Abstract
Intestinal inflammation is a major health problem which impairs the quality of life, impacts mental health and is exacerbated by stress and psychiatric disturbances which, in turn, can affect disease prognosis and response to treatment. Accumulating evidence indicates that the immune system is an important interface between intestinal inflammation and the enteric, sensory, central and autonomic nervous systems. In addition, the neuroimmune interactions originating from the gastrointestinal tract are orchestrated by the gut microbiota. This article reviews some major insights into this complex homeostatic network that have been achieved during the past two years and attempts to put these advances into perspective with novel opportunities of pharmacological intervention.
Keywords: Cannabinoids, colitis, corticotropin-releasing factor, cytokines, enteric nervous system, gastrointestinal immune system, gut microbiota, inflammatory bowel disease, intestinal permeability, irritable bowel syndrome, mental health, neuropeptide Y, opioid peptides, pain, primary afferent neurons, stress, transient receptor potential ion channels, vagal antiinflammatory pathway, vagus nerve
Introduction
Inflammatory bowel disease (IBD) is a major health problem especially in the Western countries where its prevalence is more than 200 per 100,000 inhabitants [1]. The disease impairs the quality of life not only in terms of gastrointestinal symptoms, discomfort and pain but also in terms of impaired mental health. The deterioration of well-being is exacerbated in the presence of psychiatric disturbances [2], and several mental disorders including major depression, panic and generalized anxiety are more common in IBD patients than in community controls [3]. Moreover, psychiatric disorders can affect disease prognosis and response to treatment, given that infliximab has reduced efficacy in Crohns’ disease patients with major depression [4]. In addition, stressful life events are risk factors for IBD development, exacerbation and relapse [5].
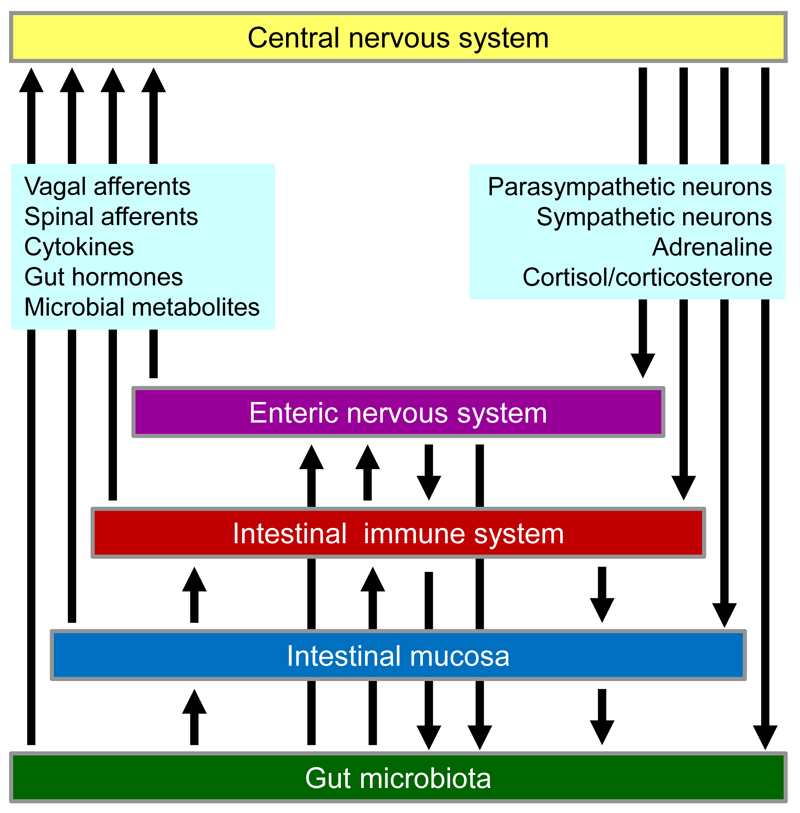
There is ample evidence that immune cells constitute an important interface between intestinal inflammatory processes and the nervous system. This is true for the interaction between inflammation and intrinsic enteric and extrinsic afferent neurons and between autonomic neurons and immune cells as exemplified by the vagal antiinflammatory pathway. These communication lines advocate an integrated view of gastrointestinal inflammation in which the gut microbiota, the intestinal mucosa, the local immune system and the enteric, sensory, central and autonomic nervous system play an interconnected role (Figure 1). For instance, stress impacts on the gut microbiota, and intestinal dysbiosis disturbs mucosal, immune and enteric nerve function. This article reviews major insights into this complex homeostatic network that have been made during the past two years, puts these advances into perspective with the physiology and pathophysiology of neuroimmune interactions and points to emerging opportunities of pharmacological intervention.
Figure 1.
Neuroimmune interactions involving the intestinal microbiota, epithelium and immune system, enteric, afferent (vagal and spinal), cerebral and autonomic (parasympathetic and sympathetic) neurons, as well as circulating microbial metabolites, gut hormones, cytokines, adrenaline and cortisol/corticosterone.
Neuroimmune interactions in the gut: impact of mucosal permeability, microbiota-neuronal and enteroendocrine-neuronal interactions
With the recognition of the intestinal microbiota as an important factor in gastrointestinal homeostasis the role of the intestinal barrier and its permeability in health and disease is increasingly appreciated [6]. Encompassing both structural and functional entities, the intestinal barrier prevents the invasion of microbes and toxins but at the same time is open to absorption of nutrients, ions and water [6]. Mucosal permeability is regulated by intrinsic and extrinsic factors including diet, microbiota composition, infection, hypoperfusion of the gut, drugs, toxins, stress and other lifestyle factors. Many maladies such as critical illness, IBD, irritable bowel syndrome (IBS), coeliac disease, food allergy, obesity, metabolic diseases and stress-related disorders are associated with enhanced intestinal permeability which facilitates translocation of luminal components, activation of the mucosal immune system and development of inflammation [6].
The relevance of the gut microbiota to the pathophysiology and treatment of IBD is dealt with in another article of this issue. However, neuroimmune interactions in the gut are seemingly impossible to consider without reference to the impact of the gut microbiota in health and disease [7–11]. Apart from being a target in the preventive and therapeutic treatment of gastrointestinal disorders, the gut microbiota also impacts drug pharmacokinetics and pharmacodynamics and produces compounds that may be seen as drugs in their own right. Targeted prebiotics, probiotics and synbiotics receive increasing attention as treatment modalities to correct the dysbiosis which may contribute to the disease [7,10].
As the implications of the gut microbiota unfold, interest focuses on the communication of the microbial community with intestinal epithelial, immune and neuronal cells [12] as is illustrated in Figure 2. It is particularly worth noting that several functional aspects of enteric neurons are altered in germ-free mice [13,14]. For instance, the input resistance and excitability of myenteric afterhyperpolarization neurons (intrinsic primary afferent neurons) is decreased in germ-free mice while the slow afterhyperpolarization following an action potential is prolonged, relative to what is seen in specific pathogen-free mice or germ-free mice that have been conventionalized with intestinal bacteria [13]. As the intrinsic primary afferent neurons signal to extrinsic primary afferent neurons via a junction involving nicotinic acetylcholine receptors [15,16], microbiota-related changes in enteric neuron function extend to extrinsic primary afferent neurons (Figure 2). Thus, excitation of vagal afferent nerve fibres in mesenteric nerve bundles by the potassium channel blocker TRAM-34 is virtually absent in germ-free mice [14]. These observations indicate that microbial factors drive the activity of both intrinsic and extrinsic primary afferent neurons. One such factor is polysaccharide A derived from Bacteroides fragilis which can activate sensory neurons of the myenteric plexus [17]. Components of Lactobacillus rhamnosus (JB-1) have a similar stimulant effect on vagal afferent neurons [15,17]. Such microbe-driven transmission processes are likely to participate in the vagus nerve-dependent effects of probiotics on brain function and behaviour [18,19].
