Abstract
Purpose
African American women are more likely to be diagnosed with metastatic breast cancer at the time of presentation than whites, and have shorter survival once diagnosed. This study examines racial differences in clinical outcomes in the setting of two large cooperative group randomized clinical trials.
Patients and Methods
The study cohort consisted of 787 white (80%) and 195 African American (20%) patients with metastatic breast cancer enrolled in two successive Cancer and Leukemia Group B (CALGB) trials using taxanes in the metastatic setting. Differences in overall survival (OS), response incidence, and time to treatment failure (TTF) were examined by race. In addition, differences in the incidence of baseline and treatment-related toxicities were examined.
Results
With 779 deaths (166 African Americans and 613 whites), median OS was 14.3 months for African Americans and 18.75 months for whites (hazard ratio [HR] = 1.37; 95% CI, 1.15 to 1.63). When adjusted for prognostic factors, African Americans had a 24% increase in the hazard of death compared with whites (HR = 1.24; 95% CI, 1.02 to 1.51). No significant differences in TTF or overall response to therapy were seen. No clinically significant toxicity differences were seen.
Conclusion
African Americans with metastatic breast cancer have an increased hazard of death compared with whites despite the receipt of similar per-protocol treatment, but experience no differences in TTF or overall response to therapy. We hypothesize that more direct and robust measures of comorbidities, and perhaps other factors such as receipt of subsequent therapy could help further explain the observed survival difference.
INTRODUCTION
It is well established that African American women have a lower incidence of but higher mortality rate from breast cancer than whites.1,2 Much of this survival disparity has been attributed to more advanced stage at diagnosis.3 Although differences in stage at diagnosis are important in understanding the survival disparity, even when analyses are controlled for stage, African Americans continue to have poorer long-term survival rates. The 5-year relative survival for patients diagnosed with metastatic disease between 1996 and 2002 was 28% for whites and only 16% for African Americans. Of particular concern is that this survival disparity is growing compared with the 1975 to 1979 period, when the 5-year cancer-specific survival was 18% for whites and 15% for African Americans.2
Understanding this stage-specific survival disparity is challenging. Previous researchers have pointed to racial differences in tumor-related factors such as hormone-receptor status and tumor histology.3–6 Others have explored the importance of patient-related characteristics such as socioeconomic status and competing comorbidities among African American women.3,7–9 Finally, treatment-related factors such as inadequate therapy or higher rates of toxicity, perhaps leading to lower intensity of treatment, have been explored as reasons for the poorer stage-specific survival for African American women.10–15
One way to analyze and isolate the reasons for stage-specific survival differences is to look at outcomes in the setting of a clinical trial. The advantage of analyzing clinical trial data is that eligibility criteria, treatments, and response data are standardized and quantified. Also, information on tumor biology, treatment-related toxicities, and some patient-related characteristics are prospectively collected and can be analyzed to determine whether they have an impact on observed racial differences in survival. This type of analysis has been performed in patients with nonmetastatic breast cancer with several cooperative group studies and demonstrated no differences in breast cancer–specific survival between African Americans and whites after adjusting for demographic and prognostic tumor variables.16–18 However, analysis of the Southwest Oncology Group (SWOG) adjuvant breast cancer trials did find that African Americans had lower overall survival (OS) and cancer-specific survival compared with whites even after controlling for demographics and prognostic tumor variables.19 In the metastatic breast cancer setting, no large cooperative group analysis has been performed. A small study of metastatic breast cancer patients participating in five Piedmont Oncology Association trials found no difference in response rates between African Americans and whites; however, white patients had a statistically significant 6-month longer median survival.20
The metastatic breast cancer setting offers a unique setting to explore racial differences in survival in that the analysis begins at time when all patients are established to have incurable disease. In this analysis, we examined racial differences in clinical outcomes and the potential reasons behind those differences in the setting of two large cooperative group metastatic breast cancer trials.
PATIENTS AND METHODS
Study Population
The study cohort consisted of patients enrolled in Cancer and Leukemia Group B (CALGB) trials 9342 and 9840. Details of these trials have been reported previously.21,22 In brief, between January 15, 1994, and July 31, 1997, CALGB 9342 randomly assigned patients with measurable metastatic breast cancer or inoperable breast cancer and zero to one prior treatments for locally advanced or metastatic disease to three different doses of paclitaxel (175 mg/m2, 210 mg/m2, or 250 mg/m2) administered over 3 hours every 3 weeks. Between January 15, 1998, and November 14, 2003, CALGB 9840 randomly assigned patients with measurable metastatic breast cancer and zero to one prior chemotherapy regimens for locally advanced or metastatic breast cancer to paclitaxel 175 mg/m2 administered every 3 weeks or paclitaxel 100 mg/m2 administered weekly. Weekly dose was subsequently amended to 80 mg/m2. Patients with HER-2–positive tumors (immunohistochemistry 3+) received trastuzumab; patients with HER-2–negative tumors (immunohistochemistry 0 to 2+) were randomly assigned to receive trastuzumab or not. After excluding 61 patients who were neither white nor African American and 13 patients with unknown ethnicity, the final cohort consisted of 787 white and 194 African American patients.
