Abstract
The Wnt signalling cascades have essential roles in development, growth and homeostasis of joints and the skeleton. Progress in basic research, particularly relating to our understanding of intracellular signalling cascades and fine regulation of receptor activation in the extracellular space, has provided novel insights into the roles of Wnt signalling in chronic arthritis. Cartilage and bone homeostasis require finely tuned Wnt signalling; both activation and suppression of the Wnt–β-catenin cascade can lead to osteoarthritis in rodent models. Genetic associations with the Wnt antagonist encoded by FRZB and the transcriptional regulator encoded by DOT1L with osteoarthritis further corroborate the essential part played by Wnts in the joint. In rheumatoid arthritis, inhibition of Wnt signalling has a role in the persistence of bone erosions, whereas Wnts have been associated with the ankylosing phenotype in spondyloarthritis. Together, these observations identify the Wnt pathway as an attractive target for therapeutic intervention; however, the complexity of the Wnt signalling cascades and the potential secondary effects of drug interventions targeting them highlight the need for further research and suggest that our understanding of this exciting pathway is still in its infancy.
Introduction
The complexity of Wnt signalling
Wnt signalling cascades modulate biological processes from early embryonic development to organogenesis, to growth and postnatal tissue homeostasis, and are involved in a number of diseases.1 The Wnt family comprises at least 19 members that interact with distinct receptor subtypes and activate several downstream cascades. The complexity of Wnt signalling, the limited understanding of specific ligand–receptor interactions, the unusual downstream cascades and the seemingly limitless involvement of this pathway in different biological processes have attracted the attention of an ever-growing research community. The identification of key roles for Wnts in skeletal and joint development triggered research into the involvement of these molecules in chronic arthritis, particularly osteoarthritis (OA) but also inflammatory diseases such as rheumatoid arthritis (RA) and spondyloarthritis (SpA).
Properties of Wnt proteins
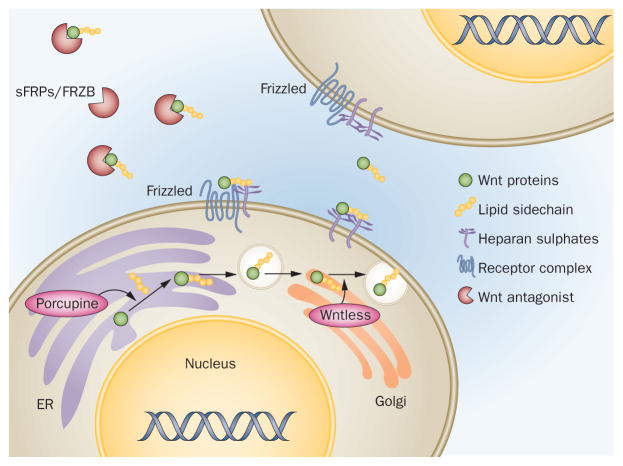
Wnts are glycoproteins that are further modified through addition of a lipid moiety, which influences the interactions of these proteins with receptors and affects their mobility, as well as their use in experimental settings.1 Until recently, Wnts could not be successfully purified or synthesized as recombinant molecules, limiting their availability for in vitro experiments. The post-translational modification of Wnt proteins depends on intracellular processes regulated by enzymes such as porcupine in the endoplasmic reticulum and Wntless in the Golgi apparatus (Figure 1).2,3 Porcupine is a protein-cysteine N-palmitoyltransferase that catalyses the covalent bonding of a palmitoyl lipid molecule to the Wnt protein, whereas Wntless has essential roles in the cytoplasmic shuttling and secretion of Wnts.
Figure 1.
The complex synthesis and signalling properties of Wnt molecules. Wnts are glycosylated and palmitoylated in the ER. The latter process results in covalent attachment of a fatty acid chain to the Wnt protein, catalyzed by the enzyme porcupine. Wntless is a Golgi apparatus molecule essential for further trafficking of Wnts and their secretion. Membrane bound heparan sulphate proteoglycans on the surface of the Wnt-producing source cell and the target cells can interact with Wnt, thereby contributing to establishment of concentration gradient of Wnt expression. Wnt antagonists such as FRZB (also known as sFRP3) and other sFRPs can act as direct antagonist, sequestering secreted Wnts, but in doing so can also increase the signalling range of Wnts by acting as molecular shuttles that carry the ligand away from the source cells. Abbreviations: ER, endoplasmic reticulum; sFRPs, secreted frizzled-related proteins.
Developmental processes depend on concentration gradients of morphogens, which determine the signal intensity in a particular cell—based on the localization of the cell in the gradient—and thus offer spatial and temporal control of cellular differentiation patterns.4 The lipid modification of Wnts is likely to complicate the control of their signalling range, particularly considering the hydrophobic nature of the palmitoyl moiety. Interactions with heparan sulfate glycoproteins on the cell surface and in the extracellular matrix, as well as with lipid membranes and various Wnt-binding molecules with antagonizing and shuttling properties all influence the range of activity of secreted Wnts and the cells targeted for activation (Figure 1).5–7 These features could be of particular importance in tissues rich in extracellular matrix, such as cartilage and bone, and in interactions with synovial fluid molecules such as hyaluronan and lubricin.
Wnt signalling cascades
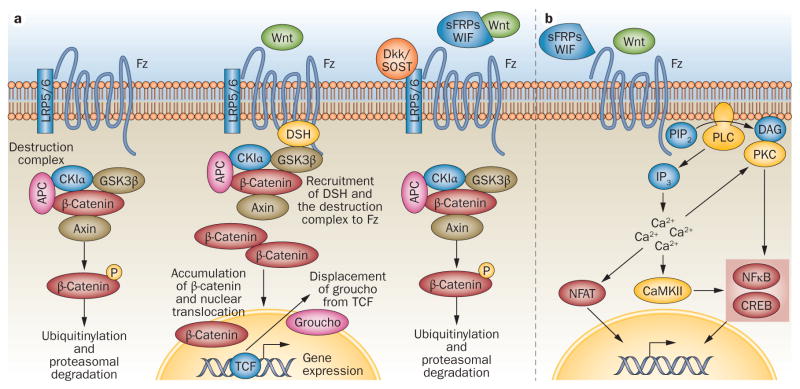
Wnt proteins activate a number of different signalling cascades (Figure 2).1,8 The best understood pathway is the so-called ‘canonical’ Wnt signalling pathway (Figure 2a), which results in the translocation of β-catenin to the nucleus. Other cascades are collectively classified as ‘non-canonical’ (Figure 2b). The difficulties associated with production of recombinant Wnts contribute to the persistence of gaps in our knowledge of specific ligand–receptor interactions involved in these pathways. To date the main cell surface Wnt receptors that have been identified are the Frizzled (Fz) receptors. Fz receptors are G-protein-coupled seven-transmembrane domain proteins with a Wnt interacting cysteine-rich extracellular domain. The Fz receptors are promiscuous, and a single Fz receptor can bind to a range of Wnt proteins and vice versa.
Figure 2.
Wnt signalling cascades. a | The canonical Wnt signalling cascade is dependent on intracellular signalling molecule β-catenin. In the absence of Wnt binding to Fz receptors, β-catenin is sequestered into a destruction complex composed of Axin, CK1α, APC and GSK3β, phosphorylated, ubiquitinylated and subsequently degraded by the proteasome. Upon binding of Wnt to Fz receptors and LRP5/6 co-receptors, DSH interacts with the receptor complex and recruits the destruction complex to the cell membrane allowing newly synthesized β-catenin to accumulate within the cytoplasm and to translocate to the nucleus. Nuclear β-catenin can displace the transcriptional co-repressor groucho from TCF transcription factors and promote activation of a gene transcription program. Both Wnt-binding antagonists (sFRPs/WIF) and Wnt receptor antagonists (Dkk/SOST) inhibit the canonical cascade. b | In the noncanonical Wnt signalling cascade, different phosphorylation cascades are activated by specific ligand–receptor interactions, seemingly without engagement of the LRP co-receptors. Many of these cascades are triggered by an increase in intracellular Ca2+ concentrations secondary to PLC- and DAG production. Subsequently, PKC and CaMKII can activate transcription factors like NFκB and CREB, mediated IP3 and calmodulin is involved in the activation of NFAT. Only the Wnt-binding antagonists inhibit the noncanonical cascade. Abbreviations: APC, adenomatous polyposis coli; CaMKII, calcium/calmodulin-dependent protein kinase type II; CK1α, caseine kinase 1-α; CREB, cyclic AMP-responsive element-binding protein; DAG, diacylglycerol; Dkk, Dickkopf; DSH, disheveled; GSK3β, glycogen synthase kinase-3 β; IP3, inositol 1,4,5-triphosphate; LRP, low-density lipoprotein receptor-related protein; NFAT, nuclear factor of activated T cells; NFκB, nuclear factor κB; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PLC, phospholipase C; sFRPs, secreted frizzled-related proteins; SOST, sclerostin; WIF, Wnt inhibitory factor.