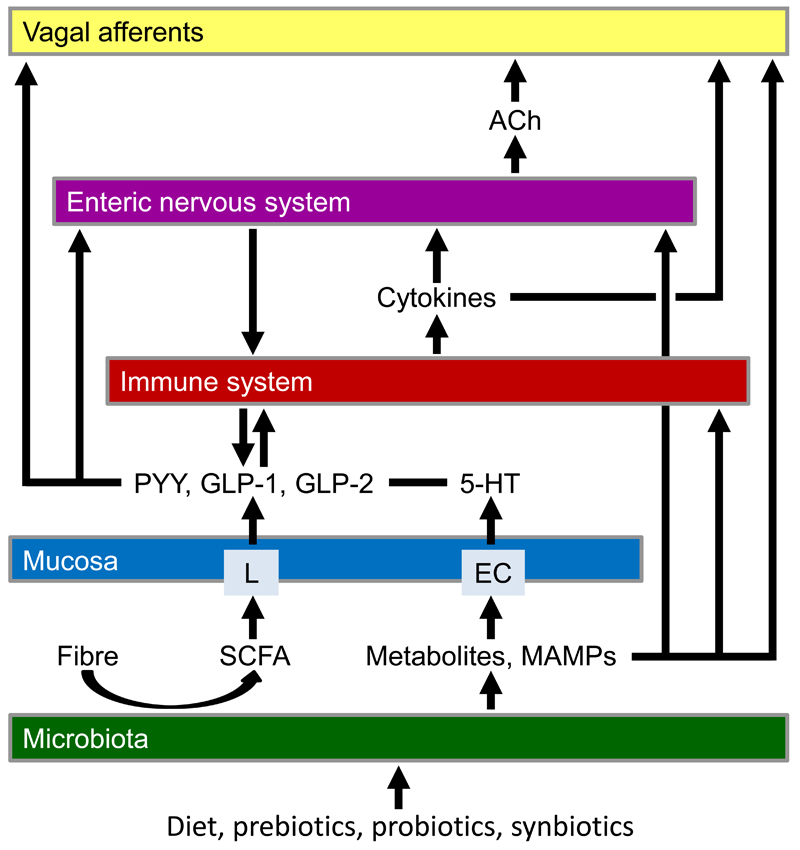
Figure 2.
Local neuroimmune interactions in the gut. The graph shows interactions between microbiota, mucosa, immune system, enteric nervous system and vagal afferent nerve endings in the gastrointestinal wall and some of the mediators involved. Abbreviations: ACh, acetylcholine; EC, enterochromaffin cells; GLP, glucagon-like peptide; 5-HT, 5-hydroxytryptamine; L, L cells; MAMPs, microbe-associated molecular patterns; PYY, peptide YY; SCFA, short chain fatty acids.
The transmission of luminal stimuli to immune and neuronal cells in the gut wall depends on specialized epithelial cells such as enteroendocrine cells. Endowed with a plethora of receptors, enteroendocrine cells have the capacity to operate as biosensors that communicate with other cells through release of gut hormones. For instance, metabolites produced by spore-forming bacteria of the gut microbiota promote host serotonin biosynthesis in enterochromaffin cells (Figure 2) and in this way influence gastrointestinal motility and platelet function [20]. Other bacterial metabolites such as short chain fatty acids trigger glucagon-like peptide-1 secretion from L cells in the lower gut, which in turn impacts gastrointestinal and metabolic functions [21]. In addition, enteroendocrine cells are capable of direct neurotransmission through specialized cell-nerve contacts termed neuropods where basic elements of neurotransmission such as pre-, post-, and transsynaptic proteins are expressed [22]. Gut hormones released from enteroendocrine cells may also impact on intestinal immune processes (Figure 2), given that proinflammatory prostaglandins acting via EP4 receptors enhance the release of glucagon-like peptide-1 and -2 as well as peptide YY (PYY) [23] and enteroendocrine cell activity is enhanced in Crohn’s disease affecting the small bowel [24].
Neuroimmune interactions in the gut: impact of Mas-related gene receptors, mast cells, opioids, nociceptin and cannabinoids
Mas-related gene (Mrg) receptors are G protein-coupled receptors that have been implicated in the regulation of inflammatory responses to non-immunological activation of mast cells, in mast cell-neuron communication and in nociception [25]. MrgE and MrgF are down-regulated in trinitrobenzene sulfonic acid (TNBS)-induced ileitis, whereas MrgA4, MrgB2 and MrgB8 are up-regulated in enteric sensory neurons and nerve fibres of the lamina propria. In addition, de novo expression of MrgB10 occurs in enteric sensory neurons and in newly recruited mucosal mast cells which are in close contact with nerve fibres in the lamina propria [25]. Neuroimmune interactions involving mast cells seem to contribute to IBS symptom generation both in children and adults [26]. Analysis of 21 paediatric patients (versus 10 healthy controls) has disclosed that the number of mast cells in close proximity to nerve fibres is significantly enhanced in the ileum and colon. Both the abdominal pain intensity and frequency correlates with the mast cell count in the ileum and right colon [26].
Opioid peptides occur in enteric neurons, epithelial cells and immune cells [27] and are thus likely to be involved in neuroimmune communication. Experimental colitis induced by dextran sulfate sodium (DSS) or by T cell transfer to immune-deficient mice leads to accumulation of colitogenic CD4+ T cells (Th1 and Th17 cells) in the inflamed intestine and expression of high levels of opioid peptides by these cells [28]. The local release of opioids from colitogenic CD4+ T cells reduces inflammation-associated visceral hypersensitivity as assessed by the visceromotor response to colorectal distension [28]. Abundant production of opioids by T cells during intestinal inflammation thus plays a homeostatic role in dampening inflammation-associated visceral pain. In addition, opioids may control the inflammatory process itself, given that a μ-opioid receptor agonist attenuates colitis-related disease activity and expression of myeloperoxidase, cytokines, caspases and nuclear factor-κB, whereas the antiapoptotic factor Bcl-xL is upregulated [29]. However, the antiinflammatory effect of μ-opioid receptor activation affects only the acute but not delayed phase of DSS-induced colitis [29].
