Abstract
Background
The analysis of phosphatidylethanol, a promising direct ethanol metabolite, in dry blood spots (PEth-DBS) is advantageous due to ease of storage, transportation and minimal invasiveness of capillary blood collection. One potential application of PEth-DBS is to confirm prenatal alcohol exposure in newborns suspected of FASD; however, stability of PEth-DBS is largely unknown.
Methods
Phlebotomized samples from 31 adults with a history of alcoholism, admitted to the University of New Mexico Emergency Department, were analyzed for blood alcohol content and pipetted onto DBS cards (13 spots per patient). The first spot was analyzed within 2 weeks of collection for a baseline PEth; the remaining 12 spots were allocated into three temperature conditions (room temperature, 4°C, −80°C) for the repeated measures analysis. In addition, 5 newborn DBS samples with a baseline PEth>LOD were obtained from a prospective cohort at UNM and re-analyzed at 4 months after storage at −80°C. A mixed linear model was fitted to examine the effects of temperature, time and temperature–time interaction on PEth degradation over the first 9 months.
Results
The baseline PEth levels were 592.8 ± 86.7 ng/ml and 18.3 ± 4.8 ng/ml in adult and newborn samples, respectively. All DBS samples remained positive in successive samples in all temperature conditions. Results of mixed linear model demonstrated a significant effect of temperature (P < 0.001) on PEth degradation over 9 months.
Conclusions
PEth-DBS appears to be relatively stable, especially when stored at lower temperatures. These initial results are encouraging and highlight the PEth-DBS potential in retrospective assessment of alcohol exposure.
INTRODUCTION
Recently, Fetal Alcohol Syndrome (FAS) was estimated to affect 6–9 per 1000 live births in a representative Midwestern U.S. town (May et al., 2014). Fetal Alcohol Spectrum Disorder (FASD) affects ∼10 per 1000 live births (May and Gossage, 2001; Anderson et al., 2006). While FASD is associated with life-long disabilities, the majority of children do not present with birth defects but have severe neurocognitive deficits, such as a demonstrable decrease in reaction times and inattentiveness, deficits in sensory processing, executive functioning and working memory suggesting a generalized deficit in the speed of central processing and integration of information (Jacobson, 1998; Kodituwakku, 2009). Given the lack of physical features indicative of alcohol exposure in children who do not have full FAS, accurate information about maternal drinking is needed to confirm the diagnosis of FASD and to allow access to earlier interventions. Obtaining this information can often be difficult as maternal self-reporting, especially in a busy clinic environment, may be unreliable due, in part, to the stigma associated with alcohol use during pregnancy. As such, ethanol biomarkers may be an objective method to identify additional patients missed by questionnaires and provide opportunities for point-of-care interventions. Unfortunately, biomarkers of ethanol-induced pathology, such as gamma glutamyltransferase (GGT), mean corpuscular volume (MCV) and carbohydrate-deficient transferrin (CDT), and even some direct ethanol metabolites (e.g. fatty acid ethyl esters [FAEEs]) can be time consuming, expensive, and often ineffective in confirming moderate or episodic prenatal alcohol exposure (PAE) (Bakhireva and Savage, 2011).
Phosphatidylethanol (PEth) has emerged as a novel non-oxidative direct ethanol metabolite persisting in erythrocyte cell membranes for 3–4 weeks following moderate ethanol exposure (Varga et al., 2000; Hannuksela et al., 2007; Isaksson et al., 2011). The half-life of PEth was reported as 4.0 ± 0.7 days (Varga et al., 2000) and it might be detectable up to 20 days since the last ethanol consumption (Gnann et al., 2014). In the presence of ethanol, PEth is formed via phospholipase D. In comparison to indirect more traditional ethanol biomarkers, PEth has increased specificity and might be 2-fold more sensitive than indirect ethanol biomarkers in the detection of latent ethanol use (Maenhout et al., 2013). For example, PEth testing has been found to be 94.5–99% sensitive in detecting alcohol exposure in non-pregnant patients with alcohol dependence (Aradottir et al., 2006a; Hartmann et al., 2007). In another study of non-pregnant women, PEth levels were detectable in 93% of subjects who consumed two drinks or more per day and in 53% of subjects who consumed less than or equal to one drink per day (Stewart et al., 2010). A recent study performed by Kwak et al. noted that about 5% of pregnant women in their first trimester with self-reported alcohol abstinence tested positive for PEth (Kwak et al., 2014).