The canonical cascade
Canonical cascade activation is dependent on further interaction of the Wnt–Fz complex with co-receptors including low-density lipoprotein receptor-related protein (LRP) 5/6. Unlike many signalling cascades, ligand– receptor interaction does not result in a consecutive cascade of downstream phosphorylation and kinase activation, but in the stoichiometric titration of a signalling inhibition complex (Figure 2a).9
The key mediator within the canonical cascade is β-catenin, a molecule with multiple functions, including a role in cell adhesion. In the absence of Wnt activation, cytoplasmic β-catenin is caught in a ‘destruction complex’ composed of adenomatous polyposis coli protein (APC), glycogen synthase kinase-3 β (GSK3β), casein kinase 1-α (CKIαs) and the axin proteins, which results in phosphorylation and subsequent ubiquitin-mediated degradation of β-catenin by the proteasome (Figure 2a). Upon Wnt–Fz interaction and phosphorylation of LRP co-receptors, the destruction complex is tethered away from the cytoplasm through interaction with the receptor complex at the cell membrane allowing newly synthesized β-catenin to accumulate in the cytoplasm and translocate to the nucleus.9 Dishevelled, which interacts with the cytoplasmic region of Fz as well as a number of members of the destruction complex, is a key protein in this process. This stoichiometric view of β-catenin accumulation is novel; previously, it was theorized that ligand–receptor interaction resulted in breakdown of the destruction complex thereby freeing β-catenin.1 β-catenin is not itself a transcription factor but binds to a number of transcription factors, co-activators or co-repressors. After translocating to the nucleus, β-catenin displaces the transcriptional co-repressor groucho from TCF transcription factors. With TCF-bound β-catenin functioning as a scaffold, a multiprotein complex is formed and acts to fine tune the gene transcription machinery and thus gene expression.1
The noncanonical cascades
Although the noncanonical Wnt signalling cascades have important roles in the regulation of cell shape, orientation, mobility, differentiation and communication,8,10,11 the elements of these pathways are less well defined. In the non-canonical cascades Fz receptors seem to function similarly to other G-protein-coupled receptors, activating enzymatic cascades such as those involving the mitogen activated kinases (MAPKs) c-Jun N-terminal kinase (JNK) and p38, phospholipase-C, phosphodiesterase, and protein kinase C (Figure 2b).8 Subsequent events can include JNK interacting with the actin cytoskeleton in cell migration. Other cascades converge on intracellular calcium release, which leads to activation of calcium/calmodulin-dependent protein kinase II (CaMKII) and/or the transcription factor nuclear factor of activated T cells (NFAT) via calcineurin. These downstream processes probably inhibit canonical Wnt signalling, adding another level of complexity. Noncanonical signals can also be mediated by alternative receptors, such as tyrosine-protein kinase transmembrane receptor ROR2,12 and co-receptors, such as syndecan-4.13
Key points.
Wnts are lipid-modified glycoproteins that activate a variety of signalling pathways in an autocrine or paracrine manner; target cell activation is dependent on the formation of complex signalling centres
Wnts are essential for articular cartilage and bone homeostasis, but excessive Wnt signalling is associated with loss of the stable articular chondrocyte phenotype and can lead to cartilage damage
In rheumatoid arthritis, healing of erosions can be inhibited by elevated local concentrations of Wnt antagonists such as Dickkopf-related protein 1 and secreted frizzled-related protein 1
Wnts are associated with ankylosis in spondyloarthritis, and data from developmental biology suggest that specific Wnts stimulate direct bone formation and not the initial steps in endochondral ossification
Various Wnt antagonists have been proposed as biomarkers in patients with spondyloarthritis, but further validation is required
Wnt pathway antagonists
Many additional factors play a part in the activation of receptors for Wnts, including a number of antagonists. For example, Dickkopfs (Dkks) and sclerostin inhibit LRP co-receptors and are considered specific antagonists of the canonical cascades. Additionally, the Wnt inhibitory factor (WIF) and the secreted frizzled-related protein (sFRP) families comprise secreted molecules that bind to Wnts in the extracellular space and can interfere with both canonical and noncanonical cascades. Views regarding WIF and sFRPs are rapidly evolving, with evidence suggesting that they are not pure antagonists but can also contribute to receptor activation, both directly and indirectly, by acting as molecular shuttles that extend the range of Wnt signalling (Figure 1).5,6 Other proteins, belonging to the Norrin and R-spondin families, interact with Fz and/or LRP receptors and act as Wnt agonists.14,15 R-spondins do not trigger a direct activation of the canonical cascade, but potentiate Wnt signalling through interaction with leucine-rich repeat containing G-protein-coupled receptors.15
Wnts in bone and joint development
The Wnt signalling cascade is essential from early development, through organogenesis, to tissue homeostasis. On the basis of the hypothesis that recapitulation of embryonic signalling has a role in remodelling and repair responses in arthritic disease, rheumatological research has increasingly paid attention to advances in developmental biology. Our current understanding of the role of Wnts and Wnt antagonists in skeletal development and joint disease, as discussed in the following sections, are summarized in Tables 1 and 2, respectively. The Wnt pathway demonstrates an extraordinary degree of complexity and fine-tuning, and many aspects remain unclear, in particular, the roles of specific ligands and receptors and the balance between canonical and noncanonical cascades. The spatiotemporal context and the differentiation status of the cell involved are factors that define cell behaviour on activation of Wnt signalling cascades (Figure 3).16,17
Table 1.
Wnts associated with skeletal development and joint disease
| Protein | Association with skeletal development and growth | Role in joint disease |
|---|---|---|
| Wnt1 | Not found in developing mouse limb24 | Unknown |
| Wnt2a | Expressed in developing muscle in mice; not associated with skeletal differentiation24 | Unknown |
| Wnt2b | Expressed in growth plate chondrocytes92 | Upregulated in the synovium in mouse models of OA49 |
| Wnt3 | Expressed in the AER in mice19,24 | Upregulated in the synovium in mouse models of OA49 |
| Wnt3a | Expressed in the AER in chickens93 | Can stimulate both canonical and noncanonical signalling dependent on concentration and context65 Often used as a reagent in the experimental setting |
| Wnt4 | Expressed in developing joints19,24,94,95 | Downregulated in the synovium during the early phase of mouse collagenase-induced OA49 |
| Wnt5a | Expressed in the AER, developing chondrocytes and the perichondrium; involved in chondrocyte and osteoblast differentiation24,95,96 | Associated with proinflammatory and tissue-destructive processes, including osteoclast differentiation53,54,76 |
| Wnt5b | Expressed in the AER, developing chondrocytes and the perichondrium; involved in chondrocyte and osteoblast differentiation24,95,96 | Upregulated in the synovium during the early phase of mouse collagenase-induced OA49 |
| Wnt6 | Expressed in the AER and the ectoderm; not associated with skeletal differentiation24 | Upregulated in the synovium during the early phase of mouse collagenase-induced OA49 |
| Wnt7a | Expressed in the ectoderm; not associated with skeletal differentiation24 | Unknown |
| Wnt7b | Expressed in the ectoderm and the perichondrium24 | Upregulated by IL-1 in human chondrocytes, activating canonical Wnt signalling67 Upregulated in the synovium during the late phase of disease in the STR/ort mouse model of OA49 |
| Wnt8a | Not found in developing mouse limb24 | Unknown |
| Wnt8b | Not found in developing mouse limb24 | Unknown |
| Wnt9a | Expressed in developing joints24,94,95 | Upregulated in the cartilage during the late phase of disease in the STR/ort mouse model of OA49 |
| Wnt9b | Expressed in the ectoderm; not associated with skeletal differentiation24 | Upregulated in the synovium during the early phase of disease in the mouse STR/ort model of OA49 |
| Wnt10a | Expressed in the AER and the ectoderm; not associated with skeletal differentiation24 | Downregulated in cartilage during the intermediate phase of disease in the STR/ort mouse model of OA49 |
| Wnt10b | Expressed in the AER and the ectoderm; not associated with skeletal differentiation24 | Upregulated during the resolution/healing phase of serum-induced arthritis in mice68 |
| Wnt11 | Expressed in the AER, the mesenchyme close to condensations, prehypertrophic chondrocytes and developing joints24 | Upregulated in the synovium in the STR/ort mouse model of OA49 |
| Wnt16 | Expressed in developing joints, perichondrium and periosteum24,94 | Upregulated in articular cartilage after injury and in the synovium of mouse OA models48,49 |
Abbreviations: AER, apical ectodermal ridge; OA, osteoarthritis.
Table 2.
