Abstract
With the advent of high-throughput molecular technologies, several precision medicine (PM) studies are currently ongoing that include molecular screening programs and PM clinical trials. Molecular profiling programs establish the molecular profile of patients’ tumors with the aim to guide therapy based on identified molecular alterations. The aim of prospective PM clinical trials is to assess the clinical utility of tumor molecular profiling and to determine whether treatment selection based on molecular alterations produces superior outcomes compared with unselected treatment. These trials use treatment algorithms to assign patients to specific targeted therapies based on tumor molecular alterations. These algorithms should be governed by fixed rules to ensure standardization and reproducibility. Here, we summarize key molecular, biological, and technical criteria that, in our view, should be addressed when establishing treatment algorithms based on tumor molecular profiling for PM trials.
Precision medicine (PM), also called “personalized medicine,” is defined by the National Cancer Institute as “a form of medicine that uses information about a person’s genes, proteins, and environment to prevent, diagnose, and treat disease.” PM in oncology emerged with the advent of molecularly targeted agents (MTAs) almost two decades ago and is mainly based today on the DNA molecular information of the patients’ tumors. While cytotoxic agents destroy rapidly dividing cells by triggering DNA and cell division machinery, MTAs modulate the function of specific molecular targets in cell signaling, proliferation, apoptosis, angiogenesis, metabolism, migration, or invasion. It is now established that a majority of deleterious molecular alterations are shared by various tumor types (1). There have been multiple examples of MTAs being effective in several tumor types harboring a same molecular alteration (ie, trastuzumab and lapatinib for HER2 amplified and overexpressed in 10% to 15% of breast and gastric cancers). In solid tumors, molecular alterations are often noted in a very small proportion of patients (ie, ROS1 and ALK translocations in lung cancer) and therefore it is challenging to set up clinical trials to demonstrate the benefit of these drugs in small subgroups of patients (2).
Advances in high-throughput technologies now allow the identification of actionable molecular alterations in a single assay for an affordable cost in and a timeframe compatible with clinical practice (3). Clinical trials have been launched in which several MTAs are included in the same protocol and patients are assigned to a specific MTA based upon the molecular alterations identified their tumors. Some of these trials are not stratified on the drugs used nor on the tumor types in their designs, but they assess the overall strategy of using MTAs based on the identified molecular alterations (3). These latter clinical trials actually evaluate the algorithm that has been set up to allocate treatments to patients. Examples of these trials include metastatic disease from all cancer types trials such as the SHIVA trial (4), MPACT (5), and the WINTHER trial (6), as well as disease-specific trials such as the SAFIR02 trials (7). These trials, often named PM trials, evaluate the efficiency of the treatment algorithm used to guide therapy (3). Therefore, treatment algorithms are essential for these PM trials but no guidelines exist for their establishment.
In order to produce interpretable and reproducible results, several key aspects of these treatment algorithms should be thoughtfully defined prior to starting the trial. The aim of this review is to stress the importance of treatment algorithms in PM trials. We describe, in the present manuscript, some of the criteria that should be taken into account to design treatment algorithms for future PM trials (8). Key aspects include the specification of the technology used for molecular profiling, the definition of “targetable” molecular alterations and targeted agents, and the prioritization of targetable molecular alterations in patients whose tumors have more than a single alteration.
Fundamental Hypothesis: Cancer Is a Genomic Disease
The advent of next-generation sequencing (NGS) led in the last few years to a massive increase in cancer molecular profiling, allowing the characterization of DNA sequence variants in tumor tissues to better understand cancer progression and to index cancer genomes ultimately aiming to inform therapeutic decision. NGS techniques are still expensive (although prices are decreasing considerably) for the sequencing of the entire human genome (3 Gb) and are outside of the current reach of clinical diagnostic laboratories. Targeted sequencing, including exome sequencing (coding regions [ie, 1% of the human genome 28Mb]), or sequencing of a subset of known genes or mutation hotspots (targeted NGS) are more routinely for clinical testing and research. Targeted NGS allows analyzing several hundreds of mutation hotspots located in oncogenes and in tumor suppressor genes (TSG) using dedicated cancer panel kits, thus expediting molecular diagnosis.
The pathogenesis of cancer involves a multistep dynamic process that includes clonal expansion, genetic diversification, and clonal selection (Supplementary Figure 1, available online) (9). Therapeutic interventions may destroy cancer cell clones but may also provide a potent selective pressure for the expansion of resistant variants clones. Genetic alterations in cancer include abnormal activation of oncogenes (gain-of-function) or inactivation of TSG (loss-of-function). Oncogenes and TSG (Table 1) differ in their mechanisms of actions (Supplementary Figures 2 and 3, available online). Gain-of-function oncogene alterations act dominantly (ie, alteration of only one of the two alleles is a sufficient molecular step for activation and potential cancer induction) (Table 1). On the other hand, TSG loss-of-function follows a recessive mode of action; ie, both alleles need to be altered to participate in tumorigenesis (Table 1; Supplementary Figures 2 and 3, available online). Consequently, these differences in the identified alterations should be considered in designing treatment algorithms for PM trials.
Table 1.
