Abstract
Background
Use of electronic cigarettes (e-cigarettes) and other nicotine containing products is increasing among women of reproductive age. The short- and long-term effects of these products on both mother and fetus are unknown.
Methods
Because e-cigarettes are nicotine delivery systems, we sought to conduct a comprehensive review of the effects of nicotine on the fetus.
Results
In utero nicotine exposure in animal models is associated with adverse effects for the offspring lung, cardiovascular system and brain. In the lung, this included reduced surface area, weight, and volume, as well as emphysema-like lesions. In adulthood, exposed offspring demonstrate elevated blood pressure and increased perivascular adipose tissue. In the brain, exposure alters offspring serotonergic, dopaminergic, and norepinephrine networks, which in turn are associated with behavioral and cognitive impairments. We also review current data on the lack of efficacy of nicotine replacement therapy in pregnant women, and highlight different nicotine containing products such as snuff, snus, and hookah.
Conclusion
We conclude that no amount of nicotine is known to be safe during pregnancy, and studies specifically addressing this risk are crucial and an imminent public health issue.
Keywords: nicotine, electronic cigarettes, pregnancy
Introduction
Despite public health warnings, the incidence of smoking during pregnancy remains higher than 10% in the United States (Martin et al., 2007; Tong et al., 2013). This is a major public health issue because smoking during pregnancy results in a higher risk of perinatal and obstetric complications such as preterm birth, premature rupture of membranes, placental abruption and placenta previa, as well as impaired lung function of the developing fetus (Hofhuis et al., 2003; Suter et al., 2010, 2013). The association between maternal tobacco smoke exposure and restricted fetal growth has been well studied. Tobacco exposure in utero is associated with a greater risk of lower birth-weight babies (Suter et al., 2013). Pregnant women may consider switching to electronic cigarettes (e-cigarettes) in an attempt to quit smoking or as a proposed safer option to the harmful chemicals in combustible tobacco. Previous works hypothesize that carbon monoxide-mediated hypoxia and nicotine-mediated vasoconstriction of vessels in the utero-placental circulation may result in fetal growth delay (England et al., 2012). The advent of electronic cigarettes brings new questions to the field of perinatal medicine. Are there similar adverse effects of e-cigarette exposure on the developing fetus? If so, what are they? Are electronic cigarettes a “better” alternative for pregnant women who currently smoke? Are the risks for harm significantly less, different, or greater?
While the safety of e-cigarettes is under heated debate, their use among women of reproductive age is steadily increasing. According to the Centers for Disease Control, approximately 10% of American high school students have tried e-cigarettes at least once in 2012, and one in five smokers have reported using them (Corey et al., 2013). Similarly, the use of hookah is increasing among high school students, especially among adolescents of higher socioeconomic status (Palamar JJ et al., 2014). According to Forbes, the sale of e-cigarettes generates over $1 billion in revenue per year. Therefore, it is a priority to move the study of e-cigarette use during pregnancy to the forefront of perinatal research (including industry-sponsored research) to determine the attributable risk of adverse effects of their use on the developing fetus. Given both the increasing prevalence of use among reproductive aged women and the potential for a lack of stigma of e-cigarette use in pregnancy, this is likely to become a major public health issue with critical ramifications for child development.
Because of their relative novelty, little to no data exist on the short- and long-term health effects of e-cigarette use. There are more than 400 brands of e-cigarettes but testing has been performed in only a few brands. The inhaled compounds of e-cigarettes and the vapor contain variable amounts of nicotine, propylene glycol, and in some cases diethylene glycol (Riker et al., 2012). Despite increasing prevalence no data exist on the consequences of e-cigarette use on reproductive health, nor on e-cigarette exposure to the fetus. This is a serious gap in our clinical, scientific, and public health knowledge. It is very possible, if not probable, that e-cigarette use is going to become an important issue with regard not only to perinatal health, but to the health and risk of addiction in generations to come.
In this article, we review the literature on e-cigarette use and prevalence including the limited studies of e-cigarettes as a smoking cessation aid. We describe the process involved in nicotine metabolism. We review the literature from studies using animal models which gives us insight into the effects of in utero exposure to nicotine on the offspring. We also summarize other nicotine containing products that are gaining popularity among women of reproductive age.
What is an Electronic Cigarette?
E-cigarettes are hand-held battery operated devices whose use closely mimics the act of smoking (Cahn and Siegel, 2011). It consists of three separate parts. A battery is used to power an atomizer, which vaporizes liquid nicotine that is stored in a replaceable cartridge. The user can purchase different flavors of liquid nicotine which come in disposable cartridges and attach it to the atomizer. The atomizer senses a change in pressure when the user inhales, and delivers a “hit” of vaporized nicotine. While e-cigarettes contain nicotine, which is derived from tobacco, they contain no tobacco (Cahn and Siegel, 2011). Studies which use gas chromatography mass spectrometry have identified the main components of e-cigarettes as nicotine, propylene glycol, and glycerine; additionally trace amounts of tobacco specific nitrosamines and diethylene glycol have been detected (Cahn and Siegel, 2011; Burstyn, 2014).
Who is Using Electronic Cigarettes?
Since 2009, the awareness of electronic cigarettes has steadily increased (Tan and Bigman, 2014). The sale of e-cigarettes has also been steadily increasing since their introduction to the U.S. markets in 2007. Until recently, e-cigarettes have not been regulated by the U.S. Food and Drug Administration. Because of this, there have been no federal regulations on e-cigarette sales to minors. Similarly, e-cigarette marketing has been less stringently regulated than marketing of combustible tobacco products. As the awareness and use of e-cigarettes continues to increase, this begs the question, who are the main users of these electronic nicotine delivery systems?
One unfortunate answer to this question appears to be middle and high school students. A major study from the Centers for Disease Control, the National Youth Tobacco Survey, revealed that from 2011 to 2012, ever use of e-cigarettes among middle and high school students went from 3.3% to 6.8% while current or past month use went from 1.1% to 2.1% (Corey et al., 2013). Of the middle schoolers who reported using electronic cigarettes within the past month, 20% had never tried a tobacco cigarette. This survey revealed that e-cigarette use may actually encourage traditional cigarette use in adolescents (Dutra and Glantz, 2014).
