Abstract
Background
Surveillance for acute flaccid paralysis with laboratory confirmation has been a key strategy in the global polio eradication initiative, and the laboratory platform established for polio testing has been expanded in many countries to include surveillance for cases of febrile rash illness to identify measles and rubella cases. Vaccine-preventable disease surveillance is essential to detect outbreaks, define disease burden, guide vaccination strategies and assess immunization impact. Vaccines now exist to prevent Japanese encephalitis (JE) and some etiologies of bacterial meningitis.
Methods
We evaluated the feasibility of expanding polio–measles surveillance and laboratory networks to detect bacterial meningitis and JE, using surveillance for acute meningitis-encephalitis syndrome in Bangladesh and China and acute encephalitis syndrome in India. We developed nine syndromic surveillance performance indicators based on international surveillance guidelines and calculated scores using supervisory visit reports, annual reports, and case-based surveillance data.
Results
Scores, variable by country and targeted disease, were highest for the presence of national guidelines, sustainability, training, availability of JE laboratory resources, and effectiveness of using polio–measles networks for JE surveillance. Scores for effectiveness of building on polio–measles networks for bacterial meningitis surveillance and specimen referral were the lowest, because of differences in specimens and techniques.
Conclusions
Polio–measles surveillance and laboratory networks provided useful infrastructure for establishing syndromic surveillance and building capacity for JE diagnosis, but were less applicable for bacterial meningitis. Laboratory-supported surveillance for vaccine-preventable bacterial diseases will require substantial technical and financial support to enhance local diagnostic capacity.
Keywords: Vaccine preventable diseases, VPD, Surveillance, Meningitis, Japanese encephalitis
1. Background
Strong systems for vaccine-preventable disease (VPD) surveillance are essential for making evidence-based decisions concerning the introduction of new vaccines. To provide a strategic approach for enhancing VPD surveillance and immunization program monitoring, the World Health Organization (WHO) and other global partners developed a Global Framework for Immunization Monitoring and Surveillance (GFIMS) [1]. One principle of the framework was to link epidemiologic and laboratory surveillance systems for all VPDs and leverage the investment for poliomyelitis surveillance.
Since 1985, identification of poliomyelitis cases has relied on active surveillance for acute flaccid paralysis (AFP) and laboratory testing of stool specimens for polioviruses [2–4]. Beginning in the early 1990s, many countries expanded viral VPD surveillance to facilitate measles case detection by adding a rash-fever syndrome case definition. Measles diagnostic testing capacity has been developed within poliovirus reference laboratories, and a network of sub-national laboratories. This polio–measles surveillance infrastructure has provided a platform for establishing laboratory-supported surveillance for other VPDs of viral etiology, including rubella and yellow fever. A survey in countries in the WHO African Region found that adding other VPDs to the AFP surveillance program did not compromise polio surveillance [5].
The availability of vaccines against diseases caused by Haemophilus influenzae type b (Hib), Neisseria meningitidis (Nm), and Streptococcus pneumoniae (Sp) has created the need to include bacterial disease surveillance in the VPD surveillance framework. Diagnosis of bacterial diseases, however, is more complex than that of viral diseases because of the need for specimen collection before starting antibiotic treatment, an invasive specimen collection procedure, quality laboratory capability at the specimen collection site, and immediate specimen transportation from the collection site to diagnostic laboratories. Surveillance for invasive bacterial disease (IBD) has been conducted in many countries primarily through sentinel hospital networks [6–10], but these systems have not been linked to VPD surveillance.
In 2005, the United States Centers for Disease Control and Prevention (USCDC) and WHO proposed using polio–measles surveillance networks to establish case-based acute encephalitis syndrome (AES) surveillance in India and acute meningitis-encephalitis syndrome (AMES) surveillance in Bangladesh and China. These countries were selected because of strong networks for surveillance and laboratory confirmation of polio and measles; these syndromes were selected because they may be manifestations of several VPDs, including those caused by Japanese encephalitis (JE) virus, Sp, Hib or Nm. Available vaccines that protect against these diseases include multiple formulations of JE vaccine, multivalent pneumococcal conjugate vaccines (PCV), monovalent and combination Hib vaccines, and polysaccharide and conjugate meningococcal vaccines [11–14]. None of the countries had introduced PCV, JE, Hib or meningococcal vaccines into their routine immunization programs, although China provided routine and/or campaign vaccination in some provinces.
