based on gender and height according to the equipment’s default normal references values as recommended by the manufacturer based on existing reference values. 26–28
Spirometry
A Jaeger Masterscreen Spirometry system was used to determine flow-volume loop. Forced expiratory volume, forced vital capacity, forced expiratory flow (25%-75%) and peak flow were measured. The system was calibrated in accordance with ATS standards. All testing procedures were in performed accordance with ATS guidelines for determination of expiratory flow volume measurements.29 Patients were directed to exert maximal effort during forced expiratory and inspiratory maneuvers. The best of three acceptable recorded trials was reported. Percent predicted values were based on age, height, gender and ethnicity. One research nurse that was trained in both procedures performed spirometry and IOS measurements.
Statistical analysis
Two separate one-way ANOVAs of each patient’s mean Z5 amplitude and their Z5 amplitude standard deviation was used to compare Z5 (cmH2O/L/sec) profiles of healthy, asthmatic and VCD patients. Post-hoc comparisons of the healthy, asthmatic and VCD groups were made using Sidak’s multiple comparisons test.
Results
Subject Characteristics
As shown in Table 1, all patients suspected of having VCD carried the diagnosis of asthma and had been placed on controller medications. Only patient #3 was symptomatic for asthma at the time of our evaluation. Three of six patients (patients 1–3) suspected of having VCD had evidence of vocal cord dysfunction at baseline using IOS; the remaining 3 patients did not and underwent either treadmill exercise challenge or inhalation challenge to induce airway obstruction that had occurred under similar circumstances by history. Of these, none were positive for VCD as determined by IOS and laryngoscopy. Patient #1 alone had findings on spirometry consistent with VCD. Thus, only patients with evidence on IOS at baseline for VCD were found to have VCD by laryngoscopy.
Table 1.
Patient Characteristics and VCD Results*
| Patient | Age | Gender | Dx Asthma | Medications | Challenge | IOS | Spirometry | Laryngoscopy |
|---|---|---|---|---|---|---|---|---|
| 1 | 12 | F | Yes | ICS, Atrovent, Xopenex, Singulair, Antihistamines | none | + | + | + |
| 2 | 14 | M | Yes | ICS, LABA, OCS | none | + | − | + |
| 3 | 48 | F | Yes | ISC, LABA, Omalizimab | none | + | − | + |
| 4 | 45 | F | Yes | ICS, Singulair, Antihistamines | TM | − | − | − |
| 5 | 55 | F | Yes | ICS, LABA | Smell | − | − | − |
| 6 | 60 | F | Yes | ICS, LABA, Omalizimab | TM | − | − | − |
All patients had IOS, spirometry and laryngoscopy performed. + indicates results were consistent with the diagnosis of VCD.
Other abbreviations: ICS, inhaled corticosteroid; LABA, long-acting Beta2 agonist; OCS, oral corticosteroids; TM, treadmill
Normal impedance measurements are unaffected by exercise challenge
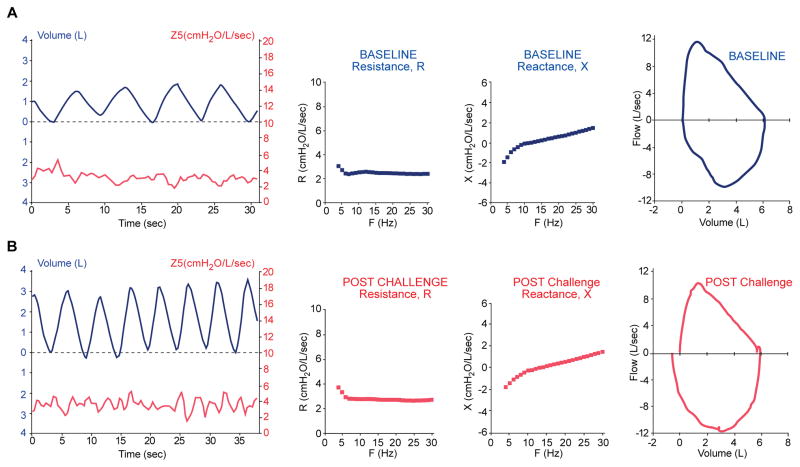
Impedance, Z in the normal lung may increase minimally during tidal volume breathing. Figure 1A shows the pattern of frequency independent impedance vs. time in relationship to lung volume in addition to R, X and the spirometric flow volume loop in a representative non-atopic, 21 year old male with a baseline FEV1 of 127%. After exercise challenge of the healthy subject, (Figure 1B) there is an increase in respiratory rate and a small increased variability of impedance. R, X and FVL loop remain similar which was a consistent finding with the remaining 6 controls challenged.