Dry blood spot (DBS) cards were introduced by Dr Guthrie in 1963 to test for phenylketonuria, and are routinely collected from more than 95% of infants in the United States for routine screening for genetic disease and often banked for extended periods. Recent development of liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay (Faller et al., 2011; Jones et al., 2011) now allows for measuring PEth in DBS specimens. This analysis offers a unique opportunity to confirm or rule out PAE in children suspected to have FASD. Results of our recent studies demonstrated that PEth analysis in DBS offers unique advantages over other biomarkers (such as PEth in liquid specimens or FAEE in meconium), including ease of collection and storage, cost effectiveness and high specificity (Bakhireva et al., 2013a; Bakhireva et al., 2014a). However, PEth in DBS is likely to identify alcohol exposure only during the last 2–4 weeks prior to delivery. While it is conventionally believed that risky drinking early in gestation is likely to result in structural defects characteristic of FAS, PAE later in pregnancy is likely to result in neurocognitive deficits without facial dysmorphia (Feldman et al., 2012). While PEth in DBS cards cannot capture PAE during the first or second trimesters, a positive test result most likely will identify a continuous risky drinker throughout pregnancy.
PEth in whole blood liquid specimens might be affected by storage conditions. That is, a decrease in PEth concentrations has been observed in liquid blood specimens during storage at −20°C (but not 2–8°C or −80°C) (Aradottir et al., 2004). In addition, de novo synthesis of PEth in liquid specimens in the presence of ethanol has been previously described (Varga and Alling, 2002; Aradottir et al., 2004; Helander and Zheng, 2009), which limits clinical utility of this biomarker in liquid specimens. Post-collection synthesis in dry blood specimens is highly unlikely, but stability of PEth in DBS cards is largely unknown. A small recent study of five adult subjects, however, demonstrated promising stability of PEth in DBS cards stored at both −20 and 20°C (Faller et al., 2013). Using degradation of >15% of the initial measured PEth concentration as the definition for instability, Faller et al. were able to show that PEth was stable for 30 days in DBS cards at all tested temperature conditions in this small sample.
We have previously demonstrated that the analysis of PEth levels in banked DBS cards offers a unique and cost effective opportunity when compared with testing PEth in liquid blood specimens or analysis of meconium biomarkers to confirm PAE in newborns (Bakhireva et al., 2013b; Bakhireva et al., 2014b). The ability to detect moderate PAE via PEth in DBS cards may facilitate the diagnosis of FASD in infants that would otherwise go undiagnosed in the absence of the physical characteristics of FAS, thus promoting the prospect of earlier interventions. The implementation of this assessment is currently limited by the absence of published information on the chemical stability of PEth in DBS cards stored beyond 30 days. The objective of this study was to (a) examine the degree of PEth degradation in DBS cards stored for 9 months (with intermediate time points); and (b) examine the effect of three temperature conditions (room temperature, +4°C fridge, and −80°C freezer) on the degree of PEth degradation over time. Temperature storage conditions of room temperature and +4°C were selected to simulate storage conditions of many existing state repositories, with −80°C chosen as a previously described stable control temperature for liquid specimens (Faller et al., 2013).
METHODS
The study utilized a hybrid design with cross-sectional collection of specimens with repeated analysis in a prospective time course to evaluate the stability of PEth over time. This study included specimens from two studies at the University of New Mexico (UNM) to evaluate the stability of PEth up to 9 months.