Data on JE and bacterial meningitis incidence in Bangladesh, China and India are limited. In Bangladesh, JE virus appears to be endemic throughout the country [15,16]. In China, approximately 33,900 cases of JE occur annually [17]. JE is endemic throughout much of India with large seasonal outbreaks documented in several states in northern India [18,19]. Although meningitis caused by Sp, Hib and Nm has been greatly reduced in countries where vaccines against these pathogens are routinely used [20], cases continue to occur in Bangladesh, China, and India. In Bangladesh, meningitis caused by Sp and Hib has been monitored through sentinel surveillance networks [10,21,22]. Outbreaks of meningococcal meningitis and septicemia were reported in China from 2003 to 2005 [23]. In Vellore District in the Indian state of Tamil Nadu, the estimated annual Hib meningitis incidence was 7.1 cases per 100,000 children <5 years of age, comparable to rates reported from Europe before Hib vaccine introduction [24].
AMES surveillance was launched by Ministries of Health (MOH) in China in May 2006 and in Bangladesh in October 2007 using the infrastructure of the polio–measles surveillance and laboratory networks. Because of India’s priority to identify best practices to improve AES surveillance, meningitis surveillance was not included in India; AES surveillance started in May 2007. Project areas included three sentinel hospitals (one in each of three districts) in Bangladesh, 24 (six in each of four prefectures) in China and four (one in each of four JE-endemic states) in India (Fig. 1). Criteria for selection of project areas included the absence of wild poliovirus circulation and meeting the global indicators for rates of non-polio AFP and adequate stool collection6 [25]. Details and data of the AMES–AES surveillance systems are described elsewhere [16,26–31]. The purpose of this paper is to evaluate the feasibility of expanding polio–measles surveillance and laboratory networks to detect bacterial meningitis and JE, using surveillance for acute meningitis-encephalitis syndrome in Bangladesh and China and acute encephalitis syndrome in India.
Fig. 1.
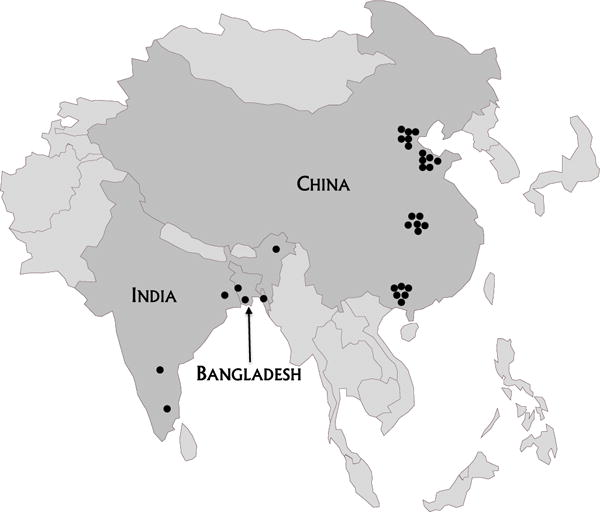
Location of AMES surveillance sites in Bangladesh and China, and AES surveillance sites in India.
2. Methods
Countries established active surveillance for clinically recognizable characteristics and symptoms at sentinel hospitals. An AMES case was defined as the acute onset of fever in a person of any age at any time of year, accompanied by a change in mental status, or new onset seizures (excluding simple febrile seizures), or meningeal signs or neck stiffness [32]. The AES case definition did not include meningeal signs or neck stiffness. Cerebrospinal fluid (CSF) and blood specimens were collected from all identified AMES and AES case–patients and tested for JE IgM by enzyme-linked immunosorbent assay (ELISA). CSF specimens from AMES case–patients were also tested for bacterial meningitis by bacterial culture and latex agglutination (for Hib, Nm serogroups and Sp) where available, and CSF and sera by real-time polymerase chain reaction (PCR). If specimen quantity permitted, the primary sentinel site laboratory referred aliquots to secondary and tertiary laboratories for quality control and advanced testing.
To evaluate AMES and AES surveillance, we developed post hoc nine qualitative indicators to assess implementation of surveillance and six quantitative indicators to evaluate performance (Box 1). Five of the nine qualitative indicators (national guidelines, monitoring, feedback, representativeness and sustainability) assessed the overall implementation of syndromic surveillance, irrespective of the target syndrome. Implementation of surveillance to detect JE or bacterial meningitis was evaluated through the four remaining qualitative indicators—training, availability of laboratory resources, data standardization and effectiveness of building on polio–measles networks (how readily polio–measles standard operating procedures [SOPs], staff, specimen shipping, engagement with hospital clinicians were adaptable to AMES/AES). Six quantitative indicators (data completeness, data validity, timeliness, specimen collection, specimen referral to secondary and tertiary laboratories) evaluated performance based on core surveillance attributes and activities.