Figure 1. Pulmonary testing in normal volunteer.
21 yo normal volunteer. (A) At baseline. Frequency independent variability of impedance, Z5 with tidal volume is observed. R5–20 and X plots are smooth with low frequency dependent variability. Flow-volume loop is normal. (B) After exercise challenge. Expected increases in respiratory rate, tidal volume and Z5 variability, are seen otherwise other parameters remain unchanged.
Impedance in asthmatics
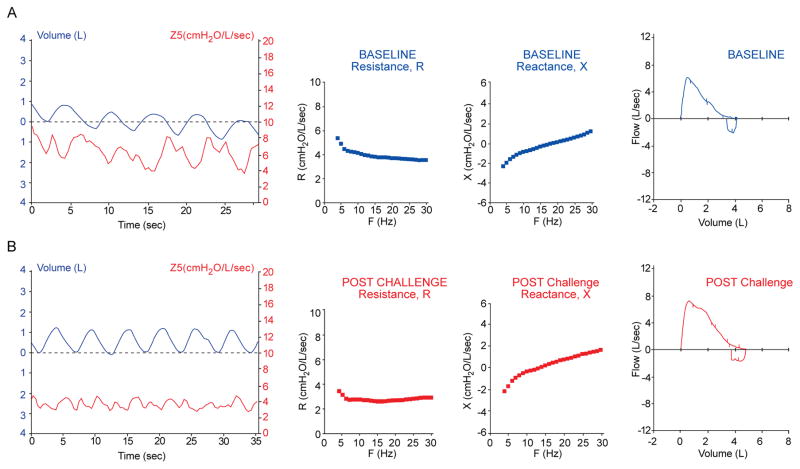
Symptomatic asthmatics tend to have a decrease in lung impedance with inspiration as the IOS pressure pulse signal is less impeded with increase lung volume expansion, as seen in the example impedance tracing in Figure 2A of an asthmatic without a history suggestive of VCD. Following administration of a β2 agonist, the variability in impedance is diminished during quiet breathing; and obstruction to airflow as determined by R and X and in the FVL improves (Figure 2B).
Figure 2. Pulmonary testing in asthmatic.
47 yo asthmatic without a history suggestive of VCD. (A) Symptomatic and (B) post bronchodilator treatment with β2 agonist which shows less variability of Z5 with quiet breathing and significant reduction in R5–20, area of X and improvement in FVL parameters.