Statistical Analysis
The primary study end points were tumor response, OS, and time to treatment failure (TTF). OS was calculated as the time from study entry to date last known alive or to date of death resulting from any cause. TTF was measured from study entry to date of first disease progression or to date of death for those patients who died without progression. Patients who were alive and without progression were censored at the date they were last known to be progression free. Tumor response was defined as complete or partial response. Complete response was defined as the disappearance of all lesions. Partial response was defined as a reduction of at least 50% of the sum of the product of all lesions with bidimensional measurements, and with stable or improving nonmeasurable lesions. Patients whose tumors could not be assessed for response because of excessive toxicity or early death were considered nonresponders.
Logistic regression was used to model the relation of tumor response with race and other covariables.23 Proportional hazards regression was used to model the relation of OS and TTF, respectively, with race and other covariables.24 Each of the two clinical trials whose populations are included herein was stratified by line of therapy. Therefore all models include indicator variables for line of therapy and for study. For each end point, we constructed two sets of models. The base model included race, study and line of therapy. In addition to the variables in the base model, the full model included other covariables. To assess the relation of race on time to onset of selected toxicity with a severity of at least grade 3, we constructed proportional hazards regression models for each toxicity that had at least a 20% incidence of grade 3 or higher severity. We used the National Cancer Institute Common Toxicity Criteria version 2.0 to grade toxicity.
Covariables of interest were patient demographics (age, menopausal status), clinicopathologic features (tumoral estrogen-receptor [ER] status, number of measurable metastatic sites, performance score, prior adjuvant therapy, pretreatment blood counts) and treatment factors (dose schedule and paclitaxel dose) available at study entry. Race was determined by patient self-report. The following variables were analyzed as dichotomous variables: race (African American v white), study (9342 v 9840), line of therapy (first v second), ER status (negative v positive), menopausal status (pre v post), prior adjuvant therapy (yes v no), and dose schedule (weekly v standard). Paclitaxel dose (80, 100, 175, 210, 250), performance score (0 to 3) and patient age were analyzed on a continuous scale. Other variables were transformed, as follows: (1) square root:number of measurable metastatic sites and (2) log10:WBC, platelets and granulocytes.
Main effects were assessed in multivariate regression models using the Wald χ2 test. Odds ratios (ORs) and hazards ratios (HRs) for main effects and their respective 95% CIs were taken from the corresponding regression model. ORs and their 95% CIs for interaction terms were calculated using the method of Hosmer and Lemeshow.23
OS, TTF, and time to toxicity distributions were estimated using the Kaplan-Meier product limit technique.24 We used the χ2 test to compare two proportions and the Mann-Whitney U test to compare two groups on continuous variables. Analyses used a modified intention-to-treat approach, including all patients who began protocol therapy regardless of eligibility determination. There was a 5% ineligibility incidence in each racial subgroup.
All P values are two sided. We report as significant P ≤ .05. CALGB statisticians performed statistical analyses on data available in the CALGB database as of February 2005 using SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
Race in Relation to Other Patient and Disease Characteristics at Study Entry