Functional relevance of genetic alterations in cancer and available techniques for their detection*
| Type of gene or molecule | Definition | Functional alteration | Relevant alterations in cancer |
|---|---|---|---|
| Oncogene | An oncogene is a gene that when active has the potential to cause cancer | Activating alteration (gain of function) | Activating mutations (DNA & RNA) Gene amplifications (DNA) Overexpression (RNA & protein) Translocations (DNA & RNA) DNA hypomethylation (DNA) Dysregulation by microRNAs (RNA) |
| Tumor suppressor gene | A tumor suppressor gene is a gene that inactivated has the potential to cause cancer | Inactivating alteration (loss of function) | Inactivating mutations (DNA & RNA) Homozygous or heterozygous deletions (DNA) Loss of expression (RNA & protein) DNA hypermethylation (DNA) Dysregulation by microRNAs (RNA) |
| Type of molecule | Type of alteration | Routine techniques (diagnostic) | High-throughput techniques (research) |
| DNA | Mutations | Sanger sequencing & targeted NGS | WES & WGS & targeted NGS |
| Translocations | FISH (+/- IHC screening or validation) | WGS & WES | |
| Amplifications/gains or heterozygous and homozygous deletions | Gene dosage PCR FISH & CGHa (+ IHC validation) | WGS (& WES & targeted NGS) | |
| RNA | Mutations | RT-PCR & Sanger sequencing | RNA sequencing |
| Translocations (fusion transcripts) | RT-PCR & targeted NGS | RNA sequencing | |
| Overexpression | Quantitative RT-PCR | RNA sequencing | |
| Protein | Overexpression/underexpression | IHC | RPPA, LC-MS/MS & others |
* CGHa = comparative genomic hybridization array; FISH = fluorescent in situ hybridization; IHC = immunohistochemistry; LC-MS/MS = liquid chromatography–tandem mass spectrometry; NGS = next-generation sequencing; PCR = polymerase chain reaction; RPPA = reverse phase protein array; RT-PCR = reverse transcriptase PCR; WES = whole-exome sequencing; WGS = whole-genome sequencing.
Oncogenes and TSGs alterations could be detected at the DNA, RNA, or protein levels using different techniques (Table 1). A large proportion of the genetic alterations found in any tumor are considered “passengers” ie, not resulting in a deleterious effect. In genomically complex tumors, it is not trivial to decipher the “driver” alterations, including those that have a major deleterious impact and those that are the determinants of inherent or acquired resistance to specific MTAs.
Precision Medicine Trials and Treatment Algorithms
The Cancer Genome Atlas and the International Cancer Genomics Consortium have identified recurrent point mutations, copy number variations, translocations, and potential new therapeutic targets in more than 50 cancer subtypes, respectively (10). These pioneering projects leveraged new high-throughput techniques and paved the way for academic cancer centers and pharmaceutical companies to translate molecular data to clinical practice. The co-evolution of innovative PM trial designs and sequencing technologies holds the promise of linking tumor genomics to therapeutic effectiveness. The molecular alterations included in the PM algorithm and the techniques used to assess these alterations, the computational pipelines, parameters, thresholds, and guidelines for the interpretation of these alterations, should be defined. Changes in techniques should be described, and molecular platforms should be validated. Despite the lack of guidelines, major clinical trials use US Food and Drug Administration (FDA)–certified platforms to ensure reproducibility of their results. In addition, the National Cancer Institute, in collaboration with scientists representing multiple areas of expertise relevant to ‘omics’-based test development, has developed a checklist of criteria that can be used to determine the use of omics-based tests for guiding patient care in clinical trials (11).
Definitions of Identified Molecular Alterations and Their Biological Consequences
Oncogene Activation
PM trials may include gene copy number and mutational assessments to guide therapy. The following key points should be considered for interpretation of the functional relevance of the alteration. The specific definition for each alteration should be specified in the frame of the PM trial algorithm.
Comparative genomic hybridization arrays (CGHa) provide the information on gene copy number alterations. The number of copies of a specific oncogene that reflects its potential amplification should be interpreted by taking into account tumor cellularity and ploidy. We suggest, as reported in the SHIVA trial, that only focal amplifications with six or more copies and less than 10Mb in size could be considered relevant (8). These thresholds were previously suggested using fluorescent in situ hybridization (FISH) assay to estimate the minimal level amplification detected by CGHa to be validated as true DNA amplicon in clinical practice (12,13). Further validations and correlations with protein expression levels using immunohistochemistry (IHC) techniques are encouraged. A 10Mb amplicon comprises approximately 100 genes, among which driver genes are expected to be amplified and overexpressed at the RNA and protein levels. In contrast, large amplifications or gains (with only few additional copies 3 to 5 and >10Mb) should not be considered (Table 2). The amplitude of the gain/amplification needs to be corrected based on the tumor cell content. For example, we believe that a focal gain of five copies in samples with 50% of tumors cells should be considered a focal amplification since reevaluated to more than six copies within tumor cells (8). CGHa techniques may also indicate potential chromosomal translocations, which require further confirmation by FISH or reverse transcriptase polymerase chain reaction (RT-PCR).
Table 2.
Gene copy number alterations: technical aspects and interpretation of the Comparative Genomic Hybridization array analyses
| Alteration | Proposed technical considerations | Proposed interpretation depending on the type of gene | ||
|---|---|---|---|---|
| Type of alteration | Fold change* | Size of amplicon | Oncogene | Tumor suppressor gene |
| DNA copy number gain | DNA copy number >2 and <6 for diploid tumors | No specific size to be considered | Not considered | Not considered |
| Focal amplification | DNA copy number ≥ 6 for diploid tumors | ≤10 Mb† | Considered only if: known in the literature/ databases to be of theranostic interest Potentially validated by IHC |
Not considered |
| Heterozygous deletion | 1 copy for diploid tumors | No specific size to be considered | Not considered | Considered only if associated to an inactivating mutation of the second allele or loss of the tumor suppressor protein validated by IHC |
| Homozygous deletion | 0 copy for diploid tumors | No specific size to be considered | Not considered | Considered |
| Somatic uniparental isodisomy | 2 identical copies for diploid tumors | No specific size to be considered | Not considered | Considered only if associated to an inactivating mutation of the remaining allele or loss of the tumor suppressor protein validated by IHC |
* Fold change to be interpreted by taking into account tumor cellularity and ploidy. IHC = immunohistochemistry.