Vocal cord dysfunction detected by IOS
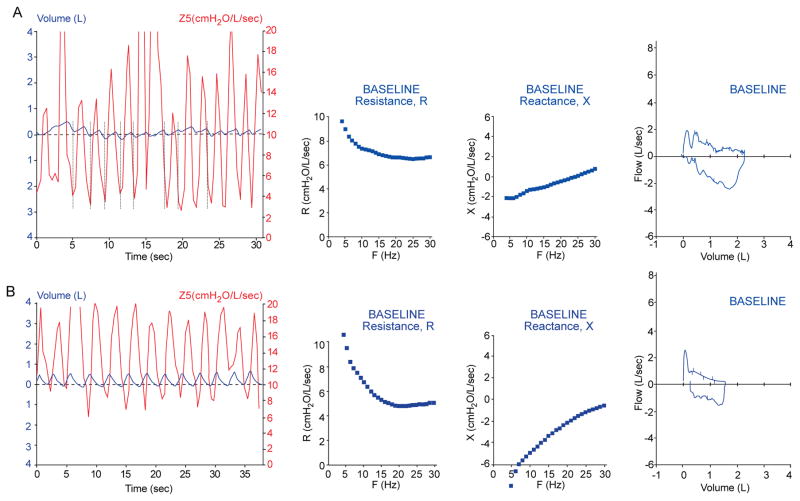
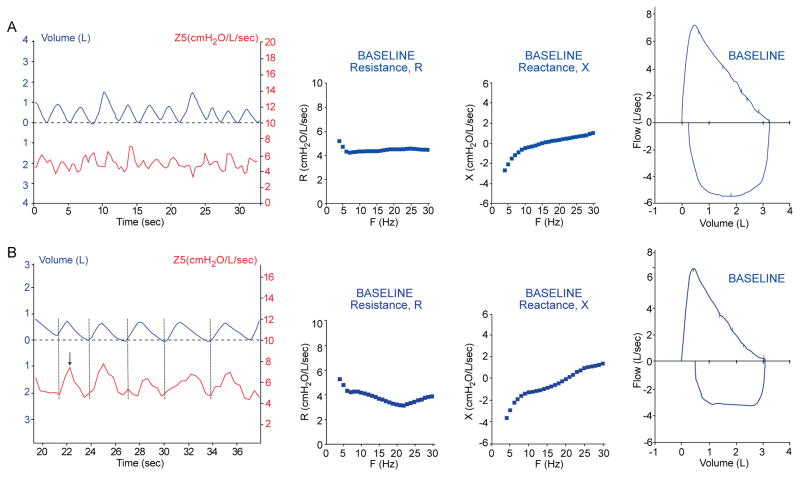
Figure 3A displays the tracings of patient #2, a 14 year old male, with undefined breathing difficulties for several years until VCD was diagnosed by laryngoscopy. This patient adducts his vocal cords during inspiration, which is typical of patients with VCD. As can be seen, this adduction of the vocal cords was detected by IOS in the Z5 vs. tidal volume curve, which exhibits a characteristic pattern that appears as a steep rise in impedance with inspiration (see Figure 3 (compare to impedance curves in Figure 1)). In the IOS impedance graph there are dramatic high amplitude spikes with inspiration consistent with dynamic proximal obstruction to air flow. Figure 3B shows similar IOS findings in an asthmatic adult with endoscopically proven VCD. The FV curve in both of these patients reveals a flow volume trapezium pattern consistent with the inspiratory and expiratory obstruction of VCD. Repeat IOS studies to coincide with triggering events are warranted. Figure 4A (Patient #1) displays baseline tracings of a 12 year old female with a history of intermittent dyspnea that had been diagnosed as having VCD via laryngoscopy. All initial tracings appear normal. However, during the evaluation, the patient had an acute anxiety attack associated with dyspnea. Repeat studies immediately following this event are displayed in Figure 4B. The truncation of the inspiratory loop of the F-V curve was then detected. Modest peaks in impedance of significant variability during inspiration were seen in the Z5 curve from IOS. Here, IOS also displayed a plateau of the reactance spectrum near resonant frequency, consistent with upper airway obstruction. Multiple trials were performed to exclude the possibility of upper airway obstruction due to tongue or mouthpiece artifact.
Figure 3. Characteristic impedance curve of VCD.
A) Patient#2, 14 yo male with VCD and muscular tension dysphonia. Z5 curve shows dramatic spike (> 10 cmH2O/L/sec) in impedance with onset of inspiration (vertical lines) correlated with vocal cord closure and flattening in X. Severe respiratory impairment detected by spirometry. B) Patient#3, 48 yo female with VCD and asthma diagnosed by endoscopy. Spikes in Z5 are also seen during inspiration. Spirometry shows biphasic trapezoidal truncation.
Figure 4. Anxiety- induced VCD.
Patient #1, 12 yo female with a history of asthma and VCD, (A) at baseline with normal pulmonary function. (B) During a panic attack, the classic truncation of the inspiratory loop of the FV curve was detected and modest peaks of impedance (Z5) of significant variability are seen during inspiration. Flattening of the X curve is consistent with extrathoracic obstruction.
