Abstract
Background
The prevalence of diabetes mellitus (DM) is expected to increase in sub-Saharan Africa. Patients with HIV are at particular risk. We investigated the DM burden among antiretroviral therapy (ART)-naïve patients with HIV in Guinea-Bissau.
Methods
Patients were consecutively included. Demographic and lifestyle data were collected and one fasting blood glucose (FBG) measurement was used to diagnose DM (FBG≥7.0 mmol/L) and impaired fasting glucose (IFG) (FBG≥6.1 and <7.0 mmol/L).
Results
By June 2015, 953 newly diagnosed ART-naïve patients with HIV had been included in the study of whom 893 (93.7%) were fasting at the time of inclusion. Median age among the fasting patients was 37 years (IQR 30–46 years) and 562 (62.9%) were women. The prevalence of DM was 5.8% (52/893) while 5.6% (50/893) had IFG. DM was associated with family history of DM (OR 3.92, 95% CI 1.78 to 8.63), being 41–50 years (OR 2.98, 95% CI 1.18 to 7.49) or older than 50 years (OR 3.14, 95% CI 1.09 to 9.07) and Fula ethnicity (OR 2.72, 95% CI 1.12 to 6.62).
Conclusions
DM prevalence was higher among younger patients compared with the background population in Bissau. Traditional risk factors for DM such as advancing age and a family history of DM apply also for ART-naïve patients with HIV.
Keywords: Diabetes mellitus, Guinea-Bissau, HIV, Impaired fasting glucose
Introduction
Globally, an estimated 36.9 million people were living with HIV in 2014. Seventy percent of all new HIV infections in 2014 occurred in sub-Saharan Africa.1 The majority of individuals with HIV-2 live in West Africa, where 1–2 million people are infected.2 The West African country Guinea-Bissau holds the world's highest prevalence of HIV-2, and at the same time the country has been experiencing a rise in the prevalence of HIV-1.3
Non-communicable diseases have become increasingly important in low-income countries. Globally, an estimated 366 million people were living with diabetes mellitus (DM) in 2011 with a projected increase to 552 million by 2030.4 The prevalence of DM is rising in sub-Saharan Africa and ranges from 0.6% in the general population in rural Uganda to 13% among urban black South Africans.5,6
A potential association between HIV infection and DM is sparsely investigated and is further complicated by differences in the prevalence of risk factors for DM in individuals with HIV, compared with uninfected individuals.7,8 Antiretroviral treatment (ART) with protease inhibitors (PIs) and nucleoside analogues have both been reported to be associated with hyperglycemia.9,10 Although the nucleoside analogue zidovudine (AZT) has been phased out in Western guidelines, it is still a part of the recommended first line treatment regimen used in Guinea-Bissau. HIV infection itself may also contribute to glucose abnormalities. Insulin resistance markers were higher in all groups of patients with HIV compared with HIV uninfected controls, even among those who were not receiving ART,10 suggesting a detrimental effect of HIV infection itself. Furthermore, secondary infections in patients with HIV are known to induce an increased cortisol and stress hormone response, resulting in insulin resistance.11
Risk factors for DM such as increasing age, hepatitis C virus (HCV) coinfection and body mass index (BMI) have been reported to have a more profound effect upon the risk of diabetes among patients with HIV suggesting that HIV and other risk factors for DM interact in a synergistic manner.12
In sub-Saharan Africa, DM has previously received a low priority, partly due to the perception that with a high prevalence of HIV, relatively few will live long enough to develop DM.13 The improved survival due to ART will likely affect the DM burden. However, the magnitude of this effect is yet to be determined.14
Few studies have reported the prevalence and the associated risk factors for DM among newly diagnosed patients with HIV in sub-Saharan Africa without ART exposure. Accordingly, the current study aimed to determine the prevalence and risk factors of DM among ART-naïve patients with HIV-1, HIV-2 and HIV-1/2 in Bissau, Guinea-Bissau. Impaired fasting glucose was also investigated, as this condition is often a precursor of DM development.15
Materials and methods
Setting and study population
The outpatient HIV clinic at Hospital National Simão Mendes (HNSM) opened in 2005 and is the largest ART centre in Guinea-Bissau. The clinic provides care for citizens of Bissau, while it is also a reference center for the other HIV clinics in the country. The Bissau HIV cohort is the world's largest cohort of HIV-2 infected patients.16 Challenges, treatment adherence and loss-to-follow-up at the HIV clinic have previously been described.17,18
This cross-sectional cohort study was conducted at HNSM between 4 May 2011 and 2 June 2015. At the first visit to the clinic, patients were tested for HIV and demographic information was collected. Blood samples were usually drawn the following day. Blood samples were drawn between 08:00 and 10:00 h. Fasting was defined as not having ingested food or caloric fluids after midnight, thus patients were fasting for at least 8 hours. Questioning about fasting was done by a well-trained assistant using a standardized questionnaire. A questionnaire regarding demography and lifestyle was filled in immediately after the blood sample was drawn. All ART-naïve newly diagnosed HIV positive patients older than 15 years of age and not pregnant were included in the study when having a blood sample drawn for laboratory analyses. Individuals were considered as newly diagnosed if the glucose measurement was performed within 30 days of HIV diagnosis.