†10Mb locus size include an average of 100 genes (1 gene per 100 Kb).
NGS techniques provide information on single-nucleotide variants (SNV) resulting in point mutations or in-frame deletions/insertions that can induce gain-of-function of “targetable” oncogenes (Table 3). Key criteria for NGS analysis are the coverage and sequencing depth (redundancy of coverage, depth of coverage) (14). These two terms are used interchangeably. Coverage refers to the expected coverage based on the number and the length of high-quality reads before or after alignment to the reference. Sequencing depth reflects the average number of times a given region has been sequenced by independent reads. For example, a genome sequencing study may sequence a genome to 30X average depth and achieve a 95% breadth of coverage of the reference genome at a minimum depth of 10 reads (14).
Table 3.
Technical aspects and mutations’ interpretation of the targeted gene panel NGS analyses
| Mutation | Technical considerations | Proposed Interpretation | Interpretation depending on the type of gene | ||
|---|---|---|---|---|---|
| Type | Proposed depth | Proposed frequency | Alteration to be considered | Oncogene | Tumor suppressor gene |
| Single-nucleotide variant | >300X | (>4%) strand ratio (>0.2) | Missense mutation (SNV ≤ 0.1% in 1000 genome and/or ESV) | Considered only if known in the literature/ databases to be activating mutation (within or close to a functional domain or hot spot) or with positive predictive scoring (eg, SIFT & PolyPhen-2) | Considered only if known in the literature/databases to be inactivating mutation (within or close to a functional domain or hot spot) |
| Nonsense mutation (SNV ≤ 0.1% in 1000 genome and/or ESV) | Not considered | Considered | |||
| Splice-site mutation (SNV ≤ 0.1% in 1000 genome and/or ESV) | Considered only if known in the literature/ databases to be activating (deleted exon in-frame) | Considered when resulting in a frameshift mutation | |||
| Synonymous mutation | Not considered | Not considered | |||
| Single-nucleotide polymorphism (SNV > 0.1% in 1000 genome and/or ESV) | Not considered | Not considered | |||
| Nucleotide(s) insertion deletion | >300X | (>4%) | In-frame | Considered only if known in the literature/ databases to be activating | Not considered |
| Frameshift | Not considered | Considered | |||
* 1000 genome: www.1000genomes.org. ESV = EBI structural variant (http://dgv.tcag.ca/dgv/app/faq); NGS = next-generation sequencing; PolyPhen-2 = Polymorphism Phenotyping v2, a tool that predicts possible impact of an amino acid substitution on the structure and function of a human protein using straightforward physical and comparative considerations (http://genetics.bwh.harvard.edu/pph2/); SIFT = predicts whether an amino acid substitution affects protein function (www.sift.jcvi.org); SNP = single-nucleotide polymorphism; SNV = single-nucleotide variant
Detection of somatic alterations in tumor biopsy samples is complicated by both the presence of normal cells as well as the presence of multiple clonal subpopulations within tumor cells. These factors affect the required depth of sequencing to call clonal mutations at sufficient power (>80%) in each sample, with greater than 100X required to detect mutations that may be present in around 20% of tumor cells (15). The sequencing depth (>300X in clinical routine of somatic genetics) and frequency of SNV (>4%) should be well defined at the start of the trial (8). Once the technical parameters are set, only missense (ie, PIK3CA mutations), splice-site mutation revealing in-frame exon skipping (ie, exon 14 skipping in MET mRNA), in-frame micro deletions (EGFR exon 19 deletion), or micro insertions that are well established to be activating mutations, should be considered functionally relevant. Other SNVs, nonsense and synonymous mutations, single-nucleotide polymorphisms (SNP), frame-shift insertion/deletion, etc., are not functionally relevant for oncogene activation and should not be considered as targetable alterations (Table 3). Notably, some mutations in oncogenes are resistance biomarkers (ie, T790M in exon 20 of EGFR). The functional relevance of a molecular alteration should be assessed in relevance with the MTA.
Tumor Suppressor Genes Inactivation
Inactivation of TSG implies that the two alleles that code for a particular protein are altered. Loss of one allele of a TSG can be considered relevant only if the second allele has an inactivating mutation or if further validation (for instance using IHC) can prove the loss of the tumor suppressor protein’s expression (Tables 2 and 3). More specifically, only loss-of-function homozygous mutations/deletions and somatic uniparental isodisomy (associated with an inactivating mutation) are considered deleterious. For TSG, nonsense mutations, splice-site mutation, or insertion/deletions resulting in a frameshift mutation are considered. Missense mutations are considered relevant if they are established inactivating mutations in the literature. In the near future, TSG inactivation by DNA hypermethylation should be analyzed in PM trials (ie, BRCA1 and BRCA2 promoter hypermethylation, as biomarker of sensitivity to PARP inhibitors).
Of note, several reports suggest that certain TSG show “haplo-insufficiency,” ie, the loss of one copy of the gene is insufficient for proper function and may contribute to tumorigenesis (16). Although the biological impact of haplo-insufficiency in cancer requires further investigation, there is strong evidence that a single-copy mutation or loss of one allele of a TSG like PTEN could be sufficient for cancer progression in humans and mice (17).
