Abstract
Stress is defined as an adverse condition that disturbs the homeostasis of the body and activates adaptation responses. Among the many pathways and mediators involved, neuropeptide Y (NPY) stands out due to its unique stress-relieving, anxiolytic and neuroprotective properties. Stress exposure alters the biosynthesis of NPY in distinct brain regions, the magnitude and direction of this effect varying with the duration and type of stress. NPY is expressed in particular neurons of the brainstem, hypothalamus and limbic system, which explains why NPY has an impact on stress-related changes in emotional-affective behaviour and feeding as well as on stress coping. The biological actions of NPY in mammals are mediated by the Y1, Y2, Y4 and Y5 receptor, Y1 receptor stimulation being anxiolytic whereas Y2 receptor activation is anxiogenic. Emerging evidence attributes NPY a role in stress resilience, the ability to cope with stress. Thus there is a negative correlation between stress-induced behavioural disruption and cerebral NPY expression in animal models of post-traumatic stress disorder. Exogenous NPY prevents the negative consequences of stress, and polymorphisms of the NPY gene are predictive of impaired stress processing and increased risk of neuropsychiatric diseases. Stress is also a factor contributing to, and resulting from, neurodegenerative diseases such as Alzheimer’s, Parkinson’s and Huntington’s disease, in which NPY appears to play an important neuroprotective role. This review summarizes the evidence for an implication of NPY in stress-related and neurodegenerative pathologies and addresses the cerebral NPY system as a therapeutic target.
Keywords: Anxiety, neurodegenerative diseases, neuropeptide Y, post-traumatic stress disorder, stress, stress-induced feeding changes, stress resilience, Y1, Y2, Y5
1. NPY and its receptors
Neuropeptide Y (NPY) is a 36-amino acid peptide which belongs to the so-called NPY family of biologically active peptides, together with two other members, peptide YY and pancreatic polypeptide (PP) (1). Originally NPY was isolated from brain extracts (2) and found to be one of the most abundant neuropeptides within the brain (3). NPY has a pivotal role in many physiological functions such as food intake, energy homeostasis, circadian rhythm, and cognition (4–7). In addition, the peptide has been suggested to be a key component in the stress response, having anxiolytic properties (3,8).
The numerous and diverse NPY effects are related to its expression in a multitude of brain areas. Mapping studies investigating NPY mRNA production within the rodent brain identified 4 regions as the main sources of cerebral NPY synthesis (9). These regions include the hypothalamic arcuate nucleus (ARC), the locus coeruleus (LC), the nucleus tractus solitarii (NTS) and the septohippocampal nucleus (9). NPY is, in addition, present in many cortical interneurons, amygdala, hippocampus, nucleus accumbens (NAc), periaqueductal gray, basal ganglia and thalamus (1,9). An important fibre tract containing NPY exists between the ARC, a major source of NPY, and the paraventricular nucleus of the hypothalamus (PVH), the major source of corticotropin-releasing hormone (CRH), allowing a crosstalk between these two neuropeptide systems (10).
To date seven different Y receptors (Y1-Y8) have been described in vertebrates, of which up to 5 (Y1, Y2, Y4, Y5, y6) are present in mammals (11). While the Y1, Y2, Y4 and Y5 receptors are functional in all mammals, the y6 receptor is non-functional in several mammals including humans and has been lost in rats (12). The receptor originally identified as the Y3 receptor has been characterized as CXC chemokine receptor type 4 and is thus included in the chemokine receptor family (13). NPY shows strong affinity for the Y1, Y2 and Y5 receptor, while PP is the preferential agonist at the Y4 receptor (13). Like NPY itself, the Y1 and Y2 receptors are widely distributed throughout the brain. Brain areas expressing Y1 and Y2 receptor immunoreactivity include the frontal cortex, lateral septum, NAc, bed nucleus of the stria terminalis, PVH, ARC, lateral hypothalamus, amygdala, hippocampus, NTS and area postrema (9,14,15). In contrast, Y4 receptor expression is restricted to only a few brain regions including the medial preoptic area, PVH, NTS and area postrema (9). Finally, the Y5 receptor occurs in several limbic brain areas but is less abundant than the Y1 or Y2 receptor (16,17). Interestingly, Y5 receptor expression coincides in most cases with Y1 receptor expression, but not vice versa (18,19).
2. Role of NPY in stress processing
Stress is a term deeply rooted and frequently used in everyday language. Originally described as acute nonspecific nocuous agents affecting the body by Hans Selye in the 1930s (20), stressors are now defined by their ability to disturb homeostasis (21). These disturbances are detected by the sensory systems and the information is transmitted to the brain. Depending on the magnitude of homeostatic alterations, the perception of stress activates adaptation systems. A number of reports indicates that NPY is crucial for the stress adaptation process, besides other major biological pathways such as the hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system. Accordingly, a concept has been developed, which implicates NPY in the termination of the stress response and its interaction with the HPA axis (22). This concept holds that NPY counteracts the biological actions of CRH, a 41-amino acid neuropeptide which is transcribed and released upon stress exposure in the PVH (23). On the behavioural level, stress-induced CRH expression, CRH overexpression as well as central CRH administration have been found to induce anxiety in rodents (24). In contrast, NPY, the functional counter player of CRH, has anxiolytic effects. Intracerebroventricular (icv) administration of NPY renders mice less anxious (25), an effect that is also seen after NPY injection directly into the amygdala or hippocampus, indicating that these regions are key regions in the anxiolytic effect of the peptide (26,27). The findings that NPY knockout mice are more anxious, and hippocampal or amygdalar NPY overexpression renders animals less anxious, confirm the anxiolytic properties of endogenous NPY and the importance of the limbic system in this effect (28–31).
Stress potently influences NPY expression in the brain, but the magnitude and even the direction of stress-induced NPY alterations heavily depend on the type and duration of stress, time point of NPY measurement as well as brain region examined (Table 1). For instance, NPY gene expression in the amygdala, a brain region important for the processing of fear and anxiety, is increased after footshock stress (32), unaltered by acute water avoidance stress (WAS) (33), but decreased by acute restraint stress (34). These findings indicate that the type of stressor has a large influence on the reaction of the NPY system in this brain area. A similar variability in amygdalar NPY expression has been observed with chronic stress paradigms (35–38).
Table 1:
Stress-induced effects on NPY gene expression in selected brain areas.
| Brain region |
Type of stress | Effect on NPY gene expression |
Species | Ref |
|---|---|---|---|---|
| Amy | 60-min restraint stress | ↓ 1+2h post-stress | Sprague-Dawley rats | (34) |
| 60-min restraint stress (10 days) | ↑ 2h post-stress | Sprague-Dawley rats | (35) | |
| 60-min water avoidance stress (7 days) | ↓ 1d post-stress | C57BL/6N mice | (38) | |
| 30-min water avoidance stress | No effect | C57BL/6N mice | (33) | |
| BLA | 15-min footshock stress | ↑ 2w post-stress | Wistar rats | (32) |
| 12 trials in Morris Water Maze | ↓ 5h post-stress | Sprague-Dawley rats | (37) | |
| MeA | 60-min restraint stress (1,3 or 10 days) | ↑ 0min post-stress (after 3 sessions) | Wistar-Kyoto rats | (36) |
| HT | 60-min restraint stress | No effect | Sprague-Dawley rats | (34) |
| 60-min restraint stress (10 days) | No effect | Sprague-Dawley rats | (35) | |
| 60-min water avoidance stress (7 days) | No effect | C57BL/6N mice | (38) | |
| 30-min water avoidance stress | ↑ 0min post-stress | C57BL/6N mice | (33) | |
| 30-min restraint stress | ↑ 4h post-stress | CD1 mice | (41) | |
| 60-min immobilization stress | ↑ 0h, 24h + 48h post-stress | Wistar rats | (42) | |
| ARC | 60-min restraint stress | ↑ 24h post-stress | Sprague-Dawley rats | (39) |
| 60-min restraint stress (1,3 or 10 days) | ↑ 0min post-stress (after 1 or 3 sessions) | Wistar-Kyoto rats | (36) | |
| 15-min footshock stress | ↑ 2h post-stress | Wistar Utrecht:Wistar Unileve rats | (40) | |
| Air puff (1 or 10 days) | No effect | Wistar-Kyoto rats | (43) | |
| Hip | 30-min water avoidance stress | No effect | C57BL/6N mice | (33) |
| 60-min water avoidance stress (7 days) | No effect | C57BL/6N mice | (38) | |
| 60-min restraint stress (1,3 or 10 days) | ↓ 0min post-stress (after 10 sessions) | Wistar-Kyoto rats | (36) | |
| DG | 60-min restraint stress | ↑ 6h post-stress | Sprague-Dawley rats | (39) |
| 12 trials in Morris Water Maze | ↑ 5h post-stress | Sprague-Dawley rats | (37) | |
| LC | 15-min footshock stress | ↓ 2w post-stress | Wistar rats | (32) |
| 60-min restraint stress (1,3 or 10 days) | No effect | Wistar-Kyoto rats | (36) | |
| Air puff (1 or 10 days) | ↑ 30min post-stress (after 10 days) | Wistar-Kyoto rats | (43) |
↑ or ↓ denote increased or decreased NPY mRNA levels at the given time point after stress exposure; Amy, amygdala; ARC, arcuate nucleus; BLA, basolateral amygdala; DG, dentate gyrus; Hip, hippocampus; HT, hypothalamus; LC, locus coeruleus; MeA, medial amygdala
As regards NPY expression measured across the whole hypothalamus or within the ARC, most evidence attests to increased NPY transcription after exposure to acute stressors (33,36,39–42) but not chronic stress paradigms (35,38,43), indicating habituation after repeated stress exposure. However, the pertinent evidence is somewhat contradictory as acute air puff stress does not change hypothalamic NPY expression whereas 3 days of restraint stress elevates NPY in the ARC (36,43). Importantly, the changes in NPY expression in the hypothalamus depend on the interval between stress exposure and measurement, as is evident from the data shown in table 1.
Like in the hypothalamus, the kind and duration of stress is decisive for its effect on the NPY system in the hippocampus. While WAS does not change hippocampal NPY transcription, acute restraint stress and Morris Water Maze training increase, whereas chronic restraint stress decreases NPY mRNA levels in the hippocampus (33,36–39). Likewise, NPY expression in the LC is increased, unaltered or decreased, depending on the type and length of stress exposure (32,36,43).
Besides its sensitivity to external stressors, cerebral NPY expression is also affected by the internal stress of visceral inflammation. For instance, trinitrobenzene sulfonic acid-induced colitis, an animal model of Crohn’s disease, increases the NPY concentration in brain and plasma (44). Furthermore, in mice with dextran sulfate sodium-evoked colitis, a model of ulcerative colitis, amygdalar and hippocampal NPY expression is decreased, while hypothalamic NPY production is increased (33,38). Especially the NPY changes within the limbic system are noteworthy since both colitis and the related disturbance of the microbiota-gut-brain axis induce behavioural changes which may involve NPY (1,45). However, not all experimental models of peripheral inflammation affect the central NPY system. Thus, after, Helicobacter pylori infection, the expression of NPY mRNA in the brain remains unaltered (46).
3. Role of NPY in anxiety
Increased anxiety is a distinct component of the stress response, with CRH exerting an anxiogenic effect that is counteracted by NPY-mediated anxiolysis (25–27). A number of reports indicates that the anxiolytic activity of NPY is primarily mediated by the Y1 receptor. For instance, intraamygdalar administration of the Y1 receptor antagonist BIBP 3226 is anxiogenic, while icv administration of a selective Y1 receptor agonist is anxiolytic (30,47). Direct pharmacological evidence corroborates that the anxiolytic effect of NPY is mediated by the Y1 receptor, since administration of a selective Y1 receptor antagonist before or together with NPY abolishes the anxiolytic effect (48,49). In line with these findings, conventional Y1 receptor gene knockout as well as selective Y1 receptor knockout in forebrain neurons are anxiogenic (50,51), while hippocampal Y1 receptor overexpression renders mice less anxious (52). This implication of Y1 receptors is contradicted by a study in which intraamygdalar administration of a Y1 receptor agonist failed to alter conditioned fear (53). Additionally, Y1 receptor antagonism did not prevent the anxiolytic effect of NPY, suggesting the involvement of other Y receptors.