Clinical procedures
Screening for HIV was done at the HIV clinic with the rapid test Determine HIV-1/2 assay (Abbott Laboratories, Abbott Park, ILL, USA). Confirmation of HIV infection and discrimination between HIV types were performed using SD Bioline HIV 1/2 3.0 (Standard Diagnostics Inc, Kyonggi-do, South Korea). Since June 2012, the rapid test First Response HIV Card 1-2.0 (PMC Medical, Mumbai, India) has also been used for HIV type discrimination. CD4 cell count analyses were performed at the National Public Health Laboratory by Partec CyFlow® SL_3 (Cyflow SL, Partec, Munster, Germany).
Venous blood samples were collected in serum clot-activator tubes. The tubes were centrifuged within a few hours after which serum was collected and glucose levels analysed using BA-88 Mindray Biochemistry Analyzer at the laboratory at Hospital Nacional Simão Mendes.
DM definitions
Glucose levels were measured in venous serum but is referred to as fasting blood glucose (FBG). Patients had a FBG measurement irrespective of prior DM diagnosis. Individuals with FBG ≥6.1 mmol/l (110 mg/dl) were categorized as dysglycemic. Patients with a FBG ≥6.1 mmol/l (110 mg/dl) and <7.0 mmol/l (126 mg/dl) were classified as having impaired fasting glucose (IFG), while those with FBG ≥7.0 mmol/l (≥126 mg/dl) had DM.19 Individuals on anti-diabetic treatment were classified as diabetes patients, irrespective of FBG level.
Statistical analysis
Questionnaires were entered by a data entry clerk using Access software (Microsoft, Redmond, WA, USA). Stata IC 13.0 (StataCorp, College Station, TX, USA) was used for statistical analysis. Comparisons were performed using the χ2 test for categorical variables. Continuous variables were compared using the two-sample t-test (normal distribution) or Wilcoxon rank-sum test (non-normal distribution). For the analysis of risk factors for DM, we used logistic regression and included variables in a multivariable logistic regression if the univariable regression had a p-value below 0.10.
The variables included in the multivariable regression were age, CD4, BMI, ethnicity, ‘family history of DM’ and HIV type. Since patients with HIV-1 were younger than patients with HIV-2 and older age was associated with DM, an association between HIV-1 and DM might have been masked. Therefore, we also included HIV type in the multivariable analysis. In case of missing data, we created missing data groups and included these groups in the multivariable regression in order to maintain power. The HIV type was unknown for 188 patients due to lack of confirmatory HIV tests at some periods at the HIV clinic. The patients with unknown HIV type were included in the multivariable regression. No HIV patients with diabetes had missing information about age and ethnicity whereas six patients with normal FBG had missing information about age and nine patients with normal FBG had missing information about ethnicity. These 15 patients had to be excluded from the multivariable regression for which reason the multivariable regression included 828 patients.
Results were presented as odds ratios (ORs) with 95% CIs. We considered the multivariable model our primary risk factor analysis. A p-value below 0.05 was considered significant.
Ethics
Written information was given in the official language, Portuguese, and oral information was given to all eligible patients in the widely spoken language, Portuguese Creole. Informed written consent or a fingerprint if illiterate was kept together with case report forms. The Bissau HIV cohort has been approved by the National Ethics Committee in Guinea-Bissau (Parecer NCP/No.15/2007).
Results
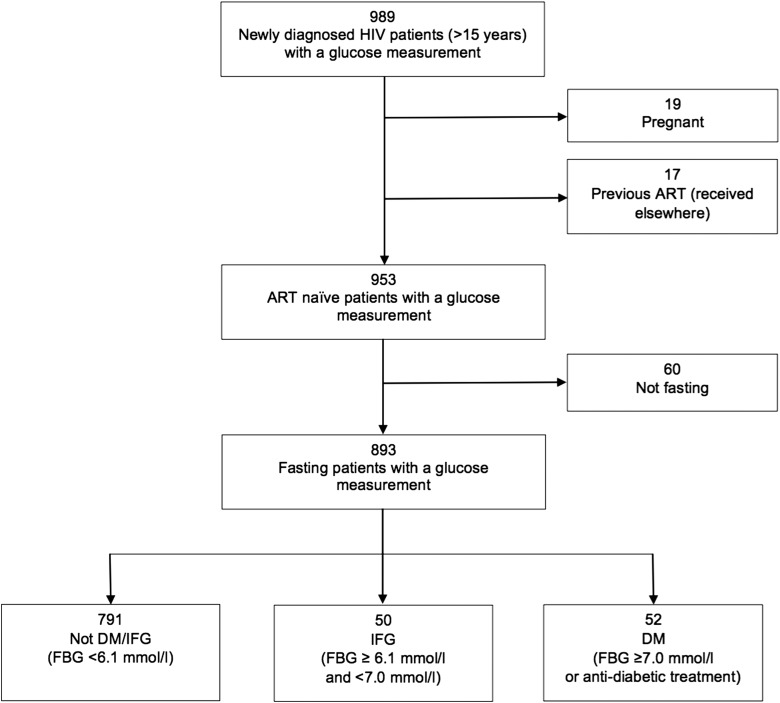
By 2 June 2015, 989 patients were included by being diagnosed with HIV, and had had a blood sample drawn for biochemistry analyses at the clinic (Figure 1). Blood glucose was measured on 953 of the newly diagnosed non-pregnant ART-naïve patients with HIV, of whom 588 (61.7%) were female. The median CD4 cell count was 226 cells/µl (IQR 106-402 cells/µl).