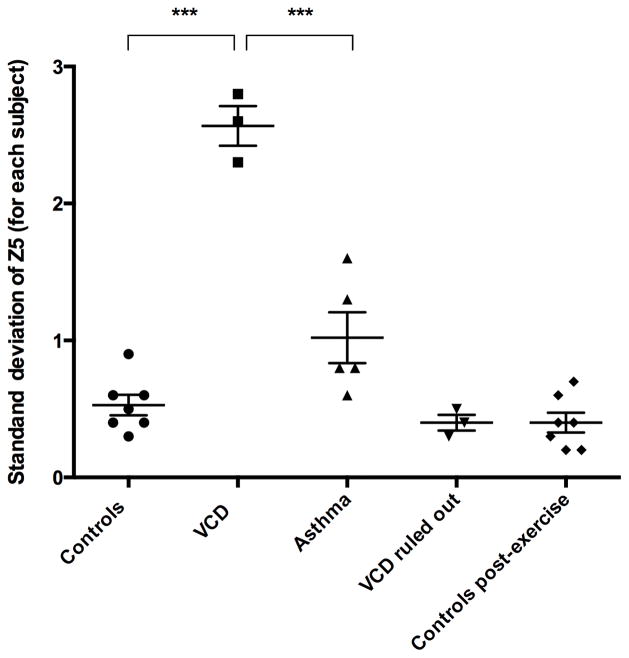
As shown in Table 2 and using one-way ANOVA comparisons, the group severity (mean Z5 amplitude [cm H20/L/s]) and variability (amplitude SDs) in patients with VCD (mean 9.3, SD 4.3) was significantly greater in comparison to healthy controls (mean 1.8, P<0.0001; SD 0.7), asthmatics (mean 4.2, P<0.0028; SD 1.2) and those patients where VCD was ruled out (mean 2.0P<0.0002; SD 0.8). The variability in impedance for each individual is shown graphically in Figure 5. Significantly greater variability was seen in individual patients with VCD in comparison to all other groups (P<0.0001). This analysis of Z5 provides an objective basis to distinguish patients with VCD from others that do not have VCD.
Table 2.
Group Mean Amplitude and Variability of Impedance
| Subjects | Mean amplitude of Z5 (cm H20/L/s) | SD (amplitude) |
|---|---|---|
| Controls (n=7) | 1.8 | 0.7 |
| VCD diagnosed (n=3) | 9.3 | 4.8 |
| Asthma (n=5) | 4.2 | 1.2 |
| VCD ruled out (n=3) | 2.0 | 0.8 |
| Controls Post exercise (n=7) | 1.5 | 0.5 |
Figure 5. Individual Variability of Impedance.
Display of the variability of impedance in the repetitive testing of each individual as represented by a dot/symbol. The mean bar indicates the average of this variability. Parametric ANOVA comparison of variability indicates significant difference (p < 0.0001) between individual patients with VCD (n=3) and controls (n=7) and asthmatics (n=5). (*** P<0.0001)
Voluntary Glottic Closure
To illustrate the distinction between VCD and voluntary glottic closure, 5 healthy controls performed self-induced glottic closure during IOS. A typical tracing at baseline (Figure S2A) and following glottic closure (Figure S2B) shows a spike in impedance with respiration that is uniformly associated with an interruption and truncation in IOS tidal volume respiration. Patients with VCD do not display this truncation in inspiratory flow (compare with Figure 3). This observation provides an approach using IOS impedance to distinguish between VCD and upper airway artifact.