The study population included 787 white (80%) and 195 African American (20%) patients. Table 1 shows patient and disease characteristics and treatment variables by race and line of therapy. Fifty-one percent of African American patients compared with 41% of white patients were receiving second line therapy for metastatic breast cancer (P = .014). Restricting to line of therapy, a larger proportion of African American patients than white patients were younger (first line, P < .0001; second line, P = .0050) and premenopausal (first line, P = .0004; second line, P = .033). Additionally, a higher proportion of African American patients had ER-negative tumors (first line, P = .0045; second line, P = .055). At study entry African Americans had significantly lower hemoglobin levels (first line, P = .0001; second line, P = .0021) and significantly higher platelet counts (first line, P = .0001; second line, P = .0057) than their white counterparts.
Table 1.
Patient and Tumor Characteristics by Race and Line of Therapy
| Characteristic | First Line
|
Second Line
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient Race
|
P | Patient Race
|
P | |||||||
| White
|
African American
|
White
|
African American
|
|||||||
| No. | % | No. | % | No. | % | No. | % | |||
| All patients | 464 | 100 | 96 | 100 | 323 | 100 | 99 | 100 | ||
|
| ||||||||||
| Study | .058 | .35 | ||||||||
| 9342 | 78 | 17 | 24 | 25 | 250 | 77 | 81 | 82 | ||
| 9840 | 386 | 83 | 72 | 75 | 73 | 23 | 18 | 18 | ||
|
| ||||||||||
| Age, years* | .0001 | .0050 | ||||||||
| < 40 | 33 | 7 | 14 | 15 | 20 | 6 | 12 | 12 | ||
| 40–49 | 100 | 22 | 32 | 33 | 61 | 19 | 19 | 19 | ||
| 50–59 | 139 | 30 | 31 | 32 | 99 | 31 | 36 | 36 | ||
| 60–69 | 114 | 25 | 15 | 16 | 100 | 31 | 24 | 24 | ||
| 70+ | 78 | 17 | 4 | 4 | 43 | 13 | 8 | 8 | ||
|
| ||||||||||
| Menopausal status | .0004 | .033 | ||||||||
| Premenopausal | 84 | 18 | 33 | 34 | 33 | 10 | 18 | 18 | ||
| Postmenopausal | 380 | 82 | 63 | 66 | 290 | 90 | 81 | 82 | ||
|
| ||||||||||
| Prior adjuvant therapy | .15 | .84 | ||||||||
| No | 221 | 48 | 37 | 39 | 143 | 44 | 43 | 43 | ||
| Yes | 236 | 51 | 55 | 57 | 168 | 52 | 53 | 54 | ||
| Missing | 7 | 2 | 4 | 4 | 12 | 4 | 3 | 3 | ||
|
| ||||||||||
| Performance score* | .077 | .49 | ||||||||
| 0 | 222 | 48 | 38 | 40 | 156 | 48 | 45 | 45 | ||
| 1 | 209 | 45 | 49 | 51 | 144 | 45 | 43 | 43 | ||
| 2 | 22 | 5 | 6 | 6 | 19 | 6 | 8 | 8 | ||
| 3 | 1 | < 1 | 2 | 2 | 0 | 0 | 1 | 1 | ||
| Missing | 10 | 2 | 1 | 1 | 4 | 1 | 2 | 2 | ||
|
| ||||||||||
| Tumoral estrogen receptor | .0045 | .055 | ||||||||
| Negative | 180 | 39 | 51 | 53 | 117 | 36 | 48 | 48 | ||
| Positive | 243 | 52 | 35 | 36 | 170 | 53 | 44 | 44 | ||
| Missing | 41 | 9 | 10 | 10 | 36 | 11 | 7 | 7 | ||
|
| ||||||||||
| No. of measurable sites* | .24 | .22 | ||||||||
| 0–1 | 274 | 59 | 54 | 56 | 189 | 59 | 52 | 53 | ||
| 2–3 | 167 | 36 | 35 | 36 | 119 | 37 | 37 | 37 | ||
| 4–6 | 18 | 4 | 7 | 7 | 9 | 3 | 6 | 6 | ||
| Missing | 5 | 1 | 0 | 0 | 6 | 2 | 4 | 4 | ||
|
| ||||||||||
| Paclitaxel dose,* mg/m2 | .38 | .72 | ||||||||
| 80/100 | 230 | 50 | 47 | 49 | 42 | 13 | 12 | 12 | ||
| 175 | 185 | 40 | 32 | 33 | 113 | 35 | 38 | 38 | ||
| 210 | 23 | 5 | 10 | 10 | 83 | 26 | 26 | 26 | ||
| 250 | 26 | 6 | 7 | 7 | 85 | 26 | 23 | 23 | ||
|
| ||||||||||
| Dose schedule | .91 | .82 | ||||||||
| Weekly | 234 | 50 | 49 | 51 | 281 | 87 | 87 | 88 | ||
| Standard | 230 | 50 | 47 | 49 | 42 | 13 | 12 | 12 | ||
|
| ||||||||||
| Median pretreatment counts | ||||||||||
| WBC, k/μL* | 6.6 | 6.2 | .20 | 6.1 | 6.2 | .75 | ||||
| Hemoglobin, g/dL* | 13.0 | 11.9 | .0001 | 12.4 | 11.9 | .0021 | ||||
| Platelets, k/μL* | 264 | 317 | .0001 | 251 | 275 | .0057 | ||||
| Granulocytes, k/μL* | 4.4 | 3.9 | .036 | 4.3 | 4.1 | .58 | ||||
Comparison used data on a continuous scale.
Influence of Race on Tumor Response, OS, and TTF
Observed effect: Base models
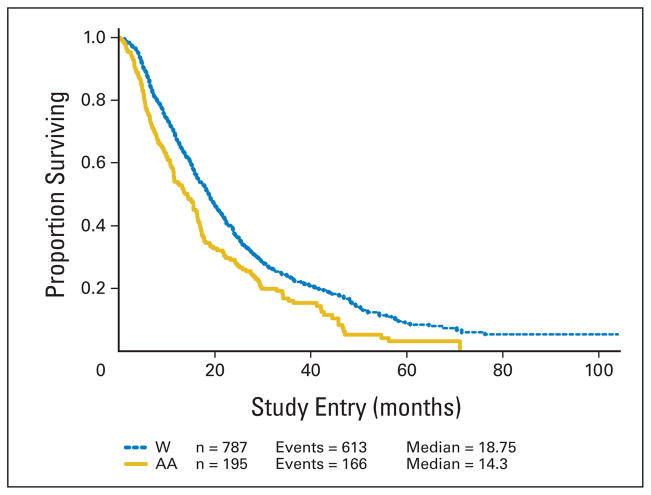
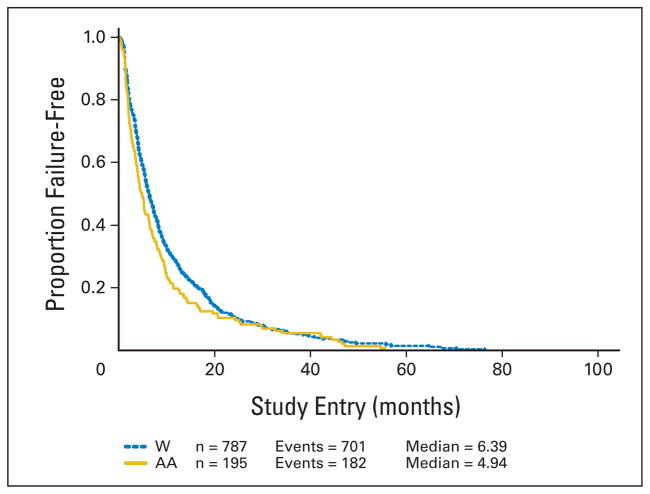
African American patients had a median OS of 14.3 versus 18.7 months for white patients. African American patients had a median TTF of 4.9 versus 6.4 months for white patients. This translates into a 37% increase in the hazard of death (HR = 1.37; 95% CI, 1.15 to 1.63) for African American patients and a 17% increase in the hazard of failure (HR = 1.17; 95% CI, 0.99 to 1.38) for African American patients over white patients. Tumor response did not differ by race overall (30% v 31%). The results are shown in Table 2. The Kaplan-Meier curves for OS and TTF are shown in Figures 1 and 2.