The majority of e-cigarette users, regardless of sex or age, are former or current smokers. One study determined that being a current tobacco smoker is the strongest predictor of current e-cigarette use in adolescents (Camenga et al., 2014). In adults, three studies show that males are more likely to use e-cigarettes than women and they were more likely to be former smokers (Foulds et al., 2011). Another study of over 4000 college students in the North Carolina area revealed that e-cigarette use was most common among current smokers; however, use was not associated with the desire to quit smoking in this population (Sutfin et al., 2013).
However, many surveys have found that adults are using electronic cigarettes as a smoking cessation aid (Etter, 2010; Etter and Bullen, 2011; Goniewicz et al., 2013b; Vickerman et al., 2013). While the data supporting e-cigarettes as an effective tool to quit smoking are lacking, some studies are reporting positive findings. Participants in an online survey of electronic cigarettes users (222 participants total) self reported a decrease in cigarette use after trying e-cigarettes (Siegel et al., 2011). One small pilot study recruited current smokers (≥15 cigarettes/day) who were not interested in quitting and offered them electronic cigarettes. They followed up with the subjects with 5 study visits up to 6 months. At week 24, 32.5% (p < 0.001) of participants decreased their cigarette consumption from a median of 25 cigarettes/day to 6 cigarettes/day (Polosa et al., 2011). A larger, 12-month, randomized control trial of 300 smokers who were not intending to quit found that a significant reduction in cigarette/ day use from baseline was observed at each study visit up to a year (Caponnetto et al., 2013). A surprising finding of this study is that this reduction was even observed in the group whose e-cigarettes contained no nicotine. Electronic cigarette use has also been shown to reduce the craving for traditional cigarettes (Bullen et al., 2010). Together, these studies seem to suggest that this may be a potential tool for cessation of traditional cigarette usage in adults.
Are Electronic Cigarettes Safer Than Combustible Tobacco Cigarettes? A Topic Under Heated Debate
It is well known that there are over 4000 chemicals in tobacco cigarettes, and at least 55 of them are known carcinogens (Hecht, 1999; Suter et al., 2010, 2009). Because e-cigarettes undoubtedly contain fewer chemicals than combustible tobacco cigarettes, they are viewed as being a safer alternative to smoking. However, there are conflicting reports on the levels of specific toxins found in the vapor. One study found that levels of arsenic, chromium, cadmium, nickel, and lead were at trace amounts not harmful to humans, while another study reported that levels of these trace metals were sometimes higher than those found in combustible tobacco products (Cobb and Abrams, 2011; Williams et al., 2013). The potential source of the contamination is likely the atomizer, which creates the aerosol from the liquid in the cartridge. Because e-cigarettes had not been regulated by the U.S. Food and Drug Administration until recently, there was no regulation on the chemicals used in the cartridges.
One thing is for certain: e-cigarette vapor does not have the same chemical make-up as combustible tobacco smoke. Therefore, the question arises, is there a potential for exposure to “secondhand vapor?” Schober et al. performed a comprehensive study on the air quality in a ventilated room where nine people used e-cigarettes for 2 hour. They found that the polycyclic aromatic hydrocarbons in the air increased by 20% and that the amount of aluminum in the air increased 2.4-fold (Schober et al., 2014). However, two studies report not finding any increase in toxicants that would pose a risk to human health (McAuley et al., 2012; Czogala et al., 2014), but one of them did find that second hand vapor does contain nicotine (Czogala et al., 2014). Czogala et al. reported that levels of nicotine in secondhand vapor were 10 times lower than those of combustible cigarettes (Czogala et al., 2014). Notable is the fact that second hand vapor indeed contains nicotine, and while the concentration is lower than from second hand tobacco smoke, the potential for persistent exposure raises the possibility that total levels of exposure may not significantly differ. In fact, increasing numbers of agencies and companies ban e-cigarette use under their “smoke free” policy due to the lack of evidence that the e-cigarettes or their emissions are safe (Riker et al., 2012).
How Much Nicotine Does an Electronic Cigarette Deliver?
Not all e-cigarettes are made equally, and different products deliver differing amounts of nicotine per puff. This is because the liquid cartridges contain varying amounts of nicotine by design. Some cartridges are even sold without nicotine and being marketed as a smoking cessation device. One study measured the amount of nicotine in the vapor of 16 different e-cigarette brands using an automated smoking machine. They found that nicotine levels from 15 puffs of an e-cigarette differed from 0.5 to 15.4 mgs of nicotine (Goniewicz et al., 2013a). Adding complexity to the issue, more experienced e-cigarette users are able to get more nicotine per puff, and can absorb the nicotine similar to conventional cigarette users (Grana et al., 2014). E-cigarette use is associated with longer puff durations compared with the duration of smokers using tobacco cigarettes (Evans and Hoffman, 2014).
Nicotine Metabolism
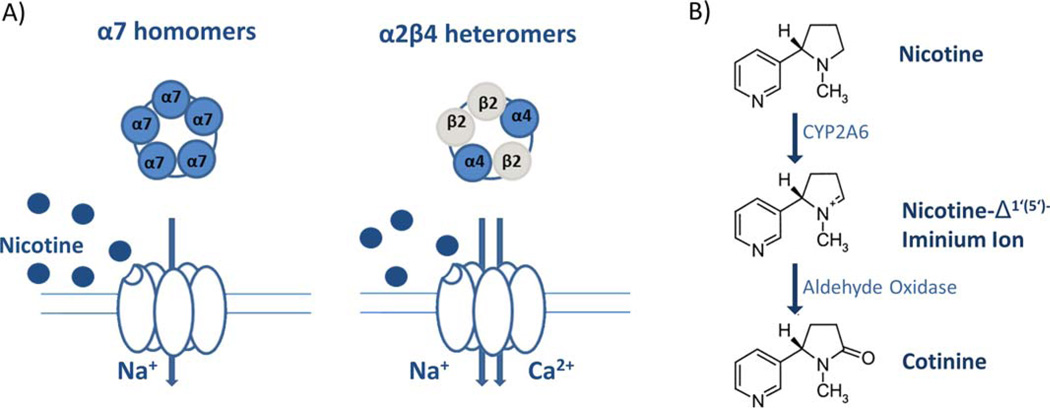
When inhaled, nicotine is able to reach the brain within seconds (Berridge et al., 2010). Nicotine, the major addictive component of tobacco, binds to nicotinic acetylcholine receptors. Nicotinic acetylcholine receptors are ligand gated ion channels activated by the endogenous neurotransmitter acetylcholine, and are composed of differing combinations of α and β subunits (α2, and β2–4) (Dani and Bertrand, 2007; De Biasi and Salas, 2008; Gotti et al., 2009) (Fig. 1). α7 homomers and α4β2 heteromers represent the two major nicotinic acetylcholine receptor subtypes found throughout the brain (Salas et al., 2003, 2004).