Figure 1.
Flowchart of enrolment. ART: antiretroviral treatment; DM: diabetes mellitus; FBG: fasting blood glucose; IFG: impaired fasting glucose.
Comparison between fasting and non-fasting individuals
Among the included patients, 893 (93.7%) reported that they were fasting at the time of the glucose measurement. Fasting and non-fasting individuals were compared with regards to HIV type, sex, age, CD4 cell count and BMI.
The non-fasting patients were more often male (p<0.01) and had a lower BMI (p=0.03), compared with the fasting patients. The median CD4 cell count in fasting individuals was higher than in non-fasting individuals, 230 cells/µl (IQR 112–409 cells/µl) vs 179 cells/µl (IQR 72–315 cells/µl) (p=0.01). The two groups were comparable with regard to HIV type and age.
Characteristics of fasting individuals
The 893 fasting patients had a mean FBG of 4.7 mmol/l (95% CI 4.6 to 4.9 mmol/l). The median age at the time of inclusion was 37 years (IQR 30–46 years), and the median CD4 cell count was 230 cells/µl (IQR 112–409 cells/µl). The fasting patients had a median BMI of 19.6 kg/m² (IQR 17.5–22.5 kg/m²). Characteristics of fasting female and male patients are summarized in Table 1.
Table 1.
Characteristics of fasting female and male patients
| Female n=562 | Male n=330 | ||
|---|---|---|---|
| n (%) | n (%) | p-value | |
| HIV-type | NS | ||
| HIV-1 | 346 (61.6) | 210 (63.6) | |
| HIV-2 | 63 (11.2) | 32 (9.7) | |
| HIV-1/2 | 41 (7.3) | 13 (3.9) | |
| Unknown | 112 (19.9) | 75 (22.7) | |
| Age | <0.01 | ||
| ≤30 years | 195 (34.7) | 42 (12.7) | |
| 31–40 years | 190 (33.8) | 132 (40.0) | |
| 41–50 years | 101 (18.0) | 95 (28.8) | |
| ≥51 years | 72 (12.8) | 59 (17.9) | |
| Unknown | 4 (0.7) | 2 (0.6) | |
| CD4 | <0.01 | ||
| ≤200 cells/µl | 216 (38.4) | 159 (48.2) | |
| 201–350 cells/µl | 123 (21.9) | 86 (26.1) | |
| ≥351 cells/µl | 201 (35.8) | 71 (21.5) | |
| Unknown | 22 (3.9) | 14 (4.24) | |
| Body mass index | 0.03 | ||
| ≤18.5 kg/m² | 196 (34.9) | 128 (38.8) | |
| >18.5–20 kg/m² | 86 (15.3) | 52 (15.8) | |
| >20–25 kg/m² | 190 (33.8) | 118 (35.8) | |
| >25 kg/m² | 73 (13.0) | 22 (6.7) | |
| Unknown | 17 (3.0) | 10 (3.0) |
NS: not significant.
IFG and DM diagnosis
Fifty-two (5.8%) of the fasting patients were classified as diabetic, and 50 (5.6%) were classified as having IFG, thus 102 (11.4%) patients were considered dysglycemic. Of the diabetic patients, 15 (29%) had known diabetes and received ongoing treatment for DM whereas 37 (71%) were newly diagnosed. Of the 15 patients with known diabetes, six had a FBG ≥7.0 mmol/l (≥126 mg/dl), two had a FBG ≥6.1 mmol/l (110 mg/dl) and <7.0 mmol/l (126 mg/dl) and seven had a FBG <6.1 mmol/l (110 mg/dl). The number of patients who received anti-diabetic treatment or had a FBG measurement above 5.6 mmol/l (100 mg/dl) was 161 (18.0%).
The crude DM prevalence was 22/556 (4.0%) among patients with HIV-1, 6/95 (6.3%) among patients with HIV-2 and 3/54 (5.6%) among patients with HIV-1/2 dual infection (p=0.53). The DM prevalence was 8/237 (3.4%) among patients younger than 31 years of age, while the prevalence among individuals between 31 and 40 years was 16/323 (5.0%). In the age group 41–50 years the prevalence of DM was 18/196 (9.2%) while patients older than 50 years had a DM prevalence of 10/131 (7.6%) (p=0.05). Characteristics of patients with DM, IFG and normal blood glucose are summarized in Table 2.
Table 2.