Table 2.
Observed Effect of Race on Response, OS, and TTF: Base Model
| Patient Group | No. of Patients | OS
|
TTF
|
Tumor Response
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (months) | HR* | 95% CI | Median (months) | HR† | 95% CI | Incidence (%) | OR‡ | 95% CI | ||
| All | 982 | 1.37 | 1.15 to 1.63 | 1.17 | 0.99 to 1.38 | 1.04 | 0.73 to 1.47 | |||
|
| ||||||||||
| African American | 195 | 14.3 | 4.9 | 30 | 23 to 37 | |||||
|
| ||||||||||
| White | 787 | 18.8 | 6.4 | 31 | 28 to 34 | |||||
NOTE. OR and HR are adjusted for study and line of therapy only.
Abbreviations: OS, overall survival; TTF, time to treatment failure; HR, hazard ratio; OR, odds ratio.
Ratio of African American:white hazard of death.
Ratio of African American:white hazard of failure.
Ratio of African American:white odds of response.
Fig 1.
Overall survival by race. Kaplan-Meier analysis of the effect of race on overall survival for patients enrolled in Cancer and Leukemia Group B metastatic breast cancer trials 9342 and 9840. W, white; AA, African American.
Fig 2.
Time to treatment failure by race. Kaplan-Meier analysis of the effect of race on time to treatment failure for patients enrolled in Cancer and Leukemia Group B metastatic breast cancer trials 9342 and 9840. W, white; AA, African American.
Adjusted observed effect: Full models
Table 3 shows the observed effect of race on OS and TTF after adjusting for all predictor variables. African American patients continue to have an increased hazard of death compared with whites, albeit of a lower magnitude than seen in the base model (HR = 1.24; 95% CI, 1.02 to 1.51; P = .030). The effect of race on TTF remains nonsignificant (HR = 1.11; 95% CI, 0.92 to 1.33; P = .29). In addition to race, other variables significantly related to survival were study, line of therapy, ER status, performance score, pretreatment platelet count, and age. Other variables significantly related to TTF were study, performance score, age, ER status, number of metastatic sites at enrollment, and dosing schedule.
Table 3.
Observed Effect of All Predictor Variables on OS and TTF
| Variable | Comparison for HR (higher v lower) | OS*
|
TTF†
|
||||
|---|---|---|---|---|---|---|---|
| P | HR | 95% CI for HR | P | HR | 95% CI for HR | ||
| Race | African American v white | .030 | 1.24 | 1.02 to 1.51 | .29 | 1.11 | 0.92 to 1.33 |
|
| |||||||
| Study | 9342 v 9840 | < .0001 | 1.74 | 1.33 to 2.28 | .0013 | 1.52 | 1.18 to 1.96 |
|
| |||||||
| Line of treatment | Second v first | .0022 | 1.36 | 1.12 to 1.65 | .14 | ||
|
| |||||||
| Age, years | 45 v 65 | .044 | 1.20 | 1.00 to 1.44 | .0041 | 1.27 | 1.08 to 1.50 |
|
| |||||||
| ER status | Negative v positive | < .0001 | 1.54 | 1.31 to 1.81 | .011 | 1.21 | 1.04 to 1.40 |
|
| |||||||
| Menopausal status | .063 | .40 | |||||
|
| |||||||
| Performance score | 2 v 1 | < .0001 | 1.38 | 1.21 to 1.58 | < .0001 | 1.29 | 1.14 to 1.46 |
|
| |||||||
| Prior adjuvant chemotherapy | .37 | .67 | |||||
|
| |||||||
| No. of metastatic sites | 5 v 3 | .61 | .028 | 1.01 | 1.00 to 1.02 | ||
|
| |||||||
| Pretreatment WBC | .58 | .84 | |||||
|
| |||||||
| Pretreatment platelet count, ×10−6 k/μL | 325 v 225 | .018 | 1.13 | 1.02 to 1.25 | .16 | ||
|
| |||||||
| Pretreatment granulocytes | .46 | .46 | |||||
|
| |||||||
| Density | Standard v dense | .076 | .0018 | 1.79 | 1.24 to 2.58 | ||
|
| |||||||
| Dose | .32 | .060 | |||||
NOTE. HR for OS and TTF are adjusted for all other variables in the model.
Abbreviations: OS, overall survival; TTF, time to treatment failure; HR, hazard ratio; ER, estrogen receptor.
n = 840, 78% with events.
n = 840 with 89% events.
Influence of Race on Treatment-Related Toxicities
Table 4 shows the incidence of grade 3+ selected toxicities by race. Compared with white patients, African American patients had a significantly higher incidence of anemia (8% v 3%) and a slightly higher incidence of thrombocytopenia (2% v < 1%) and neutropenia (42% v 35%). No differences were seen with respect to grade 3+ leucopenia, lymphocytopenia, infection, or neuropathy.
