Dear Editor
The Children’s Oncology Group (COG) focuses on developing new pediatric cancer treatments. For osteosarcoma, the 5-year event free survival (EFS) has been stalled near 65% for patients with localized tumors, and about 20% for patients with metastatic and relapsed disease, for 30 years [1]. Since the late 1980s, methotrexate, cisplatin and doxorubicin have been the cornerstone of therapy and no new active agents have been identified.
The COG Bone Tumor Committee instituted an approach described by Khanna et al. to identify agents of interest for phase II trials [2]. The cumulative relative merit for eribulin, a novel microtubule inhibitor, was higher than that of other agents considered for a Phase II trial in osteosarcoma. The Pediatric Preclinical Testing Program found complete responses in 3 of 6 osteosarcoma xenografts[3]. The Phase II study AOST1322 was designed with an expected enrollment of 1.3 patients per month, based on accrual of osteosarcoma patients to four previous COG Phase II solid tumor studies [4–6].
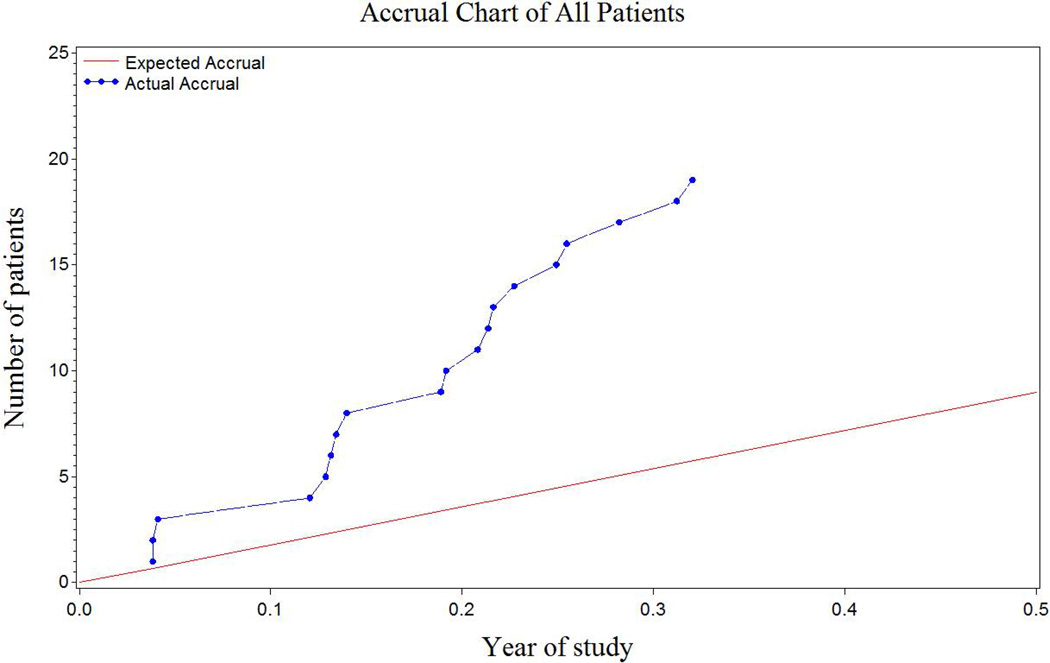
Activated in late August, 2014, AOST1322 opened at 97 North American centers and accrued at a rate of 5.5 patients per month, meeting the stage I accrual goal of 19 subjects within 3.5 months rather the expected 1.5 years (Figure 1). The study outcome will be reported separately.
Figure 1.
Actual accrual on AOST1322 as compared to the expected accrual.
There are likely multiple factors contributing to the faster-than-expected accrual, including: Very few agents are effective against osteosarcoma, so patients, families, and physicians are eagerly awaiting opportunities to participate in trials that may offer hope; A long gap between available clinical trials may have increased the demand; An early phase clinical trial designed specifically for osteosarcoma, rather than solid tumors generally, may have better attracted the attention of physicians caring for osteosarcoma patients; The treatment had a low burden for patients and families, as eribulin was given as an IV push on days 1 and 8 of a 21 day cycle, and has low toxicity; The study was open to all COG sites when there were very few competing trials, and was easily activated because of the Pediatric Central IRB; Finally, this trial, unlike some prior studies, had both survival and response endpoints, which may have encouraged accrual.
Before activation the age limit for eligibility was lowered from 16 to 12 years based on FDA recommendations following review of adult pharmacokinetic data. Although the accrual predictions were based on studies that also used this expanded age range, we did enroll nine patients younger than 16.
Though this study was designated an NCTN trial and was posted on the CTSU, the rapid accrual gave the four other NCTN cooperative groups no opportunity to enroll patients.
Regardless of these considerations, the rapid accrual on this study supports the development of additional phase II trials specifically for the treatment of osteosarcoma, based both on the need for novel agents and the ability to complete such studies rapidly. Of course, the development of more Phase II trials in osteosarcoma depends upon the availability of new agents to test.
More generally, those designing phase II clinical trials may wish to consider adjusting accrual expectations based on the breadth of the target population and the state of competing trials. Certainly, study design changes should be made with caution.
References
- 1.Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O'Leary M, Smith FO, Reaman GH. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khanna C, Fan TM, Gorlick R, Helman LJ, Kleinerman ES, Adamson PC, Houghton PJ, Tap WD, Welch DR, Steeg PS, Merlino G, Sorensen PH, Meltzer P, Kirsch DG, Janeway KA, Weigel B, Randall L, Withrow SJ, Paoloni M, Kaplan R, Teicher BA, Seibel NL, Smith M, Uren A, Patel SR, Trent J, Savage SA, Mirabello L, Reinke D, Barkaukas DA, Krailo M, Bernstein M. Toward a drug development path that targets metastatic progression in osteosarcoma. Clin Cancer Res. 2014;20(16):4200–4209. doi: 10.1158/1078-0432.CCR-13-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolb EA, Gorlick R, Reynolds CP, Kang MH, Carol H, Lock R, Keir ST, Maris JM, Billups CA, Desjardins C, Kurmasheva RT, Houghton PJ, Smith MA. Initial testing (stage 1) of eribulin, a novel tubulin binding agent, by the pediatric preclinical testing program. Pediatric blood & cancer. 2013;60(8):1325–1332. doi: 10.1002/pbc.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaty O, 3rd, Berg S, Blaney S, Malogolowkin M, Krailo M, Knight R, Schaiquevich P, Stewart C, Chen Z, Nelson M, Voss S, Ivy SP, Adamson PC. A phase II trial and pharmacokinetic study of oxaliplatin in children with refractory solid tumors: a Children's Oncology Group study. Pediatr Blood Cancer. 2010;55(3):440–445. doi: 10.1002/pbc.22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs S, Fox E, Krailo M, Hartley G, Navid F, Wexler L, Blaney SM, Goodwin A, Goodspeed W, Balis FM, Adamson PC, Widemann BC. Phase II trial of ixabepilone administered daily for five days in children and young adults with refractory solid tumors: a report from the children's oncology group. Clin Cancer Res. 2010;16(2):750–754. doi: 10.1158/1078-0432.CCR-09-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warwick AB, Malempati S, Krailo M, Melemed A, Gorlick R, Ames MM, Safgren SL, Adamson PC, Blaney SM. Phase 2 trial of pemetrexed in children and adolescents with refractory solid tumors: a Children's Oncology Group study. Pediatr Blood Cancer. 2013;60(2):237–241. doi: 10.1002/pbc.24244. [DOI] [PMC free article] [PubMed] [Google Scholar]