Discussion
The etiology and diagnosis of VCD remains a challenge to the clinician. The diagnosis may either not be considered, as it may resemble asthma in presentation, or it may be missed on examination if it is transient in nature and with a strong psychological component. 30 Standard evaluation includes detection of an inspiratory truncation in the spirometric FV loop (not specific for VCD) and the observation of vocal cord adduction during inspiration using laryngoscopy. We herein present an analysis of patients with suspected VCD where IOS provided rapid and accurate results that were consistent with endoscopic findings. In 3 patients diagnosed with VCD, IOS detected upper airway obstruction during inspiration where it exhibited a characteristic signature consisting of a spike in lung impedance (Z5) with inspiration (Figures 3–4). This is made possible because IOS delivers a frequency dependent pressure signal to the pulmonary system during passive breathing and accurately detects acute obstruction to flow over a sampling time of seconds to minutes (unlike spirometry). Patients with asthma and normal subjects examined in this study did not display these findings at baseline and following exercise challenge (Figures 1 and 2). We were also able to show the utility of IOS in a real-time evaluation of episodic VCD heightened by anxiety (patient #1, Figure 4). In support of our findings on IOS this patient also displayed a truncation in the spirometric FV loop in one trial (Figure 4).
Using the mean amplitude as an indication of severity and SD for variability we have shown the characteristic spike in amplitude is of significantly greater magnitude and variability in those with VCD in comparison to control subjects, those with a history of VCD and our cohort of asthmatics. (Figure 5, Table 2) In addition we have shown that a flattening at the peak of inspiration on the IOS flow volume curve when accompanied by an impedance peak can indicate voluntary glottic closure (Figure S2). Thus, IOS can provide diagnostic insights in the evaluation of patients with respiratory symptoms that do not respond adequately to standard therapy, in a setting where there may be a dissociation between the severity of symptoms and the results of standard lung function testing and/or in patients with unexplained upper airway symptoms at baseline, or provoked by inhalant irritants, stress or exercise. This study as also presented clinical findings consistent with the conclusion that careful attention to the Z5 tracings, in terms of spikes during inspiration, high amplitude impedance and great variability with each breath is suggestive of upper airway obstruction as seen in VCD. In addition to the objective measurements obtained from IOS in the evaluation of VCD, the ease of testing would permit the assessment of very young children that cannot perform spirometry or may not comply with invasive laryngoscopy. 16, 31–33 The limitations of this study include small VCD sample size and that all patients where the diagnosis was made had a history of asthma. Although larger studies are warranted, the findings presented in this report are consistent with the conclusion that IOS may provide a non-invasive approach to the diagnosis of VCD. 34
Supplementary Material
Clinical Implications.
Vocal cord dysfunction (VCD) is characterized by inappropriate vocal cord adduction during inspiration. The diagnosis is challenging, as expected flow volume loop abnormalities are may not be present and laryngoscopy must be performed during symptoms.
This study has determined that impulse oscillometry (IOS) exhibits a characteristic impedance pattern in patients with VCD verified by laryngoscopy.
IOS may offer a rapid and non-invasive adjunct to the assessment and diagnosis of patients with suspected VCD.
Acknowledgments
This work was supported by the Division of Intramural Research, NIAID, NIH. Support by M.Y. for this project was funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
We thank Dr. Gary Larsen for his critical review of this manuscript and helpful suggestions and Jeff Skinner for his creative contributions to the statistical analysis.
Abbreviations
- IOS
Impulse Oscillometry
- VCD
Vocal Cord Dysfunction
- GERD
Gastroesophageal reflux disease
- FVL
Flow-volume loop
- PFTs
Pulmonary function tests
- ERS/ATS
European Respiratory Society/American Thoracic Society
- Z
Impedance
- R
Resistance
- X
Reactance
- Hz
Hertz
Footnotes
Authors state no conflict of interest.