Table 4.
Incidence of Highest Grade of Selected Toxicity Observed During Entire Course of Protocol Therapy (grades 3+)
| Group* | Grade 3+
|
P | |
|---|---|---|---|
| No. | % | ||
| WBC | |||
| White | 184 | 24 | .80 |
| African American | 42 | 23 | |
|
| |||
| Platelets | |||
| White | 5 | < 1 | .053 |
| African American | 4 | 2 | |
|
| |||
| Hemoglobin | |||
| White | 21 | 3 | .0014 |
| African American | 14 | 8 | |
|
| |||
| Granulocytes | |||
| White | 267 | 35 | .080 |
| African American | 76 | 42 | |
|
| |||
| Lymphocytes | |||
| White | 302 | 39 | .82 |
| African American | 70 | 38 | |
|
| |||
| Infection | |||
| White | 41 | 5 | .22 |
| African American | 14 | 8 | |
|
| |||
| Sensory neuropathy | |||
| White | 140 | 18 | .89 |
| African American | 34 | 19 | |
|
| |||
| Motor neuropathy | |||
| White | 60 | 8 | .95 |
| African American | 14 | 8 | |
White patients, n = 767; African American patients, n = 182.
In total, 20% or more of all patients experienced grade 3 or higher leucopenia, neutropenia, and lymphocytopenia. We assessed the relation of race, after adjusting for study and line of therapy, with time to onset of grade 3+ of each of these three toxicities. Compared with African American patients, white patients had a slightly higher hazard of developing grade 3+ lymphocytopenia (HR = 1.30; 95% CI, 1.00 to 1.69; P = .048). Time to onset of grade 3+ leucopenia or neutropenia did not differ by race (data not shown).
DISCUSSION
Consistent with national epidemiologic data, we found that African Americans participating in these two large cooperative group meta-static breast cancer trials have a shorter OS than white patients. We did not, however, see any significant difference in response to therapy or TTF by race. The advantage of clinical trial data is that eligibility and treatment are standardized and several known prognostic tumor and patient-related factors are prospectively collected and can be accounted for in the analyses. However, even after adjusting for these factors, a persistent survival disparity was seen. The critical question is, why?
Race is a complex variable that is likely acting as a proxy for factors associated with lower socioeconomic status, culture, discrimination, and other factors. In addition the potential that race is also serving as surrogate for underlying differences in tumor and host biology cannot be dismissed. Any time survival differences by race are documented in the presence of what seems to be equal treatment, the issue of potential differences in underlying tumor and patient biology surfaces. Previous studies have documented racial differences in tumor biology such as hormone-receptor status, tumor grade, and S-phase fraction, which may lead to a more aggressive tumor phenotype in African Americans versus whites.6,10,25,26 Indeed, a subset analysis of patients enrolled on CALGB 9342 suggests that African American patients were more than twice as likely to have so called triple-negative tumors (ER, progesterone receptor, and HER-2 negative), and that this phenotype was associated with poorer OS.27 Consistent with this observation, recently published work by Carey et al28 suggests that the poorer prognosis basal-like breast tumor phenotype is over-represented in premenopausal African American women. Finally, racial differences in the expression of cell-cycle regulatory proteins,29–31 as well as differences in steroid-metabolizing genes, have been noted.32,33
Can differences in tumor biology explain the poorer OS observed in this study? The lack of any significant differences in overall response rates or TTF argues against the predictive importance of racial differences in underlying tumor biology in terms of response to paclitaxel-based therapies. It is, of course, possible that biologic differences that influence survival after disease progression are present, and therefore would not be reflected in lack of TTF or response differences. Also, although the hazard of failure was not statistically significant and diminished in magnitude and significance in the multivariate model, the direction favored an increased hazard of failure for African Americans. Although we feel it is unlikely, we cannot rule out the possibility that a true difference in TTF is present.
In terms of survival, a survival disparity remains even after controlling for measured differences in tumoral ER status. In addition, the interaction between race and tumoral ER status was not of statistical significance for either OS (P = .27) or TTF (P = .47) suggesting that survival disparities persisted even when only ER-positive or ER-negative patients were considered. Harris et al27 recently published a more detailed analysis of the biologic features of the tumors for some of the patients included in this analysis and their relationship to disease outcome. Although it might have been interesting to examine the effect that these variables had on racial differences in outcome, such a subset analysis would have limited statistical power. However, it is possible that accounting for more detailed tumor biology information such as grade, HER-2 status or gene-expression classifications would have allowed us to explain more of the survival disparity. Of note, in the study by Carey et al, African American women with breast cancer continued to have a poorer OS even after accounting for differences in proportion of women who had ER-negative and basal-like tumors.28
An area that is receiving increased attention is the impact that underlying comorbidities may have in explaining cancer outcome disparities. A recently published study of breast cancer patients suggests that African American patients do have higher rates of comorbidities and that controlling for them could explain 50% of the survival disparity between African Americans and whites.8 In the current study, we were able to account for the combined effects of comorbidities and tumor only minimally through an adjustment for performance score. Because there was no significant difference in baseline performance status between African Americans and whites, it is not surprising that this adjustment had little impact on the observed survival disparities. More robust and direct measures of comorbidities may have allowed us to explain more of the survival disparity. Although it is true that patients with debilitating comorbidities would not have been allowed on these trials, little is known about the impact that nonsymptomatic comorbidities such as hypertension, coronary artery disease, and diabetes may have on the survival of metastatic breast cancer patients receiving chemotherapy. This last explanation points to the need to prospectively collect this comorbidity data as part of the clinical trial process.