Wnt antagonists associated with skeletal development and joint disease
| Protein | Association with skeletal development and growth | Role in joint disease |
|---|---|---|
| sFRP1 | Expressed in developing muscle tendon insertions and proliferating and prehypertrophic chondrocytes24 | Expressed at bone erosions and downregulated during resolution/healing phase of serum-induced arthritis in mice68,69 Reduced levels in articular cartilage in STR/ort mice97 |
| sFRP2 | Expressed in mesenchymal condensations and developing joints24,98 | Downregulated during resolution/healing phase of serum-induced mouse arthritis68 |
| sFRP3/FRZB | Expressed in prehypertrophic and hypertrophic chondrocytes, the perichondrium and the periosteum24,59,98 | Genetically associated with hip shape and hip OA in humans41–45,90 Frzb knockout mice had increased cartilage damage in different OA models59 |
| sFRP4 | Expressed in areas of hypertrophic chondrocyte regression and osteoblast invasion24 | Unknown |
| sFRP5 | Expressed in proliferating and prehypertrophic chondrocytes24 | Unknown |
| Dkk1 | Expressed in the perichondrium and the periosteum24 | Inhibition shifts the phenotype of mouse arthritis from destruction to new bone formation73 Associated with reduced healing of joint erosions in RA69 Genetic variants are associated with joint destruction in RA71 Positive and negative effects in animal models of OA61,62 Proposed as biomarker in SpA and OA86,90 |
| Dkk2 | Expressed in areas surrounding developing cartilage24 | Upregulated during resolution/healing phase of serum-induced arthritis in mice68 Downregulated in human OA cartilage62 |
| Dkk3 | Expressed in carpal condensations and in the developing joint24 | Unknown |
| Dkk4 | Not found in developing mouse limb24 | Unknown |
| Sclerostin | Expressed in the perichondrium and the periosteum99 | Expressed in articular cartilage, but no confirmed effect in animal models of OA100,101 Proposed as biomarker in SpA86,88 |
Abbreviations: Dkk, dickkopf-related protein; OA, osteoarthritis; RA, rheumatoid arthritis; sFRP, secreted frizzled-related protein; SpA, spondyloarthritis.
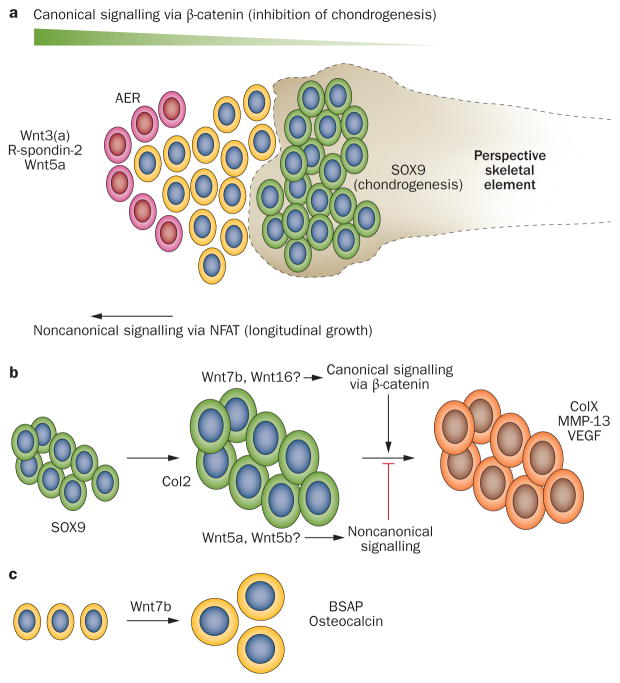
Figure 3.
The varied roles of Wnts in skeletal development. a | At the early phase of skeletogenesis, expression of Wnts, such as Wnt3 (known as Wnt3a in some organisms) and Wnt5a, at the AER keeps the progenitor cells (red cells) in a proliferative state and prevents differentiation into chondrocytes (yellow cells). Absence of sufficient Wnt signalling in cells further along the concentration gradient allows them to express the chondrogenic transcription factor SOX9 (green cells). Condensations of these chondrocytes occur distant from the Wnt source and form the cartilage template for the prospective skeletal elements. Canonical, β-catenin-dependent Wnt signalling by Wnt3a, with co-activation by R-spondin-2, has been linked to inhibition of chondrogenesis, whereas noncanonical signalling, induced by Wnt5a and involving NFAT, has been associated with proximal–distal growth. b | During endochondral bone formation, Wnt signalling is critical in the final differentiation steps towards chondrocytes hypertrophy when cells start expressing ColX, MMP-13 and VEGF (orange cells); canonical Wnt signalling stimulates this process. Chondrocyte differentiation might be triggered by Wnt7b and Wnt16 that are expressed in developing chondrocytes, which are positive for Col2 (large green cells). By contrast, noncanonical cascades triggered by Wnt5a or Wnt5b might inhibit chondrocytes hypertrophy. c | During direct bone formation, Wnts stimulate the differentiation from osteoblast precursors towards mature osteoblasts, which produce BSAP and osteocalcin. Wnt7b has been specifically associated with this process. Abbreviations: AER, apical ectodermal ridge; BSAP; bone-specific alkaline phosphatase; Col2, collagen type II; ColX, collagen type X; MMP-13, matrix metalloproteinase-13 (also known as collagenase 3); NFAT, nuclear factor of activated T cells; VEGF, vascular endothelial growth factor.
Wnts in endochondral ossification
The outline of the skeleton is mainly produced through a process called endochondral ossificiation.18 At the site of the presumptive skeletal elements, cells aggregate, condense and adhere after a proliferation phase necessary for limb outgrowth. Within this cell condensation, chondrogenic differentiation goes through different stages whilst a cartilage ‘model’ of the skeleton is laid down, with cells proliferating and further differentiating towards hypertrophy. Hypertrophy is a terminal differentiation state as the cells subsequently go into apoptosis, but not before attracting osteoprogenitor and vascular progenitor cells that ultimately replace the cartilage model with bone. After the initial developmental patterning, this process is further organized in the growth plates and remains active until the epiphyseal plates fuse. At the outer surfaces of the cartilage template and the growing bone, osteoprogenitor cells directly differentiate into osteoblasts and form a bone collar through direct bone formation, supporting the growth of the new bone. Together, these processes define the structure of the bones.
Wnt signalling in early skeletogenesis
During skeletogenesis, Wnt signalling has distinct regulatory roles defined by the differentiation status of the cells (Figure 3). At the early phase of limb growth, a distal cell population at the apical ectodermal ridge (AER) shows active Wnt signalling (Figure 3a), which keeps the cells in a proliferative and undifferentiated state that is essential for limb outgrowth.16 Wnt proteins usually have a short range, and cells distant from the AER source of Wnt are subject to less Wnt-induced signalling. As canonical Wnt signalling suppresses the expression of chondrogenic master regulator Sox9, these distal cells condense and commit to chondrogenic differentiation.