FIGURE 1.
Nicotine metabolism. A: Nicotine binds two major subtypes of nicotinic acetylcholine receptors inducing opening of ligand gated ion channels. Subsequent Na+ influx through α7 homomers, Na+, and Ca2+ influx through α2β4 heteromers. B: CYP2A6-mediated nicotine metabolism to inactive cotinine. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Many drugs and endogenous compounds are lipophilic and have to be metabolized to become more water soluble before renal excretion by the cytochrome p450s (CYPs) family of proteins. The human genome contains well over 100 CYP genes, of which 57 are functional and the remaining are pseudogenes. Among CYP families, there are 22 different P450 isoforms, and among these, a high degree of polymorphism has been identified (Aagaard-Tillery et al., 2010). The polymorphic xenobiotic-metabolizing CYP enzymes can be divided into two classes with respect to penetrance for inter-individual susceptibility for xenobiotics. The Class II CYPs include CYP2A6, which is the primary metabolizer of nicotine to cotinine.
However, variable expression of alternate cytochrome P450 enzymes (e.g., CYP2A6) has been shown to modify daily cigarette consumption (Pianezza et al., 1998; Raunio et al., 2001; Schoedel et al., 2004; Thorgeirsson et al., 2010). CYP2A6 is a highly polymorphic allele, and functions as the rate-limiting enzyme in the metabolism of nicotine to cotinine (Pianezza et al., 1998; Raunio et al., 2001; Schoedel et al., 2004; Thorgeirsson et al., 2010). Individuals with diminished activity of CYP2A6 at the CYP2A6*2 allele (CYP2A6 Lys160His T>A) inherit the slowest metabolism of nicotine and have been associated with lower cigarette consumption, shorter duration of smoking, and increased ability to quit smoking (Hong et al., 2003; Oscarson et al., 1999; Pianezza et al., 1998; Raunio et al., 2001; Schoedel et al., 2004; Thorgeirsson et al., 2010). This is attributed to the fact that CYP2A6 is a major catalyst of nicotine metabolism, and carriers of the defective CYP2A6*2 allele would require fewer cigarettes to achieve sufficient levels of nicotine in the blood. Such a relationship was recently confirmed in a genome-wide association study where the smoking behavior versus genetics in 87,000 individuals was analyzed where a very significant association to the CYP2A6 and CYP2B6 loci were found, whereas the association with lung cancer was less evident (Thorgeirsson et al., 2010).
Nicotine Exposure During Pregnancy
Currently, there are no data regarding electronic cigarette use in pregnant women. We do not know the effects on either maternal or fetal health. However, there are data on nicotine exposure in pregnancy. Here we summarize data from animal models that reveal the adverse effects of nicotine exposure in the fetus.
Animal Models: Insight Into the Effects of Nicotine on the Fetus
Animal models have provided data on the neurological and behavioral changes that occur in the offspring due to nicotine exposure throughout gestation. Importantly, these model systems enable us to examine the effects of nicotine specifically, versus exposure to maternal tobacco smoke. There are two main methods of nicotine administration using a rat model system; infusion through a subcutaneous osmotic pump, and daily nicotine injections. While the daily injections will cause a spike in nicotine levels which may closely mimic the dose from smoking, the injections stress the animal which may act as a confounder (Mantella et al., 2013). Use of the osmotic pump provides steady state levels of nicotine in the bloodstream, which may closer resemble use of a nicotine containing patch, rather than the changing levels from smoking. These data reveal that nicotine exposure is indeed deleterious to the offspring (Table 1).
TABLE 1.
Effects of In Utero Nicotine Exposure on Offspring: Insight from Animal Models
| Offspring phenotype | Reference |
|---|---|
| Alterations to the structure of the offspring lung |
(Maritz, 2013, Sekhon, 2001) |
| Increased risk of asthma in adulthood | (Maritz, 2013) |
| Elevated blood pressure in adulthood | (Gao, 2008) |
| Increased perivascular adipose accumulation |
(Gao, 2005) |
| Increased beta cell apoptosis | (Holloway, 2005) |
| Increased dopamine in the fetal offspring forebrain |
(Ribary, 1989) |
| Decreased norepinephrine levels in postnatal offspring |
(Seidler, 1992) |
| Alterations in nicotinic and dopaminergic signaling |
(Etter, 2010, Sutfin, 2013) |
| Reduced fertility in female offspring | (Holloway, 2006, Petrik, 2009) |
| Increased risk of stress induced cardiac defects | (Bruin, 2010, Lawrence, 2008) |
Nicotine readily crosses the placenta and enters fetal circulation. It interacts with nicotinic acetylcholine receptors in the fetal lung, which in turn become upregulated in the presence of maternal nicotine exposure (Sekhon et al., 2004). In utero nicotine exposure reduces the internal surface area of the lungs, reduces lung weight and volume, and induces emphysema like lesions among other negative consequences in a rat model system (Sekhon et al., 2001; Maritz, 2013). Work in a rhesus model, has revealed an alteration of offspring lung development and nicotinic receptor development with in utero nicotine exposure (Sekhon HS, 1999). In our laboratory, we have shown that in utero exposure to nicotine induces epigenetic changes in the offspring lung and brain (unpublished data). Because of these changes to the fetal lung, it is unsurprising that these offspring are at risk later in life to pulmonary disorders including asthma (Maritz, 2013).