Targeted Signaling Pathways and Sensitivity/Resistance Biomarkers
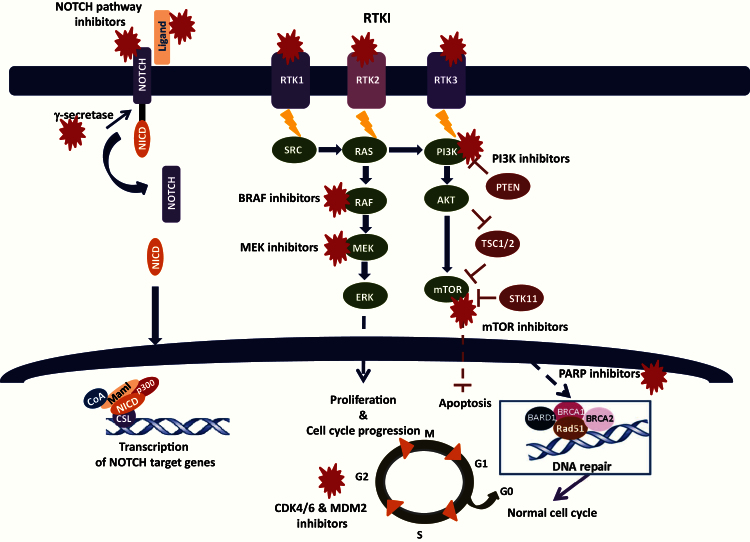
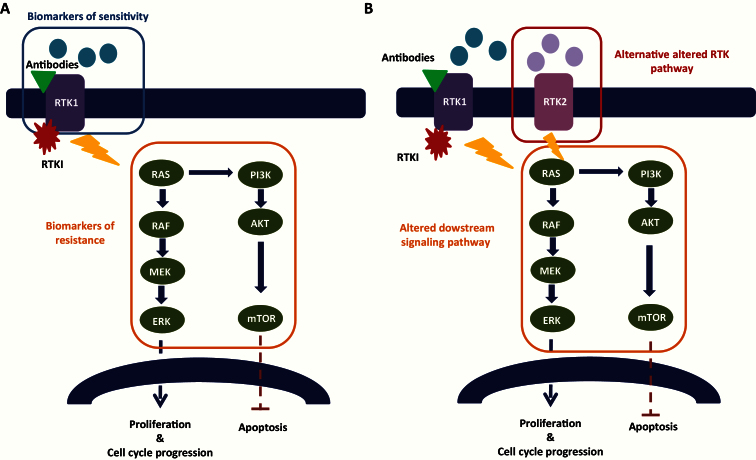
Once potentially driver alterations are validated in the context of their deleterious effect, another important consideration is their relevance as biomarkers of sensitivity (and resistance) for a specific MTA in the context of the drugs under consideration in the PM trial. The level of evidence of a predictive biomarker should be also carefully considered in the context of the continuous increase of identified molecular alterations in the era of high-throughput molecular profiling. Levels of evidence of prognostic biomarkers have been clearly defined since the early 90s and are continuously updated (18). On the other hand, the ranking is not as straightforward for theranostic biomarkers. A three-tier scale for level of evidence for associations between genomic alterations and response (sensitivity/resistance) to therapy was recently published based on the strength of available clinical data (19). Several studies demonstrate that MTA are active in tumors with mutations in well-characterized driver genes, such as imatinib in BCR/ABL-positive chronic myeloid leukemia, trastuzumab in ERBB2-positive breast cancers (20), and vemurafenib in BRAF V600E–mutated melanoma (21). Several other MTAs are currently being investigated in advanced phase clinical trials or recently received FDA approval (vismodegib, hedgehog pathway inhibitor in basal cell carcinoma). The major signaling pathways targeted in cancer treatment are summarized in Figure 1. Supplementary Table 1 presents a nonexhaustive list of potential drugs, protein targets, and possible predictive molecular biomarkers that should be considered when establishing a treatment algorithm. Predictive biomarkers of sensitivity are mainly molecular alterations of the MTA target gene or alterations upstream the signaling pathway (Figure 2A). Primary (Figure 2A) and secondary (Figure 2B) resistance biomarkers are crucial to predict treatment response and constitute alterations downstream the target gene and/or potential alternative signaling pathways. One of the major genes, when mutated, involved in the resistance to receptor tyrosine kinase inhibitors (TKI) is KRAS. Anti-EGFR antibodies (ie, cetuximab or panitumumab) are not efficient in presence of resistant KRAS mutations in patients with colorectal cancer (22), therefore restricting the use of these antibodies to patients with wild-type HRAS, NRAS, or KRAS tumors (23).
Figure 1.
Major signaling pathways and therapeutic targets in cancer.
M = mitosis; RTK = receptor tyrosine kinase; RTKI = receptor tyrosine kinase inhibitor; S = synthesis.
Figure 2.
A) Primary resistance biomarkers of receptor tyrosine kinase (RTK) inhibitors/antibodies. B) Secondary resistance biomarkers of receptor tyrosine kinase (RTK) inhibitors/antibodies.
Prioritization of Molecular Alterations in Precision Medicine Trials
Several tumor molecular alterations are identified but few are “drivers”, including some that have substantial diagnostic, prognostic, or therapeutic implications. Alterations that are targetable either directly or indirectly with approved or investigational therapies are potentially “actionable” (19).
A treatment algorithm should clearly define prioritization criteria in case of multiple tumor targetable alterations. The algorithm should include specific rules of prioritization that should not be modified during the study. Table 4 summarizes our proposed prioritization for treatment assignment, taking into consideration the following: 1) hierarchy of signaling pathways (RTK pathways, cell cycle, etc.), 2) direct target vs indirect target of the MTA, 3) oncogene activation vs TSG inactivation, 4) activating mutation vs focal amplification of oncogene, 5) size vs amplitude of focal amplifications of two oncogenes, and 6) frequency, depth, and level of validation between two mutations in two different oncogenes.
Table 4.