In the first study, adult patients (n = 31) were selected from those presenting to the UNM Hospital Emergency Department (ED). Adult patients were used due to: (a) uncertainties in the degree of PEth concentration over time, thus (b) the need to recruit patients with high enough blood alcohol content (BAC) and PEth concentrations, typically observed in adults with risky alcohol consumption patterns, to have detectable PEth after 9 months of storage. Eligibility criteria were patients: (a) with clinical signs of alcohol intoxication or a history of alcohol abuse; (b) who were 18 years of age or older; and (c) who required routine phlebotomy for a BAC for clinical evaluation. The study was approved by the UNM Human Research Review Committee (protocol: 13–352) with a waiver of consent since no personal health identifiers were collected and the study presented a minimal risk.
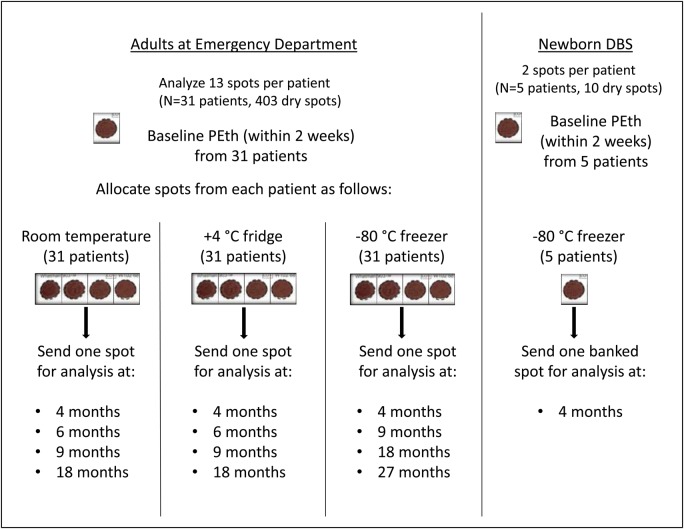
The study design is outlined in Fig. 1. From each eligible patient, 1 ml of whole blood was collected into a separate EDTA vial during routine phlebotomy at admission or triage to the ED. To minimize volumetric bias, whole blood was pipetted by one of the ED physician investigators in 45 µl aliquots onto each circle on the DBS cards. The vial was gently twirled during pipetting to avoid sedimentation of blood cells. Three DBS cards from each patient (13 total blood spots) were collected. A unique identifier was assigned to each patient and their DBS cards. BAC, obtained from liquid blood specimens for clinical purposes, was recorded on the DBS cards for each patient. Thereafter the link between the study ID and the PHI, which had been maintained only by ED physicians on the study (MB, DS), was destroyed. Cards were left to dry at room temperature for 3 h on a flat surface per manufacturer's protocol and picked up by study coordinators. Each blood spot was cut from the set of 3 cards, individually labeled with the corresponding study ID, and allocated to one of three temperature storage conditions. The first blood spot, which was designated for a baseline PEth value, was stored at room temperature and analyzed within 2 weeks from collection. Four spots were stored at room temperature, four spots were stored at +4°C, and the four remaining spots were stored at −80°C. A total of 93 cards (403 spots) were collected from adult subjects. The spots stored at room temperature and +4°C were sent for PEth analysis at 4, 6 and 9 months after collection with the remaining spots currently being banked for future analysis at 18 months. The spots stored at −80°C were sent at 4 and 9 months with the remaining two spots per patient being currently banked for future analysis at 18 and 27 months. Given that we hypothesized that PEth-DBS stored at −80°C will be the most stable, a longer follow-up period was chosen for that temperature condition. This report presents the PEth degradation data over the time course of the first 9 months.
Fig. 1.
Overview of the study design.
In the second study, DBS cards obtained from 5 newborns (10 dry spots total) enrolled in a prospective cohort study at UNM were subjected to a 4-month time course at −80°C. The study is ongoing and is approved by UNM HRRC (protocol: 12–390). A detailed methodology of this cohort is described elsewhere (Bakhireva et al., 2015). Briefly, study specific DBS cards were collected from newborns of cohort participants within 24 h of delivery at the time of the routine hospital newborn screening heel prick. DBS spots from infants born to cohort participants were selected for this stability study if they met the following inclusion criteria: (a) the baseline spot, analyzed within 2 weeks from the date of collection, had detectable (above LOD) PEth concentration; (b) at least one additional blood spot was available for the time course. Given that most cohort participants had only one additional blood spot available for the time course, the stability of PEth was largely unknown, and the baseline PEth concentration in newborn samples was much lower than in adult samples, a 4-month rather than 9-month assessment time point was selected for this sub-study (Fig. 1).