In search for the biological relevance of Y1 receptor activation, the effect of NPY on the excitability of amygdalar neurons was investigated as a potential explanation for NPY-induced behavioural changes (54). The Y1 receptor agonist [Leu31,Pro34]-NPY reduced the amplitude of NMDA-evoked excitatory postsynaptic currents but increased the amplitude of GABAA receptor-mediated inhibitory postsynaptic currents. This dual effect resulted in overall inhibition of amygdalar glutamatergic pyramidal neurons (54), which is consistent with the anxiolytic effect of NPY. Another mechanism whereby the Y1 receptor can influence anxiety involves calcineurin, a protein phosphatase, which is implicated in synaptic plasticity and shows high colocalization with the Y1 receptor in the basolateral amygdala (55). Cells expressing both the enzyme and the receptor are primarily pyramidal neurons and only to a small percentage interneurons. Antagonism of calcineurin activity prevents the long-term anxiolytic effect of NPY, suggesting an involvement of calcineurin-induced plasticity in the behavioural effect of NPY and a calcineurin-induced synaptic remodelling of pyramidal neurons in the basolateral amygdala (27,55).
In contrast to the Y1 receptor, stimulation of the Y2 receptor has an anxiogenic effect, which is related to the presynaptic localization of this receptor and its function as an autoreceptor reducing NPY release (56,57). For instance, NPY13-36, a selective Y2 receptor agonist, increases the preference of mice for the closed arms of the elevated plus maze (EPM), which is consistent with anxiogenic behaviour (58). In line with this finding, icv or intraamygdalar blockade of the Y2 receptor with BIIE0246 (59,60) as well as intraamygdalar deletion of the receptor is anxiolytic (61). In addition, the brain-penetrant Y2 receptor antagonist JNJ31020028 has also anxiolytic properties. JNJ31020028 reverses the anxiogenic effect of alcohol withdrawal and nicotine-induced social anxiety-like behaviour (62–64). Surprisingly, conventional Y2 receptor knockout has yielded mixed results. While 3 studies observed antidepressant and antianxiety effects of Y2 receptor knockout, another study failed to confirm these findings (65–68). It has been suggested that the genetic background has a strong influence on the behavioural effects of Y2 receptor knockout and may be the reason for these divergent findings.
The role of the Y2 receptor in anxiety is further obscured by an anxiolytic effect of local Y2 receptor stimulation in the brain. Administration of NPY3-36 into the region of the LC increases entries to and time spent on the open arms of the EPM (69), and intraseptal infusion of the same compound increases open arm exploration and decreases the proportion of rats which bury in the shock-probe burying test (70). A potential explanation for these apparently paradox observations relates to brain-region dependent interactions of the Y2 receptor with major neurotransmitter systems. Thus, Y2 receptor activation has been described to inhibit the release of glutamate, GABA, dopamine and noradrenaline (68,71,72). It is hence conceivable that the behavioural effects of local Y2 receptor stimulation vary depending on the neurotransmitter repertoire of specific brain regions.
Data on the role of the Y5 receptor in anxiety are limited and controversial. The first study using a Y5 receptor antagonist failed to modify anxiety in the social interaction (SI) and EPM tests (73). However, two consecutive studies revealed an anxiolytic effect of a selective Y5 receptor agonist in the EPM, open field (OF) and SI tests (47,74). These discrepancies may not only be due to different selectivity of the Y5 receptor ligands but also to different interactions with other neurotransmitter systems of the brain. Quarta et al. (75) described that a Y5 receptor agonist enhances dopamine and noradrenaline release in the NAc and medial prefrontal cortex. The implication of the Y5 receptor in anxiety is further blurred by the anxiolytic effect of a selective Y5 receptor antagonist in the SI test (76). Genetic studies did not help to solve this riddle as both Y5 receptor knockout mice and mice with hippocampal or amygdalar Y5 receptor overexpression do not differ in anxiety tests (77,78). Recently it has been postulated that an interaction of the Y5 receptor with the Y1 receptor plays a role in regulating anxiety. Specifically, conditional knockout of the Y1 receptor in Y5 receptor-expressing neurons resulted in an anxiogenic phenotype of mice (79). In another study (80) it was demonstrated that this effect is reproducible and not affected by differential housing conditions (isolation vs. group housing). A small sample size human study provides preliminary support for an involvement of the Y5 receptor gene in panic disorder (81). Furthermore, the finding of a negative correlation between Y5 receptor mRNA in the central amygdala of rhesus monkeys and anxious temperament suggests a role of this receptor in the neural processes underlying temperament (82).
Relative to NPY and peptide YY, PP has the highest affinity for the Y4 receptor (13). In the first investigation on the role of the Y4 receptor in anxiety it was found that icv administration of PP altered food intake but not anxiety (83). In contrast, genetic deletion of the Y4 receptor has an anxiolytic effect in the OF, EPM and light-dark box tests (67,84,85). In addition, Y4 receptor knockout impairs fear extinction (86). The anxiolytic effect of Y4 receptor deletion is amplified by Y2/Y4 receptor double knockout, suggesting a positive interaction of Y2 and Y4 receptors (85). Unfortunately, the elucidation of the role of Y4 receptors in anxiety and other physiological functions is hampered by the lack of Y4 receptor-selective non-peptide antagonists. It is also unclear which endogenous agonist is acting via the Y4 receptor in the brain. There is little evidence that PP is produced in the brain, given that the immunoreactivity which was once believed to reflect PP turned out to be NPY (87,88). Accordingly, central NPY, although about 100 times less potent than PP in Y4 receptor activation (89,90), or peripheral PP crossing the blood-brain barrier (BBB) (91) are probably responsible for Y4 receptor-mediated effects on anxiety.
4. NPY as a mediator between stress and food intake
The HPA axis, a stress effector pathway, and food intake have been linked with each other, based on the findings of a glucocorticoid response to food intake and fasting-induced stimulation of the HPA axis (92). Since then a multitude of studies have suggested that stress alters food intake in a bidirectional manner since both stress-induced increases and decreases of feeding have been described (93). The direction of change depends on many factors such as the severity of stress and the levels of stress hormones (glucocorticoids, CRH, urocortin), metabolism-related hormones (leptin, insulin, ghrelin) and feeding-related neuropeptides (alpha-melanocyte stimulating hormone, agouti-related protein, melanocortins, NPY) (94).
In the central nervous system the interaction between stress and food intake is regulated by neuroanatomical pathways in the hypothalamus, in which NPY has a key role. As described above, hypothalamic NPY in the ARC is altered by a variety of stressors, and the projection of NPY neurons from the ARC to the PVH (10) allows for a crosstalk of the NPY system with the HPA axis in the course of stress. NPY itself is a potent orexigenic peptide (95), which suggests that stress-induced NPY increases lead to increased stress-induced food intake. In line with this idea, Krolow et al. (96) report that calorie consumption and hypothalamic NPY expression increase in male rats subjected to isolation stress. Furthermore, after a 30-min restraint stress period, food intake is lower in female NPY knockout mice compared to female WT mice, which suggests that stress-induced food intake depends on NPY (97). Similarly, food intake in female as well as male NPY knockout mice is decreased during a 4-hour exposure to novel environment stress (97).
It has been suggested that the orexigenic effect of NPY is primarily mediated by Y1 and Y5 receptors (6). In a stress setting this has been confirmed by Goebel-Stengel et al. (98) who report that acute tail pinch increases food intake in rats, an effect inhibited by the Y1 receptor antagonist BIBP-3226. However, there is also evidence for a role of the Y2 receptor in the interaction between stress and food intake. JNJ-31020028, a Y2 receptor antagonist, attenuates the effect of stress to reduce food intake in fasted rats (99). In contrast, to our knowledge, a potential role of the Y4 and Y5 receptor in the interaction between stress and feeding has not yet been disclosed.
Although the available evidence speaks for an involvement of NPY in the stress-feeding connection, the relation between stress, NPY and food intake is not as clear-cut as suggested above, because stress can increase or decrease food intake without accompanying NPY alterations. For instance, early life stress increases food intake and body weight in adult rats, but does not influence the hypothalamic NPY content (100). Likewise, chronic immobilization stress alters food intake and body weight gain in rats, which does not depend on NPY alterations in the ARC but is accompanied by increases in leptin and leptin receptor levels (101). In another study, acute restraint stress and forced swim stress have been found to reduce cumulative food intake, which is accompanied by an increase in hypothalamic proopiomelanocortin expression while NPY levels stay unaltered (102). Furthermore, acute crowding stress inhibits food intake in fish, but does not change central NPY expression (103).
These findings are at variance with other studies in which a stress-induced reduction of food intake is associated with increased NPY expression. For example, Melhorn et al. (104) report that in the visible burrow system, a rodent model of chronic social stress, food intake is decreased in both dominant and submissive animals compared to controls, with the submissive animals showing the largest feeding reductions. NPY expression in the ARC is enhanced in both submissive and dominant animals, which may be a counterregulatory mechanism to reverse the stress-induced diminution of calorie intake (104). In line with this finding, acute restraint stress in rats also reduces food intake and increases NPY expression in the PVH (105). Furthermore, heat stress suppresses food intake in chicken, which is associated with increased hypothalamic NPY mRNA expression (106).
In humans, chronic stress-induced increases in food intake can be detrimental as they may facilitate the development of pathological conditions such as obesity. Preclinical evidence indicates that NPY favours this transition via multiple effects. Specifically, NPY released from sympathetic nerves in the course of stress activates Y2 receptors on visceral white adipose tissue, which results in an increased growth of abdominal fat (107). Additionally, stress-induced glucocorticoid release, which increases hypothalamic NPY expression and food intake, may constitute a central mechanism as to how NPY promotes obesity (108). In another experimental setting, prenatal stress has been found to predispose the female offspring to diet-induced obesity in adulthood which is associated with a decrease in the hypothalamic expression of NPY (109).
5. Role of NPY in stress resilience: evidence from animal studies
The involvement of NPY in the stress reaction of the brain and its anxiolytic properties raise the question whether NPY also promotes stress resilience, the ability to cope with stress. Indeed, several lines of evidence attest to resilience-promoting properties of the peptide.
An initial study in transgenic rats with hippocampal NPY overexpression found that these animals are protected from the negative consequences of restraint stress (29). Precisely, the transgenic rats were insensitive to stress-induced anxiety on the EPM and did not show fear suppression in a punished drinking test. This finding is in line with another study which reported NPY as a resilience factor for restraint stress-induced behavioural disturbances. Repeated administration of NPY into the basolateral amygdala rendered mice resilient to stress as they were protected from the negative consequences of restraint stress in the SI test, an effect present for up to 8 weeks (27). A role for NPY in stress resilience was also corroborated through analysis of stress-induced disturbances of NPY expression in distinct brain regions (110). After chronic variable stress NPY peptide levels in the amygdala were reduced, while NPY levels in the prefrontal cortex were increased. These molecular changes may provide an explanation for the exaggerated fear response and delayed expression of fearful arousal seen in rats subjected to chronic variable stress (111). Interestingly, both reduced amygdalar NPY and increased prefrontal NPY synthesis may have the same functional consequence, namely to impair stress resilience. Attenuated NPY signalling in the amygdala has negative behavioural consequences (anxiety), and the increase of NPY formation in cortical interneurons may dampen the output from the prefrontal cortex, disinhibit the amygdala, and thus cause negative behavioural effects. Finally, NPY has also been suggested as a key mediator of the resilience effect of repeated WAS which prevents behavioural disturbances due to experimental colitis (38). Repeated WAS, a psychological stressor for mice, reversed colitis-induced behavioural disturbances in the OF and SI tests, an effect associated with an increase in hypothalamic NPY mRNA. Although it is at first glance surprising that chronic stress is beneficial for mice suffering from colitis, it has been previously suggested that repeated mild predictable stress during adolescence can attenuate the behavioural response to stress in adulthood (112). Thus, the right daily “dose” of stress can be beneficial, may even be necessary to develop adequate coping strategies for unexpected traumatic events and may involve recruitment of the central NPY system.