Neuroimmune interactions also contribute to the adverse effects of opioid drugs in the gut such as opioid-induced constipation and the risk of gut-derived infections. Morphine can induce bacterial translocation from the ileum to murine mesenteric lymph nodes and liver, an effect that is attenuated in Toll-like receptor (TLR)-2 and TLR4 knockout mice [30]. Bacterial translocation is facilitated because morphine and TLR agonism disrupt tight junction protein organization in the ileum in a myosin light chain kinase-dependent fashion [30]. In addition, TLR4 is involved in the inhibitory effect of morphine on colonic peristalsis, which takes place because of opioid-induced disruption of enteric nerve activity [27]. Thus, the morphine-induced decrease in propulsive motor activity in the guinea-pig and murine colon is mitigated by the TLR4 antagonist TAK-242 [31].
Nociceptin receptors (NOPs) are expressed by gastrointestinal muscle cells, neurons and immune cells that infiltrate the mucosa. A beneficial role of NOPs in intestinal inflammation has been envisaged from a decrease of NOP mRNA in patients with IBD [32]. Indeed, the NOP agonist SCH 221510 is able to attenuate TNBS-induced murine colitis following peroral and intraperitoneal, but not intracolonic, administration of the compound [32]. In addition, SCH 221510 inhibits the pain response to acute intracolonic administration of mustard oil, which indicates that nociceptin possesses both antiinflammatory and antinociceptive activities.
Cannabinoids (CBs) constitute another class of antiinflammatory neuroimmune mediators. The fatty acid amide hydrolase inhibitor PF-3845, which blocks the breakdown of endocannabinoids, has been found to inhibit TNBS-induced colitis in mice along with alterations in the colonic levels of arachidonic and oleic acid derivatives and prostaglandins [33]. Intraperitoneal administration of the CB receptor agonist AM841 reduces both DSS- and TNBS-evoked colitis in mice when administered before or after induction of inflammation, an effect that is absent in CB1 and CB2 receptor knockout mice [34]. In contrast, the peripherally restricted CB receptor agonist CB13 does not alleviate colitis when given intraperitoneally but decreases inflammation after central administration [34]. Taken all data together, both peripheral and cerebral CB receptors appear to participate in the cannabinoid-mediated control of intestinal inflammation.
In a prospective placebo-controlled trial in 21 Crohn’s disease patients who did not respond to established therapy, treatment with a standardized Δ9-tetrahydrocannabinol preparation for 8 weeks led to complete remission in 5 of 11 subjects (compared with 1 of 10 in the placebo group), with no significant side effects [35]. A clinical response was observed in 10 of 11 subjects in the cannabis group and 4 of 10 in the placebo group [35]. Further controlled clinical trials of the efficacy and safety of CB therapy in IBD are also advocated by a retrospective survey of patients with IBD (n = 313) [36]. In the subjects using CBs to relieve IBD symptoms (17.6%), Cannabis was reported to reduce abdominal pain (83.9%), abdominal cramping (76.8%), joint pain (48.2%) and diarrhoea (28.6%), although side effects were frequent [36].
Neuroimmune interactions in the gut: impact of transient receptor potential channels
Approximately 20 of the 30 mammalian transient receptor potential (TRP) channel subunits are expressed by neuronal, epithelial and other cells within the alimentary canal. They participate in taste, chemaesthesis, mechanosensation and nociception and in the regulation of gastrointestinal motility, secretion, absorption, blood flow, and mucosal homeostasis [37]. Changes in TRP channel expression or function are associated with a variety of gastrointestinal diseases/disorders, notably gastro-oesophageal reflux disease, IBD, functional dyspepsia, IBS and cancer [37]. TRP channels are thus drug targets for the management of several gastrointestinal pathologies, TRP channels of the vanilloid (TRPV), ankyrin (TRPA) and melastatin (TRPM) subtypes being specifically associated with gastrointestinal inflammation.
Inflammation leads to upregulation of TRPV1 and TRPA1 in the sensory innervation of the gut, whereas blockade, downregulation or knockout of these TRP channels reduces or prevents inflammation-associated hypersensitivity and pain [37]. TRPA1 also acts as a sensor of enterochromaffin cells [38] and operates as secondary transducer of proinflammatory mediators such as bradykinin and protease-activated receptor-2 agonists [37]. Combined activation of TRPV1 and TRPA1 synergizes in inflammation-associated disturbances of the central nervous system, given that the increase in the visceromotor response to colorectal distension associated with TNBS-evoked colitis is inhibited by combined TRPV1 and TRPA1 blockade to a larger extent than by blockade of either channel alone [39]. TRPV4 is another proinflammatory and pronociceptive TRP channel, activation of which increases mucosal permeability and induces colitis. This property, the activation of TRPV4 by proinflammatory mediators and inflammation-evoked upregulation of TRPV4 recommends the channel for therapeutic exploitation in IBD [40].
TRPM8 expression is likewise upregulated in both human and murine colitis [41]. TRPM8 knockout mice are hypersusceptible to DSS-induced colitis, and CD11c-positive dendritic cells from TRPM8 knockout mice are hyperresponsive to TLR stimulation [42]. A similar phenotype is found in calcitonin gene-related peptide (CGRP) receptor-deficient mice, which points to a functional link between TRPM8 and CGRP [42]. CGRP is upregulated in TRPM8 knockout mice, and administration of exogenous CGRP to TRPM8 knockout mice reverses their hyperinflammatory phenotype [42]. In addition, TRPM8 stimulation counteracts the TNBS-evoked cytokine and chemokine formation and inflammation in the murine colon. TRPM8 signalling via CGRP [42] thus appears to be an innate antiinflammatory mechanism that represents a novel therapeutic target in colitis [41]. The endogenous activators of TRM8 in colitis remain to be identified; potential candidates include phosphatidylinositol 4,5-bisphosphate, endovannilloids, endocannabinoids and phospholipase A2-derived lysophospholipids [41].
Neuroimmune communication from the inflamed gut to the brain: impact of blood-brain barrier, cytokines and vagal afferents
Neuroimmune interactions impact not only the gastrointestinal tract but also the bidirectional communication between the gut and brain and the cross-talk of the gut microbiota with the immune, endocrine and nervous system [8,11,43]. The microbiota-gut-immune-brain communication network (Figure 1) uses 5 information carriers from the gut to the brain (vagal and spinal afferent neurons; immune mediators; gut hormones; gut microbiota-derived molecules) and 4 information carriers from the central nervous system to the gut (sympathetic and parasympathetic efferent neurons; neuroendocrine factors involving the adrenal medulla and cortex) [8]. Additional factors include the blood-brain barrier (BBB) and the processing of peripheral information in distinct brain circuits.
Recent work has shown that the BBB is under the regulatory influence of the gut microbiota, given that BBB permeability is increased in germ-free mice from intrauterine life to adulthood and associated with reduced expression of the BBB tight junction proteins occludin and claudin-5 [44]. Exposure of germ-free mice to pathogen-free gut microbiota normalizes BBB permeability and upregulates expression of tight junction proteins [44]. Since the BBB is essential for brain development, function and homeostasis, the control of BBB permeability seems to be a particular mechanism whereby the gut microbiota controls brain activity and behaviour. Circulating bacterial metabolites, microbe-associated molecular patterns (MAMPs) and cytokines are likely to act as humoural messengers at BBB-dependent and BBB-independent blood-brain interfaces.