BAC and PEth analyses
Blood alcohol was determined at the Tricore Reference Laboratories (Albuquerque, NM) by the Dimension® procedure. This is an enzymatic diagnostic test for the quantitative measurement of ethyl alcohol in human serum on the Dimension Vista® System. Dry blood spots were prepared on Whatman 903 Specimen Collection Paper (Florham Park, NJ, USA) and analyzed for PEth levels at the U.S. Drug Testing Laboratory (USDTL; Des Plaines, IL) via LC/MS/MS, as previously described (Jones et al., 2011). Three punches were taken from each spot using a McGill Basic Series GEM 1/8 (3.175 mm) round hand held punch, subjected to methanol extraction, and then reconstituted into a mobile phase composed of 20% 2 mM ammonium acetate, 58% acetonitrile and 22% isopropanol. The liquid phase was analyzed by LC/MS/MS and compared with a standard curve. Separation was achieved using an Agilent Zorbax Eclipse Plus C-8 column with an Agilent Technologies 6460 tandem mass spectrometer detector using electro-spray ionization (ESI) in the negative mode. The most prevalent homolog of PEth, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanol (POPE or PEth 16:0/18:1) was evaluated in the study (Gnann et al., 2014). The USDTL performed LC/MS/MS method validation with the following parameters: limit of detection (LOD) was 2.0 ng ml−1, limit of quantification (LOQ) was 8.0 ng ml−1, and intra-assay coefficient of variation ranged from 1.6 to 6.6%. The intra- (n = 5) and inter-assay (4 days, n = 20) accuracy and precision were determined at three PEth concentrations (20, 100 and 200 ng/ml). All accuracy measurements were within 12% of target value and all precision measurements (%CV) were <9%. The % recovery was 56, 76 and 71% at 20, 50 and 100 ng/ml, respectively, as previously described elsewhere (Jones et al., 2011).
Data analysis and power calculations
Numerical and graphical summaries [mean, standard error of the mean (SEM), % change from baseline] were computed for PEth concentrations at each time point and BAC measurements at baseline. Pearson's correlation between PEth and BAC at baseline was estimated. For each storage condition, the baseline and 9 months final concentrations were compared by paired t-test. To examine the effect of temperature and time on PEth degradation, a 3 × 4 factorial design (3 temperature conditions and 4 time points) was utilized. Since temperature and time are conditions defined in the same subject, these two factors were considered repeated factors. The analysis was carried out by the PROC MIXED procedure in SAS version 9.3 (Gary, NC). Both time and temperature were treated as categorical variables to allow for non-linear responses. To account for the potential effect of the baseline PEth concentration on the rate of degradation, the outcome variable for the mixed linear model was percent change in PEth at each time point from the baseline. In addition to examining the main effects of temperature and time on PEth percent change, a temperature–time interaction was introduced to determine if there was a higher degree of degradation over time at higher temperatures.
For a power analysis, comparison of the average percent of PEth concentration retained of the baseline concentration at 9 months (percent PEth retained) between the room temperature and the refrigerator temperature conditions was performed. Power calculations were based on preliminary data provided by the USDTL, which indicated that in 5 DBS cards, 69% (±15.5) of PEth concentration was retained (or 31% degradation) at 9 months at room temperature (Joe Jones, USDTL, personal communication). A sample size of 20 patients per condition was adequate to detect a 14.1% decline from the baseline PEth concentration (83.1% of PEth retained) over 9 months, with an 80% power and an alpha of 0.05.