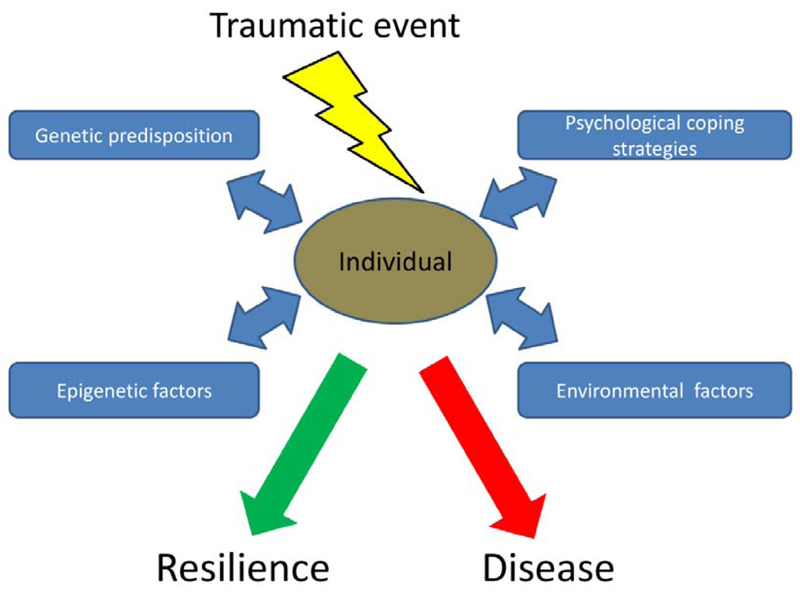
Stress resilience has turned out to be an individual trait, which advocates the study of stress reactions in individuals in addition to group-based protocols. This translational approach is based on the aetiology and pathophysiology of post-traumatic stress disorder (PTSD). It is well established that the response of individuals to the same psychological trauma varies widely, ranging from severe psychological impairments to complete resilience. Although individual and environmental factors relevant for coping with traumatic events have been identified, appropriate animal models are necessary to uncover the biological basis of stress resilience (Figure 1).
Figure 1. Key factors for the individual response to an extreme traumatic event.
Various genetic, epigenetic, psychological and environmental susceptibility and/or resilience factors determine whether individuals develop disease or stay healthy (resilience).
To delineate individual differences in the behavioural stress reaction, Bardi et al. (113) investigated the coping style of rats during gentle restraint. To this end they quantified the number of escape bouts during 2 sessions of holding the rats in supine position for periods of 1 min. Active coping was defined as 7 or more escape trials during the test while passive coping was defined as six or fewer escape trials. Variable copers were defined as rats exhibiting passive coping during one test session and active coping in another session. Importantly, there was a correlation between the coping style of rats during gentle restraint and hippocampal NPY levels. Immunohistochemical analysis revealed that rats with a variable coping strategy had higher hippocampal NPY levels than rats with a passive or active coping style only (113). Moreover, variable copers exhibited a high level of adaptive responsiveness in three successive forced swim tests along with increased NPY levels in the amygdala and the bed nucleus of the stria terminalis (114). Behavioural flexibility in response to a stressful task may thus be a correlate of stress resilience mediated by NPY.
Cohen et al. (115) adopted a different model in which the behavioural reaction of rats to predator scent stress was used to evaluate individual stress coping. Based on cut-off behavioural criteria, animals were differentiated into an extreme behavioural response group, a partial behavioural response group and a minimal behavioural response group (115). Based on this paradigm, a correlation between behavioural disruption of animals after predator scent stress and brain NPY levels was disclosed (116). Animals whose behaviour was extremely disrupted had the lowest brain NPY levels. Treatment of animals with NPY shortly after stress exposure significantly reduced the prevalence of extremely disrupted behaviour while administration of the Y1 receptor antagonist BIBO3304 exaggerated behavioural disruption. These findings suggest that NPY acting via the Y1 receptor is a stress resilience factor (116). A consecutive study found that the behavioural and NPY response to predator scent stress in rats depends on the phase of the light/dark cycle (117). If stress was applied at the onset of the inactive phase (light phase), rats were more anxious and displayed more disruption of behaviour than rats exposed to stress at the onset of the dark phase. NPY and Y1 receptor immunoreactivity decreased in response to stress in the PVH and ARC, a change that was more pronounced in rats stressed at the onset of the light phase, indicating a circadian influence on the stress-induced disruption of the NPY system (117). NPY administration before stress exposure had an anxiolytic effect, which was accompanied by upregulation of NPY and the Y1 receptor in the brain (117). In another study from the same group NPY was also implicated in the resilience effects of physical exercise (118). Rats which underwent a 6-week treadmill training before predator scent stress exposure did not suffer from the adverse behavioural consequences of the stressor (reduced time spent on the open arms of the EPM, increased acoustic startle response). This exercise-induced behavioural resilience was related to NPY, as stress exposure decreased NPY expression in the hippocampus, an effect that was absent in animals with treadmill exercise (118).
Finally, NPY was suggested to be a protective factor in the stress-induced memory impairment on the radial maze memory task (119). Rats subjected to chronic immobilization stress performed significantly worse during the memory test, an effect associated with enhanced NPY expression in the ARC. Detailed analysis revealed that rats with little or no stress-induced memory impairment (i.e., stress-resilient individuals) had the highest NPY levels in the ARC and habenula. It thus seems that stress-induced NPY expression is blunted in susceptible individuals, indicating a maladaptive stress response system (119).
6. Role of NPY in stress resilience: evidence from human studies
Stress resilience in humans has often been addressed in connection with the study of PTSD patients. In the aftermath of trauma these patients re-experience the traumatic event (distressing memories or dreams), develop strategies to avoid confrontation with the trauma (avoidance of conversations about the incident, avoidance of places associated with the trauma) and show symptoms of increased arousal such as sleep disturbances (120). Interestingly, PTSD develops only in a subpopulation of trauma-exposed subjects, while the majority stays healthy. It appears as if individual coping strategies determine how humans react to extreme stressful situations. The stress-resilient individuals seem to be protected by genetic, molecular and/or environmental factors (e.g., family, social contacts) preventing the development of disease (Figure 1).
Already 15 years ago the anxiolytic properties of NPY in rodent studies sparked interest in investigating the role of this peptide in PTSD patients. Rasmusson et al. (121) found that basal and yohimbine-stimulated plasma levels of NPY are lowered in PTSD subjects. The effect of yohimbine, an α2 adrenoceptor antagonist, to raise circulating NPY arises from its ability to increase sympathetic activity due to its presynaptic site of action. Moreover, the baseline NPY levels correlated negatively with combat exposure scale scores and panic (121). Reduced baseline plasma levels of NPY were also found in combat-exposed individuals with PTSD in another study (122). However, the authors noted that NPY levels were diminished also in combat-exposed individuals without PTSD, indicating that trauma itself is the major factor for plasma NPY regulation. In contrast, motor vehicle accident survivors with depression or PTSD do not present with alterations in serum NPY levels, which is at variance with the afore-mentioned hypothesis (123). A further study investigating combat-related PTSD came up with an opposite result, with plasma NPY levels in combat-exposed veterans without PTSD being higher than in non-exposed veterans (124). Interestingly, a subanalysis of combat-exposed veterans without PTSD revealed that those with a history of past PTSD had the highest NPY levels, suggesting that the ability to overcome trauma-induced disease (i.e. developing resilience) is related to high levels of NPY expression.
As concerns were raised whether plasma NPY accurately reflects NPY levels in the CNS (125), other studies focused on NPY levels in the cerebrospinal fluid (CSF). A study employing this approach showed that combat-exposed veterans with PTSD have lower CSF NPY levels than healthy men (126). Furthermore, a recent report from the same group showed that combat-exposed veterans with PTSD have lower CSF NPY than combat-exposed veterans without PTSD (127). This is in line with the above-mentioned rodent studies suggesting that high cerebral levels of NPY represent an important resilience factor for stress-induced disturbances, while low NPY levels promote behavioural disruption (116,117). It appears as if high NPY concentrations in the CSF can prevent the development of trauma-induced PTSD.
The stress resilience-promoting properties of NPY were also investigated in relation to psychological hardiness, a personality trait that refers to the three interrelated personality characteristics known as commitment, control and challenge (128). Together these personality characteristics appear to protect individuals from the negative health effects of stress. In a cohort of Norwegian navy cadets with overall high psychological hardiness scores it was found that “unbalanced” hardiness subjects (with low scores in the challenge parameter) had lower blood NPY levels in the context of a highly stressful military exercise than “balanced” individuals, which points to an impaired ability to cope with the stressful situation (128). Furthermore, the balanced hardiness group showed a more pronounced NPY rise in the face of stress, indicating a more adaptive stress response. Another study investigated ways to improve stress resilience in relation to NPY (129). Specifically, marines completing a mindfulness-based mind fitness training (an 8-week series of psychological instructions and individual practices with the aim to enhance stress resilience) had lower plasma NPY levels after a stressful military exercise than soldiers without a similar training. The authors explain the beneficial effect of this training scheme by an improvement in the efficient use of neural processing resources and thus an altered autonomic response to stress (129).
A role of NPY as a stress resilience factor in humans is also supported by genetic evidence. Analysis of various single nucleotide polymorphisms (SNPs) of the NPY gene revealed NPY genotype-dependent alterations in the central processing of stress or emotional events. Accordingly, individuals with a low-expression NPY genotype show exaggerated amygdala reactivity to threat-related facial expressions, which may reflect an overreaction to a stressful event (130). Similarly, the rostral anterior cingulate cortex is activated in low-expression NPY genotypes when reading negatively valenced words, while high-expression NPY genotype individuals show the opposite reaction (131). Furthermore, individuals with a low-expression NPY genotype experience enhanced negative affect before and after a pain stimulus. It thus appears that a low-expression NPY genotype predisposes individuals to hyper-responsiveness to negative stimuli and potentially to subsequent disease development (131). In line with these findings, carriers of the C allele of the NPY SNP rs16147 present with increased bilateral amygdala activation while viewing angry faces and a significant association with depression and anxiety when exposed to early life psychological adversity (132,133). Other studies found that variation of the NPY gene confers disease risk in the context of extreme stress. For instance, the NPY rs16147 SNP increases the likelihood to develop a generalized anxiety disorder after hurricane exposure (134). Individuals with high hurricane exposure and the “TT” genotype of the SNP were 3.6 times more at risk for being diagnosed with the disorder. Furthermore, in another report, carriers of the same SNP displayed HPA axis dysregulation in response to a psychological stress task dependent on early life adversity (135). Likewise, the risk for anxiety susceptibility after childhood adversities depends on the NPY genotype, with three SNPs of the NPY gene (rs16142, rs2023890, and rs17374047) increasing the risk (136). The relevance of NPY as a stress resilience factor has been confirmed in a study with monkeys. Macaques exposed to early life adversity exhibit enhanced arousal during stress and lowered CSF levels of NPY when carrying a SNP in the NPY promoter region (137).
7. NPY as a potential therapeutic for PTSD
Treatment of human primary psychiatric diseases with neuropeptide therapeutics such as NPY is challenging as it faces several technical limitations. To date these include systemic route of administration, short in vivo stability, poor BBB penetration as well as complexity and costs of manufacture (138). Particularly the inability of neuropeptides to pass the BBB and the disadvantages of oral or systemic administration (absorption, first-pass metabolism, plasma protein binding, time to reach the brain, peripheral and central side effects) slowed the progress in the development of neuropeptide therapeutics (139). Meanwhile research has focused on a promising non-invasive way to circumvent problems associated with systemic neuropeptide administration, namely intranasal drug delivery. This administration route has been shown to achieve direct CNS delivery of various compounds with rapid increases in CNS levels and, in parallel, to avoid drawbacks of peripheral drug treatment (139).
NPY application via the intranasal administration route has been repeatedly shown to exert beneficial behavioural effects. After exposure to single prolonged stress (SPS), a rat model of PTSD, Serova et al. (140) observed a reduction of anxiety and depression-like behaviour in animals receiving a single pre-stress dose of intranasal NPY. Furthermore, intranasal NPY reduced the perceived severity of stress and prevented stress-induced dysregulation of the HPA axis and noradrenergic activity (140). In a therapeutic study design, NPY was able to ameliorate the SPS-induced behavioural disturbances when administered immediately or even 7 days after stress exposure (140–142). Similarly to pre-stress NPY administration, post-stress intranasal NPY application also prevented HPA axis dysregulation (143).