We developed indicator scores by adapting quantitative criteria and targets from published polio–measles surveillance guidelines and recommendations and two authors (KFC, HSS) awarded points as described below [32–34]. Each indicator included 1–7 components. Components whose achievement could be measured as “yes” or “no”, were awarded points as follows: 1 point for “yes” and 0 points for “no” (for example, “were guidelines based on international standards?”). Components that could be measured for each year of surveillance, or had multiple criteria were worth up to 2 points (for example, “conducted biannual visits to sentinel sites”). For representativeness, if all health facilities in the project area (province, district, or state) reported cases, 2 points were awarded; if an indirect measure of representativeness was used, such as catchment survey, 1 point was awarded.
To compute the percentages used to calculate scores, we used the number of cases that met the indicator target as the numerator, and the number of AMES cases detected in China (May 2006–September 2008), or in Bangladesh (October 2007–December 2008); or the number of AES cases detected in India (May 2007–December 2008) as the denominator. Timeliness of reporting was scored according to country guidelines for preliminary case reporting. It was not possible to evaluate sensitivity, as we did not have data on active case searches.
We used reports from supervisory visits and annual reports to calculate the indicator scores. In China, we reviewed semi-annual and annual case-based surveillance data, and in Bangladesh and India, we examined computerized epidemiological and laboratory data. We also analyzed a convenience sample of observations collected during field visits in India (autumn 2008) and Bangladesh (winter 2009) to evaluate specimen collection and transport practices and to assess laboratory data flow. Statistical Package for the Social Sciences (SPSS, IBM Corporation) was used for data analysis.
3. Results
3.1. Implementation of syndromic surveillance
In all three countries, national AMES–AES surveillance guidelines were developed based on WHO standards, and copies were present at sentinel sites in China and India; in Bangladesh, guidelines were developed at the national level, but were not distributed (Table 1). National program officers in all countries conducted and documented monitoring and supervisory visits to sentinel sites at least semiannually; international partners participated annually, although standard monitoring protocols were not used. Feedback to sentinel sites was delayed in all countries, primarily because of policies requiring the national level to wait for confirmation from tertiary laboratories before reporting results. In India, feedback was also delayed because epidemiologic and laboratory data were not readily linkable in the national database. China applied a population-based approach consisting of active surveillance at all hospitals in the prefecture and laboratory confirmation only at sentinel sites. Bangladesh undertook catchment surveys in project districts to provide a basis for adjusting surveillance data, and India conducted only sentinel surveillance. All three countries developed credible plans to sustain AMES and AES surveillance in at least some sentinel sites, using national and donor resources and funding.
Table 1.
Evaluation of implementation of syndromic surveillance for AMES–AESa Bangladesh (BNG), China (CHN) and India (IND), using four qualitative indicators.
| Indicator | Component | Scores for syndromic surveillance
|
|||
|---|---|---|---|---|---|
| Max scorec | BNG | CHN | IND | ||
| 1. National guidelines | Guidelines based on international standards | 1 | 1 | 1 | 1 |
| Guidelines used at all administrative levels | 1 | 0 | 1 | 1 | |
| Total | 2 | 1 | 2 | 2 | |
| 2. Monitoring and supervision | Conducted biannual visits to sentinel sites | 2 | 2 | 2 | 2 |
| Used standard protocol for monitoring and supervisory visits | 2 | 0 | 0 | 0 | |
| Documented site visits | 2 | 2 | 2 | 2 | |
| Total | 6 | 4 | 4 | 4 | |
| 3. Feedback | Monthly feedback to sentinel sites | 2 | 0 | 0 | 0 |
| Total | 2 | 0 | 0 | 0 | |
| 4. Representativeness | Population-based datad | 2 | 1 | 2 | 0 |
| 5. Sustainability | MOHb plan to sustain surveillance | 1 | 1 | 1 | 1 |
| MOH budget to sustain surveillance | 1 | 1 | 1 | 1 | |
| Total | 2 | 2 | 2 | 2 | |
| Total | 14 | 8 | 10 | 8 | |
AMES–AES, acute meningitis-encephalitis syndrome/acute encephalitis syndrome.