RESULTS
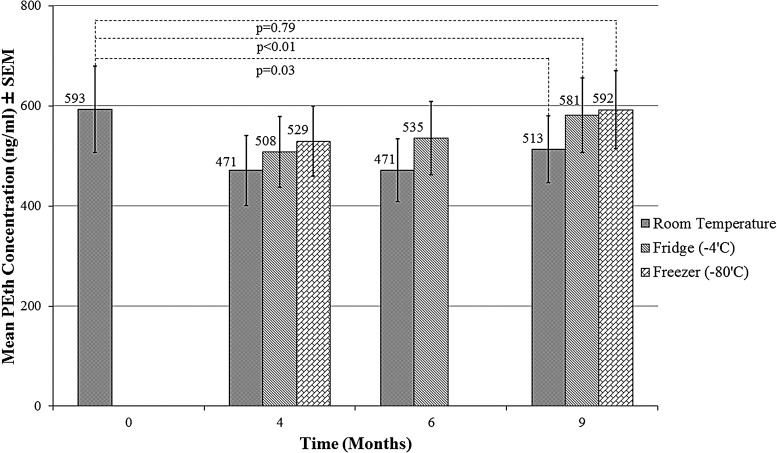
In the adult sample, the mean baseline PEth concentration was 592.8 ± 86.7 ng/ml and mean BAC was 295.8 ± 15.5 mg/dl. There was a moderate correlation at baseline between BAC and PEth (r = 0.35; P = 0.05). Mean PEth concentrations at each time point are shown in Fig. 2. After 9 months of storage, PEth concentration remained largely unchanged at −80°C (592.2 ± 77.7 ng/ml) or 4°C (581.4 ± 74.5 ng/ml). At room temperature, PEth concentration decreased to 512.9 ± 66.7 ng/ml—a 13.5% mean degradation over 9 months (Fig. 2). The only significant difference in mean PEth concentrations between baseline and 9 months was observed for the room temperature condition (P = 0.03), while the mean differences at the 4°C and −80°C storage conditions were not significant (P = 0.71 and P = 0.99, respectively, for a paired t-test).
Fig. 2.
PEth degradation over 9 months in DBS cards obtained from adults (n = 31). Note: baseline samples were stored at room temperature for 2 weeks prior to analysis.
Results of the repeated measures mixed linear model demonstrated a significant effect of temperature (P < 0.001), but not time (P = 0.23) on the PEth percent change over the entire time course. A temperature-by-time interaction of borderline statistical significance (P = 0.047) was observed indicating that PEth degradation over time varied by storage temperature conditions. Pairwise comparisons demonstrated significant differences (P < 0.05) between both lower temperature conditions and room temperature at each time point (note: −80°C is not available at 6 months), but not between −80°C and 4°C.
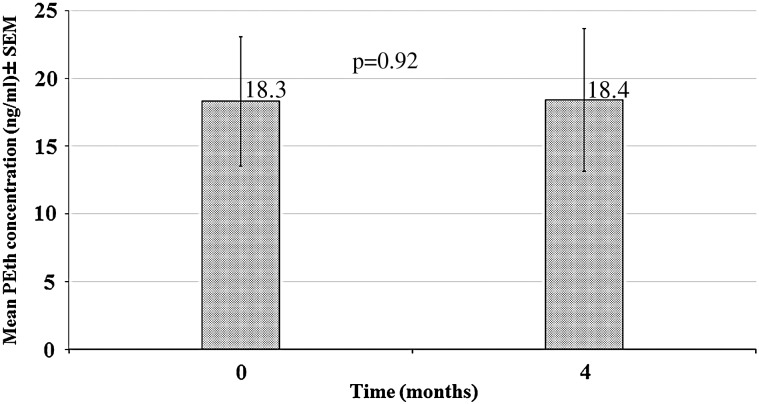
The newborn sample included mostly Hispanic (60%) male (80%) infants, four of whom were full-term and one preterm. The mean baseline PEth concentration in this sample was 18.3 ± 4.8 ng/ml (Fig. 3). At 4 months, the mean concentration was unchanged (18.4 ± 5.3 ng/ml; P = 0.92). Of note, all adult and newborn samples had detectable PEth levels at the end of the time course, thus all patients enrolled would be considered ‘positive’ for alcohol exposure after several months.