In utero nicotine exposure also has metabolic consequences for the offspring. Using a rat model, Gao et al. showed that nicotine exposed offspring have elevated blood pressure in adulthood, possibly due to the increase in brown adipocytes in aortic perivascular adipose tissue (Gao et al., 2008). These offspring had an increase in postnatal body weight and perivascular adipose accumulation (Gao et al., 2005). Offspring also had decreased serum insulin and increased beta cell apoptosis in adulthood revealing that in utero nicotine exposure may predispose the offspring to a diabetic phenotype (Holloway et al., 2005).
Even a single dose of nicotine can alter the offspring neurobiology, and the results are rapid. A single injection of nicotine to pregnant rats showed that within 30 min there was increased dopamine in the offspring forebrain at gestational day 21 (Ribary and Lichtensteiger, 1989) Daily administration of nicotine in pregnancy alters the serotonin transporter expression in offspring forebrain at postnatal day 22 (Muneoka et al., 2001) and decreases brain norepinephrine levels at postnatal day 3 (Seidler et al., 1992). Prenatal nicotine exposure reduced dopamine turnover in the forebrain and serotonin turnover in the midbrain of postnatal day 15 and 22 offspring (Muneoka et al., 1997). The effects of in utero nicotine have been demonstrated out to postnatal day 60, in a rat model in which histopathological changes were evident in the cerebellum and hippocampus of nicotine exposed offspring (Abdel-Rahman et al., 2005). Other studies reveal that nicotine exposure affects the development and function of nicotinic acetylcholine receptors (Slotkin et al., 1987) and dopaminergic signaling (Richardson and Tizabi, 1994), and decreases cingulate cortical volume (Zhu et al., 2012) in the offspring.
In addition to the signaling and histological changes in the brain of offspring exposed to nicotine, behavioral and cognitive changes in the offspring have also been reported. In both mice and rats, prenatal nicotine exposure is associated with memory and learning deficits using an elevated maze task (Ernst et al., 2001). There is also evidence for gender specific responses to nicotine exposure. After being trained in an active avoidance paradigm at postnatal day 60, prenatal nicotine exposure is associated with reduced learning in the male offspring and enhanced learning in the female (Genedani et al., 1983).
Animal models have helped to demonstrate that nicotine exposure is associated with adverse metabolic, pulmonary, neurological, and cognitive outcomes. Others have shown that nicotine exposure is associated with reduced fertility in female offspring (Holloway et al., 2006; Petrik et al., 2009). Offspring have increased risk of stress induced cardiac defects (Lawrence et al., 2008; Bruin et al., 2010) It is with this information in mind that clinicians must decide how to manage their patients’ smoking habits during pregnancy as well as lactation, as nicotine is found in the breast milk of smokers (Ferguson Lawrence et al., 2008, 1976).
MULTIGENERATIONAL EFFECTS?
When a woman exposes her female offspring in utero to nicotine, she is exposing both her daughter and her grandchildren as well. This is due to the fact that gametogenesis occurs during fetal development in females (Tilly and Sinclair, 2013). One study, using a rat model of prenatal nicotine administration, showed that not only did the immediate offspring develop symptoms of childhood asthma, but the grandchildren did as well, even though the grandchildren were not exposed to nicotine in utero (Rehan et al., 2012). Longitudinal studies of e-cigarette use would be ideal to determine if offspring and grand-offspring are similarly affected.
Use of Nicotine Replacement Therapies in Pregnancy as a Smoking Cessation Tool: Need for a Better Alternative
Because smoking is known to have high risks for the fetus, including stillbirth, growth restriction, and placental abruption and previa, women are advised to quit smoking while pregnant and to minimize second hand smoke exposure. To do so, many turn to nicotine replacement therapies (NRTs), which include the nicotine containing patch, and nicotine gum. Here we highlight randomized-controlled trials (RCTs) studying the efficacy of NRTs in pregnancy.
A systematic meta-analysis from 2011 of RCTs testing the efficacy of NRT with and without behavioral support in pregnant women concluded that there is insufficient evidence to conclude that the patch is either safe or effective for use in pregnancy (Coleman et al., 2011). This meta-analysis included data from five trials which had enrolled 695 pregnant smokers. Data from two recent placebo controlled RCTs similarly conclude a lack of efficacy of NRT in pregnancy measuring complete abstinence from smoking at delivery as the primary outcome. Berlin et al. enrolled 476 smokers from 23 maternity wards in France (Berlin et al., 2014). Abstinence from smoking was achieved in 5.5% of the NRT group and in 5.1% of the placebo group. They concluded that the patch was not an effective smoking cessation aid in their population. Similar results were reported from a placebo controlled RCT enrolling 1050 participants testing the efficacy of adding a nicotine patch to behavioral therapy (Coleman et al., 2012). The authors concluded that the nicotine patch did not increase the rate of abstinence from smoking. They also reported a low level of compliance as only 7.2% of nicotine patch and 2.8% of placebo control subjects used the patches for more than one month. A smaller RCT which enrolled 40 pregnant women where 20 received both the patch and counseling while the 20 women in the control group received only counseling, found that the patch was not effective for smoking cessation (Hotham et al., 2006). They reported that the patch was only used intermittently and that there was a high level of withdrawal from the study due to lack of interest. A RCT of pregnant smokers looked at the efficacy of nicotine containing gum (N = 100) compared with a placebo (N = 94). While the group with the nicotine gum did not show an increase in smoking cessation compared with placebo, they did smoke fewer cigarettes per day, had lower nicotine in the saliva and their infants had a higher birthweight and gestational age (Oncken et al., 2008).
The results of these studies reveal a need for a more efficacious alternative to smoking cessation in pregnancy besides the patch and gum. Because the results of these studies lead to the conclusion that NRT is not effective in pregnancy, many pregnant women may turn to electronic cigarettes to help them abstain from tobacco cigarettes. While the evidence of e-cigarettes as a smoking cessation device is currently lacking (Bhatnagar et al., 2014), current smokers have reported initiating their use to reduce tobacco cigarette consumption (Etter and Bullen, 2011; Kralikova et al., 2013). However, given the absence of demonstration of efficacy, alongside a lack of studies on the potential long-term risks of e-cigarette use in pregnancy, such advocacy is not supported by current evidence.