In humans evidence for a therapeutic effect of intranasal NPY is limited. A small pilot study found that intranasal NPY application is associated with very low systemic absorption rates, inducing local vasoconstriction but failing to modify mean arterial pressure or heart rate, which indicates a good safety profile (144). The ability of NPY to influence CNS function when applied via the nasal route was inferred from an investigation of central mechanisms of food intake. Specifically, intranasal NPY attenuated electrocortical signs of meal-related satiety (145). To our knowledge there is no published study on the effects of NPY or NPY analogues on neuropsychiatric disease in humans. However, the potential of intranasal NPY administration as a treatment option for PTSD is currently under investigation in a Phase I trial (clinicaltrials.gov).
8. Role of NPY in neurodegenerative diseases
Neurodegenerative diseases represent a leading health issue and comprise wide-spread pathologies such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and Huntington’s disease (HD). Genetic, environmental as well as toxic factors contribute to the pathogenesis of these neurodegenerative diseases to a differential degree. Stress, on the one hand, may trigger or exacerbate the disease course while the disease symptoms, on the other hand, are a source of stress to the patients (146,147). NPY has been implicated in all of these disorders either as a biomarker, a neuroprotective factor or a potential therapeutic.
NPY and Alzheimer’s disease
Reductions of NPY levels in plasma, CSF and brain of AD patients sparked interest in the relation of this neuropeptide with AD (148,149). Soon afterwards a negative correlation between NPY levels in the CSF and duration of the disease was described (150). In parallel it was found that cortical NPY neurons are severely damaged during AD (151). The use of transgenic AD mouse models confirmed that especially cortical but also hippocampal neurons expressing NPY are strongly affected by the AD pathology, which points to a particular vulnerability of these neurons and a role of NPY in AD pathophysiology (152–154). Importantly, hippocampal NPY neurons are affected already in the early stages of AD. NPY may thus constitute a potential biomarker of treatment efficacy during AD drug development (152).
Additionally, several reports attest to the therapeutic efficacy of NPY in various experimental AD models. Infusion of NPY C-terminal fragments into the brains of APP (amyloid precursor protein) transgenic mice ameliorates neurodegenerative pathology, and pretreatment of cultured primary human neurons with NPY fragments prevents the cytotoxic effects of beta-amyloid(1-42) (Aß(1-42)) (155). A number of in vitro experiments also suggests a neuroprotective effect of NPY. For instance, the toxic effect of Aß(25-35) on SH-SY5Y neuroblastoma cell viability is prevented by a 24 h pretreatment with NPY (156). In a similar setting involving primary cortical neurons of rats, NPY is likewise able to prevent the deleterious effects of Aß(25-35) (157). The neuroprotective effect of NPY in these studies is associated with changes in nerve growth factor and brain-derived neurotrophic factor gene expression (156–159).
In-vivo experiments show that NPY plays a role in the behavioural changes associated with AD pathology. In the colchicine-induced rat model of AD, memory impairments in the Morris Water Maze are ameliorated by NPY and the Y1 receptor agonist [Leu31,Pro34]-NPY, whereas the Y1 receptor antagonist BIBP3226 produces the opposite effect (160). While this study suggests that the Y1 receptor is crucial in the AD-related cognitive impairment, beneficial effects of NPY may also be mediated by Y2/Y5 receptors which are able to prevent excitotoxicity by inhibiting glutamate release (161). In line with this finding, NPY prevents Aß(1-42)-induced depression-like behaviour and spatial memory impairments in mice, but fails to do so after pretreatment with BIIE0246, a Y2 receptor antagonist (162).
In contradiction of these findings there are also reports that question the involvement of NPY in the AD disease course. For instance, a human study investigating NPY single nucleotide polymorphisms did not find an association with increased AD risk (163), and plasma levels of NPY were found unaltered in a more recent study of AD patients (164)These findings do not necessarily negate an involvement of cerebral NPY in the AD pathology, which needs to be proven by clinical trials of pharmacological interventions in the NPY system.
NPY and Huntington’s disease
In contrast to AD which causes a reduction of NPY-expressing cells, the number of NPY-expressing cells in HD is increased in the basal ganglia, cortex and the subventricular zone (165–167). It thus appears as if NPY-containing interneurons are spared from the degenerative process, which in the striatum affects primarily medium-sized spiny projection neurons expressing GABA (168), and the increase in the number of cerebral NPY neurons reflects an attempt to balance the loss of other neurons. The enhanced NPY levels in the subventricular zone may be of further clinical significance, given that the cell proliferation rate in this region, one of the few brain areas capable of adult neurogenesis, correlates with HD disease severity (169). The HD-related enhanced neurogenesis is likely to be a counterregulatory mechanism to compensate for the progressive neuronal cell loss during the disease course. NPY is known to increase neurogenesis (170), and a further increase in cerebral NPY expression by pharmacological interventions may be a promising treatment strategy in HD. In fact, NPY has already been shown to be of therapeutic value in mice with HD pathology. Specifically, icv NPY administration increases survival time and ameliorates motor performance as well as cerebral atrophy of R6/2 mice (171). A recent human study disclosed a modest association of HD onset age with two NPY promoter variations and a strong association with a Y2 receptor single nucleotide polymorphism (172). Patients with the high expression Y2 receptor genotype had a later age of disease onset. In addition, NPY and the Y2 receptor agonist NPY(3-36) were found to protect PC12 cells expressing mutant huntingtin (htt) exon 1 against mutant htt-induced cell death (172).
NPY and Parkinson’s disease
An association of NPY with PD is supported by findings that disruption of the nigrostriatal pathway by 6-hydroxydopamine (6-OHDA) or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine results in an increased number of striatal NPY-expressing neurons (173–175). In line with this finding, post-mortem analysis of brain tissue from PD patients reveals an increase of NPY-expressing neurons in the striatum (176), which may be related to the loss of dopaminergic tone on the striatal NPY interneurons. Interestingly, NPY levels in the CSF of PD patients appear to be lower than in healthy individuals (177), which may point to a reduction of NPY release or increased NPY turnover. A recent study suggests that NPY exerts a neuroprotective action in both in-vivo and in-vitro models of PD (178). In vitro, NPY protects SH-SY5Y dopaminergic neuroblastoma cells from 6-OHDA-induced toxicity, while in vivo NPY preserves the nigrostriatal dopaminergic pathway from neurodegeneration. These neuroprotective effects are blocked by BIIE0246, a Y2 receptor antagonist, which suggests that the NPY effects are mediated via this receptor. Besides its potential therapeutic effect, NPY has also been implicated in the weight gain associated with deep brain stimulation of the subthalamic nucleus, a current treatment option of PD. Two studies found increased circulating NPY levels as a potential factor relevant to this side effect (179,180).
9. Conclusions
Since its discovery and isolation from porcine brain extracts, NPY has gained significant attention for its anti-stress and anxiolytic properties. Exciting new lines of research implicate the peptide as an important mediator of stress resilience, the ability to cope with stress, and as a neuroprotective factor in neurodegenerative diseases. These implications are supported by genetic, molecular and pharmacological studies. Extensive analysis identifies the amygdala-hippocampus network and associated limbic brain areas as key components in NPY-mediated stress resilience. While several animal models demonstrate the therapeutic efficacy of NPY in the context of neuropsychiatric and neurodegenerative disease, data from human studies are still inconclusive. Due to the unfavourable kinetics of neuropeptide drugs there is a need for novel therapeutic strategies that enable neuropeptide therapeutics to bypass or cross the BBB. Following this approach, intranasal NPY represents a promising strategy for the treatment of PTSD.
10. Acknowledgements
This study was supported by the Austrian Science Fund (FWF grants P23097-B18, P25912-B23 and W1241-B18).
References
- (1).Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012 Dec;46(6):261–274. doi: 10.1016/j.npep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982 Apr 15;296(5858):659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- (3).Sajdyk TJ, Shekhar A, Gehlert DR. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004 Aug;38(4):225–234. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- (4).White JD. Neuropeptide Y: a central regulator of energy homeostasis. Regul Pept. 1993 Dec 10;49(2):93–107. doi: 10.1016/0167-0115(93)90431-7. [DOI] [PubMed] [Google Scholar]
- (5).Eaton K, Sallee FR, Sah R. Relevance of neuropeptide Y (NPY) in psychiatry. Curr Top Med Chem. 2007;7(17):1645–1659. doi: 10.2174/156802607782341037. [DOI] [PubMed] [Google Scholar]
- (6).Zhang L, Bijker MS, Herzog H. The neuropeptide Y system: pathophysiological and therapeutic implications in obesity and cancer. Pharmacol Ther. 2011 Jul;131(1):91–113. doi: 10.1016/j.pharmthera.2011.03.011. [DOI] [PubMed] [Google Scholar]
- (7).Morin LP. Neuroanatomy of the extended circadian rhythm system. Exp Neurol. 2013 May;243:4–20. doi: 10.1016/j.expneurol.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004 Aug;38(4):213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- (9).Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002 May;26(3):259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- (10).Broberger C, Visser TJ, Kuhar MJ, Hokfelt T. Neuropeptide Y innervation and neuropeptide-Y-Y1-receptor-expressing neurons in the paraventricular hypothalamic nucleus of the mouse. Neuroendocrinology. 1999 Nov;70(5):295–305. doi: 10.1159/000054490. [DOI] [PubMed] [Google Scholar]
- (11).Sundstrom G, Larsson TA, Xu B, Heldin J, Larhammar D. Interactions of zebrafish peptide YYb with the neuropeptide Y-family receptors Y4, Y7, Y8a, and Y8b. Front Neurosci. 2013 Mar 15;7:29. doi: 10.3389/fnins.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Bromee T, Sjodin P, Fredriksson R, Boswell T, Larsson TA, Salaneck E, et al. Neuropeptide Y-family receptors Y6 and Y7 in chicken. Cloning, pharmacological characterization, tissue distribution and conserved synteny with human chromosome region. FEBS J. 2006 May;273(9):2048–2063. doi: 10.1111/j.1742-4658.2006.05221.x. [DOI] [PubMed] [Google Scholar]