MOH = Ministry of Health.
Maximum score—if the component could be answered in a “yes/no” fashion, the maximum score was 1 point (e.g., components for national guidelines and sustainability); if the component could be measured for each year of surveillance, a maximum score of 2 points were possible (e.g., components for monitoring and supervision and feedback).
If all health facilities in the project area (province, district or state) reported cases, 2 points were awarded; if an indirect measure of representativeness was used, such as catchment survey, 1 point was awarded.
3.2. Implementation of surveillance for JE and bacterial meningitis
Comprehensive training agendas for disease-specific surveillance were developed in all countries (Table 2). These included (1) surveillance workshops for staff at sentinel, intermediate and national levels; (2) laboratory workshops on WHO- and USCDC-recommended techniques for JE IgM ELISA, bacterial culture, and, in China, on real-time PCR for diagnosis of bacterial meningitis; and (3) training at USCDC for senior surveillance and laboratory specialists in epidemiology and molecular techniques.
Table 2.
Evaluation of implementation of AMES–AESb surveillance targeting Japanese encephalitis and bacterial meningitis in Bangladesh (BNG), China (CHN) and India (IND), using four qualitative indicators.
| Indicator | Component | JE surveillance
|
Bacterial meningitis surveillancea
|
|||||
|---|---|---|---|---|---|---|---|---|
| Max scoreb | BNG | CHN | IND | Max score | BNG | CHN | ||
| 1. Training | Content based on technical protocol | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Target audience relevantc | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Timing appropriated | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Total | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| 2. Availability of laboratory resources | Appropriate equipment in placee | 2 | 2 | 2 | 2 | 2 | 1 | 1 |
| Appropriate reagents in placee | 2 | 1 | 2 | 1 | 2 | 1 | 1 | |
| Total | 4 | 3 | 4 | 3 | 4 | 2 | 2 | |
| 3. Data standardization | Electronic data managementf | 2 | 1 | 1 | 2 | 2 | 1 | 1 |
| Surveillance, laboratory data linkableg | 2 | 2 | 2 | 1 | 2 | 2 | 2 | |
| Total | 4 | 3 | 3 | 3 | 4 | 3 | 3 | |
| 4. Effectiveness of building on polio–measles (PM) networks | Surveillance protocols, tools adaptedh | 2 | 1 | 1 | 1 | 2 | 1 | 1 |
| PM district staff oversaw sentinel sites | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| PM national staff oversee surveillance | 2 | 2 | 2 | 1 | 2 | 2 | 2 | |
| PM regional bulletins adapted | 1 | 1 | 0 | 1 | 1 | 0 | 0 | |
| Laboratory protocols, tools adaptedi | 2 | 2 | 2 | 2 | 2 | 0 | 0 | |
| PM laboratory staff utilized | 2 | 2 | 2 | 1 | 2 | 0 | 0 | |
| PM regional staff oversaw laboratory | 2 | 2 | 2 | 2 | 2 | 0 | 0 | |
| Total | 13 | 12 | 11 | 10 | 13 | 5 | 5 | |
| Total | 24 | 21 | 21 | 19 | 24 | 13 | 13 | |
AMES–AES, acute meningitis-encephalitis syndrome/acute encephalitis syndrome.
India did not conduct surveillance for bacterial meningitis.
Components that could be answered in a “yes/no” fashion, were each worth 1 point (e.g., all components for training and one component of effectiveness of building on polio–measles networks); components that could be measured for each year of surveillance, were each worth 2 points (e.g., components for availability of laboratory resources, data standardization and other components for effectiveness of building on polio–measles networks).
Surveillance, laboratory and clinical staff at all levels.
Introductory training prior to launch and refresher training as needed.
For encephalitis surveillance, equipment and reagents to perform JE IgM ELISA at all laboratories; for meningitis surveillance, equipment and reagents to perform Gram stain and bacterial culture at sentinel site laboratories, and to perform real-time PCR at secondary laboratories in all three countries and tertiary laboratories in China.
Electronic data base used for local database for surveillance (1 point) and laboratory (1 point) data.
National level surveillance data easily linked with laboratory data.
Protocols and tools for case investigation, line listing, reporting, monitoring, data management and feedback.
Protocols and tools for specimen transport, laboratory reporting, monitoring and data management.