Characteristics of patients with diabetes mellitus, impaired fasting glucose and normal blood glucose
| Normal blood glucose n=791 | Impaired fasting glucose n=50 | Diabetes mellitus n=52 | ||
|---|---|---|---|---|
| n (%) | n (%) | n (%) | p-value | |
| HIV-type | NS | |||
| HIV-1 | 504 (63.7) | 30 (60.0) | 22 (42.3) | |
| HIV-2 | 80 (10.1) | 9 (18.0) | 6 (11.5) | |
| HIV-1/2 | 48 (6.1) | 3 (6.0) | 3 (5.8) | |
| Unknown | 159 (20.1) | 8 (16.0) | 21 (40.4) | |
| Sex | NS | |||
| Female | 496 (62.7) | 33 (66.0) | 33 (63.5) | |
| Male | 294 (37.2) | 17 (34.0) | 19 (36.5) | |
| Unknown | 1 (0.1) | 0 (0.0) | 0 (0.0) | |
| Age | 0.05 | |||
| ≤30 years | 218 (27.6) | 11 (22.0) | 8 (15.4) | |
| 31–40 years | 293 (37.0) | 14 (28.0) | 16 (30.8) | |
| 41–50 years | 164 (20.7) | 14 (28.0) | 18 (34.6) | |
| ≥51 years | 110 (13.9) | 11 (22.0) | 10 (19.2) | |
| Unknown | 6 (0.8) | 0 (0.0) | 0 (0.0) | |
| CD4 | 0.02 | |||
| ≤200 cells/µl | 346 (43.7) | 13 (26.0) | 17 (32.7) | |
| 201–350 cells/µl | 186 (23.5) | 10 (20.0) | 13 (25.0) | |
| ≥351 cells/µl | 228 (28.8) | 24 (48.0) | 20 (38.5) | |
| Unknown | 31 (3.9) | 3 (6.0) | 2 (3.9) | |
| Body mass index | <0.01 | |||
| ≤18.5 kg/m² | 300 (37.9) | 8 (16.0) | 17 (32.7) | |
| >18.5–20 kg/m² | 126 (15.9) | 6 (12.0) | 6 (11.5) | |
| >20–25 kg/m² | 269 (34.0) | 22 (44.0) | 17 (32.7) | |
| >25 kg/m² | 72 (9.1) | 12 (24.0) | 11 (21.2) | |
| Unknown | 24 (3.0) | 2 (4.0) | 1 (1.9) | |
| Ethnicity | <0.01 | |||
| Pepel | 67 (8.5) | 9 (18.0) | 8 (15.4) | |
| Fula | 158 (20.0) | 17 (34.0) | 19 (36.5) | |
| Mandinga | 85 (10.8) | 2 (4.0) | 2 (3.9) | |
| Manjaco | 64 (8.1) | 5 (10.0) | 5 (9.6) | |
| Mancanha | 62 (7.8) | 5 (10.0) | 2 (3.9) | |
| Balanta | 178 (22.5) | 7 (14.0) | 9 (17.3) | |
| Othera | 168 (21.2) | 5 (10.0) | 7 (13.5) | |
| Unknown | 9 (1.1) | 0 (0.0) | 0 (0.0) | |
| Attended schoolb | NS | |||
| No | 272 (34.4) | 20 (40.0) | 15 (28.9) | |
| Primary | 234 (29.6) | 12 (24.0) | 17 (32.7) | |
| Secondary | 130 (16.4) | 9 (18.0) | 9 (17.3) | |
| Tertiary | 146 (18.5) | 8 (16.0) | 11 (21.2) | |
| Unknown | 9 (1.1) | 1 (2.0) | 0 (0.0) | |
| Smoking | NS | |||
| No | 670 (84.7) | 46 (92.0) | 44 (84.6) | |
| Yes | 113 (14.3) | 4 (8.0) | 7 (13.5) | |
| Previouslyc | 7 (0.9) | 0 (0.0) | 0 (0.0) | |
| Unknown | 1 (0.1) | 0 (0.0) | 1 (1.9) | |
| Family history of diabetes | <0.01 | |||
| No | 620 (78.4) | 40 (80.0) | 29 (55.8) | |
| Yes | 81 (10.2) | 7 (14.0) | 14 (26.9) | |
| Unknown | 90 (11.4) | 3 (60) | 9 (17.3) |
NS: not significant.
a Other ethnicities: Beafada, Bijago, Caboverdeano, Felupe, other and mixed.
b Koran school not included.
c No patients reporting previous smoking were diabetic.
Risk factors for DM
The findings from univariable and multivariable logistic regression analyses comparing patients classified as diabetic with individuals with normal FBG are summarized in Table 3. In the multivariable analysis, a family history of DM (OR 3.92, 95% CI 1.78 to 8.63, p<0.01), being 41–50 years of age (OR 2.98, 95% CI 1.18 to 7.49, p=0.02) or older than 50 years of age (OR 3.14, 95% CI 1.09 to 9.07, p=0.04) and Fula ethnicity (OR 2.72, 95% CI 1.12 to 6.62, p=0.03) were associated with DM. The mean age was significantly higher among patients with DM (41.9 years, 95% CI 38.9 to 45.0), compared with patients with normal FBG (38.1 years, 95% CI 37.3 to 38.9), p=0.02. A BMI above 25 kg/m2 showed a tendency to be associated with DM (OR 2.33, 95% CI 0.74 to 7.36, p=0.15)
Table 3.