- (13).Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013 Dec;170(8):1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, et al. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111(3):443–532. doi: 10.1016/s0306-4522(01)00463-8. [DOI] [PubMed] [Google Scholar]
- (15).Eva C, Serra M, Mele P, Panzica G, Oberto A. Physiology and gene regulation of the brain NPY Y1 receptor. Front Neuroendocrinol. 2006 Sep;27(3):308–339. doi: 10.1016/j.yfrne.2006.07.002. [DOI] [PubMed] [Google Scholar]
- (16).Dumont Y, Jacques D, Bouchard P, Quirion R. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. J Comp Neurol. 1998 Dec 21;402(3):372–384. [PubMed] [Google Scholar]
- (17).Morales-Medina JC, Dumont Y, Quirion R. A possible role of neuropeptide Y in depression and stress. Brain Res. 2010 Feb 16;1314:194–205. doi: 10.1016/j.brainres.2009.09.077. [DOI] [PubMed] [Google Scholar]
- (18).Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999 Apr;11(4):1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- (19).Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol. 2003 Sep 22;464(3):285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- (20).Selye H. A syndrome produced by diverse nocuous agents. 1936. J Neuropsychiatry Clin Neurosci. 1998 Spring;10(2):230–231. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- (21).Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009 Jul;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- (22).Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994 Feb;17(2):80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- (23).Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997 Feb;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- (24).Bakshi VP, Kalin NH. Corticotropin-releasing hormone and animal models of anxiety: gene-environment interactions. Biol Psychiatry. 2000 Dec 15;48(12):1175–1198. doi: 10.1016/s0006-3223(00)01082-9. [DOI] [PubMed] [Google Scholar]
- (25).Heilig M, Soderpalm B, Engel JA, Widerlov E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 1989;98(4):524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- (26).Smialowska M, Wieronska JM, Domin H, Zieba B. The effect of intrahippocampal injection of group II and III metobotropic glutamate receptor agonists on anxiety; the role of neuropeptide Y. Neuropsychopharmacology. 2007 Jun;32(6):1242–1250. doi: 10.1038/sj.npp.1301258. [DOI] [PubMed] [Google Scholar]
- (27).Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, et al. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008 Jan 23;28(4):893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Bannon AW, Seda J, Carmouche M, Francis JM, Norman MH, Karbon B, et al. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000 Jun 16;868(1):79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- (29).Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R, et al. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12852–12857. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Primeaux SD, Wilson SP, Cusick MC, York DA, Wilson MA. Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology. 2005 Sep;30(9):1589–1597. doi: 10.1038/sj.npp.1300705. [DOI] [PubMed] [Google Scholar]
- (31).Lin EJ, Lin S, Aljanova A, During MJ, Herzog H. Adult-onset hippocampal-specific neuropeptide Y overexpression confers mild anxiolytic effect in mice. Eur Neuropsychopharmacol. 2010 Mar;20(3):164–175. doi: 10.1016/j.euroneuro.2009.08.004. [DOI] [PubMed] [Google Scholar]
- (32).de Lange RP, Wiegant VM, Stam R. Altered neuropeptide Y and neurokinin messenger RNA expression and receptor binding in stress-sensitised rats. Brain Res. 2008 May 30;1212:35–47. doi: 10.1016/j.brainres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- (33).Reichmann F, Hassan AM, Farzi A, Jain P, Schuligoi R, Holzer P. Dextran sulfate sodium-induced colitis alters stress-associated behaviour and neuropeptide gene expression in the amygdala-hippocampus network of mice. Sci Rep. 2015 Jun 12;5:9970. doi: 10.1038/srep09970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Thorsell A, Svensson P, Wiklund L, Sommer W, Ekman R, Heilig M. Suppressed neuropeptide Y (NPY) mRNA in rat amygdala following restraint stress. Regul Pept. 1998 Sep 25;75–76:247–254. doi: 10.1016/s0167-0115(98)00075-5. [DOI] [PubMed] [Google Scholar]
- (35).Thorsell A, Carlsson K, Ekman R, Heilig M. Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport. 1999 Sep 29;10(14):3003–3007. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- (36).Sweerts BW, Jarrott B, Lawrence AJ. The effect of acute and chronic restraint on the central expression of prepro-neuropeptide Y mRNA in normotensive and hypertensive rats. J Neuroendocrinol. 2001 Jul;13(7):608–617. doi: 10.1046/j.1365-2826.2001.00674.x. [DOI] [PubMed] [Google Scholar]
- (37).Hadad-Ophir O, Albrecht A, Stork O, Richter-Levin G. Amygdala activation and GABAergic gene expression in hippocampal sub-regions at the interplay of stress and spatial learning. Front Behav Neurosci. 2014 Jan 21;8:3. doi: 10.3389/fnbeh.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Hassan AM, Jain P, Reichmann F, Mayerhofer R, Farzi A, Schuligoi R, et al. Repeated predictable stress causes resilience against colitis-induced behavioral changes in mice. Front Behav Neurosci. 2014 Nov 6;8:386. doi: 10.3389/fnbeh.2014.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Conrad CD, McEwen BS. Acute stress increases neuropeptide Y mRNA within the arcuate nucleus and hilus of the dentate gyrus. Brain Res Mol Brain Res. 2000 Jun 23;79(1–2):102–109. doi: 10.1016/s0169-328x(00)00105-4. [DOI] [PubMed] [Google Scholar]
- (40).Kas MJ, Bruijnzeel AW, Haanstra JR, Wiegant VM, Adan RA. Differential regulation of agouti-related protein and neuropeptide Y in hypothalamic neurons following a stressful event. J Mol Endocrinol. 2005 Aug;35(1):159–164. doi: 10.1677/jme.1.01819. [DOI] [PubMed] [Google Scholar]
- (41).Ferenczi S, Zelei E, Pinter B, Szoke Z, Kovacs KJ. Differential regulation of hypothalamic neuropeptide Y hnRNA and mRNA during psychological stress and insulin-induced hypoglycemia. Mol Cell Endocrinol. 2010 Jun 10;321(2):138–145. doi: 10.1016/j.mce.2010.02.036. [DOI] [PubMed] [Google Scholar]
- (42).Chigr F, Rachidi F, Tardivel C, Najimi M, Moyse E. Modulation of orexigenic and anorexigenic peptides gene expression in the rat DVC and hypothalamus by acute immobilization stress. Front Cell Neurosci. 2014 Jul 18;8:198. doi: 10.3389/fncel.2014.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).McDougall SJ, Widdop RE, Lawrence AJ. Differential gene expression in WKY and SHR brain following acute and chronic air-puff stress. Brain Res Mol Brain Res. 2005 Feb 18;133(2):329–336. doi: 10.1016/j.molbrainres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- (44).Baticic L, Detel D, Kucic N, Buljevic S, Pugel EP, Varljen J. Neuroimmunomodulative properties of dipeptidyl peptidase IV/CD26 in a TNBS-induced model of colitis in mice. J Cell Biochem. 2011 Nov;112(11):3322–3333. doi: 10.1002/jcb.23261. [DOI] [PubMed] [Google Scholar]
- (45).Farzi A, Reichmann F, Holzer P. The homeostatic role of neuropeptide Y in immune function and its impact on mood and behaviour. Acta Physiol (Oxf) 2015 Mar;213(3):603–627. doi: 10.1111/apha.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Bercik P, Verdu EF, Foster JA, Lu J, Scharringa A, Kean I, et al. Role of gut-brain axis in persistent abnormal feeding behavior in mice following eradication of Helicobacter pylori infection. Am J Physiol Regul Integr Comp Physiol. 2009 Mar;296(3):R587–94. doi: 10.1152/ajpregu.90752.2008. [DOI] [PubMed] [Google Scholar]
- (47).Sorensen G, Lindberg C, Wortwein G, Bolwig TG, Woldbye DP. Differential roles for neuropeptide Y Y1 and Y5 receptors in anxiety and sedation. J Neurosci Res. 2004 Sep 1;77(5):723–729. doi: 10.1002/jnr.20200. [DOI] [PubMed] [Google Scholar]
- (48).Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999 Mar 5;368(2–3):143–147. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- (49).Lach G, de Lima TC. Role of NPY Y1 receptor on acquisition, consolidation and extinction on contextual fear conditioning: dissociation between anxiety, locomotion and non-emotional memory behavior. Neurobiol Learn Mem. 2013 Jul;103:26–33. doi: 10.1016/j.nlm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- (50).Karl T, Burne TH, Herzog H. Effect of Y1 receptor deficiency on motor activity, exploration, and anxiety. Behav Brain Res. 2006 Feb 15;167(1):87–93. doi: 10.1016/j.bbr.2005.08.019. [DOI] [PubMed] [Google Scholar]
- (51).Bertocchi I, Oberto A, Longo A, Mele P, Sabetta M, Bartolomucci A, et al. Regulatory functions of limbic Y1 receptors in body weight and anxiety uncovered by conditional knockout and maternal care. Proc Natl Acad Sci U S A. 2011 Nov 29;108(48):19395–19400. doi: 10.1073/pnas.1109468108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Olesen MV, Christiansen SH, Gotzsche CR, Nikitidou L, Kokaia M, Woldbye DP. Neuropeptide Y Y1 receptor hippocampal overexpression via viral vectors is associated with modest anxiolytic-like and proconvulsant effects in mice. J Neurosci Res. 2012 Feb;90(2):498–507. doi: 10.1002/jnr.22770. [DOI] [PubMed] [Google Scholar]
- (53).Fendt M, Burki H, Imobersteg S, Lingenhohl K, McAllister KH, Orain D, et al. Fear-reducing effects of intra-amygdala neuropeptide Y infusion in animal models of conditioned fear: an NPY Y1 receptor independent effect. Psychopharmacology (Berl) 2009 Oct;206(2):291–301. doi: 10.1007/s00213-009-1610-8. [DOI] [PubMed] [Google Scholar]
- (54).Molosh AI, Sajdyk TJ, Truitt WA, Zhu W, Oxford GS, Shekhar A. NPY Y1 receptors differentially modulate GABAA and NMDA receptors via divergent signal-transduction pathways to reduce excitability of amygdala neurons. Neuropsychopharmacology. 2013 Jun;38(7):1352–1364. doi: 10.1038/npp.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Leitermann RJ, Sajdyk TJ, Urban JH. Cell-specific expression of calcineurin immunoreactivity within the rat basolateral amygdala complex and colocalization with the neuropeptide Y Y1 receptor. J Chem Neuroanat. 2012 Oct;45(1–2):50–56. doi: 10.1016/j.jchemneu.2012.07.005. [DOI] [PubMed] [Google Scholar]
- (56).Caberlotto L, Fuxe K, Hurd YL. Characterization of NPY mRNA-expressing cells in the human brain: co-localization with Y2 but not Y1 mRNA in the cerebral cortex, hippocampus, amygdala, and striatum. J Chem Neuroanat. 2000 Dec;20(3–4):327–337. doi: 10.1016/s0891-0618(00)00107-1. [DOI] [PubMed] [Google Scholar]
- (57).Sajdyk TJ, Schober DA, Smiley DL, Gehlert DR. Neuropeptide Y-Y2 receptors mediate anxiety in the amygdala. Pharmacol Biochem Behav. 2002 Mar;71(3):419–423. doi: 10.1016/s0091-3057(01)00679-7. [DOI] [PubMed] [Google Scholar]
- (58).Nakajima M, Inui A, Asakawa A, Momose K, Ueno N, Teranishi A, et al. Neuropeptide Y produces anxiety via Y2-type receptors. Peptides. 1998;19(2):359–363. doi: 10.1016/s0196-9781(97)00298-2. [DOI] [PubMed] [Google Scholar]
- (59).Bacchi F, Mathe AA, Jimenez P, Stasi L, Arban R, Gerrard P, et al. Anxiolytic-like effect of the selective neuropeptide Y Y2 receptor antagonist BIIE0246 in the elevated plus-maze. Peptides. 2006 Dec;27(12):3202–3207. doi: 10.1016/j.peptides.2006.07.020. [DOI] [PubMed] [Google Scholar]