Circumventricular organs (e.g., area postrema and subfornical organ) lie outside the BBB, contain neurons and immune cells in close proximity and allow for a direct communication between circulating factors and brain structures [45]. In contrast, communication via the BBB is tightly controlled by receptors and transport mechanisms which regulate the responsiveness of the BBB to immunomodulatory molecules (e.g., cytokines, chemokines, prostaglandins) and the exchange of chemokines, cytokines and even immune cells across the BBB [46]. Peripheral immune activation and cytokine signalling across the BBB evokes a neuroinflammatory reaction in the central nervous system that contributes to the pain, hyperalgesia and behavioural disturbances associated with peripheral inflammation. For instance, peripheral injection of the TLR4 agonist lipopolysaccharide (LPS) is associated with increased expression of proinflammatory cytokines in the periphery and brain [45,47,48]. The peripheral and cerebral immune and sickness response to LPS is markedly boosted if the immune system is primed by nucleotide-binding and oligomerization domain (NOD)-1 or NOD2 agonists [48]. In addition, peripheral leukocytes can exacerbate brain damage by release of cytotoxic mediators (e.g., hypochlorous acid formed via the myeloperoxidase system) that disrupt BBB function [49].
MAMPs and cytokines can sensitize and/or stimulate vagal and spinal afferent neurons and thus contribute to a rapid propagation of immune signals to the brain [47,50–53]. Vagal afferents are particularly sensitive to immune as well as microbial factors produced, for instance, by Bacteroides fragilis and Lactobacillus rhamnosus (JB1) [15,17]. For instance, tumour necrosis factor-α and LPS are capable of directly activating vagal afferent neurons in culture [50]. In addition, LPS can stimulate sensory neurons via activation of TRPA1 [51] and sensitize afferent fibres in mesenteric nerves to serotonin, bradykinin and gut distension, an effect in which mast cells and cyclooxygenase-2 play a role [52]. Spinal afferent neurons supplying the colon are also responsive to proinflammatory cytokines such as tumour necrosis factor-α and interleukin-1β, which may have a bearing on the pathogenesis of IBS [53]. Thus, the mechanical hypersensitivity of mouse colonic nerve fibres evoked by peripheral blood mononuclear cell supernatants of IBS patients is reduced by infliximab and that evoked by tumour necrosis factor-α is inhibited by a TRPA1 blocker [53]. Sensitization and other long-term alterations in brain circuitry and activity are ultimately responsible for the hyperalgesia, pain and disturbances of mental health that occur in IBD and IBS, a condition that entails low-grade inflammation or IBD in remission [43].
Neuroimmune communication from the brain to the gut: stress and gut inflammation
Stress can cause disease exacerbation and relapses in IBD and IBS patients, a relationship that has been confirmed in most experimental models of intestinal inflammation [54]. Both physical and psychological stressors cause formation of proinflammatory cytokines in the periphery [55], which may be due to ‘sterile inflammation’ [56]. In this phenomenon, stress releases damage-associated molecular pattern molecules such as heat shock protein-72, uric acid and ATP which in turn activate various pattern recognition receptors (TLRs, NODs) that stimulate cytokine production [56]. Importantly, there are pre-existing individual differences in the sensitivity of the peripheral immune system that predict and promote vulnerability to social stress [57]. Of the cytokines regulated by stress, interleukin (IL)-6 is most highly upregulated in mice that respond to chronic stress with exaggerated social avoidance behavior [57]. A similar rise of serum IL-6 is seen in patients with treatment-resistant major depression [57]. Individual differences in IL-6 levels from ex vivo stimulated murine leukocytes also predict susceptibility versus resilience to a social stressor. These pre-existing differences in stress-responsive IL-6 release have been confirmed by genetic experiments in which IL-6 knockout mice as well as mice treated with an IL-6 monoclonal antibody turned out to be resilient to social stress [57].
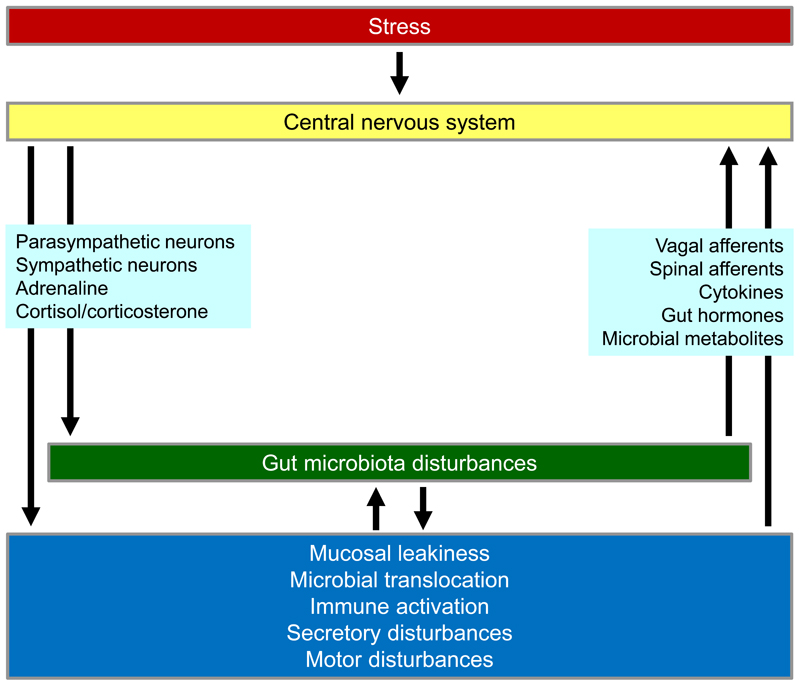
The hypothalamic-pituitary-adrenal (HPA) axis is an important interface between stress and the gut, with corticotropin-releasing factor (CRF) as a mediator operating both in the brain and gut [58]. Glucocorticoids released from the adrenal cortex (cortisol in humans, corticosterone in rodents) dampen immune processes and are likely to interfere with neuroimmune processes during stress. CRF may contribute to the stress-induced exacerbation of IBS symptoms, given that two experimental stressors (hand immersion in ice water or administration of CRF) constrict the small bowel in healthy volunteers, mimicking the effect of stress seen in patients with diarrhoea-predominant IBS [59]. In the gut, stress leads to disruption of mucosal tight junctions, which enhances mucosal permeability, facilitates microbial translocation, induces an immune response and promotes inflammation (Figure 3). In addition, stress disrupts the community structure of the gut microbiota [60], which also contributes to gut leakiness. Intestinal decontamination by oral antibiotics blocks stress-induced bacterial translocation and, consequently, TLR activation and neuroinflammation in the brain [61]. Conversely, enforcement of the gut microbiota structure with a probiotic counteracts stress-induced gut leakiness [62] and reduces stress-induced activation of brain neurons and stress-induced depression of hippocampal neurogenesis [63]. These findings emphasize that gut leakiness is an important factor in the negative impact of stress on gut, immune and brain function.
Figure 3.
Effect of stress on neuroimmune interactions in the brain and gut.