Experiments utilizing the chick limb model and transgenic mice have enabled the identification of the specific Wnts involved in early endochondral ossification. The AER is defined and shaped by complex interactions between Wnt and fibroblast growth factor (FGF) signalling.19,20 FGF-10 has been shown to induce the production of Wnt3 in mice and Wnt3a in chickens, which lead to FGF-8 gene expression via activation of the canonical pathway.19,20 In addition, the Wnt agonist R-spondin-2 (Rspo2) plays a part in this process by maintaining FGF expression, as demonstrated by particular skeletal malformations that primarily affect hindlimb development in Rspo2−/− mice.21 The signalling gradient established by Wnt3 and Wnt3a in mice and chickens, respectively, determines skeletal cell differentiation along the proximodistal axis. By contrast, activation of the non-canonical ROR2–JNK signalling cascade by WNT5A expression stimulates distal-directed growth of the AER cells.22 In the chick limb model, other Wnts (Wnt2b and Wnt8c) seem to contribute specifically to definition of the AER by upregulating expression of FGF10.20
In early skeletal progenitor cells, the Wnt pathway decides between osteogenic or chondrogenic differentiation. The accumulated in vitro and in vivo data are sometimes confusing, as overexpression studies or the use of cell lines might not be representative of spatiotemporally regulated processes that occur in vivo. Nonetheless, overexpression of WNT1 and WNT7A has been shown to block chondrogenesis in the chick limb model.23 However, Wnt1 does not appear to be expressed in developing mouse limb, whereas Wnt7a is restricted to the ectoderm.24 By contrast, Wnt2b, Wnt4, Wnt5a, Wnt5b, Wnt7b Wnt9a, Wnt11 and Wnt16 mRNA expression has been detected during limb skeletal development (Table 1).24 In general, activation of canonical Wnt cascades inhibits the early stages of chondrogenesis,25–27 and Wnt5a and Wnt5b promote early chondrogenesis through activation of noncanonical Wnt signalling.28
The roles of Wnts in bone formation
At later stages of endochondral bone formation, the impact of Wnt signalling is different as the pathway stimulates cells to undergo terminal differentiation, resulting in the production of so-called hypertrophic chondrocytes expressing type X collagen, vascular endothelial growth factor (VEGF) and matrix metallo-proteinase-13 (MMP-13), which are subsequently replaced by bone (Figure 3b).18 Expression data demonstrate the presence of Wnt5a, Wnt5b and Wnt11 in pre-hypertrophic chondrocytes.24 Chondrocyte hypertrophy is stimulated by canonical Wnt signalling through inhibition of parathyroid hormone related protein (PTHrP) signalling.26,29–32 By contrast, Wnt5a and Wnt5b are able to inhibit chondrocyte hypertrophy by activating nuclear factor κB (NFκB) and JNK signalling, respectively, as demonstrated in models of chondrogenesis.33,34
During the final stages of endochondral bone formation and in the bone collar where direct bone formation occurs, Wnts act as agonists to osteoprogenitor cell differentiation, promoting osteoblast maturation (Figure 3c). Furthermore, active Wnt signalling in chondrocytes seems to stimulate direct perichondrial bone formation;32 in vivo evidence supports an essential role for canonical Wnt signalling and Wnt7b has been specifically associated with this process.25,35
Wnt signalling in joint development
Cells in the joint interzone do not differentiate into cartilage thereby interrupting the shaping rods of the developing limb. The dense cell layers at the joint interzone that eventually evolve into the synovial joint and its cavity are associated with expression of Wnt9a (also know as Wnt14) and Wnt4;36 these Wnts have been associated with activation of the canonical cascade in this process, limiting the chondrogenic potential of the joint interzone chondrosynovioprogenitor cells and probably prompting them to become synovial connective tissue.37,38
Wnt signalling in bone growth and repair
The direct impact of Wnts on bone is particularly important in growth and remodelling of the skeleton. The balance between the activities of bone-forming osteoblasts and bone-resorbing osteoclasts is orchestrated by mechanosensing osteocytes. Osteocytes produce Wnts and canonical Wnt signalling stimulates osteoblast maturation; however, osteocytes can also produce sclerostin, which antagonizes LRP co-receptors for Wnt and is a critical mediator of the remodelling process due to its inhibition of osteoblast maturation and thus bone formation.39,40
The initial bone morphogenic functions of Wnt signalling might influence an individual’s response to weight loading and repair. A nested case–control study of white women, aged ≥65 years, from the Study of Osteoporotic Fractures cohort, indicated that individuals with specific genetic variants of FRZB, which encodes the Wnt antagonist sFRP3, had proximal femur shapes comprising wider femoral necks and smaller head diameters (termed ‘mode 2’) than control individuals.41 Subjects with a particular FRZB variant and mode 2 proximal femur shape had an increased risk of developing radiographic hip OA.41 This finding suggests that FRZB and Wnt signalling could serve an important role in determining hip shape and that variation in these factors can influence the relationship between hip shape and OA.41
Wnt research in arthritis
Genetic studies
Translational evidence for a role of Wnt signalling in chronic arthritis has come from genetic studies and from analysis of tissues for expression of molecules comprising the Wnt pathway. Nonsynonymous polymorphisms in FRZB were associated with severe hip OA in a family-based study and this association was subsequently confirmed in other patient populations including elderly women from a community-based population with hip OA.42–44 Functional assessment of the effect of the polymorphisms in FRZB suggested that disease-associated variants result in reduced Wnt inhibitory activity.42 The evidence for this genetic association remains strongest for individuals with hip OA, with a meta-analysis—including novel datasets from genome-wide association studies (GWAS)—failing to confirm the association between FRZB polymorphisms and OA at other joints (knee and hand).45 The inconsistencies observed in studies investigating the association between FRZB alleles and OA might result from differences in the definitions of OA phenotypes used, the quality of the scoring and the number of cases and controls included in different cohorts.41
Additional genetic evidence of a role for Wnt signalling in susceptibility to OA came from a GWAS that demonstrated a link between DOT1L and radiographic joint-space width, used as a proxy for cartilage thickness and hip OA, in a population-based study.46 The association was confirmed in a smaller cohort of hip OA subjects. DOT1L encodes histone H3-K79 methyltransferase, a protein that has been linked to variety of cellular pathways but seems to be active mainly in association with TCF transcription factors of the canonical Wnt cascades.47 Functional assays demonstrated that knockdown of Dot1l in mouse chondrogenic cells interferes with the dynamics of chondrogenic differentiation through a mechanism involving Wnt and TCF signalling.46
Wnt expression analysis in model systems
Further indications that the Wnt pathway can contribute to the development of OA have come from analysis of gene expression in models of acute articular cartilage damage. Dell’Accio et al.48 found that FRZB expression was downregulated in parallel with upregulation of Wnt16 and downstream target genes, and nuclear localization of β-catenin after cartilage injury was induced in adult human cartilage explants. Furthermore, Wnt16 and β-catenin were readily detected in tissue taken from regions of joints with moderate-to-severe OA-related damage, but were almost undetectable in areas where cartilage was preserved.48 Wnts genes for which mRNA was detected in ≥20% of samples but which were not differentially regulated after cartilage damage were WNT2B, WNT3, WNT5A, WNT5B, WNT10A and WNT10B;48 Wnt antagonists genes expressed but without marked changes in abundance after injury included SFRP1, SFRP4 and DKK1–3.48 Another study examined the Wnts present in cartilage in two different mouse models of OA: Blom et al.49 reported expression of Wnt2b, Wnt4, Wnt5a, Wnt6, Wnt7b, Wnt9a, Wnt10a, Wnt10b, Wnt11 and Wnt16 in articular cartilage. In the collagenase-induced OA model, none of these genes seems to be differentially regulated, and only subtle changes at late stages of disease were noted for Wnt6, Wnt9a, Wnt10a, Wnt10b and Wnt16 in the STR/ort model.49 Interestingly, the Wnt target gene Wisp1 was nonetheless found to be strongly upregulated in these analyses.49
Blom et al.49 also reported consistent expression of Wnt2, Wnt2b, Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt9, Wnt10a, Wnt10b, Wnt11 and Wnt16 in the synovium of mice during the course of both OA models used. Upregulation of Wnt16 was most striking, particularly in the collagenase-induced OA model.49 Earlier work suggested that Wnts are expressed in the synovium of patients with chronic arthritis. Sen et al.50 demonstrated expression of WNT1, WNT5A, WNT11, WNT10B and WNT13 in the RA synovium; in particular, WNT5A was expressed at an increased level in RA tissue compared with samples from patients with OA and healthy controls.50 In addition, Nakamura et al.51 detected mRNAs from WNT3, WNT3A, WNT4, WNT5A, WNT5B, WNT7B, WNT8B, WNT9A, WNT9B, WNT10A, WNT10B, WNT11 and WNT13 in a more comprehensive analysis of RA and OA synovial samples; in particular, WNT7B was found to be upregulated in RA synovial samples.51 However, these results were only partially replicated in another study of seven RA synovial samples: expression of WNT10B was detected in five, WNT5A in three, and WNT3A, WNT5B and WNT9A in only one sample.52 Expression of WNT5A, WNT10B, WNT11 and WNT9A was detected in one or two out of the seven OA synovial tissues tested.52 In a series of experiments, Sen et al.50,53,54 provided evidence that over-expression of WNT5A in synovial fibroblasts was associated with the production of proinflammatory cytokines IL-6 and IL-15, and tissue destructive enzymes, invoking a phenotype reflective of RA fibroblast-like synoviocytes. This finding could provide an explanation for the associations observed between expression of WNT5A and chronic arthritis.
Wnts and OA: a challenging network
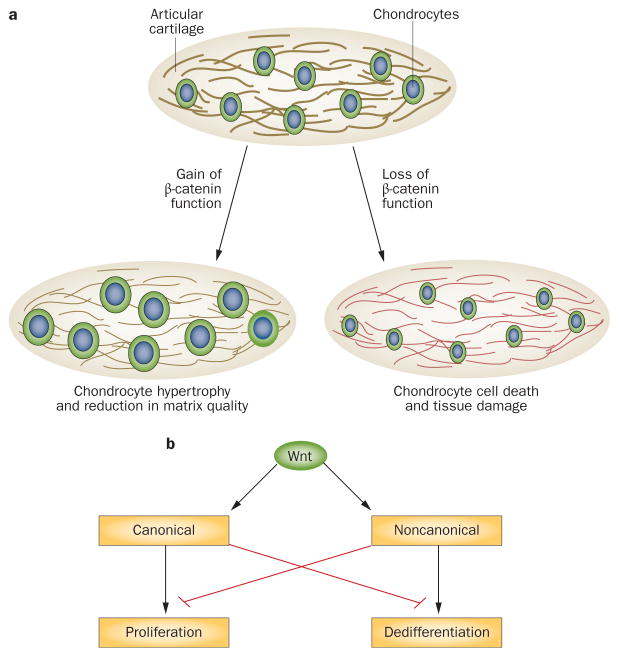
Gaining an understanding of Wnt signalling in OA based on data from disease models has proven difficult, as the results a number of studies, at first sight, seem contradictory.55–57 Cartilage-specific tamoxifen-induced activation of β-catenin in mice aged 3–6 months resulted in joint destruction, with loss of the articular chondrocyte stable phenotype and progression towards terminal hypertrophy associated with OA-like lesions.56 In a different mouse model, tamoxifen-driven activation of β-catenin signalling in cartilage initially induced acute loss of proteoglycans, which was followed by increases in cartilage thickness and cell proliferation.57 Consistent with the observation that the activity of β-catenin regulates chondrocyte proliferation, conditional ablation of β-catenin in chondrocytes led to hypocellularity in articular cartilage.57 Furthermore, transgenic expression of a dominant negative form of β-catenin within the articular cartilage resulted in chondrocyte apoptosis and OA-like disease in mice.55 Thus, mice seem to develop OA-like disease both when β-catenin is activated and inhibited (Figure 4a).