- (60).Kallupi M, Vendruscolo LF, Carmichael CY, George O, Koob GF, Gilpin NW. Neuropeptide YY(2)R blockade in the central amygdala reduces anxiety-like behavior but not alcohol drinking in alcohol-dependent rats. Addict Biol. 2014 Sep;19(5):755–757. doi: 10.1111/adb.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Tasan RO, Nguyen NK, Weger S, Sartori SB, Singewald N, Heilbronn R, et al. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci. 2010 May 5;30(18):6282–6290. doi: 10.1523/JNEUROSCI.0430-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Cippitelli A, Rezvani AH, Robinson JE, Eisenberg L, Levin ED, Bonaventure P, et al. The novel, selective, brain-penetrant neuropeptide Y Y2 receptor antagonist, JNJ-31020028, tested in animal models of alcohol consumption, relapse, and anxiety. Alcohol. 2011 Sep;45(6):567–576. doi: 10.1016/j.alcohol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- (63).Aydin C, Oztan O, Isgor C. Effects of a selective Y2R antagonist, JNJ-31020028, on nicotine abstinence-related social anxiety-like behavior, neuropeptide Y and corticotropin releasing factor mRNA levels in the novelty-seeking phenotype. Behav Brain Res. 2011 Sep 23;222(2):332–341. doi: 10.1016/j.bbr.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Aydin C, Oztan O, Isgor C. Hippocampal Y2 receptor-mediated mossy fiber plasticity is implicated in nicotine abstinence-related social anxiety-like behavior in an outbred rat model of the novelty-seeking phenotype. Pharmacol Biochem Behav. 2014 Oct;125:48–54. doi: 10.1016/j.pbb.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Tschenett A, Singewald N, Carli M, Balducci C, Salchner P, Vezzani A, et al. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur J Neurosci. 2003 Jul;18(1):143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- (66).Carvajal CC, Vercauteren F, Dumont Y, Michalkiewicz M, Quirion R. Aged neuropeptide Y transgenic rats are resistant to acute stress but maintain spatial and non-spatial learning. Behav Brain Res. 2004 Aug 31;153(2):471–480. doi: 10.1016/j.bbr.2004.01.004. [DOI] [PubMed] [Google Scholar]
- (67).Painsipp E, Herzog H, Holzer P. Implication of neuropeptide-Y Y2 receptors in the effects of immune stress on emotional, locomotor and social behavior of mice. Neuropharmacology. 2008 Jul;55(1):117–126. doi: 10.1016/j.neuropharm.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Zambello E, Zanetti L, Hedou GF, Angelici O, Arban R, Tasan RO, et al. Neuropeptide Y-Y2 receptor knockout mice: influence of genetic background on anxiety-related behaviors. Neuroscience. 2011 Mar 10;176:420–430. doi: 10.1016/j.neuroscience.2010.10.075. [DOI] [PubMed] [Google Scholar]
- (69).Kask A, Rago L, Harro J. Anxiolytic-like effect of neuropeptide Y (NPY) and NPY13-36 microinjected into vicinity of locus coeruleus in rats. Brain Res. 1998 Mar 30;788(1–2):345–348. doi: 10.1016/s0006-8993(98)00076-6. [DOI] [PubMed] [Google Scholar]
- (70).Trent NL, Menard JL. Lateral septal infusions of the neuropeptide Y Y2 receptor agonist, NPY(13-36) differentially affect different defensive behaviors in male, Long Evans rats. Physiol Behav. 2013 Feb 17;110–111:20–29. doi: 10.1016/j.physbeh.2012.12.011. [DOI] [PubMed] [Google Scholar]
- (71).Silva AP, Xapelli S, Grouzmann E, Cavadas C. The putative neuroprotective role of neuropeptide Y in the central nervous system. Curr Drug Targets CNS Neurol Disord. 2005 Aug;4(4):331–347. doi: 10.2174/1568007054546153. [DOI] [PubMed] [Google Scholar]
- (72).Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2006 Oct;51(5):1013–1022. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- (73).Kask A, Vasar E, Heidmets LT, Allikmets L, Wikberg JE. Neuropeptide Y Y(5) receptor antagonist CGP71683A: the effects on food intake and anxiety-related behavior in the rat. Eur J Pharmacol. 2001 Mar 2;414(2–3):215–224. doi: 10.1016/s0014-2999(01)00768-3. [DOI] [PubMed] [Google Scholar]
- (74).Morales-Medina JC, Dominguez-Lopez S, Gobbi G, Beck-Sickinger AG, Quirion R. The selective neuropeptide Y Y5 agonist [cPP(1-7),NPY(19-23),Ala31,Aib32,Gln34]hPP differently modulates emotional processes and body weight in the rat. Behav Brain Res. 2012 Aug 1;233(2):298–304. doi: 10.1016/j.bbr.2012.05.015. [DOI] [PubMed] [Google Scholar]
- (75).Quarta D, Leslie CP, Carletti R, Valerio E, Caberlotto L. Central administration of NPY or an NPY-Y5 selective agonist increase in vivo extracellular monoamine levels in mesocorticolimbic projecting areas. Neuropharmacology. 2011 Feb-Mar;60(2–3):328–335. doi: 10.1016/j.neuropharm.2010.09.016. [DOI] [PubMed] [Google Scholar]
- (76).Walker MW, Wolinsky TD, Jubian V, Chandrasena G, Zhong H, Huang X, et al. The novel neuropeptide Y Y5 receptor antagonist Lu AA33810 [N-[[trans-4-[(4,5-dihydro[1]benzothiepino[5,4-d]thiazol-2-yl)amino]cyclohexyl]me thyl]-methanesulfonamide] exerts anxiolytic- and antidepressant-like effects in rat models of stress sensitivity. J Pharmacol Exp Ther. 2009 Mar;328(3):900–911. doi: 10.1124/jpet.108.144634. [DOI] [PubMed] [Google Scholar]
- (77).Olesen MV, Christiansen SH, Gotzsche CR, Holst B, Kokaia M, Woldbye DP. Y5 neuropeptide Y receptor overexpression in mice neither affects anxiety- and depression-like behaviours nor seizures but confers moderate hyperactivity. Neuropeptides. 2012 Apr;46(2):71–79. doi: 10.1016/j.npep.2012.01.002. [DOI] [PubMed] [Google Scholar]
- (78).Ito M, Dumont Y, Quirion R. Mood and memory-associated behaviors in neuropeptide Y5 knockout mice. Neuropeptides. 2013 Apr;47(2):75–84. doi: 10.1016/j.npep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- (79).Longo A, Mele P, Bertocchi I, Oberto A, Bachmann A, Bartolomucci A, et al. Conditional inactivation of neuropeptide Y Y1 receptors unravels the role of Y1 and Y5 receptors coexpressing neurons in anxiety. Biol Psychiatry. 2014 Dec 1;76(11):840–849. doi: 10.1016/j.biopsych.2014.01.009. [DOI] [PubMed] [Google Scholar]
- (80).Longo A, Oberto A, Mele P, Mattiello L, Pisu MG, Palanza P, et al. NPY-Y1 coexpressed with NPY-Y5 receptors modulate anxiety but not mild social stress response in mice. Genes Brain Behav. 2015 Sep;14(7):534–542. doi: 10.1111/gbb.12232. [DOI] [PubMed] [Google Scholar]
- (81).Domschke K, Hohoff C, Jacob C, Maier W, Fritze J, Bandelow B, et al. Chromosome 4q31-34 panic disorder risk locus: association of neuropeptide Y Y5 receptor variants. Am J Med Genet B Neuropsychiatr Genet. 2008 Jun 5;147B(4):510–516. doi: 10.1002/ajmg.b.30629. [DOI] [PubMed] [Google Scholar]
- (82).Roseboom PH, Nanda SA, Fox AS, Oler JA, Shackman AJ, Shelton SE, et al. Neuropeptide Y receptor gene expression in the primate amygdala predicts anxious temperament and brain metabolism. Biol Psychiatry. 2014 Dec 1;76(11):850–857. doi: 10.1016/j.biopsych.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Asakawa A, Inui A, Ueno N, Fujimiya M, Fujino MA, Kasuga M. Mouse pancreatic polypeptide modulates food intake, while not influencing anxiety in mice. Peptides. 1999 Dec;20(12):1445–1448. doi: 10.1016/s0196-9781(99)00155-2. [DOI] [PubMed] [Google Scholar]
- (84).Painsipp E, Wultsch T, Edelsbrunner ME, Tasan RO, Singewald N, Herzog H, et al. Reduced anxiety-like and depression-related behavior in neuropeptide Y Y4 receptor knockout mice. Genes Brain Behav. 2008 Jul;7(5):532–542. doi: 10.1111/j.1601-183X.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Tasan RO, Lin S, Hetzenauer A, Singewald N, Herzog H, Sperk G. Increased novelty-induced motor activity and reduced depression-like behavior in neuropeptide Y (NPY)-Y4 receptor knockout mice. Neuroscience. 2009 Feb 18;158(4):1717–1730. doi: 10.1016/j.neuroscience.2008.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Verma D, Wood J, Lach G, Herzog H, Sperk G, Tasan R. Hunger Promotes Fear Extinction by Activation of an Amygdala Microcircuit. Neuropsychopharmacology. 2015 Jun 11; doi: 10.1038/npp.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, et al. Neuropeptide Y distribution in the rat brain. Science. 1983 Aug 26;221(4613):877–879. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- (88).DiMaggio DA, Chronwall BM, Buchanan K, O'Donohue TL. Pancreatic polypeptide immunoreactivity in rat brain is actually neuropeptide Y. Neuroscience. 1985 Aug;15(4):1149–1157. doi: 10.1016/0306-4522(85)90259-3. [DOI] [PubMed] [Google Scholar]
- (89).Yan H, Yang J, Marasco J, Yamaguchi K, Brenner S, Collins F, et al. Cloning and functional expression of cDNAs encoding human and rat pancreatic polypeptide receptors. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4661–4665. doi: 10.1073/pnas.93.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Gehlert DR, Schober DA, Gackenheimer SL, Beavers L, Gadski R, Lundell I, et al. 125I]Leu31, Pro34-PYY is a high affinity radioligand for rat PP1/Y4 and Y1 receptors: evidence for heterogeneity in pancreatic polypeptide receptors. Peptides. 1997;18(3):397–401. doi: 10.1016/s0196-9781(96)00346-4. [DOI] [PubMed] [Google Scholar]
- (91).Whitcomb DC, Taylor IL, Vigna SR. Characterization of saturable binding sites for circulating pancreatic polypeptide in rat brain. Am J Physiol. 1990 Oct;259(4 Pt 1):G687–91. doi: 10.1152/ajpgi.1990.259.4.G687. [DOI] [PubMed] [Google Scholar]
- (92).Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ. The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Ann N Y Acad Sci. 1995 Dec 29;771:730–742. doi: 10.1111/j.1749-6632.1995.tb44724.x. [DOI] [PubMed] [Google Scholar]
- (93).Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007 Jul 24;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- (94).Maniam J, Morris MJ. The link between stress and feeding behaviour. Neuropharmacology. 2012 Jul;63(1):97–110. doi: 10.1016/j.neuropharm.2012.04.017. [DOI] [PubMed] [Google Scholar]
- (95).Gehlert DR. Role of hypothalamic neuropeptide Y in feeding and obesity. Neuropeptides. 1999 Oct;33(5):329–338. doi: 10.1054/npep.1999.0057. [DOI] [PubMed] [Google Scholar]
- (96).Krolow R, Noschang C, Arcego DM, Huffell AP, Marcolin ML, Benitz AN, et al. Sex-specific effects of isolation stress and consumption of palatable diet during the prepubertal period on metabolic parameters. Metabolism. 2013 Sep;62(9):1268–1278. doi: 10.1016/j.metabol.2013.04.009. [DOI] [PubMed] [Google Scholar]
- (97).Forbes S, Herzog H, Cox HM. A role for neuropeptide Y in the gender-specific gastrointestinal, corticosterone and feeding responses to stress. Br J Pharmacol. 2012 Aug;166(8):2307–2316. doi: 10.1111/j.1476-5381.2012.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Goebel-Stengel M, Stengel A, Wang L, Tache Y. Orexigenic response to tail pinch: role of brain NPY(1) and corticotropin releasing factor receptors. Am J Physiol Regul Integr Comp Physiol. 2014 Feb 1;306(3):R164–74. doi: 10.1152/ajpregu.00335.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Shoblock JR, Welty N, Nepomuceno D, Lord B, Aluisio L, Fraser I, et al. In vitro and in vivo characterization of JNJ-31020028 (N-(4-{4-[2-(diethylamino)-2-oxo-1-phenylethyl]piperazin-1-yl}-3-fluorophenyl)-2- pyridin-3-ylbenzamide), a selective brain penetrant small molecule antagonist of the neuropeptide Y Y(2) receptor. Psychopharmacology (Berl) 2010 Feb;208(2):265–277. doi: 10.1007/s00213-009-1726-x. [DOI] [PubMed] [Google Scholar]