Other Sources of Nicotine: Smokeless Tobacco Products and Hookah
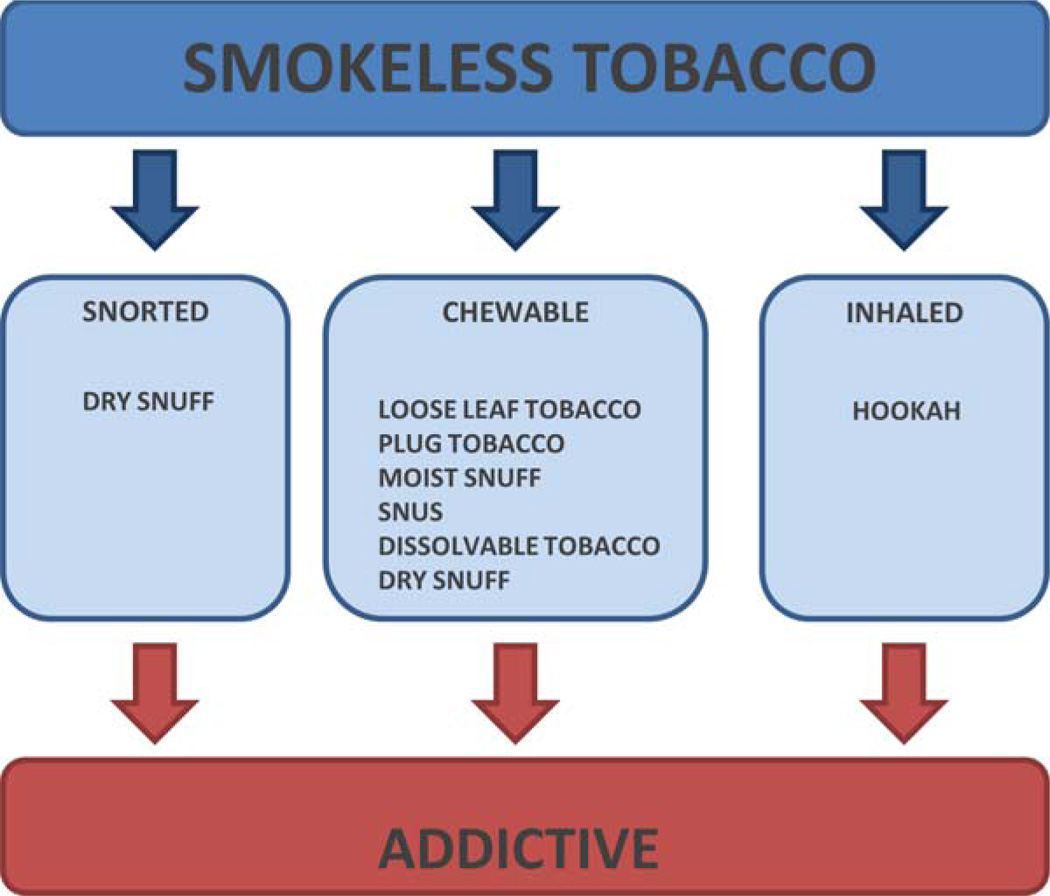
Smokeless tobacco products, also known as noncombustible nicotine products, are marketed under different names and formulations. These products consist of tobacco or a tobacco blend that containing toxicants and are marketed to individuals of all ages. Smokeless tobacco may be chewed, placed in the mouth outside of the gum, or snorted through the nose (Piano et al., 2010; Borgerding et al., 2012) (Fig. 2). Because specific tobacco containing products are becoming more popular with young women, including snus and hookah (2011), it is likely that use of these products will soon become an important public health issue with regard to fertility and pregnancy.
FIGURE 2.
Types of smokeless tobacco. While smokeless tobacco products differ in the mode of use, they contain nicotine, which is addictive. Many also have carcinogenic properties. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Types of Smokeless Tobacco and Hookah
Smokeless tobacco products include a variety of chewing tobaccos, moist and dry snuff, and dissolvable tobaccos (Borgerding et al., 2012).
CHEW OR LOOSE LEAF TOBACCO
Loose leaf or chewing tobacco is air-cured, processed, and flavored with sugar. It is packaged in the form of small strips of shredded tobacco, placed in foil packages, and sold “loose” in bags. Users chew the tobacco or place it between the gum and lip (Borgerding et al., 2012).
PLUG TOBACCO
Plug chewing tobacco consists of cured tobacco leaves treated with syrup and pressed together into bricks. The user may bite off or cut a portion of the brick to either chew or place between gum and lip. Different formulations include tobacco pressed into a cake or plug or twist tobacco which has rope-like strands (Borgerding et al., 2012).
MOIST SNUFF
This formulation is known as “dipping” tobacco or “dip” and is sold in cans. It is the traditional snuff and is made of finely ground and cured tobacco with a high moisture content. Tobacco for moist snuff is aged, fermented, flavored, and cut into small granules. Users may place a “pinch” or “dip” between the gum and either lip or cheek (Borgerding et al., 2012).
SNUS
Heat-treated moist snuff or “snus,” the Swedish word for snuff, is a ground tobacco that is packaged into a small pouch or sachet or is loose. Snus is placed in the mouth between the cheek and gum and is absorbed. It does not require spitting (Borgerding et al., 2012). While data on snus use during pregnancy are lacking, it has been reported that its use is associated with an increased risk of stillbirth (Wikstrom et al., 2010b) and preterm birth (Wikstrom et al., 2010a) but not with a significant reduction in birthweight (Juarez and Merlo, 2013). Prenatal exposure to snus also leads to increased tobacco (including snus) use in adolescent girls (Rydell et al., 2012).
DRY SNUFF
Dry snuff has low moisture content and consists of fermented, finely ground cured tobacco that is sniffed up the nose or placed in the mouth to dissolve like snus (Borgerding et al., 2012).
DISSOLVABLE TOBACCO
These products are “spitless” and dissolve completely in the mouth. They are made of finely milled tobacco with different flavors. Dissolvable tobaccos are packaged as strips, sticks (like toothpicks) coated with or composed of tobacco, or orbs (flavored pellets) (Borgerding et al., 2012).