References
- 1.Morris MJ, Christopher KL. Diagnostic criteria for the classification of vocal cord dysfunction. Chest. 2010;138:1213–23. doi: 10.1378/chest.09-2944. [DOI] [PubMed] [Google Scholar]
- 2.Morris MJ, Deal LE, Bean DR, Grbach VX, Morgan JA. Vocal cord dysfunction in patients with exertional dyspnea. Chest. 1999;116:1676–82. doi: 10.1378/chest.116.6.1676. [DOI] [PubMed] [Google Scholar]
- 3.Christopher KL, Wood RP, 2nd, Eckert RC, Blager FB, Raney RA, Souhrada JF. Vocal-cord dysfunction presenting as asthma. The New England journal of medicine. 1983;308:1566–70. doi: 10.1056/NEJM198306303082605. [DOI] [PubMed] [Google Scholar]
- 4.Newman KB, Mason UG, 3rd, Schmaling KB. Clinical features of vocal cord dysfunction. American journal of respiratory and critical care medicine. 1995;152:1382–6. doi: 10.1164/ajrccm.152.4.7551399. [DOI] [PubMed] [Google Scholar]
- 5.Powell DM, Karanfilov BI, Beechler KB, Treole K, Trudeau MD, Forrest LA. Paradoxical vocal cord dysfunction in juveniles. Archives of otolaryngology--head & neck surgery. 2000;126:29–34. doi: 10.1001/archotol.126.1.29. [DOI] [PubMed] [Google Scholar]
- 6.McFadden ER, Jr, Zawadski DK. Vocal cord dysfunction masquerading as exercise-induced asthma. a physiologic cause for “choking” during athletic activities. American journal of respiratory and critical care medicine. 1996;153:942–7. doi: 10.1164/ajrccm.153.3.8630577. [DOI] [PubMed] [Google Scholar]
- 7.Boulet LP. Cough and upper airway disorders in elite athletes: a critical review. British journal of sports medicine. 2012;46:417–21. doi: 10.1136/bjsports-2011-090812. [DOI] [PubMed] [Google Scholar]
- 8.Perkner JJ, Fennelly KP, Balkissoon R, Bartelson BB, Ruttenber AJ, Wood RP, 2nd, et al. Irritant-associated vocal cord dysfunction. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 1998;40:136–43. doi: 10.1097/00043764-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Kellman RM, Leopold DA. Paradoxical vocal cord motion: an important cause of stridor. The Laryngoscope. 1982;92:58–60. doi: 10.1288/00005537-198201000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Gavin LA, Wamboldt M, Brugman S, Roesler TA, Wamboldt F. Psychological and family characteristics of adolescents with vocal cord dysfunction. The Journal of asthma : official journal of the Association for the Care of Asthma. 1998;35:409–17. doi: 10.3109/02770909809048949. [DOI] [PubMed] [Google Scholar]
- 11.Earles J, Kerr B, Kellar M. Psychophysiologic treatment of vocal cord dysfunction. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2003;90:669–71. doi: 10.1016/S1081-1206(10)61874-1. [DOI] [PubMed] [Google Scholar]
- 12.Newman KB, Mason UG, 3rd, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995;152:1382–6. doi: 10.1164/ajrccm.152.4.7551399. [DOI] [PubMed] [Google Scholar]
- 13.Wood RP, 2nd, Milgrom H. Vocal cord dysfunction. The Journal of allergy and clinical immunology. 1996;98:481–5. doi: 10.1016/s0091-6749(96)70079-9. [DOI] [PubMed] [Google Scholar]
- 14.Maat RC, Roksund OD, Halvorsen T, Skadberg BT, Olofsson J, Ellingsen TA, et al. Audiovisual assessment of exercise-induced laryngeal obstruction: reliability and validity of observations. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2009;266:1929–36. doi: 10.1007/s00405-009-1030-8. [DOI] [PubMed] [Google Scholar]
- 15.Gimenez LM, Zafra H. Vocal cord dysfunction: an update. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2011;106:267–74. doi: 10.1016/j.anai.2010.09.004. quiz 75. [DOI] [PubMed] [Google Scholar]
- 16.Komarow HD, Myles IA, Uzzaman A, Metcalfe DD. Impulse oscillometry in the evaluation of diseases of the airways in children. Ann Allergy Asthma Immunol. 2011;106:191–9. doi: 10.1016/j.anai.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beraldo PS, Mateus SR, Araujo LM, Horan TA. Forced oscillation technique to detect and monitor tracheal stenosis in a tetraplegic patient. Spinal Cord. 2000;38:445–7. doi: 10.1038/sj.sc.3101005. [DOI] [PubMed] [Google Scholar]
- 18.Cao J, Que C, Wang G, He B. Effect of posture on airway resistance in obstructive sleep apnea-hypopnea syndrome by means of impulse oscillation. Respiration. 2009;77:38–43. doi: 10.1159/000114146. [DOI] [PubMed] [Google Scholar]
- 19.Dundas I, Chan EY, Bridge PD, McKenzie SA. Diagnostic accuracy of bronchodilator responsiveness in wheezy children. Thorax. 2005;60:13–6. doi: 10.1136/thx.2004.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander S. Niven, correspondence. .