Risk factors for diabetes mellitus
| Univariable analysis n=843 |
Multivariable analysis n=828 |
|||
|---|---|---|---|---|
| OR (95% CI) | p-value | aOR (95% CI) | p-value | |
| HIV-type | ||||
| HIV-1 | 1.00 | NA | 1.00 | NA |
| HIV-2 | 1.72 (0.68–4.37) | NS | 1.16 (0.41–3.27) | NS |
| HIV-1/2 | 1.43 (0.41–4.96) | NS | 1.10 (0.30–4.09) | NS |
| Sex | ||||
| Female | 1.00 | NA | ND | ND |
| Male | 0.97 (0.54–1.74) | NS | ND | ND |
| Age | ||||
| ≤30 years | 1.00 | NA | 1.00 | NA |
| 31–40 years | 1.49 (0.63–3.54) | NS | 1.53 (0.61–3.85) | NS |
| 41–50 years | 2.99 (1.27–7.05) | 0.01 | 2.98 (1.18–7.49) | 0.02 |
| ≥51 years | 2.48 (0.95–6.45) | NS | 3.14 (1.09–9.07) | 0.04 |
| CD4 | ||||
| ≤200 cells/µl | 0.56 (0.29–1.09) | NS | 0.63 (0.30–1.35) | NS |
| 201–350 cells/µl | 0.80 (0.39–1.64) | NS | 1.08 (0.49–2.37) | NS |
| ≥351 cells/µl | 1.00 | NA | 1.00 | NA |
| Body mass index | ||||
| ≤18.5 kg/m² | 1.19 (0.46–3.09) | NS | 1.22 (0.45–3.28) | NS |
| >18.5–20 kg/m² | 1.00 | NA | 1.00 | NA |
| >20–25 kg/m² | 1.33 (0.51–3.45) | NS | 1.34 (0.49–3.67) | NS |
| >25 kg/m² | 3.21 (1.14–9.04) | 0.03 | 2.33 (0.74–7.36) | NS |
| Ethnicity | ||||
| Pepel | 2.36 (0.87–6.37) | NS | 2.73 (0.94–7.98) | NS |
| Fula | 2.38 (1.05–5.41) | 0.04 | 2.72 (1.12–6.62) | 0.03 |
| Mandinga | 0.47 (0.10–2.20) | NS | 0.54 (0.11–2.69) | NS |
| Manjaco | 1.55 (0.50–4.78) | NS | 1.38 (0.41–4.60) | NS |
| Mancanha | 0.64 (0.13–3.03) | NS | 0.62 (0.12–3.09) | NS |
| Balanta | 1.00 | NA | 1.00 | NA |
| Othera | 0.82 (0.30–2.26) | NS | 0.80 (0.28–2.30) | NS |
| Attended schoolb | ||||
| No | 1.00 | NA | ND | ND |
| Primary | 1.32 (0.64–2.70) | NS | ND | ND |
| Secundary | 1.26 (0.54–2.94) | NS | ND | ND |
| Tertiary | 1.37 (0.61–3.05) | NS | ND | ND |
| Smoking | ||||
| No | 1.00 | NA | ND | ND |
| Yes | 0.94 (0.41–2.15) | NS | ND | ND |
| Previouslyc | NA | NA | ND | ND |
| Family history of diabetes | ||||
| No | 1.00 | NA | 1.00 | NA |
| Yes | 3.70 (1.87–7.28) | <0.01 | 3.92 (1.78–8.63) | <0.01 |
aOR: adjusted odds ratios; NA: not applicable; ND: not done; NS: not significant.
a Other ethnicities: Beafada, Bijago, Caboverdeano, Felupe, other and mixed.
b Koran school not included.
c No patients reporting previous smoking were diabetic.
Discussion
To obtain data on baseline prevalence of DM in an urban HIV-infected population we investigated the DM burden among ART-naïve patients with HIV Guinea-Bissau. We found a DM prevalence of 5.8% and an IFG prevalence of 5.6% among ART-naïve newly diagnosed individuals with HIV. A family history of DM and age above 40 years were strongly associated with DM.
Strengths and weaknesses
Most studies of DM in patients with HIV have only examined the effect of ART on glucose homeostasis. Thus, the fact that the patients with HIV in our study were ART-naïve makes this a valuable data source on the association of HIV infection with FBG abnormalities prior to ART. No studies have previously examined the association between HIV-2 and DM, thus our study provides new information in an unexplored area; there was no significant difference in the DM prevalence when comparing patients with different HIV types. Our study included a large number of patients, ensuring a high precision of our estimates. Other studies from sub-Saharan Africa, that examined blood glucose levels among ART-naïve patients with HIV, included a relatively smaller number of subjects.20–23
In this study, we used a single FBG measurement to diagnose DM. Diagnostic certainty would have been higher if two FBG measurements had been available, or oral glucose tolerance tests and glycosylated hemoglobin (HbA1C) values had been obtained.
We used WHO criteria for diagnosis of DM and IFG that are based on measurements of glucose in venous plasma. We, however, measured glucose levels in venous serum. It has been shown that serum values of glucose are 1.2% lower than the corresponding plasma values.24 Thus, the fact that we measured glucose levels in serum may have caused a slight underestimation of the prevalence of DM and IFG.