HOOKAH
Hookah or waterpipe tobacco smoking (hookah, narghile, shisha) is gaining popularity among adolescents and college students. Different flavored tobaccos ranging from apple to cappuccino are placed in the head of the device. Charcoal-heated air is drawn through the flavored tobacco. Smoke bubbles though the water-chamber creating a vapor that is smoked through a shared head or hose (Akl et al., 2010; Shihadeh et al., 2012). The most recent data on hookah use reveal an increase among high school seniors, with an increased likelihood among adolescents of higher socioeconomic status (Palamar JJ et al., 2014).
Summary
As the prevalence and incident use of electronic cigarettes continues to increase among reproductive aged women, an understanding of their risks during pregnancy becomes a pressing need in the public health arena. From animal studies we have learned many of the negative effects on the offspring of nicotine exposure in utero. However, we need to weigh the risks of nicotine exposure with those of combustible tobacco products. While many nonpregnant women may switch to e-cigarettes to quit smoking, there is currently no clinical knowledge of their efficacy and safety in pregnancy. In the clinic, self-query questions directly asking about e-cigarette use and that of other non-nicotine containing products (instead of just “do you smoke” questions) ought to be included for a comprehensive health and exposure history. Based on the evidence currently available, we summarily conclude that no amount of nicotine is known to be safe during pregnancy.
Acknowledgments
This work was funded by NIEHS P30ES023512 (K.A. and M.A.S.), NIDDK R01DK089201 (K.A. and M.A.S.), NINR R01NR014792 (K.A.) and NICHD K99HD075858 (M.A.S.).
Footnotes
The authors report no conflicts of interest.
References
- Committee opinion no. 503: tobacco use and women’s health. Obstet Gynecol. 2011;118:746–750. doi: 10.1097/AOG.0b013e3182310ca9. [DOI] [PubMed] [Google Scholar]
- Aagaard-Tillery K, Spong CY, Thom E, et al. Pharmacogenomics of maternal tobacco use: metabolic gene polymorphisms and risk of adverse pregnancy outcomes. Obstet Gynecol. 2010;115:568–577. doi: 10.1097/AOG.0b013e3181d06faf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Rahman A, Dechkovskaia AM, Sutton JM, et al. Maternal exposure of rats to nicotine via infusion during gestation produces neurobehavioral deficits and elevated expression of glial fibrillary acidic protein in the cerebellum and CA1 subfield in the offspring at puberty. Toxicology. 2005;209:245–261. doi: 10.1016/j.tox.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Akl EA, Gaddam S, Gunukula SK, et al. The effects of waterpipe tobacco smoking on health outcomes: a systematic review. Int J Epidemiol. 2010;39:834–857. doi: 10.1093/ije/dyq002. [DOI] [PubMed] [Google Scholar]
- Association AL. The emergence of new smokeless tobacco products. http://www.lung.org/stop-smoking/tobacco-control-advocacy/reports-resources/tobacco-policy-trend-reports/new-smokeless-tobacco-products.pdf. [Google Scholar]
- Berlin I, Grange G, Jacob N, Tanguy ML. Nicotine patches in pregnant smokers: randomised, placebo controlled, multicentre trial of efficacy. BMJ. 2014;348:g1622. doi: 10.1136/bmj.g1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MS, Apana SM, Nagano KK, et al. Smoking produces rapid rise of [11C]nicotine in human brain. Psychopharmacology (Berl) 2010;209:383–394. doi: 10.1007/s00213-010-1809-8. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A, Whitsel LP, Ribisl KM, et al. Electronic cigarettes: a policy statement from the american heart association. Circulation. 2014;130:1418–1436. doi: 10.1161/CIR.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgerding MF, Bodnar JA, Curtin GM, Swauger JE. The chemical composition of smokeless tobacco: a survey of products sold in the United States in 2006 and 2007. Regul Toxicol Pharmacol. 2012;64:367–387. doi: 10.1016/j.yrtph.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci. 2010;116:364–374. doi: 10.1093/toxsci/kfq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C, McRobbie H, Thornley S, et al. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;19:98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- Burstyn I. Peering through the mist: systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health. 2014;14:18. doi: 10.1186/1471-2458-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? J Public Health Policy. 2011;32:16–31. doi: 10.1057/jphp.2010.41. [DOI] [PubMed] [Google Scholar]
- Camenga DR, Delmerico J, Kong G, et al. Trends in use of electronic nicotine delivery systems by adolescents. Addict Behav. 2014;39:338–340. doi: 10.1016/j.addbeh.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb NK, Abrams DB. E-cigarette or drug-delivery device? Regulating novel nicotine products. N Engl J Med. 2011;365:193–195. doi: 10.1056/NEJMp1105249. [DOI] [PubMed] [Google Scholar]
- Coleman T, Chamberlain C, Cooper S, Leonardi-Bee J. Efficacy and safety of nicotine replacement therapy for smoking cessation in pregnancy: systematic review and meta-analysis. Addiction. 2011;106:52–61. doi: 10.1111/j.1360-0443.2010.03179.x. [DOI] [PubMed] [Google Scholar]
- Coleman T, Cooper S, Thornton JG, et al. A randomized trial of nicotine-replacement therapy patches in pregnancy. N Engl J Med. 2012;366:808–818. doi: 10.1056/NEJMoa1109582. [DOI] [PubMed] [Google Scholar]
- Corey C, Wang B, Johnson S, et al. Electronic cigarette use among middle and high school students — United States, 2011–2012. MMWR Morb Mortal Wkly Rep. 2013;62:729–730. [PMC free article] [PubMed] [Google Scholar]
- Czogala J, Goniewicz ML, Fidelus B, et al. Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob Res. 2014;16:655–662. doi: 10.1093/ntr/ntt203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- De Biasi M, Salas R. Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Exp Biol Med (Maywood) 2008;233:917–929. doi: 10.3181/0712-MR-355. [DOI] [PubMed] [Google Scholar]