Suggestions for prioritizing molecular alterations in precision medicine trials*
| Suggestions to prioritize detected molecular alterations | Comments |
|---|---|
| Classification of targeted pathways to be taken into account (RTK vs cell cycle etc.) | - |
| Direct target of the drug vs indirect biomarkers in the altered signaling pathway | - |
| Oncogene activation vs tumor suppressor gene inactivation | If similarly proven alterations (databases and literature) |
| Activating mutation vs focal amplification of oncogene | To be taken into account: Frequency and depth of the mutation Level of validation of the mutation in databases, literature, functional predictions (SIFT, PolyPhen-2) etc. Characteristic of the focal amplification (fold change and size of amplicon) |
| Size of amplicon vs amplitude of focal amplifications of 2 oncogenes? | To be taken into account: Fold change ≥ 6 copies (corrected to the tumor cellularity) Small amplicon (≤10Mb) |
| Activating mutations of oncogene 1 vs activating mutation of oncogene 2 | To be taken into account: Frequency and depth of the mutation Level of validation of the mutation in databases, literature, etc. (validated vs unknow mutation and score PolyPhen-2, SIFT, etc.) |
| All of the above to be considered in presence or absence of downstream resistance biomarkers | - |
* PolyPhen-2 = Polymorphism Phenotyping v2, a tool that predicts possible impact of an amino acid substitution on the structure and function of a human protein using straightforward physical and comparative considerations (http://genetics.bwh.harvard.edu/pph2/); RTK = receptor tyrosine kinase; SIFT = predicts whether an amino acid substitution affects protein function (www.sift.jcvi.org).
Direct MTA targets could be of higher impact than the indirect signaling pathway in question. Oncogenes’ alterations because of their impact on inducing cancer could be considered more deleterious than TSG alterations and consequently considered of higher impact in treatment algorithms. For example, when a tumor sample from a patient enrolled in a PM trial harbors an activating mutation of BRAF (V600E) and a homozygous deletion in PTEN, the inhibition of BRAF using vemurafenib should be considered of higher priority as compared with the use of an AKT or an mTOR inhibitor. An important criterion in prioritizing molecular alterations is also the presence of downstream resistance biomarkers. These data should be considered together with technical issues and thresholds (Table 5).
Table 5.
Key points to be addressed for a reproducible treatment algorithm*
| 1 | Technology | What technology and design (coverage of genes) should be used depending of the molecular alterations of interest? (eg, technology ap\propriate to detect somatic uniparental isodisomies) |
| 2 | Thresholds | What thresholds should be used: for amplifications/deletions: amplicon size? gene copy number? taking ploidy into account? for mutations: sequencing depth? frequency of the variant? |
| 3 | Quality controls | What kind of quality controls should be performed? Tumor cellularity check, sequencing, CGHa, bioinformatics, etc. |
| 4 | Validation of the molecular alterations identified | for amplifications/deletions (eg, by IHC) for mutations (eg, by Sanger sequencing or duplicate samples for targeted NGS) |
| 5 | Interpretation of the results | for hotspot mutations based on literature knowledge and databases use of prediction scores with fixed interpretations’ rule for nonreported mutations |
| 6 | Molecular alteration/target relation | What drugs should be given for what alteration? TKI vs antibodies? Should drug combinations be proposed? |
| 7 | Prioritization | Oncogene vs tumor suppressor gene Direct target of the drug vs vs indirect biomarkers in the pathway |
* CGHa = comparative genomic hybridization arrays; IHC = immunohistochemistry; NGS = next-generation sequencing; TKI = tyrosine kinase inhibitor.
Key Consideration for Precision Medicine Clinical Trials
The role of PM trials is to determine which molecular profiles correlate with sensitivity to specific MTAs in metastatic cancers with targetable alterations. In the near future, the design of PM trials needs to take into consideration the rapid progress of the high-throughput screening technology and the increase in the available molecular, scientific, and medical information. We will detail in the following section key issues such as tumor heterogeneity and the importance of optimizing molecular platforms via regulatory authorities’ guidelines, as well as computational pipelines and data management issues.
Tumor Heterogeneity
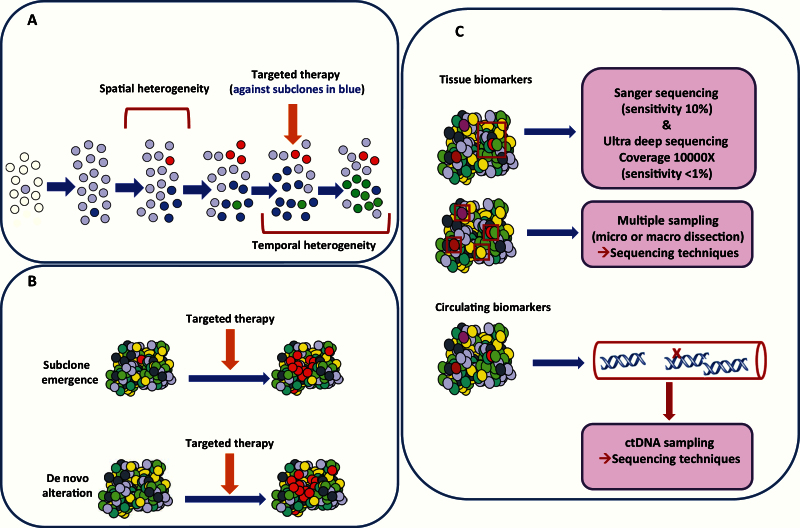
One of the major challenges in PM lies in obtaining an accurate view of the tumor molecular landscape, in order to choose the appropriate targeted therapy approach. Sequencing studies have demonstrated tumor diversity, not only among tumors from different patients (intertumor heterogeneity) but also within various metastatic sites of the same patients (intratumor heterogeneity). Tumor heterogeneity appears to be the result of a branching evolution of cancer’s molecular characteristics (24). Comparative studies suggest that molecular alterations found in metastatic samples reflect the patterns of subclones that already exist in the primary tumor. Some of these subclones may also acquire additional mutations that may be correlated to organ-specific metastasis (25). Subclonal evolution may result in metastatic tumors harboring resistance/sensitivity markers initially observed in low frequency in the primary tumor. In the clinical setting, tumor temporal heterogeneity could explain variable response to MTAs (26), emphasizing the importance of the appropriate representative biopsy for molecular analyses (Figure 3A), although a single biopsy may not necessarily be representative of the geographic landscape of the entire metastatic disease burden. A branched pattern of evolution, in which multiple distinct subclones co-exist and evolve simultaneously within a tumor, will result in a heterogeneous tumor in which there is potential for multiple subclonal driver events. Cancer therapies can act as the selection pressure to drive tumor evolution along a certain lineage if pre-existing subclones possess genotypes that confer drug resistance. Resistant tumor cells could be developed de novo on treatment, or minor resistant subclones may pre-exist below detectable limits (Figure 3B).