Due to the cross sectional nature of the study, we were able to examine potential statistical associations between DM and HIV, but we were unable to assess causation. Thus, it is not clear if HIV infection preceded elevated glucose levels or vice versa. The study adjusted for some of the confounding factors, but residual confounding factors for the association between DM and HIV cannot be ruled out. It is likely that individuals with DM are symptomatic and to a greater extent seek health care which may lead to HIV-testing.
In this study, we only included patients with HIV who were diagnosed and treated at a health care facility. A low median CD4 cell count of 226 cells/µl among the newly diagnosed patients with HIV indicates that the burden of undiagnosed HIV may be considerable in Bissau.
One important limitation of the study was the lack of an HIV negative control group, but we were able to compare the observed DM burden with previous results from the general population.25
Another limitation was the use of rapid tests for HIV type discrimination that have shown to overestimate the number of patients with HIV-1/2 dual infection,26 and a high number of patients with unknown HIV type.
As this study did not include measurements of insulin, C-peptide and glutamic acid decarboxylase (GAD) antibodies, we were not able to describe the pathophysiological characteristics of DM and IFG. Future studies should address this issue.
Comparison between fasting and non-fasting individuals
The median CD4 cell count in non-fasting individuals were lower than in fasting individuals. Thus, individuals not fasting had more progressed disease than fasting individuals had. As CD4 cell count was not associated with DM, this difference between fasting and non-fasting individuals is not suspected to have affected the prevalences found. The fasting patients had a higher BMI than the non-fasting patients. As a high BMI showed a tendency to be associated with DM, this difference between the two groups may have caused us to overestimate the prevalence of DM.
DM prevalence among HIV-infected patients compared with healthy individuals in Bissau
Haraldsdottir et al. have recently examined the prevalence of DM and IFG in a survey of population controls in the general population in Bissau.25 Controls were selected randomly from the Bandim Health Project's demographic surveillance database and visited at home. The survey included 531 controls, and the initial screening was done using random blood glucose measurements, with DM suspects subsequently investigated with two FBG measurements. The fasting controls had a mean age of 31 years and a mean BMI of 24.2 kg/m². Eleven (2.1%) individuals had DM, while eight (1.5%) individuals had IFG. The prevalences of DM in the four age groups ‘younger than 31 years’, ‘31–40 years’, ‘41–50 years’ and ‘older than 50 years’ were 0.6%, 2.4%, 4.0% and 7.7%, respectively compared with 3.4%, 5.0%, 9.2% and 7.6% in our study.
Thus, though based on a small number of DM patients, our results indicate that the DM prevalences in the age groups ‘younger than 31 years’, ‘31–40 years’ and ‘41–50 years’ are higher among ART-naïve patients with HIV, compared with healthy individuals in Bissau. A comparison of the results from our study with the results from the study by Haraldsdottir et al. shows a significant association between DM and untreated HIV infection in the age group ‘younger than 31 years’, (p=0.02). These results could indicate that patients with HIV have increased risk of DM, even in the absence of ART. However, this comparison was not adjusted for other confounders than age.
The prevalence of DM in individuals older than 50 years was similarly high in ART-naïve patients with HIV and in healthy individuals in Bissau. This suggests that advancing age is a risk factor for DM in both individuals with HIV and uninfected individuals, and that older age outweighs the effect of HIV-infection on DM risk.
DM prevalence in the general population in sub-Saharan Africa
A study from Kenya found a DM prevalence of 2.2% in the rural population and 12.2% in the urban population.27 A review including 21 reports from countries in West Africa found a prevalence of DM at approximately 4.0% in urban adults and 2.6% in rural adults.28 Approximately 80% of the individuals included in our study were living in the capital city at the time of HIV diagnosis. The International Diabetes Federation estimated the DM burden for the entire sub-Saharan Africa to be 4.9% in 2013.29 Thus, the DM prevalence in the general population in Bissau is low, compared with other countries in the region.
Prevalence of DM, IFG and dysglycemia among patients with HIV in sub-Saharan Africa
There are large inter-country variations in the prevalence of DM, IFG and dysglycemia among ART-naïve patients with HIV in sub-Saharan Africa. The prevalence of DM ranges from 3.4% in South Africa20 to 26.0% in Cameroon22 compared with 5.8% in our study. The prevalence of dysglycemia (FBG ≥6.1 mmol/l) ranges from 5.6% in Ethiopia23 to 23.6% in Nigeria30 compared with 11.4% in our study. Only some of the inter-country variations can be explained by differences in age and BMI among the included patients in the above-mentioned studies.
A number of studies used a lower FBG cut-off of 5.6 mmol/l (100 mg/dl), examining the prevalence of dysglycemia among ART-naïve patients with HIV. To accommodate the difficulties caused by different definitions of dysglycamia, we have in this study both described the prevalences of DM and IFG on the basis of the WHO criteria, and furthermore stated the prevalence of dysglycemia with a cut-off of 5.6 mmol/l (100 mg/dl). Using a cut-off of 5.6 mmol/l our results (18.0%) are comparable to the prevalence of dysglycemia in both Southern Ethiopia (21.5%)21 and South Africa (25.7%).20
In Cameroon, the prevalences of both DM and IFG were significantly higher among treatment-naïve HIV-infected patients compared with healthy controls.22 These results are consistent with our findings. Conversely, a meta-analysis comparing HIV-infected individuals from sub-Saharan Africa to individuals without HIV did not find any evidence of association between HIV infection and FBG.31
Risk factors for DM
Our results showed that traditional risk factors for DM such as advancing age and a family history of DM apply also for ART-naïve patients with HIV. Our results are consistent with Yinzhong Shen et al. who found older age to be significantly associated with an increased risk of DM among ART-naïve patients with HIV.32 With better survival because of ART these factors may contribute to the DM burden in a similar way to that of the general population. The reason for the association between Fula ethnicity and DM is unclear and needs further investigation.