- Dutra LM, Glantz SA. Electronic cigarettes and conventional cigarette use among US adolescents: a cross-sectional study. JAMA Pediatr. 2014;168:610–617. doi: 10.1001/jamapediatrics.2013.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England LJ, Kim SY, Shapiro-Mendoza CK, et al. Maternal smokeless tobacco use in Alaska Native women and singleton infant birth size. Acta Obstet Gynecol Scand. 2012;91:93–103. doi: 10.1111/j.1600-0412.2011.01273.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231. doi: 10.1186/1471-2458-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106:2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- Evans SE, Hoffman AC. Electronic cigarettes: abuse liability, topography and subjective effects. Tob Control. 2014;23(Suppl 2):ii23–ii29. doi: 10.1136/tobaccocontrol-2013-051489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BB, Wilson DJ, Schaffner W. Determination of nicotine concentrations in human milk. Am J Dis Child. 1976;130:837–839. doi: 10.1001/archpedi.1976.02120090047008. [DOI] [PubMed] [Google Scholar]
- Foulds J, Veldheer S, Berg A. Electronic cigarettes (e-cigs): views of aficionados and clinical/public health perspectives. Int J Clin Pract. 2011;65:1037–1042. doi: 10.1111/j.1742-1241.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Holloway AC, Su LY, et al. Effects of fetal and neonatal exposure to nicotine on blood pressure and perivascular adipose tissue function in adult life. Eur J Pharmacol. 2008;590:264–268. doi: 10.1016/j.ejphar.2008.05.044. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Holloway AC, Zeng ZH, et al. Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obes Res. 2005;13:687–692. doi: 10.1038/oby.2005.77. [DOI] [PubMed] [Google Scholar]
- Genedani S, Bernardi M, Bertolini A. Sex-linked differences in avoidance learning in the offspring of rats treated with nicotine during pregnancy. Psychopharmacology (Berl) 1983;80:93–95. doi: 10.1007/BF00427504. [DOI] [PubMed] [Google Scholar]
- Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013a;15:158–166. doi: 10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an internet survey. Drug Alcohol Rev. 2013b;32:133–140. doi: 10.1111/j.1465-3362.2012.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, et al. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- Hofhuis W, de Jongste JC, Merkus PJ. Adverse health effects of prenatal and postnatal tobacco smoke exposure on children. Arch Dis Child. 2003;88:1086–1090. doi: 10.1136/adc.88.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway AC, Kellenberger LD, Petrik JJ. Fetal and neonatal exposure to nicotine disrupts ovarian function and fertility in adult female rats. Endocrine. 2006;30:213–216. doi: 10.1385/ENDO:30:2:213. [DOI] [PubMed] [Google Scholar]
- Holloway AC, Lim GE, Petrik JJ, et al. Fetal and neonatal exposure to nicotine in Wistar rats results in increased beta cell apoptosis at birth and postnatal endocrine and metabolic changes associated with type 2 diabetes. Diabetologia. 2005;48:2661–2666. doi: 10.1007/s00125-005-0022-5. [DOI] [PubMed] [Google Scholar]
- Hong YC, Lee KH, Son BK, et al. Effects of the GSTM1 and GSTT1 polymorphisms on the relationship between maternal exposure to environmental tobacco smoke and neonatal birth weight. J Occup Environ Med. 2003;45:492–498. doi: 10.1097/01.jom.0000063627.37065.a1. [DOI] [PubMed] [Google Scholar]
- Hotham ED, Gilbert AL, Atkinson ER. A randomised-controlled pilot study using nicotine patches with pregnant women. Addict Behav. 2006;31:641–648. doi: 10.1016/j.addbeh.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Juarez SP, Merlo J. The effect of Swedish snuff (snus) on offspring birthweight: a sibling analysis. PLoS One. 2013;8:e65611. doi: 10.1371/journal.pone.0065611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralikova E, Novak J, West O, et al. Do e-cigarettes have the potential to compete with conventional cigarettes?: a survey of conventional cigarette smokers’ experiences with e-cigarettes. Chest. 2013;144:1609–1614. doi: 10.1378/chest.12-2842. [DOI] [PubMed] [Google Scholar]
- Lawrence J, Xiao D, Xue Q, et al. Prenatal nicotine exposure increases heart susceptibility to ischemia/reperfusion injury in adult offspring. J Pharmacol Exp Ther. 2008;324:331–341. doi: 10.1124/jpet.107.132175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella NM, Kent PF, Youngentob SL. Fetal nicotine exposure increases preference for nicotine odor in early postnatal and adolescent, but not adult, rats. PLoS One. 2013;8:e84989. doi: 10.1371/journal.pone.0084989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritz GS. Perinatal exposure to nicotine and implications for subsequent obstructive lung disease. Paediatr Respir Rev. 2013;14:3–8. doi: 10.1016/j.prrv.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2005. Natl Vital Stat Rep. 2007;56:1–103. [PubMed] [Google Scholar]
- McAuley TR, Hopke PK, Zhao J, Babaian S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal Toxicol. 2012;24:850–857. doi: 10.3109/08958378.2012.724728. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Ogawa T, Kamei K, et al. Nicotine exposure during pregnancy is a factor which influences serotonin transporter density in the rat brain. Eur J Pharmacol. 2001;411:279–282. doi: 10.1016/s0014-2999(00)00925-0. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Ogawa T, Kamei K, et al. Prenatal nicotine exposure affects the development of the central serotonergic system as well as the dopaminergic system in rat offspring: involvement of route of drug administrations. Brain Res Dev Brain Res. 1997;102:117–126. doi: 10.1016/s0165-3806(97)00092-8. [DOI] [PubMed] [Google Scholar]
- Oncken C, Dornelas E, Greene J, et al. Nicotine gum for pregnant smokers: a randomized controlled trial. Obstet Gynecol. 2008;112:859–867. doi: 10.1097/AOG.0b013e318187e1ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarson M, McLellan RA, Gullsten H, et al. Identification and characterisation of novel polymorphisms in the CYP2A locus: implications for nicotine metabolism. FEBS Lett. 1999;460:321–327. doi: 10.1016/s0014-5793(99)01364-2. [DOI] [PubMed] [Google Scholar]
- Palamar JJ, Zhou S, Sherman S, Weitzman M. Hookah Use Among US High School Seniors. Pediatrics. 2014;134:227–234. doi: 10.1542/peds.2014-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik JJ, Gerstein HC, Cesta CE, et al. Effects of rosiglitazone on ovarian function and fertility in animals with reduced fertility following fetal and neonatal exposure to nicotine. Endocrine. 2009;36:281–290. doi: 10.1007/s12020-009-9229-4. [DOI] [PubMed] [Google Scholar]