Fig. 3.
Mean PEth concentration of newborn cards (n = 5) stored for 4 months in −80°C. Note: baseline samples were stored at room temperature for 2 weeks prior to analysis.
DISCUSSION
Our results indicate that PEth appears to be stable in dry media for at least 9 months, especially when stored at +4°C or −80°C conditions. Repeated measures analysis demonstrated a significant effect of temperature and time-by-temperature interaction on PEth degradation. No significant differences between the two colder conditions were observed. Cards stored at room temperature had a higher rate of degradation (13.5% over 9 months); however, while statistically significant, this decrease is unlikely to be analytically significant. It appears that the most substantial drop occurred during the first 4 months of storage with very little decrease after that—an observation which requires careful examination in future studies with larger sample size and longer follow-up period. Storage in −80°C had minimal, if any, effect on the PEth follow-up concentrations in both adult and newborn specimens.
The moderate correlation between baseline PEth and BAC is an expected finding given very different detection windows for the two biomarkers. While PEth is typically elevated due to chronic or episodic moderate alcohol consumption and might be detectable in peripheral blood up to 3 weeks after the last drinking episode (Aradottir et al., 2006b; Marques et al., 2011; Wurst et al., 2012), BAC is a measure of alcohol consumption in the past 12 h.
To our knowledge, this is the first report examining the longer-term stability of PEth in DBS cards and the effect of different storage conditions in a systematic way. While several studies examined stability of PEth in liquid specimens (Aradottir et al., 2004; Helander and Zheng, 2009), we are not aware of any published studies examining long-term stability of PEth in DBS cards. A study by Faller et al. examined stability of PEth-DBS over a shorter period of 30 days. In that study, PEth-free heparinized fresh blood specimens spiked with a known concentration of PEth, as well as pooled EDTA blood samples from five adult subjects undergoing alcohol detoxification, were spotted onto the DBS cards and stored at either 20°C or −20°C (Faller et al., 2013). In five authentic samples, PEth was stable over 30 days in either temperature condition (<5% degradation). In spiked blood specimens, PEth was found to be less stable, especially at room temperature. This was thought to be due to free PEth having a higher susceptibility to hydrolysis than PEth bound within the erythrocyte membrane. We are also not aware of any studies which assessed stability of PEth in DBS specimens obtained from newborns. Even though our study included a limited number of newborn specimens stored only for 4 months, preliminary results indicate insignificant PEth degradation at −80°C.
Several limitations need to be acknowledged. First, in addition to PEth degradation, assay accuracy could have attributed to within-subjects variability in PEth concentrations. The USDTL reports an intra-assay coefficient of variation between 1.6 and 6.6%; however, this variability is not expected to affect one particular storage condition over another. Second, the effect of humidity and pH were not taken into account. Higher humidity can potentially accelerate PEth degradation, and needs to be examined in follow-up studies. Cards stored in state repositories at −80°C are expected to be less affected by variability in humidity as it is expected to be uniform. Third, we acknowledge that the primary application of PEth analysis in DBS cards would be in newborns as a screening tool for PAE, especially if DBS cards stored in state repositories can be used for these purposes. However, the majority of our specimens were obtained from adults with higher blood alcohol and PEth concentrations than is expected to be observed in most newborns. In addition, adult DBS specimens were obtained by pipetting blood obtained during phlebotomy on Guthrie cards. We attempted to balance this limitation by including 4-month −80°C stability data in a small sample of newborn dry blood spots. In addition, recent data presented in an abstract form demonstrated a very strong correlation (r = 0.97) between PEth in venous blood spot and finger blood (capillary) spot (Javors et al., 2015).