Experimental studies demonstrate that gastrointestinal inflammation alters the cerebral processing of stress. If mice with DSS-induced colitis are exposed to acute water avoidance stress (WAS), they present with prolonged immobility during the WAS session, which is associated with brain region-dependent alterations in neuropeptide Y (NPY), CRF and brain-derived neurotrophic factor [64]. Furthermore, the combination of colitis and stress increases IL-6 and growth regulated oncogene-α levels in the brain [64]. Experimental studies also indicate that the interaction between stress and gastrointestinal inflammation depends on context. For instance, environmental enrichment (EE) has been found to aggravate experimentally induced colitis, to enhance the WAS-induced activation of the dentate gyrus (visualized by expression of c-Fos) and to unmask an inhibitory effect of colitis on WAS-evoked c-Fos expression in the hippocampus [65]. Conversely, EE inhibits the WAS-evoked activation of the central amygdala and infralimbic cortex and attenuates the inhibitory effect of colitis on WAS-evoked c-Fos expression in these regions [65]. These data demonstrate that EE has a region-specific effect on stress-induced c-Fos expression in the corticolimbic system, which is likely to improve stress resilience [65].
Despite the seemingly adverse impact of stress on the gut, immune system and brain, this relationship cannot be generalized because psychological stress may per se provoke mild spontaneous colitis, increase or decrease the severity of chemically induced colitis, or be devoid of any effect [54]. Predictable chronic WAS, for instance, fails to modify the severity of DSS-evoked colitis in the mouse but prevents colitis-evoked anxiety and disruption of social interaction [66]. This effect of repeated WAS is associated with a rise of circulating corticosterone and an increase in hypothalamic NPY expression, which may underlie the resilience effect of predictable chronic stress [66]. In another study WAS and TNBS-evoked colitis have been found to induce short-term, but not long-term, visceral hypersensitivity and HPA axis activation without any additive interaction [67].
Neuropeptides such as CRF and NPY have proved to be important mediators of stress-related neuroimmune interactions because both peptides occur at multiple sites in the gut and brain and affect multiple functions in both organ systems [58,68]. CRF, for instance, participates in the stress-evoked inhibition of upper gastrointestinal transit and stimulation of colonic motility [58]. NPY serves a proinflammatory role in the gut, while cerebral NPY protects against distinct disturbances in response to immune challenge, enforcing stress resilience both in the brain and periphery [68,69]. During restraint stress, faecal pellet output is significantly increased in mice deficient in NPY or the gut hormone PYY, relative to wildtype mice [69,70]. CRF1 receptor blockade reduces defaecation in wild-type and NPY knockout mice but has no effect in PYY knockout mice [69]. Endogenous NPY and PYY thus appear to inhibit the colonic motor stimulation evoked by stress, the effect of NPY depending on endogenous CRF acting via CRF1 receptors [69].
Neuroimmune communication in the autonomic nervous system: the vagal antiinflammatory pathway
Vagal afferent neurons make a significant contribution to the communication between the peripheral immune system and the brain. Cytokine-stimulated vagal afferents are thought to mediate a vago-vagal antiinflammatory reflex which via cholinergic vagal efferents, release of acetylcholine and activation of α7 subunit-containing nicotinic acetylcholine receptors (α7nAChR) on macrophages and other immune cells inhibits inflammation within the gut [71]. The vagal antiinflammatory reflex is thus a particular example of the role of the central nervous system in modulating the peripheral immune system and consequently inflammation. This neuroimmune interaction offers new therapeutic opportunities to treat intestinal inflammation and improve postoperative ileus as is evident from the beneficial effects of electrical stimulation of the vagus nerve [71].
The vagal antiinflammatory reflex can be elicited by immune, surgical or electrical stimulation of vagal afferents. Inoculation of mice with either Campylobacter jejuni or Salmonella typhimurium has been found to signal to the nucleus of the solitary tract, an effect that seems to be due to excitation of capsaicin-sensitive vagal afferents by proinflammatory mediators formed in response to bacterial infection [50]. Electrical stimulation of vagal afferents has been reported to attenuate surgery-induced intestinal inflammation and to improve postoperative intestinal transit in a spleen- and T cell-independent manner, whereas α7nAChR knockout or deficiency abolishes the antiinflammatory response [72]. Anterograde labelling has shown that vagal efferents do not contact resident macrophages but that cholinergic myenteric neurons form close contacts with resident macrophages expressing α7nAChR [72]. It thus seems that cholinergic myenteric neurons constitute the postganglionic part of the efferent reflex arm and that resident macrophages in the intestinal muscularis are the ultimate target whereby vagal nerve stimulation blunts postoperative inflammation [72]. This antiinflammatory reflex seems to be called into operation whenever there is ongoing inflammation in the gut to dampen the local immune response in a homeostatic manner. In keeping with this contention, surgical lesion of the vagal branch that innervates the small intestine aggravates the surgery-induced delay of intestinal transit, causes an influx of leukocytes and increases pro-inflammatory cytokine expression in the gut muscularis [73].
Conclusions
The information reviewed here attests to multiple neuroimmune interactions in intestinal inflammation which are not restricted to the digestive tract but also affect remote organs such as the brain. It has become obvious that the immune system serves as important interface between the inflammatory process and the multiple nervous systems that have an impact on gastrointestinal physiology and pathophysiology: enteric, extrinsic sensory, central and autonomic neurons. The emerging influence of the gut microbiota in health and disease has added a further dimension of complexity to the neuroimmune network operating in gut inflammation. Microbial metabolites, gut hormones, immune mediators and neuropeptides turn out to be important factors governing neuroimmune interactions in intestinal inflammation. These factors and their receptors pose numerous opportunities for therapeutic intervention. Mast cell mediators, cannabinoids, opioids, NPY, PYY, CRF and TRP channels such as TRPA1, TRPV1, TRPV4 and TRPM8 are among those entities that have been specifically addressed in the past years.
Intestinal inflammation can severely impair the quality of life and negatively impact mental health. Conversely, gut inflammation can be exacerbated by adverse life experiences such as stress as well as psychiatric disturbances which, in turn, can aggravate disease prognosis and response to treatment. Analysis of the underlying mechanisms reveals that both circulating molecules and sensory neurons transmit information from the gut to brain to modify brain function and behaviour. The impact of stress on gut function illustrates how the central and autonomic nervous system as well as neuroendocrine messages modify intestinal inflammation. Stress also disrupts the microbial community in the gut, which in turn enhances mucosal permeability and disturbs neuroimmune interactions in the intestine. Dissection of these reciprocal interrelationships between microbiota, immune and nervous system unfolds a homeostatic network that offers unprecedented opportunities for pharmacological intervention.
Highlights.
The gut microbiota governs intestinal permeability and neuroimmune interactions.
TRPM8, cannabinoids, opioids, NPY and CRF are neuroimmune mediators in the gut.
Gut inflammation impacts brain function via immune mediators and vagal afferents.
Stress affects multiple neuroimmune interactions in the gut and brain.
Ongoing intestinal inflammation activates a vagal antiinflammatory pathway.
Acknowledgements
Work in the author’s laboratory was supported by the Austrian Science Funds (FWF grants P23097-B18, P25912-B23 and W1241-B18).