Contrary to Yinzhong Shen et al.32 we did not find an association between CD4 cell count and DM. Thus, increased DM prevalence could not be explained by lower CD4 cell count, which indicates that replication of HIV virus results in a state of chronic inflammation independently of CD4.
Conclusions
We found a DM prevalence of 5.8% and an IFG prevalence of 5.6% among ART-naïve newly diagnosed HIV infected individuals. Our results indicate that even prior to ART, younger patients with HIV could be at increased risk of DM, compared with healthy controls in the general population in Bissau. Traditional risk factors for DM such as advancing age and a family history of DM apply also for ART-naïve patients with HIV.
Acknowledgments
Bissau HIV cohort study group: Amabelia Rodrigues, David da Silva, Zacarias da Silva, Candida Medina, Ines Oliviera-Souto, Lars Østergaard, Alex Laursen, Sanne Jespersen, Peter Aaby, Anders Fomsgaard, Christian Erikstrup, Bo Langhoff Hønge and Christian Wejse (chair).
Authors' contributions: DS, SJ, ALL, CE, CW and BLH conceived the study; CM and FGC carried out clinical assessment; DS, SJ, ALL, CE, CW and BLH carried out analysis and interpretation of data. DS, SJ, CW and BLH drafted the manuscript; SJ, ALL, CW, CE, HK, AH, TH, MBA, LØ and BLH critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. BLH and CW are guarantors of the paper.
Acknowledgements: The authors are grateful to the clinic assistants Aminata, Quintino, Marques, João Paulo, Imbemba, Zé and the rest of the staff working in the HIV clinic at HNSM for their dedication to work and kindness with patients. We are also grateful to the laboratory staff working at the HIV section at the National Public Health Laboratory and to the office staff at the Bandim Health Project for making this study possible.
Funding: The HIV clinic is supported financially by its collaboration with International Epidemiologic Databases to Evaluate AIDS (IeDEA) and West African Platform for HIV Intervention Research (WAPHIR). We acknowledge The National Cancer Institute (NCI), the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) and the National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. National Institutes of Health (NIH), as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) under Award Number U01AI069919. Data collection has been supported by the European & Developing Countries Trials Partnership (EDCTP).
Competing interests: None declared.
Ethical approval: The Bissau HIV cohort has been approved by the National Ethics Committee in Guinea-Bissau (Parecer NCP/No.15/2007).
Contributor Information
Collaborators: for the Bissau HIV Cohort study group, Amabelia Rodrigues, David da Silva, Zacarias da Silva, Candida Medina, Ines Oliviera-Souto, Lars Østergaard, Alex Laursen, Sanne Jespersen, Peter Aaby, Anders Fomsgaard, Christian Erikstrup, Bo Langhoff Hønge, and Christian Wejse
References
- 1.UNAIDS. Fact sheet: 2014 statistics. http://www.unaids.org/sites/default/files/media_asset/20150714_FS_MDG6_Report_en.pdf [accessed 6 August 2015].
- 2.Arien KK, Abraha A, Quinones-Mateu ME et al. . The replicative fitness of primary human immunodeficiency virus type 1 (HIV-1) group M, HIV-1 group O, and HIV-2 isolates. J Virol 2005;79:8979–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Silva ZJ, Oliveira I, Andersen A et al. . Changes in prevalence and incidence of HIV-1, HIV-2 and dual infections in urban areas of Bissau, Guinea-Bissau: is HIV-2 disappearing? AIDS 2008;22:1195–202. [DOI] [PubMed] [Google Scholar]
- 4.Whiting DR, Guariguata L, Weil C et al. . IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311–21. [DOI] [PubMed] [Google Scholar]
- 5.Maher D, Waswa L, Baisley K et al. . Distribution of hyperglycaemia and related cardiovascular disease risk factors in low-income countries: a cross-sectional population-based survey in rural Uganda. Int J Epidemiol 2011;40:160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peer N, Steyn K, Lombard C et al. . Rising diabetes prevalence among urban-dwelling black South Africans. PloS one 2012;7:e43336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr A, Samaras K, Burton S et al. . A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 1998;12:F51–8. [DOI] [PubMed] [Google Scholar]
- 8.Goulet JL, Fultz SL, Rimland D et al. . Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis 2007;45:1593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dube MP, Johnson DL, Currier JS et al. . Protease inhibitor-associated hyperglycaemia. Lancet 1997;350:713–4. [DOI] [PubMed] [Google Scholar]
- 10.Brown TT, Li X, Cole SR et al. . Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS 2005;19:1375–83. [DOI] [PubMed] [Google Scholar]