The primary application of our study could be to confirm prenatal alcohol exposure in banked DBS cards in a child suspected to have FASD. Currently, however, the storage duration and conditions of DBS cards in state repositories greatly vary. Most states store DBS cards either at room temperature, +4°C or at −20°C, with only Iowa storing samples at −80°C for a year (and additional 4 years at room temperature (Newborn Screening Translational Research Network, 2015). The retention time among state repositories varies from one month (Kansas, South Dakota) to more than 20 years (California, Maine, Maryland, Massachusetts, Michigan, New Jersey, New York, North Dakota, Rhode Island, Vermont, Washington). In addition, state laws and regulations regarding residual DBS cards also greatly vary (Marques et al., 2011). Most states do not fully address legal and ethical issues related to the retention and use of DBS cards beyond newborn screening (Marques et al., 2011). In addition, the Newborn Screening Saves Lives Reauthorization Act H.R. 1281, section 12, which went into effect on March 18, 2015, states that research cannot be done on DBS without parental consent. The effect of this new law on specific state repositories (some states currently use an opt-out umbrella consent) is not yet fully clarified. It is also unclear whether identified DBS cards can be used for clinical purposes, such as early and reliable diagnosis of FASD. Clearly, if the state laws actually allow such use, parental/guardian consent would be required in a child suspected of FASD in order to obtain an identifiable DBS card from the state repository.
In summary, this is the largest study to date examining PEth stability in DBS cards over 9 months. While overall stability results are encouraging, the current storage conditions in most state repositories underline the need for longer real-time and accelerated (elevated stress conditions such as temperature, humidity and pH) stability tests (Magari, 2003) in newborn DBS cards. Future report will examine PEth stability in the remaining spots at 18 and 27 months. While a detailed report of legal and ethical issues related to the use of DBS cards for research and clinical purposes is beyond the scope of this manuscript, these issues are critically important and need to be addressed before any targeted screening programs are put forward.
FUNDING
This project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) through Grant Number 8UL1TR000041. Dr Bakhireva's effort is partially supported by the NIH/NIAAA R01 AA021771 and R15 AA022242 grants.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- Anderson JE, Ebrahim S, Floyd L et al. (2006) Prevalence of risk factors for adverse pregnancy outcomes during pregnancy and the preconception period—United States, 2002–2004. Mat Child Health J 10:S101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aradottir S, Seidl S, Wurst FM et al. (2004) Phosphatidylethanol in human organs and blood: a study on autopsy material and influences by storage conditions. Alcohol Clin Exp Res 28:1718–23. [DOI] [PubMed] [Google Scholar]
- Aradottir S, Asanovska G, Gjerss S et al. (2006) Phosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol Alcohol 41:431–7. [DOI] [PubMed] [Google Scholar]
- Bakhireva LN, Savage DD (2011) Focus on: biomarkers of fetal alcohol exposure and fetal alcohol effects. Alcohol Res Health 34:56. [PMC free article] [PubMed] [Google Scholar]
- Bakhireva LN, Savich RD, Raisch DW et al. (2013) The feasibility and cost of neonatal screening for prenatal alcohol exposure by measuring phosphatidylethanol in dried blood spots. Alcohol Clin Exp Res 37:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhireva LN, Leeman L, Savich RD et al. (2014) The validity of phosphatidylethanol in dried blood spots of newborns for the identification of prenatal alcohol exposure. Alcohol Clin Exp Res 38:1078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhireva LN, Lowe JR, Gutierrez HL et al. (2015) Ethanol, Neurodevelopment, Infant and Child Health (ENRICH) prospective cohort: study design considerations. Adv Pediatr Res 2 http://www.apr-journal.com/archives/360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller A, Richter B, Kluge M et al. (2011) LC-MS/MS analysis of phosphatidylethanol in dried blood spots versus conventional blood specimens. Anal Bioanal Chem 401:1163–6. [DOI] [PubMed] [Google Scholar]