Figure 4.
The complex roles of Wnts in cartilage homeostasis and disease. a | Both overexpression and loss of β-catenin in the articular cartilage lead to joint damage. Overexpression β-catenin results in chondrocytes hypertrophy and loss of matrix quality, whereas loss of β-catenin function results in tissue damage through chondrocyte death. b | Canonical and noncanonical Wnt signalling pathways keep each other in check through reciprocal inhibition. The canonical pathway appears to stimulate proliferation. The noncanonical pathway stimulates dedifferentiation.
Observations made in a surgically-induced model of OA in Lrp5−/− mice were similar to those documented in mice with conditional ablation of β-catenin.58 In mice genetically manipulated to abrogate Frzb expression, the cartilage phenotype was normal;59 however, the severity of enzymatic and inflammation-induced cartilage damage was more severe in the Frzb−/− mice compared with wild-type controls, providing further evidence that excess Wnt signalling is deleterious for the homeostasis of the joint, especially the cartilage.59 Overexpression of Wisp1, a Wnt1 target gene encoding Wnt1-inducible-signalling pathway protein 1, also resulted in increased cartilage damage in mouse models of OA and was associated with increased MMP and aggrecanase expression.49 Moveover, WISP1 expression was found to be increased in cartilage and synovial samples from patients with OA.49 In addition, activation of Wnt signalling by pharmaceutical inhibition of GSK3β increased levels of active (nuclear) β-catenin within the articular cartilage and resulted in cartilage alterations consistent with those seen in early OA, particularly cartilage fibrillation, in a rat model.60 Conversely, knockdown of the Wnt pathway inhibitor Dkk1 using antisense oligonucleotides protected cartilage and bone in a rat model of OA;61 however, overexpression of Dkk1 by adenoviral transfer or in chondrocyte-specific Dkk1 transgenic mice also protected the joint in the mouse destabilization of the medial meniscus model of OA.62
Although contrasting and confusing, most—if not all—of these data from rodent models fit well with general principles of Wnt signalling in skeletal cells. Wnts have a role in the terminal differentiation of chondrocytes, a deleterious outcome in the context of OA. Therefore, different OA models with genetic-based derepression of Wnt signalling in vivo, including the β-catenin activator and the Frzb−/− models, demonstrate more severe disease compared with wild-type controls. On the other hand, a complete absence of canonical Wnt signalling also has adverse effects, as survival of cells within cartilage seems to be impaired. This suggests that balanced control of Wnt signalling in articular cartilage, probably involving a number of Wnt antagonists such as FRZB, Dkk1 and gremlin,63 is required for joint homeostasis. Indeed, transcriptomic analysis of the cartilage–subchondral bone unit in Frzb−/− mice revealed compensatory upregulation of other sFRPs, confirming that Wnt signalling in these tissues is tightly regulated.64
Additional in vitro analyses have provided insights into some of the molecular mechanisms involved in joint homeostasis and susceptibility to arthritis. Nalesso et al.65 found that stimulation of the differentiated human articular chondrocytes with Wnt3a stimulated proliferation, but also increased dedifferentiation, as evidenced by reduced expression of mRNA for aggrecan, collagen type II and transcription factor SOX9 (a master regulator of skeletal development), and by reduced production of sulphated proteoglycans in vitro and in vivo.65 Inhibition of the canonical Wnt cascade using Dkk1 demonstrated that the dedifferentiation process is driven by the noncanonical pathway, in particular by CaMKII and calcium signalling.65 Moreover, evidence suggested that the signalling cascades mediated by CaMKII and β-catenin have reciprocal inhibitory effects (Figure 4b), as treatment of chondrocytes with Dkk1 stimulated noncanonical cascades leading to dedifferentiation.65 This landmark study, therefore, clearly identified the urgent need for detailed knowledge of the balance between canonical and noncanonical signalling to understand the potential approaches to pharmaceutical intervention targeting the Wnt pathway. For instance, although Wnt5a is typically associated with noncanonical signalling, Cailotto et al.66 demonstrated that Wnt5a might contribute to the maintenance of the differentiation status of articular chondrocytes through canonical signalling and identified a link between expression of Wnt5a and pyrophosphate transport regulated by progressive ankylosis protein homolog (ANK).
Caution should be applied when rodent and human systems are compared, as events downstream of WNTs in human cells can be different from those seen in mice.67 For example, Wnt3a or inhibition of GSK3β counteract MMP expression induced by IL-1 in human cells or cartilage explants, whereas opposite effect occur in similarly stimulated mouse or bovine cartilage explants.67
Do Wnts promote joint repair in RA?
Far less is known about Wnt signalling in RA compared with in OA. Nonetheless, two specific aspects of the disease, inflammation and erosive damage, and a role for Wnt signalling therein merit specific attention. Despite the overall progress in control of inflammation, damage to the skeletal tissues as a consequence of arthritis is still considered a largely irreversible process. Histomorphological studies of erosive lesions in humans and in rodent models suggest that sustained low-grade inflammation is responsible for local upregulation of Wnt antagonists such as sFRPs and Dkks, which limits osteoblast maturation and healing of erosions.68,69 In a mouse model of erosive arthritis, Walsh et al.69 detected expression of Dkk1–3, Sfrp1, Sfrp2 and Sfrp4 in the arthritic synovium. Dkk1 expression peaked with inflammation and coincided with the development of bone erosions, Sfrp1 and Sfrp2 were upregulated from the early stages of arthritis, whereas Dkk2, Dkk3 and Sfrp4 expression was associated with late stages of disease in which inflammation had subsided.69 Furthermore, the presence of sFRP1 and Dkk1 protein in the synovium and at sites of bone erosion was demonstrated by immunohistochemistry.69 Using a variant of the mouse serum-transfer-induced arthritis model, Matzelle et al.68 focused on alterations to bone occurring after resolution of inflammation, increased osteoblast activity and appositional repair of bone erosions, which lead to restoration of overall bone architecture. In keeping with the findings of Walsh and colleagues,69 resolution of inflammation was paralleled by a reduction in expression levels of Sfrp1 and Sfrp2;68 increased Dkk1 expression was sustained over a longer period, whereas Dkk2 mRNA levels were low at the peak of inflammation but gradually increased thereafter.68 In parallel with the downregulation of the sFRPs during resolution of inflammation, Wnt10b expression levels increased.68 In addition to the roles of Wnt signalling in the activation of osteoblasts and initiation of bone formation, Wnt activity might require fine-tuning during the late mineralization phase of bone repair,70 a process that is consistent with the differential regulation of Wnt antagonists observed in these studies.68,69
Importantly, genetic variants of DKK1 have been associated with progression of erosive disease in patients with RA and one of these variants (rs1896368) correlated with serum levels of Dkk1.71 Moreover, activation of β-catenin signalling induces osteoprotegerin, the natural antagonist of the interaction between receptor activator of NFκB (RANK) and its ligand, RANKL, and thus osteoclast differentiation and activation.72 Therefore, modulation of Wnt signalling could represent a dual target strategy used to promote healing of joints with erosions. However, the observation that treatment of a mouse model of RA with antibodies to Dkk1 resulted in excessive new bone formation, reaching beyond pre-existing skeletal boundaries and leading to osteophyte formation, urges caution in this approach.73
Wnt5a has been associated with macrophage activation and inflammation.74,75 In addition, the specific interaction between Wnt5a and the receptor ROR2 stimulates osteoclast differentiation, and treatment of mice with a decoy receptor protected against the development of bone erosions in an arthritis model.76 In the context of the complex network of chronic inflammation with multiple amplifying and regulatory feedback loops, the roles of Wnt5a and other Wnts have not yet been completely defined. Furthermore, data link the Wnt antagonist sFRP1 to differentiation of type 17 T-helper (TH17) cells,77 providing evidence that Wnt signalling could contribute to the outcome of diseases such as RA in a number of different ways.