References
- 1.Cosnes J, Gower–Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794.e4. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 2.Engelmann G, Erhard D, Petersen M, Parzer P, Schlarb AA, Resch F, Brunner R, Hoffmann GF, Lenhartz H, Richterich A. Health-related quality of life in adolescents with inflammatory bowel disease depends on disease activity and psychiatric comorbidity. Child Psychiatry Hum Dev. 2015;46:300–307. doi: 10.1007/s10578-014-0471-5. [DOI] [PubMed] [Google Scholar]
- 3.Filipovic BR, Filipovic BF. Psychiatric comorbidity in the treatment of patients with inflammatory bowel disease. World J Gastroenterol. 2014;20:3552–3563. doi: 10.3748/wjg.v20.i13.3552. [Review focussing on neural and non-neural mechanisms of acid sensing throughout the gastrointestinal tract, with emphasis on the roles played by acid-sensitive ion channels such as TRPV1 and ASIC3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Persoons P, Vermeire S, Demyttenaere K, Fischler B, Vandenberghe J, Van Oudenhove L, Pierik M, Hlavaty T, Van Assche G, Noman M. The impact of major depressive disorder on the short-and long-term outcome of Crohn's disease treatment with infliximab. Aliment Pharmacol Ther. 2005;22:101–110. doi: 10.1111/j.1365-2036.2005.02535.x. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein CN. Treatment of IBD: where we are and where we are going. Am J Gastroenterol. 2015;110:114–126. doi: 10.1038/ajg.2014.357. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability - a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [Comprehensive review of the intestinal barrier, its functional state in terms of mucosal permeability and its role in health and disease] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saulnier DM, Ringel Y, Heyman MB, Foster JA, Bercik P, Shulman RJ, Versalovic J, Verdu EF, Dinan TG, Hecht G, Guarner F. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes. 2013;4:17–27. doi: 10.4161/gmic.22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzer P, Farzi A. Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol. 2014;817:195–219. doi: 10.1007/978-1-4939-0897-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014;13:69–86. doi: 10.1111/gbb.12109. [A review of the behavioural phenotypes associated with manipulations of the gut microbiota] [DOI] [PubMed] [Google Scholar]
- 10.Cammarota G, Ianiro G, Cianci R, Bibbò S, Gasbarrini A, Currò D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: Potential for therapy. Pharmacol Ther. 2015;149:191–212. doi: 10.1016/j.pharmthera.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Nedawi K, Mian MF, Hossain N, Karimi K, Mao YK, Forsythe P, Min KK, Stanisz AM, Kunze WA, Bienenstock J. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J. 2015;29:684–695. doi: 10.1096/fj.14-259721. [Analysis of some microbial metabolites that may be relevant to the communication of gut microbiota to intestinal immune cells and neurons] [DOI] [PubMed] [Google Scholar]
- 13.McVey Neufeld KA, Mao YK, Bienenstock J, Foster JA, Kunze WA. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil. 2013;25:183–e88. doi: 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- 14.McVey Neufeld KA, Perez-Burgos A, Mao YK, Bienenstock J, Kunze WA. The gut microbiome restores intrinsic and extrinsic nerve function in germ-free mice accompanied by changes in calbindin. Neurogastroenterol Motil. 2015;27:627–636. doi: 10.1111/nmo.12534. [The excitability of intrinsic and extrinsic primary afferent neurons of the gut is grossly reduced in germ-free mice but restored by restoration of the gut microbiota] [DOI] [PubMed] [Google Scholar]
- 15.Perez-Burgos A, Wang B, Mao YK, Mistry B, McVey Neufeld KA, Bienenstock J, Kunze WA. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am J Physiol. 2013;304:G211–G220. doi: 10.1152/ajpgi.00128.2012. [Metabolites of the probiotic Lactobacillus rhamnosus (JB-1) are able to excite vagal sensory neurons] [DOI] [PubMed] [Google Scholar]
- 16.Perez-Burgos A, Mao YK, Bienenstock J, Kunze WA. The gut-brain axis rewired: adding a functional vagal nicotinic “sensory synapse”. FASEB J. 2014;28:3064–3074. doi: 10.1096/fj.13-245282. [DOI] [PubMed] [Google Scholar]
- 17.Mao YK, Kasper DL, Wang B, Forsythe P, Bienenstock J, Kunze WA. Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat Commun. 2013;4:1465. doi: 10.1038/ncomms2478. [The study data attest to the ability of a particular bacterial metabolite to increase the activity of sensory neurons in the intestine] [DOI] [PubMed] [Google Scholar]
- 18.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everard A, Cani PD. Gut microbiota and GLP-1. Rev Endocr Metab Disord. 2014;15:189–196. doi: 10.1007/s11154-014-9288-6. [DOI] [PubMed] [Google Scholar]
- 22.Bohórquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, Liddle RA. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015;125:782–786. doi: 10.1172/JCI78361. [Enteroendocrine cells possess neuropods which are specialized in chemical transmission to adjacent neurons] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coskun T, O'Farrell LS, Syed SK, Briere DA, Beavers LS, Dubois SL, Michael MD, Franciskovich JB, Barrett DG, Efanov AM. Activation of prostaglandin E receptor 4 triggers secretion of gut hormone peptides GLP-1, GLP-2, and PYY. Endocrinology. 2013;154:45–53. doi: 10.1210/en.2012-1446. [DOI] [PubMed] [Google Scholar]
- 24.Moran GW, Leslie FC, McLaughlin JT. Crohn's disease affecting the small bowel is associated with reduced appetite and elevated levels of circulating gut peptides. Clin Nutr. 2013;32:404–411. doi: 10.1016/j.clnu.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 25.Avula LR, Buckinx R, Favoreel H, Cox E, Adriaensen D, Van Nassauw L, Timmermans JP. Expression and distribution patterns of Mas-related gene receptor subtypes A-H in the mouse intestine: inflammation-induced changes. Histochem Cell Biol. 2013;139:639–658. doi: 10.1007/s00418-013-1086-9. [Mas-related gene receptors represent a subfamily of G protein-coupled receptors with a putative role in neuroimmune interactions, given that their expression is altered in intestinal inflammation] [DOI] [PubMed] [Google Scholar]
- 26.Di Nardo G, Barbara G, Cucchiara S, Cremon C, Shulman RJ, Isoldi S, Zecchi L, Drago L, Oliva S, Saulle R, et al. Neuroimmune interactions at different intestinal sites are related to abdominal pain symptoms in children with IBS. Neurogastroenterol Motil. 2014;26:196–204. doi: 10.1111/nmo.12250. [The number of mucosal mast cells in close proximity to nerve fibres is elevated in IBS patients and correlates with the abdominal pain intensity score and the frequency of abdominal pain episodes] [DOI] [PubMed] [Google Scholar]
- 27.Holzer P. Pharmacology of opioids and their effects on gastrointestinal function. Am J Gastroenterol Suppl. 2014;2:9–16. [Google Scholar]
- 28.Boué J, Basso L, Cenac N, Blanpied C, Rolli-Derkinderen M, Neunlist M, Vergnolle N, Dietrich G. Endogenous regulation of visceral pain via production of opioids by colitogenic CD4+ T cells in mice. Gastroenterology. 2014;146:166–175. doi: 10.1053/j.gastro.2013.09.020. [Abundant production of opioids by T cells during intestinal inflammation plays a homeostatic role in dampening inflammation-associated visceral pain] [DOI] [PubMed] [Google Scholar]