- Faller A, Richter B, Kluge M et al. (2013) Stability of phosphatidylethanol species in spiked and authentic whole blood and matching dried blood spots. Int J Legal Med 127:603–10. [DOI] [PubMed] [Google Scholar]
- Feldman HS, Jones KL, Lindsay S et al. (2012) Prenatal alcohol exposure patterns and alcohol-related birth defects and growth deficiencies: a prospective study. Alcohol Clin Exp Res 36:670–6. [DOI] [PubMed] [Google Scholar]
- Gnann H, Thierauf A, Hagenbuch F et al. (2014) Time dependence of elimination of different PEth homologues in alcoholics in comparison with social drinkers. Alcohol Clin Exp Res 38:322–6. [DOI] [PubMed] [Google Scholar]
- Hannuksela ML, Liisanantti MK, Nissinen AE et al. (2007) Biochemical markers of alcoholism. Clin Chem Lab Med 45:953–61. [DOI] [PubMed] [Google Scholar]
- Hartmann S, Aradottir S, Graf M et al. (2007) Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol 12:81–4. [DOI] [PubMed] [Google Scholar]
- Helander A, Zheng Y (2009) Molecular species of the alcohol biomarker phosphatidylethanol in human blood measured by LC-MS. Clin Chem 55:1395–405. [DOI] [PubMed] [Google Scholar]
- Isaksson A, Walther L, Hansson T et al. (2011) Phosphatidylethanol in blood (B-PEth): a marker for alcohol use and abuse. Drug Test Anal 3:195–200. [DOI] [PubMed] [Google Scholar]
- Jacobson SW. (1998) Specificity of neurobehavioral outcomes associated with prenatal alcohol exposure. Alcohol Clin Exp Res 22:313–20. [DOI] [PubMed] [Google Scholar]
- Javors M KN, Roache J, Dougherty D (2015) Clinical lab study to characterize phosphatidylethanol as a biomarker of ethanol consumption. Alcohol Clin Exp Res 39 No. 6 (abstract 167). [Google Scholar]
- Jones J, Jones M, Plate C et al. (2011) The detection of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanol in human dried blood spots. Anal Methods 3:1101–6. [Google Scholar]
- Kodituwakku PW. (2009) Neurocognitive profile in children with fetal alcohol spectrum disorders. Dev Disabil Res Rev 15:218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak HS, Han JY, Choi JS et al. (2014) Characterization of phosphatidylethanol blood concentrations for screening alcohol consumption in early pregnancy. Clin Toxicol 52:25–31. [DOI] [PubMed] [Google Scholar]
- Maenhout TM, De Buyzere ML, Delanghe JR (2013) Non-oxidative ethanol metabolites as a measure of alcohol intake. Clin Chimica Acta 415:322–9. [DOI] [PubMed] [Google Scholar]
- Magari RT. (2003) Assessing shelf life using real-time and accelerated stability tests: although accelerated tests are needed, real-time tests are the ultimate proof. Biopharm Int 16:36–48. [Google Scholar]
- Marques P, Hansson T, Isaksson A et al. (2011) Detection of phosphatidylethanol (PEth) in the blood of drivers in an alcohol ignition interlock program. Traffic Inj Prev 12:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP (2001) Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health 25:159–67. [PMC free article] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J et al. (2014) Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics 134:855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newborn Screening Translational Research Network. (2015) State profiles: American College of Medical Genetics and Genomics. https://www.nbstrn.org/, (24 April 2015, date last accessed).
- Stewart SH, Law TL, Randall PK et al. (2010) Phosphatidylethanol and alcohol consumption in reproductive age women. Alcohol Clin Exp Res 34:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga A, Alling C (2002) Formation of phosphatidylethanol in vitro in red blood cells from healthy volunteers and chronic alcoholics. J Lab Clin Med 140:79–83. [DOI] [PubMed] [Google Scholar]
- Varga A, Hansson P, Johnson G et al. (2000) Normalization rate and cellular localization of phosphatidylethanol in whole blood from chronic alcoholics. Clinica Chimica Acta 299:141–50. [DOI] [PubMed] [Google Scholar]
- Wurst FM, Thon N, Weinmann W et al. (2012) Characterization of sialic acid index of plasma apolipoprotein J and phosphatidylethanol during alcohol detoxification—A pilot study. Alcohol Clin Exp Res 36:251–7. [DOI] [PubMed] [Google Scholar]