- 21.Goldman MD. Clinical application of forced oscillation. Pulm Pharmacol Ther. 2001;14:341–50. doi: 10.1006/pupt.2001.0310. [DOI] [PubMed] [Google Scholar]
- 22.Rigau J, Farre R, Trepat X, Shusterman D, Navajas D. Oscillometric assessment of airway obstruction in a mechanical model of vocal cord dysfunction. Journal of biomechanics. 2004;37:37–43. doi: 10.1016/s0021-9290(03)00256-2. [DOI] [PubMed] [Google Scholar]
- 23.Hira HS, Singh A. Significance of upper airway influence among patients of vocal cord dysfunction for its diagnosis: Role of impulse oscillometry. Lung India : official organ of Indian Chest Society. 2009;26:5–8. doi: 10.4103/0970-2113.45197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304–45. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- 25.Oostveen E, MacLeod D, Lorino H, Farre R, Hantos Z, Desager K, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22:1026–41. doi: 10.1183/09031936.03.00089403. [DOI] [PubMed] [Google Scholar]
- 26.Dencker M, Malmberg LP, Valind S, Thorsson O, Karlsson MK, Pelkonen A, et al. Reference values for respiratory system impedance by using impulse oscillometry in children aged 2–11 years. Clin Physiol Funct Imaging. 2006;26:247–50. doi: 10.1111/j.1475-097X.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 27.Marchal F, Haouzi P, Peslin R, Duvivier C, Gallina C. Mechanical properties of the upper airway wall in children and their influence on respiratory impedance measurements. Pediatric pulmonology. 1992;13:28–33. doi: 10.1002/ppul.1950130108. [DOI] [PubMed] [Google Scholar]
- 28.Nowowiejska B, Tomalak W, Radlinski J, Siergiejko G, Latawiec W, Kaczmarski M. Transient reference values for impulse oscillometry for children aged 3–18 years. Pediatr Pulmonol. 2008;43:1193–7. doi: 10.1002/ppul.20926. [DOI] [PubMed] [Google Scholar]
- 29.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 30.Christopher KL. Understanding vocal cord dysfunction: a step in the right direction with a long road ahead. Chest. 2006;129:842–3. doi: 10.1378/chest.129.4.842. [DOI] [PubMed] [Google Scholar]
- 31.Benjamin BN, Gray SD, Bailey CM. Neonatal vocal cord paralysis. Head & neck. 1993;15:169–72. doi: 10.1002/hed.2880150215. [DOI] [PubMed] [Google Scholar]
- 32.Heatley DG, Swift E. Paradoxical vocal cord dysfunction in an infant with stridor and gastroesophageal reflux. International journal of pediatric otorhinolaryngology. 1996;34:149–51. doi: 10.1016/0165-5876(95)01230-3. [DOI] [PubMed] [Google Scholar]
- 33.Marotta A, Klinnert MD, Price MR, Larsen GL, Liu AH. Impulse oscillometry provides an effective measure of lung dysfunction in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol. 2003;112:317–22. doi: 10.1067/mai.2003.1627. [DOI] [PubMed] [Google Scholar]
- 34.Davis RS, Brugman SM, Larsen GL. Use of videography in the diagnosis of exercise-induced vocal cord dysfunction: a case report with video clips. J Allergy Clin Immunol. 2007;119:1329–31. doi: 10.1016/j.jaci.2007.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.