JE testing equipment and initial stocks of ELISA kits were received in all project laboratories, but frequent shortages in Bangladesh and India limited JE testing. Provision of equipment and supplies for bacterial culture and PCR in China was delayed for several months. In Bangladesh, micro-centrifuges were not available for CSF processing in some hospital laboratories, necessitating the use of conventional centrifuges, which consumed larger specimen volumes, leaving less for other tests.
Data standardization was stronger for clinical case reporting than for laboratory reporting, mainly because of differences in availability of data management software and staff skilled in their use. For example, polio–measles surveillance staff in each project district used a software application (Epi Info™ [CDC, Atlanta, USA] in India and Bangladesh; EPIDATA [EpiData Association, Odense Denmark] in China) to create a clinical AMES and AES case database, while laboratory staff at sentinel sites had limited tools and experience in data management and reporting. Hospital laboratory staff in Bangladesh and China manually recorded diagnostic results and reported to the district level, where surveillance staff entered results into the AMES database. In India, hospital laboratory staff recorded JE ELISA results into a spreadsheet (MicroSoft Excel®), but lacked capability to maintain the integrity of the unique identifiers or manage large data sets. The spreadsheets were sent to the regional reference laboratory, where the results were compiled and dispatched to the national level. Loss of unique identifiers delayed linkage of JE laboratory data and AES surveillance data resulting in delayed reporting of JE test results to clinicians (per field visits).
Overall, we found polio–measles surveillance networks to be effective for developing JE and bacterial meningitis surveillance. All countries adapted polio–measles case investigation and line list formats and software for creating an AMES–AES case database; polio–measles staff provided technical oversight to AMES–AES sentinel sites. However, there were some missed opportunities to link with polio–measles surveillance. For example, none of the countries adapted polio–measles monitoring procedures and supervisory checklists for oversight of AMES–AES surveillance. While the WHO South-East Asia Regional Office (SEARO) expanded regional polio–measles surveillance bulletins to include AES surveillance data, the WHO Western Pacific Regional Office did not. Additionally, the strong links between surveillance officers and pediatricians forged by polio–measles infrastructure were not expanded to include clinicians on adult wards, who are important for AMES–AES surveillance. In addition, a 2006–2007 polio resurgence in India in non-AES areas limited the availability of national polio–measles staff to conduct surveillance [35].
Because both measles and JE testing rely on similar indirect methods, polio–measles protocols for handling and testing specimens, and reporting results were easily adapted and used for JE IgM ELISA, and laboratory staff responsible for measles diagnostic testing readily incorporated JE testing into their work. In addition, WHO regional polio–measles laboratory experts provided technical support, oversight and external quality assessment for JE testing. In contrast, few areas of overlap existed between testing for polio and measles and for bacterial meningitis. Major differences in laboratory methods, equipment and reagents prevented use of the same staff and quality-control procedures for both JE and bacterial meningitis testing.
3.3. Surveillance performance
The major strengths in surveillance performance were data completeness, concordance in JE results by ELISA retesting at secondary and tertiary laboratories, timeliness of investigation and sentinel site reporting, collection of clinical specimens and referral of specimens to a secondary laboratory (Table 3). Only China met the target for complete reporting of discharge status and concordance of JE results by proficiency testing in both 2007 and 2008, only Bangladesh met the case reporting timeliness target during both years, and only India met the 2008 target for CSF collection. No country performed well in referral of CSF specimens to secondary and tertiary laboratories. In nine of 11 instances of annual variation in meeting performance targets, the target was met in 2007 but not in 2008. Field observations in Bangladesh and India suggested that clinicians sometimes did not report case–patients from whom CSF had not been obtained, even though the patient’s illness met the case definition. It was also anecdotally reported that pediatric AMES–AES cases were more likely than adult cases to be reported.
Table 3.
Evaluation of AMES–AESa surveillance performance in Bangladesh (BNG), China (CHN), and India (IND).