- (100).Bernardi JR, Ferreira CF, Senter G, Krolow R, de Aguiar BW, Portella AK, et al. Early life stress interacts with the diet deficiency of omega-3 fatty acids during the life course increasing the metabolic vulnerability in adult rats. PLoS One. 2013 Apr 17;8(4):e62031. doi: 10.1371/journal.pone.0062031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Wang SX, Chen JX, Yue GX, Bai MH, Kou MJ, Jin ZY. Xiaoyaosan decoction regulates changes in neuropeptide y and leptin receptor in the rat arcuate nucleus after chronic immobilization stress. Evid Based Complement Alternat Med. 2012;2012:381278. doi: 10.1155/2012/381278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Calvez J, Fromentin G, Nadkarni N, Darcel N, Even P, Tome D, et al. Inhibition of food intake induced by acute stress in rats is due to satiation effects. Physiol Behav. 2011 Oct 24;104(5):675–683. doi: 10.1016/j.physbeh.2011.07.012. [DOI] [PubMed] [Google Scholar]
- (103).Upton KR, Riley LG. Acute stress inhibits food intake and alters ghrelin signaling in the brain of tilapia (Oreochromis mossambicus) Domest Anim Endocrinol. 2013 Apr;44(3):157–164. doi: 10.1016/j.domaniend.2012.10.001. [DOI] [PubMed] [Google Scholar]
- (104).Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, et al. Meal patterns and hypothalamic NPY expression during chronic social stress and recovery. Am J Physiol Regul Integr Comp Physiol. 2010 Sep;299(3):R813–22. doi: 10.1152/ajpregu.00820.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Rybkin II, Zhou Y, Volaufova J, Smagin GN, Ryan DH, Harris RB. Effect of restraint stress on food intake and body weight is determined by time of day. Am J Physiol. 1997 Nov;273(5 Pt 2):R1612–22. doi: 10.1152/ajpregu.1997.273.5.R1612. [DOI] [PubMed] [Google Scholar]
- (106).Ito K, Bahry MA, Hui Y, Furuse M, Chowdhury VS. Acute heat stress up-regulates neuropeptide Y precursor mRNA expression and alters brain and plasma concentrations of free amino acids in chicks. Comp Biochem Physiol A Mol Integr Physiol. 2015 Sep;187:13–19. doi: 10.1016/j.cbpa.2015.04.010. [DOI] [PubMed] [Google Scholar]
- (107).Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007 Jul;13(7):803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- (108).Zakrzewska KE, Cusin I, Stricker-Krongrad A, Boss O, Ricquier D, Jeanrenaud B, et al. Induction of obesity and hyperleptinemia by central glucocorticoid infusion in the rat. Diabetes. 1999 Feb;48(2):365–370. doi: 10.2337/diabetes.48.2.365. [DOI] [PubMed] [Google Scholar]
- (109).Paternain L, Batlle MA, De la Garza AL, Milagro FI, Martinez JA, Campion J. Transcriptomic and epigenetic changes in the hypothalamus are involved in an increased susceptibility to a high-fat-sucrose diet in prenatally stressed female rats. Neuroendocrinology. 2012;96(3):249–260. doi: 10.1159/000341684. [DOI] [PubMed] [Google Scholar]
- (110).McGuire JL, Larke LE, Sallee FR, Herman JP, Sah R. Differential Regulation of Neuropeptide Y in the Amygdala and Prefrontal Cortex during Recovery from Chronic Variable Stress. Front Behav Neurosci. 2011;5:54. doi: 10.3389/fnbeh.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).McGuire J, Herman JP, Horn PS, Sallee FR, Sah R. Enhanced fear recall and emotional arousal in rats recovering from chronic variable stress. Physiol Behav. 2010 Nov 2;101(4):474–482. doi: 10.1016/j.physbeh.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Suo L, Zhao L, Si J, Liu J, Zhu W, Chai B, et al. Predictable chronic mild stress in adolescence increases resilience in adulthood. Neuropsychopharmacology. 2013 Jul;38(8):1387–1400. doi: 10.1038/npp.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Bardi M, Rhone AP, Franssen CL, Hampton JE, Shea EA, Hyer MM, et al. Behavioral training and predisposed coping strategies interact to influence resilience in male Long-Evans rats: implications for depression. Stress. 2012 May;15(3):306–317. doi: 10.3109/10253890.2011.623739. [DOI] [PubMed] [Google Scholar]
- (114).Hawley DF, Bardi M, Everette AM, Higgins TJ, Tu KM, Kinsley CH, et al. Neurobiological constituents of active, passive, and variable coping strategies in rats: integration of regional brain neuropeptide Y levels and cardiovascular responses. Stress. 2010 Mar;13(2):172–183. doi: 10.3109/10253890903144621. [DOI] [PubMed] [Google Scholar]
- (115).Cohen H, Kozlovsky N, Matar MA, Zohar J, Kaplan Z. The characteristic long-term upregulation of hippocampal NF-kappaB complex in PTSD-like behavioral stress response is normalized by high-dose corticosterone and pyrrolidine dithiocarbamate administered immediately after exposure. Neuropsychopharmacology. 2011 Oct;36(11):2286–2302. doi: 10.1038/npp.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathe AA. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012 Jan;37(2):350–363. doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Cohen S, Vainer E, Matar MA, Kozlovsky N, Kaplan Z, Zohar J, et al. Diurnal fluctuations in HPA and neuropeptide Y-ergic systems underlie differences in vulnerability to traumatic stress responses at different zeitgeber times. Neuropsychopharmacology. 2015 Feb;40(3):774–790. doi: 10.1038/npp.2014.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Hoffman JR, Ostfeld I, Kaplan Z, Zohar J, Cohen H. Exercise Enhances the Behavioral Responses to Acute Stress in an Animal Model of PTSD. Med Sci Sports Exerc. 2015 Feb 19; doi: 10.1249/MSS.0000000000000642. [DOI] [PubMed] [Google Scholar]
- (119).Sweis BM, Veverka KK, Dhillon ES, Urban JH, Lucas LR. Individual differences in the effects of chronic stress on memory: behavioral and neurochemical correlates of resiliency. Neuroscience. 2013 Aug 29;246:142–159. doi: 10.1016/j.neuroscience.2013.04.052. [DOI] [PubMed] [Google Scholar]
- (120).Benedek DM. Posttraumatic stress disorder from Vietnam to today: the evolution of understanding during Eugene Brody's tenure at the journal of nervous and mental disease. J Nerv Ment Dis. 2011 Aug;199(8):544–552. doi: 10.1097/NMD.0b013e318225f0e9. [DOI] [PubMed] [Google Scholar]
- (121).Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney DS, Southwick SM. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000 Mar 15;47(6):526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- (122).Morgan CA, 3rd, Rasmusson AM, Winters B, Hauger RL, Morgan J, Hazlett G, et al. Trauma exposure rather than posttraumatic stress disorder is associated with reduced baseline plasma neuropeptide-Y levels. Biol Psychiatry. 2003 Nov 15;54(10):1087–1091. doi: 10.1016/s0006-3223(03)00433-5. [DOI] [PubMed] [Google Scholar]
- (123).Nishi D, Hashimoto K, Noguchi H, Matsuoka Y. Serum neuropeptide Y in accident survivors with depression or posttraumatic stress disorder. Neurosci Res. 2014 Jun;83:8–12. doi: 10.1016/j.neures.2014.03.009. [DOI] [PubMed] [Google Scholar]
- (124).Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006 Apr 1;59(7):660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- (125).Baker DG, Bertram TM, Patel PM, Barkauskas DA, Clopton P, Patel S, et al. Characterization of cerebrospinal fluid (CSF) and plasma NPY levels in normal volunteers over a 24-h timeframe. Psychoneuroendocrinology. 2013 Oct;38(10):2378–2382. doi: 10.1016/j.psyneuen.2013.04.020. [DOI] [PubMed] [Google Scholar]
- (126).Sah R, Ekhator NN, Strawn JR, Sallee FR, Baker DG, Horn PS, et al. Low cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol Psychiatry. 2009 Oct 1;66(7):705–707. doi: 10.1016/j.biopsych.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Sah R, Ekhator NN, Jefferson-Wilson L, Horn PS, Geracioti TD., Jr Cerebrospinal fluid neuropeptide Y in combat veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2014 Feb;40:277–283. doi: 10.1016/j.psyneuen.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Sandvik AM, Bartone PT, Hystad SW, Phillips TM, Thayer JF, Johnsen BH. Psychological hardiness predicts neuroimmunological responses to stress. Psychol Health Med. 2013;18(6):705–713. doi: 10.1080/13548506.2013.772304. [DOI] [PubMed] [Google Scholar]
- (129).Johnson DC, Thom NJ, Stanley EA, Haase L, Simmons AN, Shih PA, et al. Modifying resilience mechanisms in at-risk individuals: a controlled study of mindfulness training in Marines preparing for deployment. Am J Psychiatry. 2014 Aug;171(8):844–853. doi: 10.1176/appi.ajp.2014.13040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008 Apr 24;452(7190):997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Mickey BJ, Zhou Z, Heitzeg MM, Heinz E, Hodgkinson CA, Hsu DT, et al. Emotion processing, major depression, and functional genetic variation of neuropeptide Y. Arch Gen Psychiatry. 2011 Feb;68(2):158–166. doi: 10.1001/archgenpsychiatry.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Domschke K, Dannlowski U, Hohoff C, Ohrmann P, Bauer J, Kugel H, et al. Neuropeptide Y (NPY) gene: Impact on emotional processing and treatment response in anxious depression. Eur Neuropsychopharmacol. 2010 May;20(5):301–309. doi: 10.1016/j.euroneuro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- (133).Sommer WH, Lidstrom J, Sun H, Passer D, Eskay R, Parker SC, et al. Human NPY promoter variation rs16147:T>C as a moderator of prefrontal NPY gene expression and negative affect. Hum Mutat. 2010 Aug;31(8):E1594–608. doi: 10.1002/humu.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, et al. NPY moderates the relation between hurricane exposure and generalized anxiety disorder in an epidemiologic sample of hurricane-exposed adults. Depress Anxiety. 2010 Mar;27(3):270–275. doi: 10.1002/da.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Witt SH, Buchmann AF, Blomeyer D, Nieratschker V, Treutlein J, Esser G, et al. An interaction between a neuropeptide Y gene polymorphism and early adversity modulates endocrine stress responses. Psychoneuroendocrinology. 2011 Aug;36(7):1010–1020. doi: 10.1016/j.psyneuen.2010.12.015. [DOI] [PubMed] [Google Scholar]
- (136).Donner J, Sipila T, Ripatti S, Kananen L, Chen X, Kendler KS, et al. Support for involvement of glutamate decarboxylase 1 and neuropeptide Y in anxiety susceptibility. Am J Med Genet B Neuropsychiatr Genet. 2012 Apr;159B(3):316–327. doi: 10.1002/ajmg.b.32029. [DOI] [PubMed] [Google Scholar]
- (137).Lindell SG, Schwandt ML, Sun H, Sparenborg JD, Bjork K, Kasckow JW, et al. Functional NPY variation as a factor in stress resilience and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2010 Apr;67(4):423–431. doi: 10.1001/archgenpsychiatry.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).McGonigle P. Peptide therapeutics for CNS indications. Biochem Pharmacol. 2012 Mar 1;83(5):559–566. doi: 10.1016/j.bcp.2011.10.014. [DOI] [PubMed] [Google Scholar]
- (139).Chapman CD, Frey WH, 2nd, Craft S, Danielyan L, Hallschmid M, Schioth HB, et al. Intranasal treatment of central nervous system dysfunction in humans. Pharm Res. 2013 Oct;30(10):2475–2484. doi: 10.1007/s11095-012-0915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Serova LI, Tillinger A, Alaluf LG, Laukova M, Keegan K, Sabban EL. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience. 2013 Apr 16;236:298–312. doi: 10.1016/j.neuroscience.2013.01.040. [DOI] [PubMed] [Google Scholar]