- 29.Anselmi L, Huynh J, Duraffourd C, Jaramillo I, Vegezzi G, Saccani F, Boschetti E, Brecha NC, De Giorgio R, Sternini C. Activation of μ opioid receptors modulates inflammation in acute experimental colitis. Neurogastroenterol Motil. 2015;27:509–523. doi: 10.1111/nmo.12521. [A μ-opioid receptor agonist reduces the acute, but not chronic, phase of chemically induced colitis in the mouse] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng J, Yu H, Ma J, Wang J, Banerjee S, Charboneau R, Barke RA, Roy S. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One. 2013;8:e54040. doi: 10.1371/journal.pone.0054040. [Morphine induces bacterial translocation from the ileum to murine mesenteric lymph nodes and liver, an effect that is attenuated in Toll-like receptor (TLR)-2 and TLR4 knockout mice] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farzi A, Halicka J, Mayerhofer R, Fröhlich EE, Tatzl E, Holzer P. Toll-like receptor 4 contributes to the inhibitory effect of morphine on colonic motility in vitro and in vivo. Sci Rep. 2015;5:9499. doi: 10.1038/srep09499. [The TLR4 antagonist TAK-242 reverses the morphine-induced inhibition of peristalsis in the guinea-pig and murine colon but does not alter morphine-induced motor inhibition in the small intestine] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobczak M, Mokrowiecka A, Cygankiewicz AI, Zakrzewski PK, Sałaga M, Storr M, Kordek R, Małecka-Panas E, Krajewska WM, Fichna J. Anti-inflammatory and antinociceptive action of an orally available nociceptin receptor agonist SCH 221510 in a mouse model of inflammatory bowel diseases. J Pharmacol Exp Ther. 2014;348:401–409. doi: 10.1124/jpet.113.209825. [DOI] [PubMed] [Google Scholar]
- 33.Sałaga M, Mokrowiecka A, Zakrzewski PK, Cygankiewicz A, Leishman E, Sobczak M, Zatorski H, Małecka-Panas E, Kordek R, Storr M, et al. Experimental colitis in mice is attenuated by changes in the levels of endocannabinoid metabolites induced by selective inhibition of fatty acid amide hydrolase (FAAH) J Crohns Colitis. 2014;8:998–1009. doi: 10.1016/j.crohns.2014.01.025. [The FAAH inhibitor PF-3845, which blocks the breakdown of endocannabinoids, attenuates chemically induced colitis in mice, an effect that is associated with alterations in the colonic levels of various biolipids] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fichna J, Bawa M, Thakur GA, Tichkule R, Makriyannis A, McCafferty DM, Sharkey KA, Storr M. Cannabinoids alleviate experimentally induced intestinal inflammation by acting at central and peripheral receptors. PLoS One. 2014;9:e109115. doi: 10.1371/journal.pone.0109115. [The cannabinoid receptor agonist AM841 reduces chemically evoked colitis in mice when administered before or after induction of inflammation] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naftali T, Bar-Lev Schleider L, Dotan I, Lansky EP, Sklerovsky Benjaminov F, Konikoff FM. Cannabis induces a clinical response in patients with Crohn's disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11:1276–1280. e1. doi: 10.1016/j.cgh.2013.04.034. [In a prospective placebo-controlled trial, treatment with a standardized Δ9-tetrahydrocannabinol preparation for 8 weeks improved the disease core or led to complete remission of Crohn’s disease] [DOI] [PubMed] [Google Scholar]
- 36.Storr M, Devlin S, Kaplan GG, Panaccione R, Andrews CN. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn's disease. Inflamm Bowel Dis. 2014;20:472–480. doi: 10.1097/01.MIB.0000440982.79036.d6. [DOI] [PubMed] [Google Scholar]
- 37.Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol Ther. 2011;131:142–170. doi: 10.1016/j.pharmthera.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulze A, Hartung P, Schaefer M, Hill K. Transient receptor potential ankyrin 1 (TRPA1) channel activation by the thienopyridine-type drugs ticlopidine, clopidogrel, and prasugrel. Cell Calcium. 2014;55:200–207. doi: 10.1016/j.ceca.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Vermeulen W, De Man JG, De Schepper HU, Bult H, Moreels TG, Pelckmans PA, De Winter BY. Role of TRPV1 and TRPA1 in visceral hypersensitivity to colorectal distension during experimental colitis in rats. Eur J Pharmacol. 2013;698:404–412. doi: 10.1016/j.ejphar.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 40.Vergnolle N. TRPV4: new therapeutic target for inflammatory bowel diseases. Biochem Pharmacol. 2014;89:157–161. doi: 10.1016/j.bcp.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Ramachandran R, Hyun E, Zhao L, Lapointe TK, Chapman K, Hirota CL, Ghosh S, McKemy DD, Vergnolle N, Beck PL, et al. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc Natl Acad Sci USA. 2013;110:7476–7481. doi: 10.1073/pnas.1217431110. [TRPM8 expression is up-regulated in both human and murine samples of inflamed colon, and TRPM8 activation counteracts the proinflammatory effect of TRPV1 activation] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Jong PR, Takahashi N, Peiris M, Bertin S, Lee J, Gareau MG, Paniagua A, Harris AR, Herdman DS, Corr M, et al. TRPM8 on mucosal sensory nerves regulates colitogenic responses by innate immune cells via CGRP. Mucosal Immunol. 2015;8:491–504. doi: 10.1038/mi.2014.82. [TRPM8 knockout mice are hypersusceptible to chemically induced colitis, a similar phenotype being found in calcitonin gene-related peptide (CGRP) receptor-deficient mice. Exogenous CGRP reverses the hyper-inflammatory phenotype of TRPM8 knockout mice] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vermeulen W, De Man JG, Pelckmans PA, De Winter BY. Neuroanatomy of lower gastrointestinal pain disorders. World J Gastroenterol. 2014;20:1005–1020. doi: 10.3748/wjg.v20.i4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Guan NL, Kundu P, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [Blood-brain barrier permeability is increased in germ-free mice, beginning with intrauterine life and maintained throughout adult life] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCusker RH, Kelley KW. Immune-neural connections: how the immune system's response to infectious agents influences behaviour. J Exp Biol. 2013;216:84–98. doi: 10.1242/jeb.073411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banks WA. The blood-brain barrier in neuroimmunology: Tales of separation and assimilation. Brain Behav Immun. 2015;44:1–8. doi: 10.1016/j.bbi.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farzi A, Reichmann F, Meinitzer A, Mayerhofer R, Jain P, Hassan AM, Fröhlich EE, Wagner K, Painsipp E, Rinner B, et al. Synergistic effects of NOD1 or NOD2 and TLR4 activation on mouse sickness behavior in relation to immune and brain activity markers. Brain Behav Immun. 2015;44:106–120. doi: 10.1016/j.bbi.2014.08.011. [The peripheral and cerebral immune and sickness response to lipopolysaccharide is markedly boosted if the immune system is primed by NOD1 or NOD2 agonists] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Üllen A, Singewald E, Konya V, Fauler G, Reicher H, Nusshold C, Hammer A, Kratky D, Heinemann A, Holzer P, Malle E, et al. Myeloperoxidase-derived oxidants induce blood-brain barrier dysfunction in vitro and in vivo. PLoS One. 2013;8:e64034. doi: 10.1371/journal.pone.0064034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riley TP, Neal-McKinney JM, Buelow DR, Konkel ME, Simasko SM. Capsaicin-sensitive vagal afferent neurons contribute to the detection of pathogenic bacterial colonization in the gut. J Neuroimmunol. 2013;257:36–45. doi: 10.1016/j.jneuroim.2013.01.009. [Single-cell calcium measurements in cultured vagal afferent neurons show that tumour necrosis factor-α and lipopolysaccharide can directly activate vagal afferent neurons] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernández-Peña C, Talavera A, Kichko T, et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014;5:3125. doi: 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue B, Kasparek MS, Müller MH, Kreis ME. Modulation of intestinal afferent nerve sensitivity to inflammatory mediators following systemic endotoxin in mice. Neurogastroenterol Motil. 2015;27:550–558. doi: 10.1111/nmo.12531. [Bacterial metabolites such as lipopolysaccharide sensitize afferent fibres in the mesenteric nerves to serotonin, bradykinin and gut distension, an effect in which mast cells and cyclooxygenase-2 play a role] [DOI] [PubMed] [Google Scholar]