Wnts as master regulators of SpA
SpA and in particular ankylosing spondylitis (AS) are characterized by chronic inflammation and extensive remodelling of joints and the spine.78 In contrast to RA, destructive changes are limited in SpA, but extensive new bone formation results in the development of spinal syndesmophytes, joint-associated osteophytes and extra-articular enthesophytes, and progressively leads to joint or spine ankylosis.79 Both inflammation and new tissue formation contribute to the burden of disease.80 At present, only first-line NSAIDs have a proven benefit on ankylosis.81,82 Although the introduction of TNF blocking strategies has dramatically advanced the clinical management of SpA, changing the course of the disease and its bearing on patients lives,78 progression of skeletal remodelling and ankylosis does not seem to abrogated by anti-TNF therapy.83 Unsurprisingly, molecular pathways associated with cartilage and bone formation have therefore been at the centre of research efforts in SpA.
In a search for molecular factors that regulate bone loss in arthritis, Diarra et al.73 discovered that administration of anti-Dkk1 antibodies not only reduced overall bone loss and joint erosions, but also changed the phenotype from destructive disease towards a bone-forming arthritis. Dkk1 blockade increased levels of osteoprotegerin as well as activation of canonical Wnt signalling.73 Similar observations were made in the sacroiliac joints of transgenic mice expressing human TNF,84 which might have been expected considering that TNF induced Dkk1 expression via activation of p38 MAPK in the mouse models used by Diarra and colleagues.73 In addition, Dkk1 and Sost (encoding sclerostin) were downregulated in the spines of mice with proteoglycan-induced spondylitis, a model characterized by spinal inflammation and ankylosis.85
These results are consistent with the absence of functional Dkk1 in the sera of patients with AS compared with control populations, as measured with an ELISA based on the binding of Dkk1 to LRP6.73 Not surprisingly given these observations, combined with the availability of ELISAs that enable systemic serum concentrations to be determined, the use of soluble Wnt antagonists as biomarkers has been further explored in different settings. Amongst patients with AS, levels of Dkk1 were reported as being higher in those without syndesmophytes compared with those with new bone formation.86 However, using a sandwich ELISA that measured the Dkk1 protein concentration rather than levels of functional Dkk1, Dkk1 expression was found to be higher in patients with AS compared with healthy and disease controls.87 Moreover Dkk1 levels were higher in patients with AS treated with anti-TNF agents, in contrast with patients with RA whose Dkk1 levels decreased after anti-TNF therapy.87 Despite the increased Dkk1 expression, Wnt signalling was increased in cells treated with serum from patients with AS compared with control serum.87 Furthermore, Dkk1 blockade enhanced Wnt signalling in cells treated with control serum but not serum from patients with AS.87 Together, these findings suggest that Dkk1 is dysfunctional in patients with AS; however, the biological explanation for these observations is not clear and further research into factors that can functionally sequester Dkk1 preventing its binding to LRP6 is essential.
In an immunohistochemical analysis of surgical samples, Appel et al.88 found that sclerostin expression was confined to osteocytes, and was decreased in OA samples and virtually absent from AS tissue, both compared with samples from patients with RA and healthy controls. The reduced expression of sclerostin in OA and AS joint tissues was further reflected in the low levels of circulating sclerostin in the serum of patients with AS compared with serum from healthy individuals;88 levels of sclerostin were lower in patients with syndesmophytes as compared with those without these features.88 Analyses in the prospective German Spondyloarthritis Inception Cohort (GESPIC) suggested that lower serum levels of functional Dkk1 were predictive of radiographic progression of AS and positively correlated with serum levels of sclerostin, but were not related to levels of C-reactive protein (CRP).86 On the other hand, lower serum levels of sclerostin at baseline, which remained low after anti-TNF therapy, correlated with increased risk of high serum CRP at 12-month follow-up in patients with AS, suggesting that levels of sclerostin are suppressed by persistent inflammation.89
These data are of great interest, as they suggest that biomarkers can be developed to predict disease progression in AS. However, further research is needed to provide biological explanations for these observations and to enable the introduction of such biomarker assays to the management of individual patients. One must also bear in mind that Wnt signalling is regulated by biomechanical loading of the skeleton, and thus differences in expression of Wnt pathway components amongst patients with AS and between patients with AS, those with other diseases or healthy individuals might reflect overall changes in the endocrine and paracrine regulation of the skeleton rather than a localized phenomenon in a small number of growing syndesmophytes. Skeletal inflammation, and changes in mobility and joint loading can contribute to low levels of Wnt antagonists in patients, as the skeletal homeostat functions to maintain bone mass. Thus, a specific impact on syndesmophyte formation might be a secondary rather than a primary effect of low Wnt antagonist levels.
Biomarkers linked to the Wnt cascade
Given their roles in skeletal biology, different members of the Wnt signalling cascades have been considered as biomarkers, as introduced previously in the context of AS. As they only travel short distances from the cells that produce them and are highly homologous, Wnts themselves have not been explored in this perspective. More attention has been given to extracellular, secreted antagonists, particularly sclerostin, Dkks, FRZB and other sFRPs. Some promising data have been put forward, but many analyses require further validation. One issue that needs to be addressed is the complex question of whether expression of these markers can be linked to local phenomena, thereby reflecting progression of a given disease in the joint microenvironment (as we discussed previously with regard to syndesmophytes), or whether levels of these factors reflect changes in overall bone metabolism. Other validation issues relate to the approaches that are used to measure of these potential biomarkers. As Wnt antagonists are linked to skeletal modelling and remodelling, information about assay reproducibility, the influence of loading and of circadian rhythms is required, as these could affect comparisons between patients and controls.
The potential of Wnt signalling antagonists, FRZB and Dkk1 to provide prognostic information about development and progression of disease has been evaluated in patients with radiographic hip OA.90 Radiographs were obtained from a cohort of elderly women at baseline and after 8.3 years follow-up, and read for evidence of prevalent, incident and progressive disease by validated methods.90 A random sample of 180 subjects was assigned to two nested case–control studies, and no differences in the mean values of either protein were found in any group.90 Nevertheless, a trend indicated that elevated levels of sFRP3 might reduce the risk of incident hip OA and higher levels of Dkk1 were associated with a reduction in the risk of worsening radiographic hip OA by nearly 50%.90 These data suggest that Wnt signalling antagonists in combination with other markers could be useful in assessing skeletal tissue turnover and disease progression.
Potential therapeutic interventions
The complexity of Wnt signalling indicates that modulation of this pathway represents a definite challenge. Overexpression of Wnt pathway components and mutations in genes encoding components of the β-catenin destruction complex are associated with cancer, indicating that care is needed in the therapeutic targeting of Wnt signalling cascades.1 Currently, the therapeutic focus has been on the secreted Wnt antagonists. The master role of the osteocytes in regulating bone remodelling and, in particular, the key role for sclerostin in the inhibition of osteoblast maturation has lead to the development of anti-sclerostin antibodies to stimulate bone formation in patients with osteoporosis. The current clinical trial data for this therapy are convincing, with gains in lumbar spine bone mineral density of up to 6% after one injection reported in a phase I trial,91 but safety concerns in the long term will require longitudinal follow-up. Data from pre-clinical studies demonstrate that blockade of Dkk1 might yield similar effects to blockade of sclerostin on bone, but little is known about the effects of this approach on cartilage. Furthermore, whether the effects of such therapeutic strategies can be constrained to the original bony elements without triggering excessive ectopic bone formation also remains to be determined.
Conclusions
Our understanding of Wnt biology is rapidly increasing, in particular as novel tools become available to study the different aspects of this pathway. Increasing evidence suggests that these molecular signalling pathways have an important role in the maintenance of joint homeostasis, particularly in cartilage. In the different forms of chronic arthritis, loss of balance in Wnts signalling thereby leads to cartilage damage. In addition, Wnts might play a part in the bony-tissue responses associated with disease. Lack of active Wnt signalling could impair repair of erosion, whereas excessive or dysregulated pathway activation can lead to joint or spine ankylosis. Further insight into many details of Wnt signalling is absolutely essential, not only to facilitate development of specific therapeutic interventions targeting this pathway, but also for the development and use of biomarkers that could help further shape a personalized medicine approach.
Review criteria.
This Review is based on a personal database of articles related to Wnt signalling, osteoarthritis, rheumatoid arthritis and spondyloarthritis collected by the authors since 2000. Additional relevant papers related to Wnt signalling, focusing on articles in English, were identified by searching PubMed using the term “Wnt* AND (osteoarthritis OR rheumatoid arthritis OR ankylosing spondylitis OR spondyloarthritis OR skeletal development OR joint development)”.
Footnotes
Competing interests
The authors declare no competing interests.
Contributor Information
Rik J. Lories, Laboratory of Tissue Homeostasis and Disease, Skeletal Biology and Engineering Research Center, Department of Development and Regeneration, KU Leuven, Herestraat 49, B3000 Leuven, Belgium
Maripat Corr, Department of Medicine, University of California San Diego, 9500 Gilman Drive, La Jolla, San Diego, CA 92093-0663, USA.