- (141).Serova LI, Laukova M, Alaluf LG, Pucillo L, Sabban EL. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur Neuropsychopharmacol. 2014 Jan;24(1):142–147. doi: 10.1016/j.euroneuro.2013.11.007. [DOI] [PubMed] [Google Scholar]
- (142).Sabban EL, Serova LI, Alaluf LG, Laukova M, Peddu C. Comparative effects of intranasal neuropeptide Y and HS014 in preventing anxiety and depressive-like behavior elicited by single prolonged stress. Behav Brain Res. 2014 Dec 24; doi: 10.1016/j.bbr.2014.12.038. [DOI] [PubMed] [Google Scholar]
- (143).Laukova M, Alaluf LG, Serova LI, Arango V, Sabban EL. Early intervention with intranasal NPY prevents single prolonged stress-triggered impairments in hypothalamus and ventral hippocampus in male rats. Endocrinology. 2014 Oct;155(10):3920–3933. doi: 10.1210/en.2014-1192. [DOI] [PubMed] [Google Scholar]
- (144).Lacroix JS, Ricchetti AP, Morel D, Mossimann B, Waeber B, Grouzmann E. Intranasal administration of neuropeptide Y in man: systemic absorption and functional effects. Br J Pharmacol. 1996 Aug;118(8):2079–2084. doi: 10.1111/j.1476-5381.1996.tb15647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Hallschmid M, Benedict C, Born J, Fehm HL, Kern W. Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man. Physiol Behav. 2004 Oct 30;83(1):55–64. doi: 10.1016/j.physbeh.2004.07.023. [DOI] [PubMed] [Google Scholar]
- (146).Ricci S, Fuso A, Ippoliti F, Businaro R. Stress-induced cytokines and neuronal dysfunction in Alzheimer's disease. J Alzheimers Dis. 2012;28(1):11–24. doi: 10.3233/JAD-2011-110821. [DOI] [PubMed] [Google Scholar]
- (147).Djamshidian A, Lees AJ. Can stress trigger Parkinson's disease? J Neurol Neurosurg Psychiatry. 2014 Aug;85(8):878–881. doi: 10.1136/jnnp-2013-305911. [DOI] [PubMed] [Google Scholar]
- (148).Beal MF, Martin JB. Neuropeptides in neurological disease. Ann Neurol. 1986 Nov;20(5):547–565. doi: 10.1002/ana.410200502. [DOI] [PubMed] [Google Scholar]
- (149).Koide S, Onishi H, Hashimoto H, Kai T, Yamagami S. Plasma neuropeptide Y is reduced in patients with Alzheimer's disease. Neurosci Lett. 1995 Sep 29;198(2):149–151. doi: 10.1016/0304-3940(95)11973-z. [DOI] [PubMed] [Google Scholar]
- (150).Minthon L, Edvinsson L, Gustafson L. Correlation between clinical characteristics and cerebrospinal fluid neuropeptide Y levels in dementia of the Alzheimer type and frontotemporal dementia. Alzheimer Dis Assoc Disord. 1996 Winter;10(4):197–203. doi: 10.1097/00002093-199601040-00005. [DOI] [PubMed] [Google Scholar]
- (151).Chan-Palay V, Lang W, Allen YS, Haesler U, Polak JM. Cortical neurons immunoreactive with antisera against neuropeptide Y are altered in Alzheimer's-type dementia. J Comp Neurol. 1985 Aug 22;238(4):390–400. doi: 10.1002/cne.902380404. [DOI] [PubMed] [Google Scholar]
- (152).Ramos B, Baglietto-Vargas D, del Rio JC, Moreno-Gonzalez I, Santa-Maria C, Jimenez S, et al. Early neuropathology of somatostatin/NPY GABAergic cells in the hippocampus of a PS1xAPP transgenic model of Alzheimer's disease. Neurobiol Aging. 2006 Nov;27(11):1658–1672. doi: 10.1016/j.neurobiolaging.2005.09.022. [DOI] [PubMed] [Google Scholar]
- (153).Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, Previti ML, Gharkholonarehe N, et al. Progression of amyloid pathology to Alzheimer's disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J Neurosci. 2008 Feb 13;28(7):1537–1545. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Albuquerque MS, Mahar I, Davoli MA, Chabot JG, Mechawar N, Quirion R, et al. Regional and sub-regional differences in hippocampal GABAergic neuronal vulnerability in the TgCRND8 mouse model of Alzheimer's disease. Front Aging Neurosci. 2015 Mar 24;7:30. doi: 10.3389/fnagi.2015.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Rose JB, Crews L, Rockenstein E, Adame A, Mante M, Hersh LB, et al. Neuropeptide Y fragments derived from neprilysin processing are neuroprotective in a transgenic model of Alzheimer's disease. J Neurosci. 2009 Jan 28;29(4):1115–1125. doi: 10.1523/JNEUROSCI.4220-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Croce N, Dinallo V, Ricci V, Federici G, Caltagirone C, Bernardini S, et al. Neuroprotective effect of neuropeptide Y against beta-amyloid 25-35 toxicity in SH-SY5Y neuroblastoma cells is associated with increased neurotrophin production. Neurodegener Dis. 2011;8(5):300–309. doi: 10.1159/000323468. [DOI] [PubMed] [Google Scholar]
- (157).Croce N, Ciotti MT, Gelfo F, Cortelli S, Federici G, Caltagirone C, et al. Neuropeptide Y protects rat cortical neurons against beta-amyloid toxicity and re-establishes synthesis and release of nerve growth factor. ACS Chem Neurosci. 2012 Apr 18;3(4):312–318. doi: 10.1021/cn200127e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Croce N, Gelfo F, Ciotti MT, Federici G, Caltagirone C, Bernardini S, et al. NPY modulates miR-30a-5p and BDNF in opposite direction in an in vitro model of Alzheimer disease: a possible role in neuroprotection? Mol Cell Biochem. 2013 Apr;376(1–2):189–195. doi: 10.1007/s11010-013-1567-0. [DOI] [PubMed] [Google Scholar]
- (159).Angelucci F, Gelfo F, Fiore M, Croce N, Mathe AA, Bernardini S, et al. The effect of neuropeptide Y on cell survival and neurotrophin expression in in-vitro models of Alzheimer's disease. Can J Physiol Pharmacol. 2014 Aug;92(8):621–630. doi: 10.1139/cjpp-2014-0099. [DOI] [PubMed] [Google Scholar]
- (160).Rangani RJ, Upadhya MA, Nakhate KT, Kokare DM, Subhedar NK. Nicotine evoked improvement in learning and memory is mediated through NPY Y1 receptors in rat model of Alzheimer's disease. Peptides. 2012 Feb;33(2):317–328. doi: 10.1016/j.peptides.2012.01.004. [DOI] [PubMed] [Google Scholar]
- (161).Decressac M, Barker RA. Neuropeptide Y and its role in CNS disease and repair. Exp Neurol. 2012 Dec;238(2):265–272. doi: 10.1016/j.expneurol.2012.09.004. [DOI] [PubMed] [Google Scholar]
- (162).dos Santos VV, Santos DB, Lach G, Rodrigues AL, Farina M, De Lima TC, et al. Neuropeptide Y (NPY) prevents depressive-like behavior, spatial memory deficits and oxidative stress following amyloid-beta (Abeta(1-40)) administration in mice. Behav Brain Res. 2013 May 1;244:107–115. doi: 10.1016/j.bbr.2013.01.039. [DOI] [PubMed] [Google Scholar]
- (163).Kolsch H, Lutjohann D, Jessen F, Urbach H, von Bergmann K, Maier W, et al. Polymorphism in neuropeptide Y influences CSF cholesterol levels but is no major risk factor of Alzheimer's disease. J Neural Transm. 2006 Feb;113(2):231–238. doi: 10.1007/s00702-005-0319-z. [DOI] [PubMed] [Google Scholar]
- (164).Proto C, Romualdi D, Cento RM, Spada RS, Di Mento G, Ferri R, et al. Plasma levels of neuropeptides in Alzheimer's disease. Gynecol Endocrinol. 2006 Apr;22(4):213–218. doi: 10.1080/09513590500519385. [DOI] [PubMed] [Google Scholar]
- (165).Dawbarn D, De Quidt ME, Emson PC. Survival of basal ganglia neuropeptide Y-somatostatin neurones in Huntington's disease. Brain Res. 1985 Aug 12;340(2):251–260. doi: 10.1016/0006-8993(85)90921-7. [DOI] [PubMed] [Google Scholar]
- (166).Mazurek MF, Garside S, Beal MF. Cortical peptide changes in Huntington's disease may be independent of striatal degeneration. Ann Neurol. 1997 Apr;41(4):540–547. doi: 10.1002/ana.410410418. [DOI] [PubMed] [Google Scholar]
- (167).Curtis MA, Faull RL, Eriksson PS. The effect of neurodegenerative diseases on the subventricular zone. Nat Rev Neurosci. 2007 Sep;8(9):712–723. doi: 10.1038/nrn2216. [DOI] [PubMed] [Google Scholar]
- (168).Perez-Navarro E, Canals JM, Gines S, Alberch J. Cellular and molecular mechanisms involved in the selective vulnerability of striatal projection neurons in Huntington's disease. Histol Histopathol. 2006 Nov;21(11):1217–1232. doi: 10.14670/HH-21.1217. [DOI] [PubMed] [Google Scholar]
- (169).Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, Dragunow M, et al. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc Natl Acad Sci U S A. 2003 Jul 22;100(15):9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Zaben MJ, Gray WP. Neuropeptides and hippocampal neurogenesis. Neuropeptides. 2013 Dec;47(6):431–438. doi: 10.1016/j.npep.2013.10.002. [DOI] [PubMed] [Google Scholar]
- (171).Decressac M, Wright B, Tyers P, Gaillard A, Barker RA. Neuropeptide Y modifies the disease course in the R6/2 transgenic model of Huntington's disease. Exp Neurol. 2010 Nov;226(1):24–32. doi: 10.1016/j.expneurol.2010.07.022. [DOI] [PubMed] [Google Scholar]
- (172).Kloster E, Saft C, Akkad DA, Epplen JT, Arning L. Association of age at onset in Huntington disease with functional promoter variations in NPY and NPY2R. J Mol Med (Berl) 2014 Feb;92(2):177–184. doi: 10.1007/s00109-013-1092-3. [DOI] [PubMed] [Google Scholar]
- (173).Kerkerian L, Bosler O, Pelletier G, Nieoullon A. Striatal neuropeptide Y neurones are under the influence of the nigrostriatal dopaminergic pathway: immunohistochemical evidence. Neurosci Lett. 1986 May 6;66(1):106–112. doi: 10.1016/0304-3940(86)90174-6. [DOI] [PubMed] [Google Scholar]
- (174).Obuchowicz E, Antkiewicz-Michaluk L, Romanska I, Herman ZS. Increased striatal neuropeptide Y immunoreactivity and its modulation by deprenyl, clonidine and L-dopa in MPTP-treated mice. J Neural Transm. 2003 Dec;110(12):1375–1391. doi: 10.1007/s00702-003-0047-1. [DOI] [PubMed] [Google Scholar]
- (175).Ma Y, Zhan M, OuYang L, Li Y, Chen S, Wu J, et al. The effects of unilateral 6-OHDA lesion in medial forebrain bundle on the motor, cognitive dysfunctions and vulnerability of different striatal interneuron types in rats. Behav Brain Res. 2014 Jun 1;266:37–45. doi: 10.1016/j.bbr.2014.02.039. [DOI] [PubMed] [Google Scholar]
- (176).Cannizzaro C, Tel BC, Rose S, Zeng BY, Jenner P. Increased neuropeptide Y mRNA expression in striatum in Parkinson's disease. Brain Res Mol Brain Res. 2003 Feb 20;110(2):169–176. doi: 10.1016/s0169-328x(02)00555-7. [DOI] [PubMed] [Google Scholar]
- (177).Martignoni E, Blandini F, Petraglia F, Pacchetti C, Bono G, Nappi G. Cerebrospinal fluid norepinephrine, 3-methoxy-4-hydroxyphenylglycol and neuropeptide Y levels in Parkinson's disease, multiple system atrophy and dementia of the Alzheimer type. J Neural Transm Park Dis Dement Sect. 1992;4(3):191–205. doi: 10.1007/BF02260903. [DOI] [PubMed] [Google Scholar]
- (178).Decressac M, Pain S, Chabeauti PY, Frangeul L, Thiriet N, Herzog H, et al. Neuroprotection by neuropeptide Y in cell and animal models of Parkinson's disease. Neurobiol Aging. 2012 Sep;33(9):2125–2137. doi: 10.1016/j.neurobiolaging.2011.06.018. [DOI] [PubMed] [Google Scholar]
- (179).Escamilla-Sevilla F, Perez-Navarro MJ, Munoz-Pasadas M, Saez-Zea C, Jouma-Katati M, Piedrola-Maroto G, et al. Change of the melanocortin system caused by bilateral subthalamic nucleus stimulation in Parkinson's disease. Acta Neurol Scand. 2011 Oct;124(4):275–281. doi: 10.1111/j.1600-0404.2010.01469.x. [DOI] [PubMed] [Google Scholar]
- (180).Markaki E, Ellul J, Kefalopoulou Z, Trachani E, Theodoropoulou A, Kyriazopoulou V, et al. The role of ghrelin, neuropeptide Y and leptin peptides in weight gain after deep brain stimulation for Parkinson's disease. Stereotact Funct Neurosurg. 2012;90(2):104–112. doi: 10.1159/000335045. [DOI] [PubMed] [Google Scholar]