- 53.Hughes PA, Harrington AM, Castro J, Liebregts T, Adam B, Grasby DJ, Isaacs NJ, Maldeniya L, Martin CM, Persson J, et al. Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut. 2013;62:1456–1465. doi: 10.1136/gutjnl-2011-301856. [DOI] [PubMed] [Google Scholar]
- 54.Reber SO. Stress and animal models of inflammatory bowel disease - an update on the role of the hypothalamo-pituitary-adrenal axis. Psychoneuroendocrinology. 2012;37:1–19. doi: 10.1016/j.psyneuen.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fleshner M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain Behav Immun. 2013;27:1–7. doi: 10.1016/j.bbi.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 57.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci USA. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [There are pre-existing individual differences in the sensitivity of the peripheral immune system (elevated serum interleukin-6) that predict and promote vulnerability to social stress and major depression] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taché Y, Million M. Role of corticotropin-releasing factor signaling in stress-related alterations of colonic motility and hyperalgesia. J Neurogastroenterol Motil. 2015;21:8–24. doi: 10.5056/jnm14162. [Review of the implications of corticotropin-releasing factor in the effects of stress on the gut and the brain-gut as well as gut-brain axis] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pritchard SE, Garsed KC, Hoad CL, Lingaya M, Banwait R, Thongborisute W, Roberts E, Costigan C, Marciani L, Gowland PA, Spiller RC. Effect of experimental stress on the small bowel and colon in healthy humans. Neurogastroenterol Motil. 2015;27:542–549. doi: 10.1111/nmo.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galley JD, Nelson MC, Yu Z, Dowd SE, Walter J, Kumar PS, Lyte M, Bailey MT. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14:189. doi: 10.1186/1471-2180-14-189. [Experimental study demonstrating that stress causes intestinal dysbiosis, which in turn contributes stress-related gut leakiness] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gárate I, Garcia-Bueno B, Madrigal JL, Caso JR, Alou L, Gomez-Lus ML, Micó JA, Leza JC. Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biol Psychiatry. 2013;73:32–43. doi: 10.1016/j.biopsych.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 62.Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 63.Ait-Belgnaoui A, Colom A, Braniste V, Ramalho L, Marrot A, Cartier C, Houdeau E, Theodorou V, Tompkins T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol Motil. 2014;26:510–520. doi: 10.1111/nmo.12295. [Treatment of mice with a probiotic not only counteracts stress-induced gut leakiness but also reduces stress-induced activation of brain neurons and stress-induced depression of hippocampal neurogenesis] [DOI] [PubMed] [Google Scholar]
- 64.Reichmann F, Hassan AM, Farzi A, Jain P, Schuligoi R, Holzer P. Dextran sulfate sodium-induced colitis alters stress-associated behaviour and neuropeptide gene expression in the amygdala-hippocampus network of mice. Sci Rep. 2015 doi: 10.1038/srep09970. [in press. Chemically induced colitis alters gut-brain signalling, which in turn causes distinct gene expression changes in the limbic system and alters the molecular and behavioural response to acute water avoidance stress] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reichmann F, Painsipp E, Holzer P. Environmental enrichment and gut inflammation modify stress-induced c-Fos expression in the mouse corticolimbic system. PLoS One. 2013;8:e54811. doi: 10.1371/journal.pone.0054811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hassan AM, Jain P, Reichmann F, Mayerhofer R, Farzi A, Schuligoi R, Holzer P. Repeated predictable stress causes resilience against colitis-induced behavioral changes in mice. Front Behav Neurosci. 2014;8:386. doi: 10.3389/fnbeh.2014.00386. [Predictable chronic water avoidance stress fails to modify the severity of chemically induced colitis but prevents the colitis-evoked increase of anxiety-like behaviour and reduction of social interaction] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deiteren A, Vermeulen W, Moreels TG, Pelckmans PA, De Man JG, De Winter BY. The effect of chemically induced colitis, psychological stress and their combination on visceral pain in female Wistar rats. Stress. 2014;17:431–444. doi: 10.3109/10253890.2014.951034. [DOI] [PubMed] [Google Scholar]
- 68.Farzi A, Reichmann F, Holzer P. The homeostatic role of neuropeptide Y in immune function and its impact on mood and behavior. Acta Physiol (Oxf) 2015;213:603–627. doi: 10.1111/apha.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forbes SC, Cox HM. Peptide YY neuropeptide Y and corticotropin-releasing factor modulate gastrointestinal motility and food intake during acute stress. Neurogastroenterol Motil. 2014;26:1605–1614. doi: 10.1111/nmo.12428. [Endogenous neuropeptide Y (NPY) and peptide YY inhibit the colonic motor stimulation evoked by stress, the effect of NPY depending on endogenous corticotropin-releasing factor (CRF) acting via CRF1 receptors] [DOI] [PubMed] [Google Scholar]
- 70.Forbes S, Herzog H, Cox HM. A role for neuropeptide Y in the gender-specific gastrointestinal, corticosterone and feeding responses to stress. Br J Pharmacol. 2012;166:2307–2316. doi: 10.1111/j.1476-5381.2012.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62:1214–1222. doi: 10.1136/gutjnl-2012-302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, Michel K, Tracey KJ, Schemann M, Boesmans W, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63:938–948. doi: 10.1136/gutjnl-2013-304676. [Cholinergic myenteric neurons constitute the postganglionic part of the vagal antiinflammatory reflex, and resident macrophages in the intestinal muscularis are the ultimate target whereby vagal nerve stimulation blunts postoperative inflammation] [DOI] [PubMed] [Google Scholar]
- 73.Costes LM, van der Vliet J, van Bree SH, Boeckxstaens GE, Cailotto C. Endogenous vagal activation dampens intestinal inflammation independently of splenic innervation in postoperative ileus. Auton Neurosci. 2014;185:76–82. doi: 10.1016/j.autneu.2014.07.006. [The antiinflammatory influence of vagal efferents is called into operation whenever there is ongoing inflammation in the gut, as shown by selective vagotomy] [DOI] [PubMed] [Google Scholar]