Figure 3.
Temporal and spatial heterogeneity and molecular techniques in cancer. A) Aspects of tumor heterogeneity. B) Models for secondary resistance emergence following targeted therapy. C) Molecular techniques used to apprehend tumor heterogeneity.
Some PM clinical trials require a biopsy from a metastatic site in order to increase the likelihood that the patients will be treated with a MTA matching a molecular alteration that still exists in the tumor (8). This procedure is not implemented in routine patients’ care, although the latest reports clearly show that taking biopsies of metastatic sites is safe and feasible (3). In addition, certain metastatic sites might not always be easily accessible for sampling, while some are not useful for high-throughput screening techniques for technical reasons such as bone metastases.
Taking into account tumor heterogeneity for targeted treatment decisions in PM trials constitutes a real challenge. Several large-scale studies are ongoing today such as Lung TRACERx (27) and Aurora (28) integrating complex genomic data with phenotypic clinical annotation and outcome in order to decipher the heterogeneity of the cancer genome and mutational pathways involved in cancer.
Addressing whether subclonal populations that have resistance to therapy are present prior to treatment or whether they are selected during therapy is hindered by the limit of detection of low-frequency cancer cell populations. Conventional Sanger sequencing cannot detect variants below the level of approximately 10% prevalence. Ultra-deep sequencing offers the potential to identify minor subclonal populations (~1%). Nevertheless, the optimal detection of low-frequency subclones might require multiregion analysis or analysis of circulating tumor DNA (liquid biopsies) to capture information from distant metastases (Figure 3C) (29).
It is clear today that a single biopsy from a single metastatic site does not seem to be representative of the metastatic cancer, and multiple biopsies are definitely not acceptable today in terms of patients’ safety and multiplication of the molecular analyses costs. Circulating tumor DNA analysis is clearly a potential alternative and/or replacement to analyses using costly, harmful, and lengthy tissue biopsies of metastases, irrespective of cancer type and metastatic site, for multiplexed mutation detection and for selecting MTAs based on the patient’s tumor genetic content (30). A major challenge remains, however, the detection of CNV on ctDNA. Of note, a large proportion of targetable molecular alterations involve amplifications of oncogenes such as RTK (ERBB2, epidermal growth factor receptor [EGFR], etc). Targeted NGS approaches are mainly used today to assess SNV on ctDNA. Using appropriate bioinformatics tools for targeting NGS analysis allows estimations of CNV using formalin-fixed, paraffinembedded samples or ctDNA (31).
Optimization of Molecular Platforms and Regulatory Authorities’ Guidelines
Internationally, some guidelines and recommendations for regulating and standardizing NGS technologies for clinical use have been produced by government, clinical, and research organizations. Clinical Laboratory Improvement Amendments (CLIA) or Good Clinical Laboratory Practice (GLP) certification is required for clinical centers and consulting biotechnology companies offering NGS-based cancer diagnostic tests in the United States. Several professional societies have equally generated guidelines for the implementation of NGS tests, with a focus on analytical validity or patient privacy rules (32).
Usually, a new test on a specific biomarker needs a specific technical validation as defined in the ISO15189 norm. It is then not possible to have a control for each marker and even each alteration. Here, the molecular screenings approach needs a global validation in the quality management of the laboratory (33). A few genome analysis devices are currently approved for diagnosis, and most of the process is based on the expertise of the laboratory (34). Laboratories performing those assays should therefore be involved in a quality recognition process as the CLIA, CAP, or ISO15189. Implementation of new technologies for molecular analyses needs determination of test conditions, including DNA quality assessment, setup of standard operating procedures, participation to interlaboratory comparative assay, determination of minimum quality criteria, and testing algorithm applications (35).
Data Integration and Bioinformatics Pipelines
The field of oncology has entered the so-called big data era because of the increasing use high-throughput genomic technologies dedicated to clinical applications. The computational pipeline needs to insure a secure storage of large data files as well as access to high-performance computing to enable rapid analysis of data appropriate for routine clinical practice (36). The accuracy of variant detection is strongly influenced by the quality of the computational pipeline to avoid “false-positive variants” detection. Once the bioinformatics analyses are finalized, the interpretation of the results and the functional validity of each alteration still require a “manual” check by expert committee in light of the latest publications. The integration of different types of data including clinical and molecular data is also a major challenge before the actual analyses and report of the results in a digest format that is expected to facilitate data interpretation and treatment decision. Maintaining an efficient bioinformatics workflow in a clinical context is challenging because of the frequent updates of the computational solutions either installed on the sequencing machine or provided as standalone applications. As a consequence, any update requires that each bioinformatics pipeline needs to be validated to ensure a high specificity and sensitivity. Any change in the data format or in the analysis methods can have critical consequences on the downstream analysis and results. Moreover, many different methods are currently available to analyze NGS data, but no consensus or standard computational tools exist so far (37). This might hinder data sharing, which is become urgent today for further identification of predictive biomarkers in a large number of patients.