Nancy E. Lane, Center for Musculoskeletal Diseases, Department of Medicine, University of California at Davis School of Medicine, 4625 2nd Avenue, Suite 1002, Sacramento, CA 95817, USA
References
- 1.Clevers H, Nusse R. Wnt/β-catenin signaling disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10:3116–3128. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- 3.Bänziger C, et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 4.Tabata T, Takei Y. Morphogens their identification and regulation. Development. 2004;131:703–712. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- 5.Mii Y, Taira M. Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development. 2009;136:4083–4088. doi: 10.1242/dev.032524. [DOI] [PubMed] [Google Scholar]
- 6.Mii Y, Taira M. Secreted Wnt “inhibitors” are not just inhibitors: regulation of extracellular Wnt by secreted Frizzled-related proteins. Dev Growth Differ. 2011;53:911–923. doi: 10.1111/j.1440-169X.2011.01299.x. [DOI] [PubMed] [Google Scholar]
- 7.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 8.Sugimura R, Li L. Noncanonical Wnt signaling in vertebrate development stem cells and diseases. Birth Defects Res C Embryo Today. 2010;90:243–256. doi: 10.1002/bdrc.20195. [DOI] [PubMed] [Google Scholar]
- 9.Li VS, et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–213. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark CE, Nourse CC, Cooper HM. The tangled web of non-canonical Wnt signalling in neural migration. Neurosignals. 2012;20:202–220. doi: 10.1159/000332153. [DOI] [PubMed] [Google Scholar]
- 12.Minami Y, Oishi I, Endo M, Nishita M. ROR-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases. Dev Dyn. 2010;239:1–15. doi: 10.1002/dvdy.21991. [DOI] [PubMed] [Google Scholar]
- 13.Matthews HK, et al. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development. 2008;135:1771–1780. doi: 10.1242/dev.017350. [DOI] [PubMed] [Google Scholar]
- 14.Cruciat CM, Niehrs C. Secreted and transmembrane Wnt inhibitors and activators. Cold Spring Harb Perspect Biol. doi: 10.1101/cshperspect.a015081. http://dx.doi.org/10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed]
- 15.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 16.Leucht P, Minear S, Ten Berge D, Nusse R, Helms JA. Translating insights from development into regenerative medicine: the function of Wnts in bone biology. Semin Cell Dev Biol. 2008;19:434–443. doi: 10.1016/j.semcdb.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492:1–18. doi: 10.1016/j.gene.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefebvre V, Bhattaram P. Vertebrate skeletogenesis. Curr Top Dev Biol. 2010;90:291–317. doi: 10.1016/S0070-2153(10)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrow JR, et al. Ectodermal Wnt3/β-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawakami Y, et al. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell. 2001;104:891–900. doi: 10.1016/s0092-8674(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 21.Nam JS, et al. Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb. Dev Biol. 2007;311:124–135. doi: 10.1016/j.ydbio.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gros J, et al. Wnt5a/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. Curr Biol. 2010;20:1993–2002. doi: 10.1016/j.cub.2010.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudnicki JA, Brown AM. Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev Biol. 1997;185:104–118. doi: 10.1006/dbio.1997.8536. [DOI] [PubMed] [Google Scholar]
- 24.Witte F, Dokas J, Neuendorf F, Mundlos S, Stricker S. Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr Patterns. 2009;9:215–223. doi: 10.1016/j.gep.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Akiyama H, et al. Interactions between SOX9 and β-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Church V, Nohno T, Linker C, Marcelle C, Francis-West P. Wnt regulation of chondrocyte differentiation. J Cell Sci. 2002;115:4809–4818. doi: 10.1242/jcs.00152. [DOI] [PubMed] [Google Scholar]
- 29.Dong YF, Soung do Y, Schwarz EM, O’Keefe RJ, Drissi H. Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J Cell Physiol. 2006;208:77–86. doi: 10.1002/jcp.20656. [DOI] [PubMed] [Google Scholar]
- 30.Guo X, Mak KK, Taketo MM, Yang Y. The Wnt/β-catenin pathway interacts differentially with PTHrP signaling to control chondrocyte hypertrophy and final maturation. PLoS ONE. 2009;4:e6067. doi: 10.1371/journal.pone.0006067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamamura Y, et al. Developmental regulation of Wnt/β-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280:19185–19195. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- 32.Dao DY, et al. Cartilage-specific β-catenin signaling regulates chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development. J Bone Miner Res. 2012;27:1680–1694. doi: 10.1002/jbmr.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley EW, Drissi MH. Wnt5a regulates chondrocyte differentiation through differential use of the CaN/NFAT and IKK/NF-κB pathways. Mol Endocrinol. 2010;24:1581–1593. doi: 10.1210/me.2010-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradley EW, Drissi MH. Wnt5b regulates mesenchymal cell aggregation and chondrocyte differentiation through the planar cell polarity pathway. J Cell Physiol. 2011;226:1683–1693. doi: 10.1002/jcp.22499. [DOI] [PubMed] [Google Scholar]
- 35.Hu H, et al. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- 36.Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104:341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 37.Später D, Hill TP, Gruber M, Hartmann C. Role of canonical Wnt-signalling in joint formation. Eur Cell Mater. 2006;12:71–80. doi: 10.22203/ecm.v012a09. [DOI] [PubMed] [Google Scholar]
- 38.Später D, et al. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development. 2006;133:3039–3049. doi: 10.1242/dev.02471. [DOI] [PubMed] [Google Scholar]
- 39.Li X, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 40.Robling AG, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 41.Baker-Lepain JC, et al. Variant alleles of the Wnt antagonist FRZB are determinants of hip shape and modify the relationship between hip shape and osteoarthritis. Arthritis Rheum. 2012;64:1457–1465. doi: 10.1002/art.34526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loughlin J, et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci USA. 2004;101:9757–9762. doi: 10.1073/pnas.0403456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lane NE, et al. Frizzled-related protein variants are risk factors for hip osteoarthritis. Arthritis Rheum. 2006;54:1246–1254. doi: 10.1002/art.21673. [DOI] [PubMed] [Google Scholar]
- 44.Min JL, et al. Association of the Frizzled-related protein gene with symptomatic osteoarthritis at multiple sites. Arthritis Rheum. 2005;52:1077–1080. doi: 10.1002/art.20993. [DOI] [PubMed] [Google Scholar]
- 45.Evangelou E, et al. Large-scale analysis of association between GDF5 and FRZB variants and osteoarthritis of the hip, knee, and hand. Arthritis Rheum. 2009;60:1710–1721. doi: 10.1002/art.24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castaño Betancourt MC, et al. Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and hip osteoarthritis. Proc Natl Acad Sci USA. 2012;109:8218–8223. doi: 10.1073/pnas.1119899109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahmoudi T, et al. The leukemia-associated Mllt10/Af10-Dot1l are TCF4/β-catenin coactivators essential for intestinal homeostasis. PLoS Biol. 2010;8:e1000539. doi: 10.1371/journal.pbio.1000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dell’accio F, De Bari C, Eltawil NM, Vanhummelen P, Pitzalis C. Identification of the molecular response of articular cartilage to injury, by microarray screening: Wnt-16 expression and signaling after injury and in osteoarthritis. Arthritis Rheum. 2008;58:1410–1421. doi: 10.1002/art.23444. [DOI] [PubMed] [Google Scholar]
- 49.Blom AB, et al. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60:501–512. doi: 10.1002/art.24247. [DOI] [PubMed] [Google Scholar]
- 50.Sen M, et al. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97:2791–2796. doi: 10.1073/pnas.050574297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura Y, Nawata M, Wakitani S. Expression profiles and functional analyses of Wnt-related genes in human joint disorders. Am J Pathol. 2005;167:97–105. doi: 10.1016/S0002-9440(10)62957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imai K, et al. Differential expression of Wnts and FRPs in the synovium of rheumatoid arthritis and osteoarthritis. Biochem Biophys Res Commun. 2006;345:1615–1620. doi: 10.1016/j.bbrc.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 53.Sen M, et al. Regulation of fibronectin and metalloproteinase expression by Wnt signaling in rheumatoid arthritis synoviocytes. Arthritis Rheum. 2002;46:2867–2877. doi: 10.1002/art.10593. [DOI] [PubMed] [Google Scholar]