- 11.Samarasinghe YP. HIV and diabetes. Prim Care Diabetes 2007;1:99–101. [DOI] [PubMed] [Google Scholar]
- 12.Butt AA, McGinnis K, Rodriguez-Barradas MC et al. . HIV infection and the risk of diabetes mellitus. AIDS 2009;23:1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panz VR, Joffe BI. Impact of HIV infection and AIDS on prevalence of type 2 diabetes in South Africa in 2010. BMJ 1999;318:1351a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kengne AP, Echouffo-Tcheugui JB, Sobngwi E et al. . New insights on diabetes mellitus and obesity in Africa-part 1: prevalence, pathogenesis and comorbidities. Heart 2013;99:979–83. [DOI] [PubMed] [Google Scholar]
- 15.Unwin N, Shaw J, Zimmet P et al. . Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002;19:708–23. [DOI] [PubMed] [Google Scholar]
- 16.Jespersen S, Honge BL, Oliveira I et al. . Cohort Profile: The Bissau HIV Cohort-a cohort of HIV-1, HIV-2 and co-infected patients. Int J Epidemiol 2014;44:756–63. [DOI] [PubMed] [Google Scholar]
- 17.Jespersen S, Honge BL, Oliveira I et al. . Challenges facing HIV treatment in Guinea-Bissau: the benefits of international research collaborations. Bull World Health Organ 2014;92:909–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyrehave C, Rasmussen DN, Honge BL et al. . Nonadherence is associated with lack of HIV-related knowledge: a cross-sectional study among HIV-infected individuals in Guinea-Bissau. J Int Assoc Prov AIDS Care. 2015. DOI:10.1177/2325957415599211. [DOI] [PubMed] [Google Scholar]
- 19.WHO. Definition, diagnosis and classification of diabetes mellitus and its complications : report of a WHO consultation. Part 1, Diagnosis and classification of diabetes mellitus. 1999. http://apps.who.int/iris/handle/10665/66040 [accessed 11 October 2015].
- 20.Dave JA, Lambert EV, Badri M et al. . Effect of nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy on dysglycemia and insulin sensitivity in South African HIV-infected patients. J Acquir Immune Defic Syndr 2011;57:284–9. [DOI] [PubMed] [Google Scholar]
- 21.Tesfaye DY, Kinde S, Medhin G et al. . Burden of metabolic syndrome among HIV-infected patients in Southern Ethiopia. Diabetes Metab Syndr 2014;8:102–7. [DOI] [PubMed] [Google Scholar]
- 22.Ngatchou W, Lemogoum D, Ndobo P et al. . Increased burden and severity of metabolic syndrome and arterial stiffness in treatment-naive HIV+ patients from Cameroon. Vasc Health Risk Manag 2013;9:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abebe M, Kinde S, Belay G et al. . Antiretroviral treatment associated hyperglycemia and dyslipidemia among HIV infected patients at Burayu Health Center, Addis Ababa, Ethiopia: a cross-sectional comparative study. BMC Res Notes 2014;7:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frank EA, Shubha MC, D'Souza CJ. Blood glucose determination: plasma or serum? J Clin Lab Anal 2012;26:317–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haraldsdottir TL, Rudolf F, Bjerregaard-Andersen M et al. . Diabetes mellitus prevalence in tuberculosis patients and the background population in Guinea-Bissau: a disease burden study from the capital Bissau. Trans R Soc Trop Med Hyg 2015;109:400–7. [DOI] [PubMed] [Google Scholar]
- 26.Honge BL, Bjarnason Obinah MP, Jespersen S et al. . Performance of 3 rapid tests for discrimination between HIV-1 and HIV-2 in Guinea-Bissau, West Africa. J Acquir Immune Defic Syndr 2014;65:87–90. [DOI] [PubMed] [Google Scholar]
- 27.Christensen DL, Friis H, Mwaniki DL et al. . Prevalence of glucose intolerance and associated risk factors in rural and urban populations of different ethnic groups in Kenya. Diabetes Res Clin Pract 2009;84:303–10. [DOI] [PubMed] [Google Scholar]
- 28.Abubakari AR, Lauder W, Jones MC et al. . Prevalence and time trends in diabetes and physical inactivity among adult West African populations: the epidemic has arrived. Public Health 2009;123:602–14. [DOI] [PubMed] [Google Scholar]
- 29.Peer N, Kengne AP, Motala AA et al. . Diabetes in the Africa Region: an update. Diabetes Res Clin Pract 2014;103:197–205. [DOI] [PubMed] [Google Scholar]
- 30.Ayodele OE, Akinboro AO, Akinyemi SO et al. . Prevalence and clinical correlates of metabolic syndrome in Nigerians living with human immunodeficiency virus/acquired immunodeficiency syndrome. Metab Syndr Relat Disord 2012;10:373–9. [DOI] [PubMed] [Google Scholar]
- 31.Dillon DG, Gurdasani D, Riha J et al. . Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol 2013;42:1754–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen Y, Wang Z, Liu L et al. . Prevalence of hyperglycemia among adults with newly diagnosed HIV/AIDS in China. BMC Infect Dis 2013;13:79. [DOI] [PMC free article] [PubMed] [Google Scholar]