Additional potentially important prognostic factors such as stage at original diagnosis, year of diagnosis, time from original diagnosis to metastatic disease, socioeconomic status, and the number of additional lines of therapy administered after patients received per-protocol treatment were not incorporated into our models because such information was not reliably available. Although we cannot exclude the possibility that such information may have modified our results, the combination of the available patient and tumor-related information and the standardization of entry criteria, treatment, and follow-up allows for a robust analysis of racial differences in outcomes in the metastatic breast cancer setting. We do believe, however, that more rigorous collection of this type of information should be undertaken in future clinical trial efforts.
Recently, there has been increased focus on the role that toxicity from therapy may play in explaining the poorer OS for African American women with breast cancer. Small studies have suggested that African Americans have higher rates of neutropenia, take longer to complete treatment, receive less intensity of therapy, and are more likely to dropout of therapy perhaps as a result of toxicities.12–14 In this study, we examined the issue of toxicity in sever always. First we examined baseline values and, consistent with previous reports, we found borderline differences in granulocytes and lower median hemoglobin levels among African Americans.14,34–36 We then examined the highest rates of grade 3+ toxicity by race and found only borderline differences in the incidence of grade 3+ neutropenia and thrombocytopenia, but did find fairly significant differences in the incidence of grade3+ anemia (hemoglobin < 8.0g/dL). Of note, there was no difference in the hazard of developing grade 3+ neutropenia, which suggests no differences in the degree of therapy-related neutropenia once factors such as study, line of therapy, and time on therapy were controlled for. Finally, we hypothesized that, if there was an association between race and tolerance of therapy that influenced survival or progression, this would be seen with the higher doses of paclitaxel used in the 9342 trial. However, we found no such interaction between race and dose of therapy. In the end, we find no evidence of a clinically meaningful interaction between race and tolerance of therapy that would explain the survival disparity between African Americans and whites reported in this study.
In conclusion, this study demonstrates a 24% increased risk in the hazard of death for African American patients with metastatic breast cancer compared with whites despite standardized eligibility and treatment after adjustments for several tumor, patient, and treatment-related factors. No significant differences in TTF or tumor response were seen. We hypothesize that more direct measures of comorbidities and perhaps other factors such as receipt of subsequent therapy could help further explain the observed differences.
Acknowledgments
Supported by National Cancer Institute Grants No. CA41287, CA33601, CA77651, CA77406, CA77658, CA47559, CA47577, and CA32291, and by Aventis through the Cancer and Leukemia Group B Foundation (B.N.P.).
The following institutions participated in the study: Christiana Care Health Services Inc CCOP, Wilmington, DE—Stephen Grubbs, MD, supported by Grant No. CA45418; CALGB Statistical Office, Durham, NC—Stephen George, PhD, supported by Grant No. CA33601; Dana-Farber Cancer Institute, Boston, MA—George P. Canellos, MD, supported by Grant No. CA32291; Dartmouth Medical School–Norris Cotton Cancer Center, Lebanon, NH—Marc S. Ernstoff, MD, supported by Grant No. CA04326; Duke University Medical Center, Durham, NC—Jeffrey Crawford, MD, supported by Grant No. CA47577; Georgetown University Medical Center, Washington, DC—Edward Gelmann, MD, supported by Grant No. CA77597; Massachusetts General Hospital, Boston, MA—Michael L. Grossbard, MD, supported by Grant No. CA12449; Medical University of South Carolina, Charleston, SC—Mark Green, MD, supported by Grant No. CA03927; Memorial Sloan-Kettering Cancer Center, New York, NY, Clifford Hudis, MD, supported by Grant No. CA77651; Missouri Baptist Medical Center, St Louis, MO, Alan P. Lyss, MD; Mount Sinai Medical Center, Miami, FL—Rogerio Lilenbaum, MD, supported by Grant No. CA45564; Mount Sinai School of Medicine, New York, NY—Lewis R. Silverman, MD, supported by Grant No. CA04457; North Shore–Long Island Jewish Medical Center, Manhasset, NY—Daniel R. Budman, MD, supported by Grant No. CA35279; The Ohio State University Medical Center, Columbus, OH—Clara D Bloomfield, MD, supported by Grant No. CA77658; Rhode Island Hospital, Providence, RI—Louis A. Leone, MD, supported by Grant No. CA08025; Roswell Park Cancer Institute, Buffalo, NY—Ellis Levine, MD, supported by Grant No. CA02599; SUNY Upstate Medical University, Syracuse, NY—Stephen L. Graziano, MD, supported by Grant No. CA21060; Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC—James N. Atkins, MD, supported by Grant No. CA45808; Southern Nevada Cancer Research Foundation CCOP, Las