| Indicator | Componenta | Max scoreb | BNGc | CHNd | INDb |
|---|---|---|---|---|---|
| 1. Data completeness | ≥90% cases with age | 2 | 2 | 2 | 2 |
| >90% cases with gender | 2 | 0 | 2 | 2 | |
| ≥90% cases with discharge status | 2 | 0 | 2 | 0 | |
| ≥90% cases with immunization history | 2 | 1 | 2 | 2 | |
| ≥90% cases with laboratory results for either JEe or BMf | 2 | 1 | 1 | 1 | |
| Total | 10 | 4 | 9 | 7 | |
| 2. Data validity | ≥90% concordance by retesting serumg | 1 | 1 | 1 | 1 |
| ≥90% concordance by PT, serumh | 1 | 0 | 1 | 0 | |
| Total | 2 | 1 | 2 | 1 | |
| 3. Timeliness | ≥80% cases were reported on timei | 2 | 2 | 0 | 0 |
| ≥80% cases were investigated on timej | 2 | 2 | 2 | 2 | |
| ≥80% sentinel sites reported on timek | 2 | 2 | 2 | 2 | |
| Total | 6 | 6 | 4 | 4 | |
| 4. Specimen collection | ≥80% cases with any specimen collectedl | 2 | 2 | 2 | 2 |
| ≥80% cases with blood collected | 2 | 1 | 2 | 0 | |
| ≥80% cases with CSF collected | 2 | 0 | 0 | 1 | |
| Total | 6 | 3 | 4 | 3 | |
| 5. Specimen referral to secondary labm | ≥80% cases with any specimen referred | 2 | 2 | 2 | 1 |
| ≥80% cases with serum referred | 2 | 1 | 0 | 0 | |
| ≥80% cases with CSF referred | 2 | 0 | 0 | 0 | |
| Total | 6 | 3 | 2 | 1 | |
| 6. Specimen referral to tertiary labn | ≥80% cases with any specimen referred | 2 | 1 | 0 | 1 |
| ≥80% cases with serum referred | 2 | 1 | 0 | 0 | |
| ≥80% cases with CSF referred | 2 | 0 | 0 | 0 | |
| Total | 6 | 2 | 0 | 1 | |
| Total | 36 | 19 | 21 | 17 |
AMES–AES, acute meningitis-encephalitis syndrome/acute encephalitis syndrome; JE, Japanese encephalitis; BM, bacterial meningitis; NA, not applicable; CSF, cerebrospinal fluid; PT, proficiency testing; ND, no or insufficient data for scoring.
For calculating percentages, the following denominators were used: 867 for Bangladesh, 2815 for China, and 1637 for India.
For all indicators with the exception of data validity, each component was worth 2 points; 1 point was awarded for having achieved the target for each year of surveillance. Each component for data validity was worth 1 point because only aggregate data were available.
Bangladesh and India results from analysis of national data set; 2007 data include cases with hospital admission from launch through December 2007; 2008 data include cases with hospital admission from January through December 2008.
2007 China data from Annual Report December 2007, which includes cases with hospital admission from launch through October 2007; 2008 data from Annual Report December 2008, which includes cases with hospital admission from launch through September 2008.
Laboratory results for JE IgM ELISA.
Laboratory results for Gram stain, latex agglutination, bacterial culture or real-time PCR for Nm, Hib, and Sp.
Based on the retesting of positive specimens (China) or of positive and negative specimens (India and Bangladesh) by tertiary laboratories.
Based on results of testing serum samples provided in a proficiency testing panel.
Each country was evaluated against the country protocol. The protocol in China specified case reporting ≤24 h after admission, while those in India and Bangladesh specified notification ≤48 h after admission.
Protocols in all three countries specified case investigation ≤48 h after notification of case.
Protocols in all three countries specify weekly reporting from sentinel sites.
Serum or CSF.
Secondary laboratories in China were prefecture Chinese CDC laboratories; in India, the National Institute of Mental Health and Neurological Sciences, Bangalore; and in Bangladesh, the Institute of Public Health, Dhaka.
The National Chinese CDC Laboratories in Beijing served as the Tertiary Laboratory in China, and the Laboratories of USCDC served as Tertiary Laboratories for India and Bangladesh.
Barriers to CSF collection and testing were found, particularly in Bangladesh and India, where many patients (or their families) did not consent to lumbar puncture, particularly if they had been referred from another facility where venipuncture had been performed. In addition, patients often bore the cost of specimen collection and testing in hospital laboratories; this resulted in some clinicians ordering specimen collection and testing based on the family’s perceived ability to pay. Family members were sometimes required to transport clinical specimens from the patient’s bedside to the laboratory, with no control or monitoring of temperature or interval from collection to receipt by the laboratory. Because JE and bacterial meningitis tests were performed on the same specimens, but in different parts of the hospital laboratory, coordination was required for aliquoting and distribution.
4. Discussion
In this evaluation of AMES–AES surveillance systems in Bangladesh, China, and India, we found that polio–measles laboratory networks were useful for the development of JE diagnostic capacity, but not for confirming bacterial meningitis, primarily because of differences in laboratory staff and viral and bacterial testing methodologies.