Conclusions and Future Considerations
A major challenge in optimizing PM trials design is the appropriate use of relevant biomarkers to the MTAs in question and, in case of several MTAs tested, the setup of an appropriate treatment algorithms. We propose several criteria that should be considered when the PM trial treatment algorithms are defined (Table 5). To date, only single molecular alterations are used to drive treatment selection (unidimensional treatment algorithm). No multidimensional treatment algorithm has been proved to be effective, taking into account coexisting molecular alterations. Research in biology, biostatistics, and bioinformatics will be critical to improve them, using systems biology approaches, along with functional validation in preclinical studies. The standardization and the choice of techniques, including tumor sampling, pre-analytical preparation of tumors, DNA extraction kits, sequencers, and bioinformatics pipelines, must be carefully selected so that the rates of false negatives and positives reach a reasonable threshold in regards to the project. Techniques evolve so rapidly that one might not have any other choice than to implement changes during a trial simply because the technique used initially is not available any longer. In any case, changes in experimental methods must be precisely described when results of clinical trials are reported.
It is clear today that functional significance of some molecular alterations may differ across tumor types. This is the case for BRAF inhibitors, which are extremely efficacious in BRAF V600E–mutated melanomas (38) but have limited activity in BRAF V600E–mutated colorectal cancer because of the activation of an EGFR feedback loop in the latter one (39). Taking into account multiple molecular alterations to predict treatment efficacy, use of “systems biology” will provide valuable tools to be used in the clinic. It remains to be proven in clinical trials that systems biology can improve patient outcomes. It may also indicate that several pathways are implicated, which raises the critical question of drug combinations that are not easy to manipulate into the constitutional genetic background of the patient and his/her immune system.
In the near future, the whole genome and whole transcriptome (RNA seq) sequencing will provide a detailed overview of the molecular alterations within a tumor. In fact, several initiatives have started worldwide, such as the Michigan Oncology Sequencing Project (MIONCOSEQ) initiative (40). The cost of NGS techniques is continuously decreasing, accelerating its implementation in routine diagnosis. From a research standpoint, whole-genome sequencing with appropriate bioinformatics’ tools may replace standard molecular diagnostic techniques such as FISH, SNP-array, CGH-array, etc. Several questions remain to be answered, including the appropriate coverage and sequencing depth required for identifying SNVs of key oncogenes/TSG, the feasibility in routine practice, and data storage and processing in real time. DNA methylation techniques may be used to elucidate the complexity of the epigenome, classify the subtype of a tumor, and provide information about sensitivity/resistance to treatment. PM stakeholders need accessible, comprehensive, and frequently updated knowledge bases that describe genomic and epigenomic changes and their clinical implications, as well as continued education of clinicians and patients (37). A continuous interaction between the different stakeholders of precision medicine including oncologists, pathologists, molecular platforms technicians, biologists, bioinformaticians, and equipment manufacturers is therefore key for the development of PM approaches. Scientific and medical societies such as the American Society of Clinical Oncology or the European Society for Medical Onocology might play a central role by endorsing such initiatives.
The presence of several molecular alterations within a single tumor as well as the emergence of secondary resistance following MTA treatments suggest that novel therapeutic strategies are urgently needed. A theoretical solution would be to target all molecular alterations with matching drugs. However, this drug combination approach is challenging because of overlapping toxicities of the drugs (41). Treatment priorities should be established based on strong preclinical or clinical data. Sequential use of drugs would be worth evaluating, particularly if pharmacodynamics markers can be easily assessed. Combinations of MTAs and novel therapies such as immunotherapy and other therapies targeting the tumor micro-environment may also have great potential to treat cancer.
The implications of the tumor microenvironment, the immune system, and their interaction with tumor molecular landscape need to be addressed in future PM studies, especially in light of the impressive clinical activity of immune-checkpoint-pathway inhibitors such as anti–CTLA-4 and anti–programmed death 1 (PD-1) or its ligand (PD-L1) monoclonal antibodies in a variety of solid tumors (42,43,44). The design of such trials is challenging with the current uncertainty regarding whether biomarkers are needed to define which patients will benefit from immune-checkpoint-pathway inhibitors.
Funding
This work is supported by the grant ANR-10-EQPX-03 from the Agence Nationale de le Recherche (Investissements d’avenir) and Site de Recherche Intégré contre le Cancer (SiRIC).
Supplementary Material
The funders had no role in the writing of the manuscript or decision to submit it for publication.