Vegas, NV—John Ellerton, MD, supported by Grant No. CA35421; Syracuse Hematology-Oncology Association CCOP, Syracuse, NY—Jeffrey Kirshner, MD, supported by Grant No. CA45389; University of Alabama Birmingham, Birmingham, AL—Robert Diasio, MD, supported by Grant No. CA47545; University of California at San Diego, San Diego, CA—Joanne Mortimer, MD, supported by Grant No. CA11789; University of California at San Francisco, San Francisco, CA—Alan P. Venook, MD, supported by Grant No. CA60138; University of Chicago Medical Center, Chicago, IL—Gini Fleming, MD, supported by Grant No. CA41287; University of Illinois at Chicago, Chicago, IL—David Gustin, MD, supported by Grant No. CA74811; University of Iowa, Iowa City, IA—Gerald Clamon, MD, supported by Grant No. CA47642; University of Maryland Cancer Center, Baltimore, MD—David Van Echo, MD, supported by Grant No. CA31983; University of Massachusetts Medical Center, Worcester, MA—Mary Ellen Taplin, MD, supported by Grant No. CA37135; University of Minnesota, Minneapolis, MN—Bruce A. Peterson, MD, supported by Grant No. CA16450; University of Missouri/Ellis Fischel Cancer Center, Columbia, MO—Michael C. Perry, MD, supported by Grant No. CA12046; University of Nebraska Medical Center, Omaha, NE—Anne Kessinger, MD, supported by Grant No. CA77298; University of North Carolina at Chapel Hill, Chapel Hill, NC—Thomas C. Shea, MD, supported by Grant No. CA47559; University of Tennessee Memphis, Memphis, TN—Harvey B. Niell, MD, supported by Grant No. CA47555; Vermont Cancer Center, Burlington, VT—Hyman B. Muss, MD, supported by Grant No. CA77406; Wake Forest University School of Medicine, Winston-Salem, NC—David D. Hurd, MD, supported by Grant No. CA03927; Weill Medical College of Cornell University, New York, NY—Michael Schuster, MD, supported by Grant No. CA07968; Walter Reed Army Medical Center, Washington, DC—John C. Byrd, MD, supported by Grant No. CA26806; and Washington University, St Louis, MO–Nancy Bartlett, MD, supported by Grant No. CA77440.
Appendix
The Appendix is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
Footnotes
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Blase N. Polite, Constance Cirrincione, Gini F. Fleming, Clifford Hudis, Eric P. Winer
Provision of study materials or patients: Gini F. Fleming, Andrew Seidman, Hyman Muss, Larry Norton, Charles Shapiro, Kamal Bakri, Kelly Marcom, Diana Lake, Joel H. Schwartz, Clifford Hudis, Eric P. Winer
Collection and assembly of data: Constance Cirrincione, Andrew Seidman, Hyman Muss, Larry Norton, Charles Shapiro, Kamal Bakri, Kelly Marcom, Diana Lake, Joel H. Schwartz, Clifford Hudis, Eric P. Winer
Data analysis and interpretation: Blase N. Polite, Constance Cirrincione, Donald A. Berry, Eric P. Winer
Manuscript writing: Blase N. Polite, Constance Cirrincione, Gini F. Fleming, Clifford Hudis, Eric P. Winer
Final approval of manuscript: Blase N. Polite, Constance Cirrincione, Gini F. Fleming, Donald A. Berry, Andrew Seidman, Hyman Muss, Joel H. Schwartz, Clifford Hudis, Eric P. Winer
References
- 1.Ries LAG, Harkins D, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2003. http://seer.cancer.gov/csr/1975_2003.
- 2.Surveillance Epidemiology, and End Results: SEER*Stat Database: Incidence: SEER 17 Regs Public-Use, Nov 2005 Sub (1973–2003 varying) Bethesda, MD: National Cancer Institute, Surveillance Research Program, Cancer Statistics Branch; 2006. [Google Scholar]
- 3.Eley JW, Hill HA, Chen VW, et al. Racial differences in survival from breast cancer: Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994;272:947–954. doi: 10.1001/jama.272.12.947. [DOI] [PubMed] [Google Scholar]
- 4.Chu KC, Lamar CA, Freeman HP. Racial disparities in breast carcinoma survival rates: Separating factors that affect diagnosis from factors that affect treatment. Cancer. 2003;97:2853–2860. doi: 10.1002/cncr.11411. [DOI] [PubMed] [Google Scholar]
- 5.Li CI, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev. 2002;11:601–607. [PubMed] [Google Scholar]
- 6.Elledge RM, Clark GM, Chamness GC, et al. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86:705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 7.Newman LA, Mason J, Cote D, et al. African-American ethnicity, socioeconomic status, and breast cancer survival: A meta-analysis of 14 studies involving over 10,000 African-American and 40,000 White American patients with carcinoma of the breast. Cancer. 2002;94:2844–2854. doi: 10.1002/cncr.10575. [DOI] [PubMed] [Google Scholar]
- 8.Tammemagi CM, Nerenz D, Neslund-Dudas C, et al. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 9.Dignam JJ, Wieand K, Johnson KA, et al. Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res Treat. 2006;97:245–254. doi: 10.1007/s10549-005-9118-3. [DOI] [PubMed] [Google Scholar]
- 10.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 11.Breen N, Wesley MN, Merrill RM, et al. The relationship of socio-economic status and access to minimum expected therapy among female breast cancer patients in the National Cancer Institute Black-White Cancer Survival Study. Ethn Dis. 1999;9:111–125. [PubMed] [Google Scholar]