- 54.Sen M, Chamorro M, Reifert J, Corr M, Carson DA. Blockade of Wnt-5a/frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum. 2001;44:772–781. doi: 10.1002/1529-0131(200104)44:4<772::AID-ANR133>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 55.Zhu M, et al. Inhibition of β-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008;58:2053–2064. doi: 10.1002/art.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu M, et al. Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult β-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuasa T, et al. Transient activation of Wnt/β-catenin signaling induces abnormal growth plate closure and articular cartilage thickening in postnatal mice. Am J Pathol. 2009;175:1993–2003. doi: 10.2353/ajpath.2009.081173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lodewyckx L, Luyten FP, Lories RJ. Genetic deletion of low-density lipoprotein receptor-related protein 5 increases cartilage degradation in instability-induced osteoarthritis. Rheumatology (Oxford) 2012;51:1973–1978. doi: 10.1093/rheumatology/kes178. [DOI] [PubMed] [Google Scholar]
- 59.Lories RJ, et al. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 2007;56:4095–4103. doi: 10.1002/art.23137. [DOI] [PubMed] [Google Scholar]
- 60.Miclea RL, et al. Inhibition of GSK3β in cartilage induces osteoarthritic features through activation of the canonical Wnt signaling pathway. Osteoarthritis Cartilage. 2011;19:1363–1372. doi: 10.1016/j.joca.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 61.Weng LH, Wang CJ, Ko JY, Sun YC, Wang FS. Control of Dkk-1 ameliorates chondrocyte apoptosis, cartilage destruction, and subchondral bone deterioration in osteoarthritic knees. Arthritis Rheum. 2010;62:1393–1402. doi: 10.1002/art.27357. [DOI] [PubMed] [Google Scholar]
- 62.Oh H, Chun CH, Chun JS. Dkk-1 expression in chondrocytes inhibits experimental osteoarthritic cartilage destruction in mice. Arthritis Rheum. 2012;64:2568–2578. doi: 10.1002/art.34481. [DOI] [PubMed] [Google Scholar]
- 63.Leijten JC, et al. Gremlin 1, frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum. 2012;64:3302–3012. doi: 10.1002/art.34535. [DOI] [PubMed] [Google Scholar]
- 64.Lodewyckx L, Cailotto F, Thysen S, Luyten FP, Lories RJ. Tight regulation of wingless-type signaling in the articular cartilage–subchondral bone biomechanical unit: transcriptomics in Frzb-knockout mice. Arthritis Res Ther. 2012;14:R16. doi: 10.1186/ar3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nalesso G, et al. Wnt-3a modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. J Cell Biol. 2011;193:551–564. doi: 10.1083/jcb.201011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cailotto F, Sebillaud S, Netter P, Jouzeau JY, Bianchi A. The inorganic pyrophosphate transporter ANK preserves the differentiated phenotype of articular chondrocyte. J Biol Chem. 2010;285:10572–10582. doi: 10.1074/jbc.M109.050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma B, van Blitterswijk CA, Karperien M. A Wnt/β-catenin negative feedback loop inhibits interleukin-1-induced matrix metalloproteinase expression in human articular chondrocytes. Arthritis Rheum. 2012;64:2589–2600. doi: 10.1002/art.34425. [DOI] [PubMed] [Google Scholar]
- 68.Matzelle MM, et al. Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum. 2012;64:1540–1550. doi: 10.1002/art.33504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walsh NC, et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res. 2009;24:1572–1585. doi: 10.1359/jbmr.090320. [DOI] [PubMed] [Google Scholar]
- 70.Li X, et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 2005;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- 71.de Rooy DP, et al. Genetic studies on components of the Wnt signalling pathway and the severity of joint destruction in rheumatoid arthritis. Ann Rheum Dis. doi: 10.1136/annrheumdis-2012-202184. http://dx.doi.org/10.1136/annrheumdis-2012-202184. [DOI] [PubMed]
- 72.Glass DA, 2nd, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 73.Diarra D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 74.Maiti G, Naskar D, Sen M. The Wingless homolog Wnt5a stimulates phagocytosis but not bacterial killing. Proc Natl Acad Sci USA. 2012;109:16600–16605. doi: 10.1073/pnas.1207789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim J, et al. Wnt5a induces endothelial inflammation via β-catenin-independent signaling. J Immunol. 2010;185:1274–1282. doi: 10.4049/jimmunol.1000181. [DOI] [PubMed] [Google Scholar]
- 76.Maeda K, et al. Wnt5a–ROR2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. 2012;18:405–412. doi: 10.1038/nm.2653. [DOI] [PubMed] [Google Scholar]
- 77.Lee YS, et al. The Wnt inhibitor secreted frizzled-related protein 1 (sFRP1) promotes 42, human TH17 differentiation. Eur J Immunol. 2012:2564–2573. doi: 10.1002/eji.201242445. [DOI] [PubMed] [Google Scholar]
- 78.Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;377:2127–2137. doi: 10.1016/S0140-6736(11)60071-8. [DOI] [PubMed] [Google Scholar]
- 79.Lories RJ, Luyten FP, de Vlam K. Progress in spondylarthritis. Mechanisms of new bone formation in spondyloarthritis. Arthritis Res Ther. 2009;11:221. doi: 10.1186/ar2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Machado P, et al. Both structural damage and inflammation of the spine contribute to impairment of spinal mobility in patients with ankylosing spondylitis. Ann Rheum Dis. 2010;69:1465–1470. doi: 10.1136/ard.2009.124206. [DOI] [PubMed] [Google Scholar]
- 81.Wanders A, et al. Nonsteroidal anti-inflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005;52:1756–1765. doi: 10.1002/art.21054. [DOI] [PubMed] [Google Scholar]
- 82.Poddubnyy D, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis. 2012;71:1616–1622. doi: 10.1136/annrheumdis-2011-201252. [DOI] [PubMed] [Google Scholar]
- 83.van der Heijde D, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum. 2008;58:1324–1331. doi: 10.1002/art.23471. [DOI] [PubMed] [Google Scholar]
- 84.Uderhardt S, et al. Blockade of Dickkopf (Dkk)-1 induces fusion of sacroiliac joints. Ann Rheum Dis. 2010;69:592–597. doi: 10.1136/ard.2008.102046. [DOI] [PubMed] [Google Scholar]
- 85.Haynes KR, et al. Excessive bone formation in a mouse model of ankylosing spondylitis is associated with decreases in Wnt pathway inhibitors. Arthritis Res Ther. 2012;14:R253. doi: 10.1186/ar4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heiland GR, et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71:572–574. doi: 10.1136/annrheumdis-2011-200216. [DOI] [PubMed] [Google Scholar]
- 87.Daoussis D, et al. Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum. 2010;62:150–158. doi: 10.1002/art.27231. [DOI] [PubMed] [Google Scholar]
- 88.Appel H, et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2009;60:3257–3262. doi: 10.1002/art.24888. [DOI] [PubMed] [Google Scholar]
- 89.Saad CG, et al. Low sclerostin levels: a predictive marker of persistent inflammation in ankylosing spondylitis during anti-tumor necrosis factor therapy? Arthritis Res Ther. 2012;14:R216. doi: 10.1186/ar4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lane NE, et al. Wnt signaling antagonists are potential prognostic biomarkers for the progression of radiographic hip osteoarthritis in elderly Caucasian women. Arthritis Rheum. 2007;56:3319–3325. doi: 10.1002/art.22867. [DOI] [PubMed] [Google Scholar]
- 91.Padhi DA, et al. The effects of multiple doses of sclerostin antibody AMG 785 in healthy men and postmenopausal women with low bone mass [abstract OP0044] Ann Rheum Dis. 2012;71(Suppl 3):67–68. [Google Scholar]
- 92.Andrade AC, Nilsson O, Barnes KM, Baron J. Wnt gene expression in the post-natal growth plate: regulation with chondrocyte differentiation. Bone. 2007;40:1361–1369. doi: 10.1016/j.bone.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kengaku M, et al. Distinct Wnt pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science. 1998;280:1274–1277. doi: 10.1126/science.280.5367.1274. [DOI] [PubMed] [Google Scholar]
- 94.Guo X, et al. Wnt/β-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18:2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- 96.Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- 97.Pasold J, et al. Reduced expression of Sfrp1 during chondrogenesis and in articular chondrocytes correlates with osteoarthritis in STR/ort mice. Exp Cell Res. doi: 10.1016/j.yexcr.2012.12.012. http://dx.doi.org/10.1016/j.yexcr.2012.12.012. [DOI] [PubMed]
- 98.Ladher RK, et al. Cloning and expression of the Wnt antagonists Sfrp-2 and Frzb during chick development. Dev Biol. 2000;218:183–198. doi: 10.1006/dbio.1999.9586. [DOI] [PubMed] [Google Scholar]
- 99.Kusu N, et al. Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J Biol Chem. 2003;278:24113–24117. doi: 10.1074/jbc.M301716200. [DOI] [PubMed] [Google Scholar]
- 100.Chan BY, et al. Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthritis Cartilage. 2011;19:874–885. doi: 10.1016/j.joca.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 101.Roudier M, et al. Sclerostin is expressed in articular cartilage but loss or inhibition does not affect cartilage remodeling during aging or following mechanical injury. Arthritis Rheum. doi: 10.1002/art.37802. http://dx.doi.org/10.1002/art.37802. [DOI] [PubMed]