Polio–measles surveillance networks provided a useful infrastructure for AMES–AES surveillance and permitted case detection, specimen collection and testing, and compilation of case-based epidemiological and laboratory data. Key features included adaptable data collection protocols, experienced surveillance field staff and national surveillance officers, and WHO regional surveillance and laboratory advisors available for guidance and oversight. In addition, the polio–measles laboratory network offered an appropriate platform for building a JE laboratory network. Similar materials and technical protocols for measles and JE testing facilitated equipping AMES–AES laboratories and training staff, and (at some laboratories) permitted use of the same physical facility and personnel for measles and JE testing. All the systems were functioning within 14–28 months, which was likely accelerated by the strong partnerships among polio–measles surveillance medical officers, pediatric clinicians and network laboratory staff.
Despite these advantages, we found that the polio–measles protocols for documenting and reporting active case searches and conducting supervision were not used for AMES–AES surveillance, and bulletins for dissemination of surveillance data were only used in SEARO. Application of these tools might have improved data completeness, timeliness, monitoring and feedback for AMES–AES surveillance.
We identified several important limitations of the polio–measles surveillance systems in conducting AMES–AES surveillance. Because of the focus on children aged <15 years in case-based polio–measles surveillance, existing partnerships between surveillance medical officers and clinicians at sentinel sites involved only pediatric clinicians, and adult ward staff required a more comprehensive orientation to AMES–AES surveillance protocols. In addition, whereas the collection, handling and indirect testing of blood for AMES and AES cases were easily integrated into polio–measles systems, the collection, handling and transport of CSF for bacterial culture posed a multitude of logistic challenges, including prior treatment with antibiotics and delayed specimen transport that may have affected culture results. Polio–measles laboratory testing is used for program monitoring, not for clinical management, permitting testing to be centralized at regional or national facilities. The identification of bacterial causes of AMES, on the other hand, is needed rapidly for case management and requires on-site laboratory services. Since bacteriology requires different skills, equipment and reagents, it was not possible to use polio–measles staff or resources for bacterial meningitis diagnostic testing. Moreover, bacteriology laboratories at all levels suffered from long-standing deficiencies in equipment, quality assurance and quality control, data management skills and training. Thus, the approach for AMES surveillance did not appear to offer greater efficiencies than the current sentinel networks that have supported IBD surveillance in many countries and regions [36,37,10,21,38], although in China this project has provided the first population-based IBD surveillance data, which has continued to be sustained by the national government. A permanent bacteriology technical advisor at SEARO might have improved CSF and data management at the sentinel sites through the development of SOPs for CSF and data handling, training, on-site visits, and laboratory indicators adapted to bacteriology.
Our evaluation had several limitations. Slight differences among national surveillance protocols posed challenges in developing indicator criteria relevant to all three countries, and because the indicators were developed after the project ended, data to calculate them were not systematically collected during the evaluation period. Because country data sources differed, we had difficulty obtaining data for some indicators. Finally, it was not always possible to separate performance at start-up from that later in the project.
This multi-country, cross-regional effort to adapt the polio–measles surveillance infrastructure for laboratory-supported surveillance of JE and bacterial meningitis adds to a growing body of evidence that supports expanding VPD surveillance networks to include additional syndromes and supports the GFIMS principle of linking systems [2] and recently updated WHO guidelines recommending integration of JE surveillance with that for AFP or meningitis [32,39]. Elements facilitating integration include similarities in target age, surveillance protocols, specimen requirements, and reporting and data management systems; commonalities among laboratory protocols enable rapid capacity development. Successful integration of JE surveillance into an established, functional bacterial meningitis surveillance system in Cambodia supports these observations [40]. However, to apply the GFIMS to the development of surveillance for bacterial VPDs, enhancement of laboratory capacity for bacteriology and appropriate data management are needed to ensure that challenges do not bias disease burden estimates downward and delay the introduction of lifesaving vaccines [41].
Box 1. Indicators used for the evaluation of acute meningitis-encephalitis syndrome (AMES) an acute encephalitis syndrome (AES) surveillance.