- 12.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23:6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 13.Griggs JJ, Sorbero ME, Stark AT, et al. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81:21–31. doi: 10.1023/A:1025481505537. [DOI] [PubMed] [Google Scholar]
- 14.Hershman D, Weinberg M, Rosner Z, et al. Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. J Natl Cancer Inst. 2003;95:1545–1548. doi: 10.1093/jnci/djg073. [DOI] [PubMed] [Google Scholar]
- 15.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 16.Dignam JJ. Differences in breast cancer prognosis among African-American and Caucasian women. CA Cancer J Clin. 2000;50:50–64. doi: 10.3322/canjclin.50.1.50. [DOI] [PubMed] [Google Scholar]
- 17.Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. J Natl Cancer Inst Monogr. 2001:36–43. doi: 10.1093/oxfordjournals.jncimonographs.a003458. [DOI] [PubMed] [Google Scholar]
- 18.Roach M, III, Cirrincione C, Budman D, et al. Race and survival from breast cancer: Based on Cancer and Leukemia Group B trial 8541. Cancer J Sci Am. 1997;3:107–112. [PubMed] [Google Scholar]
- 19.Albain KS, Unger JM, Hutchins LF. Outcome of African Americans on Southwest Oncology Group (SWOG) breast cancer adjuvant therapy trials. Presented at the 26th Annual San Antonio Breast Cancer Symposium; San Antonio, TX. December 3–6, 2003. [Google Scholar]
- 20.Kimmick G, Muss HB, Case LD, et al. A comparison of treatment outcomes for black patients and white patients with metastatic breast cancer: The Piedmont Oncology Association experience. Cancer. 1991;67:2850–2854. doi: 10.1002/1097-0142(19910601)67:11<2850::aid-cncr2820671124>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Seidman AD, Berry D, Cirrincione C, et al. CALGB 9840: Phase III study of weekly (W) pacli-taxel (P) via 1-hour (h) infusion versus standard (S) 3h infusion every third week in the treatment of metastatic breast cancer (MBC), with trastuzumab (T) for HER2 positive MBC and randomized for T in HER2 normal MBC. J Clin Oncol. 2004;22(6s) suppl; abstr 512. [Google Scholar]
- 22.Winer EP, Berry DA, Woolf S, et al. Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: Cancer and Leukemia Group B trial 9342. J Clin Oncol. 2004;22:2061–2068. doi: 10.1200/JCO.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 23.Hosmer DW, Lemeshow S. Applied logistic regression. New York, NY: John Wiley & Sons; 1989. [Google Scholar]
- 24.Collett D. Modelling Survival Data in Medical Research. 2. Boca Raton, FL: Chapman & Hall/ CRC; 2003. [Google Scholar]
- 25.Chu KC, Anderson WF, Fritz A, et al. Frequency distributions of breast cancer characteristics classified by estrogen receptor and progesterone receptor status for eight racial/ethnic groups. Cancer. 2001;92:37–45. doi: 10.1002/1097-0142(20010701)92:1<37::aid-cncr1289>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 26.Shiao YH, Chen VW, Lehmann HP, et al. Patterns of DNA ploidy and S-phase fraction associated with breast cancer survival in blacks and whites. Clin Cancer Res. 1997;3:587–592. [PubMed] [Google Scholar]
- 27.Harris LN, Broadwater G, Lin NU, et al. Molecular subtypes of breast cancer in relation to paclitaxel response and outcomes in women with metastatic disease: Results from CALGB 9342. Breast Cancer Res. 2006;8:R66. doi: 10.1186/bcr1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 29.Shiao YH, Chen VW, Scheer WD, et al. Racial disparity in the association of p53 gene alterations with breast cancer survival. Cancer Res. 1995;55:1485–1490. [PubMed] [Google Scholar]
- 30.Porter PL, Lund MJ, Lin MG, et al. Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer. 2004;100:2533–2542. doi: 10.1002/cncr.20279. [DOI] [PubMed] [Google Scholar]
- 31.Jones BA, Kasl SV, Howe CL, et al. African-American/white differences in breast carcinoma: p53 alterations and other tumor characteristics. Cancer. 2004;101:1293–1301. doi: 10.1002/cncr.20500. [DOI] [PubMed] [Google Scholar]
- 32.Taioli E, Trachman J, Chen X, et al. A CYP1A1 restriction fragment length polymorphism is associated with breast cancer in African-American women. Cancer Res. 1995;55:3757–3758. [PubMed] [Google Scholar]
- 33.Guillemette C, Millikan RC, Newman B, et al. Genetic polymorphisms in uridine diphosphoglucuronosyltransferase 1A1 and association with breast cancer among African Americans. Cancer Res. 2000;60:950–956. [PubMed] [Google Scholar]
- 34.Beutler E, West C. Hematologic differences between African-Americans and whites: The roles of iron deficiency and alpha-thalassemia on hemoglobin levels and mean corpuscular volume. Blood. 2005;106:740–745. doi: 10.1182/blood-2005-02-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine AM, Berhane K, Masri-Lavine L, et al. Prevalence and correlates of anemia in a large cohort of HIV-infected women: Women’s Inter-agency HIV Study. J Acquir Immune Defic Syndr. 2001;26:28–35. doi: 10.1097/00126334-200101010-00004. [DOI] [PubMed] [Google Scholar]
- 36.Jones-Burton C, Seliger SL, Brown J, et al. Racial variations in erythropoietic response to epoetin alfa in chronic kidney disease and the impact of smoking. Nephrol Dial Transplant. 2005;20:2739–2745. doi: 10.1093/ndt/gfi128. [DOI] [PubMed] [Google Scholar]