References
- 1. Ciriello G, Miller ML, Aksoy BA, et al. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. 2013;45(10):1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sleijfer S, Ballman K, Verweij J. The future of drug development? Seeking evidence of activity of novel drugs in small groups of patients. J Clin Oncol. 2013;31(18):2246–2248. [DOI] [PubMed] [Google Scholar]
- 3. Le Tourneau C, Kamal M, Alt M, et al. The spectrum of clinical trials aiming at personalizing medicine. Chin Clin Oncol. 2014;3(2):13. [DOI] [PubMed] [Google Scholar]
- 4. Le Tourneau C, Paoletti X, Servant N, et al. Randomised proof-of-concept phase II trial comparing targeted therapy based on tumour molecular profiling vs conventional therapy in patients with refractory cancer: results of the feasibility part of the SHIVA trial. Br J Cancer. 2014;111(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsimberidou AM, Wen S, Hong DS, et al. Personalized medicine for patients with advanced cancer in the phase I program at MD Anderson: validation and landmark analyses. Clin Cancer Res. 2014;20(18):4827–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodon J, Soria JC, Berger R, et al. Challenges in initiating and conducting personalized cancer therapy trials: perspectives from WINTHER, a Worldwide Innovative Network (WIN) Consortium trial. Ann Oncol. 2015;26(8):1791–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andre F, Bachelot T, Commo F, et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol. 2014;15(3):267–274. [DOI] [PubMed] [Google Scholar]
- 8. Le Tourneau C, Delord JP, Gonçalves A, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16(13):1324–1334. [DOI] [PubMed] [Google Scholar]
- 9. Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hudson TJ, Anderson W, Artez A, et al. International Cancer Genome Consortium. International network of cancer genome projects. Nature. 2010;464(7291):993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McShane LM, Cavenagh MM, Lively TG, et al. Criteria for the use of omics-based predictors in clinical trials. Nature. 2013;502(7471):317–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bourdeaut F, Grison C, Maurage CA, et al. MYC and MYCN amplification can be reliably assessed by aCGH in medulloblastoma. Cancer Genet. 2013;206(4):124–129. [DOI] [PubMed] [Google Scholar]
- 13. Vincent-Salomon A, Lucchesi C, Gruel N, et al. Integrated genomic and transcriptomic analysis of ductal carcinoma in situ of the breast. Clin Cancer Res. 2008;14(7):1956–1965. [DOI] [PubMed] [Google Scholar]
- 14. Sims D, Sudbery I, Ilott NE, et al. Sequencing depth and coverage: key considerations in genomic analyses. Nat Rev Genet 2014;5(2):121–132. [DOI] [PubMed] [Google Scholar]
- 15. Carter SL, Cibulskis K, Helman E, et al. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol. 2012;30(5):413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berger AH, Pandolfi PP. Haplo-insufficiency: a driving force in cancer. J Pathol. 2011; 223(2):137–146. [DOI] [PubMed] [Google Scholar]
- 17. Kwon CH, Zhao D, Chen J, et al. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 2008;68(9):3286–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayes DF, Bast RC, Desch CE, et al. Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst. 1996;16;88(20):1456–1466. [DOI] [PubMed] [Google Scholar]
- 19. Meric-Bernstam F, Johnson A, Holla V, et al. Decision Support Framework for Genomically Informed Investigational Cancer Therapy. Natl Cancer Inst. 2015;11;107(7); In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16(8):2659–2671. [DOI] [PubMed] [Google Scholar]
- 21. Kantarjian H, Sawyers C, Hochhaus A, et al. International STI571 CML Study Group. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346(9):645–652. [DOI] [PubMed] [Google Scholar]
- 22. Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2008;66(8):3992–3995. [DOI] [PubMed] [Google Scholar]
- 23. Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–1765. [DOI] [PubMed] [Google Scholar]
- 24. Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turner NC, Reis-Filho JS. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012;13(4):e178–e185. [DOI] [PubMed] [Google Scholar]
- 27. Jamal-Hanjani M, Hackshaw A, Ngai Y, et al. Tracking genomic cancer evolution for precision medicine: the lung TRACERx study PLoS Biol. 2014;8;12(7):e1001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zardavas D, Maetens M, Irrthum A, et al. The AURORA initiative for metastatic breast cancer. Br J Cancer. 2014;11;111(10):1881–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pestrin M, Salvianti F, Galardi F, et al. Heterogeneity of PIK3CA mutational status at the single cell level in circulating tumor cells from metastatic breast cancer patients. Mol Oncol. 2015;9(4):749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lebofsky R, Decraene C, Bernard V, et al. Circulating tumor DNA as a non-invasive substitute to metastasis biopsy for tumor genotyping and personalized medicine in a prospective trial across all tumor types. Mol Oncol. 2015;9(4):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boeva V, Popova T, Lienard M, et al. Multi-factor data normalization enables the detection of copy number aberrations in amplicon sequencing data. Bioinformatics. 2014;30(24):3443–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bennett NC, Farah CS.Next-generation sequencing in clinical oncology: next steps towards clinical validation. Cancers (Basel). 2014;6(4):2296–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cree IA, Deans Z, Ligtenberg MJ, et al. Quality Assurance in Molecular Pathology; Royal College of Pathologists. J Clin Pathol. 2014;67(11):923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Collins FS, Hamburg MA. First FDA authorization for next-generation sequencer. N Engl J Med. 2013;19;369(25):2369–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dubbink HJ, Deans ZC, Tops BB, et al. Next generation diagnostic molecular pathology: critical appraisal of quality assurance in Europe. Mol Oncol. 2014;8(4):830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simon R, Roychowdhury S. Implementing personalized cancer genomics in clinical trials. Nat Rev Drug Discov. 2013;12(5):358–369. [DOI] [PubMed] [Google Scholar]
- 37. Servant N, Roméjon J, Gestraud P, et al. Bioinformatics for precision medicine in oncology: principles and application to the SHIVA clinical trial. Front Genet. 2014;5:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483(7387):100–103. [DOI] [PubMed] [Google Scholar]
- 40. Everett JN, Gustafson SL, Raymond VM. Traditional roles in a non-traditional setting: genetic counseling in precision oncology. J Genet Couns. 2014;23(4):655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soria JC, Massard C, Izzedine H. From theoretical synergy to clinical supra-additive toxicity. J Clin Oncol. 2009;27(9):1359–1361. [DOI] [PubMed] [Google Scholar]
- 42. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.