- Pianezza ML, Sellers EM, Tyndale RF. Nicotine metabolism defect reduces smoking. Nature. 1998;393:750. doi: 10.1038/31623. [DOI] [PubMed] [Google Scholar]
- Piano MR, Benowitz NL, Fitzgerald GA, et al. Impact of smokeless tobacco products on cardiovascular disease: implications for policy, prevention, and treatment: a policy statement from the American Heart Association. Circulation. 2010;122:1520–1544. doi: 10.1161/CIR.0b013e3181f432c3. [DOI] [PubMed] [Google Scholar]
- Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786. doi: 10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raunio H, Rautio A, Gullsten H, Pelkonen O. Polymorphisms of CYP2A6 and its practical consequences. Br J Clin Pharmacol. 2001;52:357–363. doi: 10.1046/j.0306-5251.2001.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehan VK, Liu J, Naeem E, et al. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med. 2012;10:129. doi: 10.1186/1741-7015-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribary U, Lichtensteiger W. Effects of acute and chronic prenatal nicotine treatment on central catecholamine systems of male and female rat fetuses and offspring. J Pharmacol Exp Ther. 1989;248:786–792. [PubMed] [Google Scholar]
- Richardson SA, Tizabi Y. Hyperactivity in the offspring of nicotine-treated rats: role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacol Biochem Behav. 1994;47:331–337. doi: 10.1016/0091-3057(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Riker CA, Lee K, Darville A, Hahn EJ. E-cigarettes: promise or peril? Nurs Clin N Am. 2012;47:159–171. doi: 10.1016/j.cnur.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Rydell M, Cnattingius S, Granath F, et al. Prenatal exposure to tobacco and future nicotine dependence: population-based cohort study. Br J Psychiatry. 2012;200:202–209. doi: 10.1192/bjp.bp.111.100123. [DOI] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, et al. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217:628–637. doi: 10.1016/j.ijheh.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Schoedel KA, Hoffmann EB, Rao Y, et al. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- Seidler FJ, Levin ED, Lappi SE, Slotkin TA. Fetal nicotine exposure ablates the ability of postnatal nicotine challenge to release norepinephrine from rat brain regions. Brain Res Dev Brain Res. 1992;69:288–291. doi: 10.1016/0165-3806(92)90170-2. [DOI] [PubMed] [Google Scholar]
- Sekhon HS, Jia Y, Kuryatov A, et al. Prenatal nicotine increases pulmonary alpha 7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest. 1999;103:637–647. doi: 10.1172/JCI5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon HS, Keller JA, Benowitz NL, Spindel ER. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med. 2001;164:989–994. doi: 10.1164/ajrccm.164.6.2011097. [DOI] [PubMed] [Google Scholar]
- Sekhon HS, Proskocil BJ, Clark JA, Spindel ER. Prenatal nicotine exposure increases connective tissue expression in foetal monkey pulmonary vessels. Eur Respir J. 2004;23:906–915. doi: 10.1183/09031936.04.00069604. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Salman R, Jaroudi E, et al. Does switching to a tobacco-free waterpipe product reduce toxicant intake? A crossover study comparing CO, NO, PAH, volatile aldehydes, "tar" and nicotine yields. Food Chem Toxicol. 2012;50:1494–1498. doi: 10.1016/j.fct.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking-cessation: tool results from an online survey. Am J Prev Med. 2011;40:472–475. doi: 10.1016/j.amepre.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Orband-Miller L, Queen KL, et al. Effects of prenatal nicotine exposure on biochemical development of rat brain regions: maternal drug infusions via osmotic minipumps. J Pharmacol Exp Ther. 1987;240:602–611. [PubMed] [Google Scholar]
- Suter M, Abramovici A, Aagaard-Tillery K. Genetic and epigenetic influences associated with intrauterine growth restriction due to in utero tobacco exposure. Pediatr Endocrinol Rev. 2010;8:94–102. [PMC free article] [PubMed] [Google Scholar]
- Suter MA, Aagaard-Tillery KM. Environmental influences on epigenetic profiles. Semin Reprod Med. 2009;27:380–390. doi: 10.1055/s-0029-1237426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MA, Anders AM, Aagaard KM. Maternal smoking as a model for environmental epigenetic changes affecting birthweight and fetal programming. Mol Hum Reprod. 2013;19:1–6. doi: 10.1093/molehr/gas050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutfin EL, McCoy TP, Morrell HE, et al. Electronic cigarette use by college students. Drug Alcohol Depend. 2013;131:214–221. doi: 10.1016/j.drugalcdep.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AS, Bigman CA. E-cigarette awareness and perceived harmfulness: prevalence and associations with smoking-cessation outcomes. Am J Prev Med. 2014;47:141–149. doi: 10.1016/j.amepre.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly JL, Sinclair DA. Germline energetics, aging, and female infertility. Cell Metab. 2013;17:838–850. doi: 10.1016/j.cmet.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong VT, Dietz PM, Morrow B, et al. Trends in smoking before, during, and after pregnancy–pregnancy risk assessment monitoring system, United States, 40 sites, 2000–2010. MMWR Surveill Summ. 2013;62:1–19. [PubMed] [Google Scholar]
- Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787–1791. doi: 10.1093/ntr/ntt061. [DOI] [PubMed] [Google Scholar]
- Wikstrom AK, Cnattingius S, Galanti MR, et al. Effect of Swedish snuff (snus) on preterm birth. BJOG. 2010a;117:1005–1010. doi: 10.1111/j.1471-0528.2010.02575.x. [DOI] [PubMed] [Google Scholar]
- Wikstrom AK, Cnattingius S, Stephansson O. Maternal use of Swedish snuff (snus) and risk of stillbirth. Epidemiology. 2010b;21:772–778. doi: 10.1097/EDE.0b013e3181f20d7e. [DOI] [PubMed] [Google Scholar]
- Williams M, Villarreal A, Bozhilov K, et al. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8:e57987. doi: 10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhang X, Xu Y, et al. Prenatal nicotine exposure mouse model showing hyperactivity, reduced cingulate cortex volume, reduced dopamine turnover, and responsiveness to oral methylphenidate treatment. J Neurosci. 2012;32:9410–9418. doi: 10.1523/JNEUROSCI.1041-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]