Implementation indicators
Syndromic surveillance
National guidelines
Monitoring and supervision
Feedback
Representativeness
Sustainability
Japanese encephalitis or bacterial meningitis surveillance
Training
Availability of laboratory resources
Data standardization
Effectiveness of building on polio–measles networks
Performance indicators
Data completeness
Data validity
Timeliness
Specimen collection
Specimen referral to secondary laboratory
Specimen referral to tertiary laboratory
Acknowledgments
The authors are very grateful to Dr. Jacqueline Gindler for generous help in various stages of preparing this manuscript. The authors acknowledge Drs. Jamin Morrison and Adnan Mustafa for their field work that contributed to this evaluation.
This work was supported by the United States Centers for Disease Control and Prevention, Atlanta, Georgia.
Sources of support
Funding for this evaluation was provided by the United States Centers for Disease Control and Prevention, Atlanta, Georgia.
Abbreviations
- AES
acute encephalitis syndrome
- AFP
acute flaccid paralysis
- AMES
acute meningitis-encephalitis syndrome
- BNG
Bangladesh
- CHN
China
- CSF
cerebrospinal fluid
- ELISA
enzyme-linked immunosorbent assay
- GFIMS
Global Framework for Immunization Monitoring and Surveillance
- Hib
Haemophilus influenzae type b
- IBD
invasive bacterial disease
- IND
India
- JE
Japanese encephalitis
- MOH
Ministry of Health
- Nm
Neisseria meningitides
- PCR
polymerase chain reaction
- PM
polio–measles
- PCV
pneumococcal conjugate vaccine
- SEARO
South-East Asia Regional Office
- Sp
Streptococcus pneumonia
- SPSS
Statistical Package for the Social Sciences
- UNICEF
United Nations Children’s Fund
- USCDC
United States Centers for Disease Control and Prevention
- VPD
vaccine-preventable disease
- WHO
World Health Organization
Footnotes
Attribution
Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Conflict of interest: The authors state they have no conflict of interest.
At least one case of non-polio AFP should be detected annually per 100,000 population aged less than 15 years, and all AFP cases should have a full clinical and virological investigation with at least 80% of AFP cases having adequate stool specimens collected. Adequate stool specimens are two stool specimens of sufficient quantity for laboratory analysis, collected at least 24 h apart, within 14 days after the onset of paralysis, and arriving in the laboratory by reverse cold chain and with proper documentation.
References
- 1.World Health Organization. GIVS: global immunization vision and strategy 2006–2015. Geneva: WHO; 2005. [Google Scholar]
- 2.Dabbagh AR, Eggers R, Cochi S, Dietz V, Strebel P, Cherian T. A new global framework for immunization monitoring and surveillance. Bull World Health Organ. 2007;85:904–5. doi: 10.2471/BLT.07.048223. http://www.who.int/bulletin/volumes/85/12/07-048223/en/index.html [accessed 09.10.14]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garwood P. What will become of the polio network. Bull World Health Organ. 2007;85:87–8. doi: 10.2471/BLT.07.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heymann DL, Aylward RB. Poliomyelitis eradication and pandemic influenza. Lancet. 2006;367:1464–6. doi: 10.1016/S0140-6736(06)68624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nsubuga P, McDonnell S, Perkins B, Sutter R, Quick L, White M, et al. Polio eradication initiative in Africa: influence on other infectious disease surveillance development. BMC Public Health. 2002;2:1–6. doi: 10.1186/1471-2458-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Immunization, vaccines and biologicals 2002–2003 highlights. WHO/IVB/05.06.2005. http://apps.who.int/iris/handle/10665/69090 [accessed 17.10.14]. [PubMed]
- 7.World Health Organization, African Regional Office. Hib-paediatric bacterial meningitis (Hib-PBM) surveillance network. Surveill Man. 2001 Jul;:1–51. [Google Scholar]
- 8.Hajjeh R. Accelerating introduction of new vaccines: barriers to introduction and lessons learned from the recent Haemophilus influenzae type b vaccine experience. Philos Trans R Soc B: Biol Sci. 2011;366:2827–32. doi: 10.1098/rstb.2011.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Child Health Research Project. The India invasive bacterial infections surveillance (IBIS) project. Synopsis. 1999 http://www.childhealthresearch.org/doc/synop5.pdf [accessed 17.10.14].
- 10.Levine OS, Cherian T, Hajjeh R, Knoll MD. Progress and future challenges in coordinated surveillance and detection of pneumococcal and Hib disease in developing countries. Clin Infect Dis. 2009;48:S33–6. doi: 10.1086/596479. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Meningococcal vaccines: WHO position paper. Wkly Epidemiol Rec. 2011 Nov;86:521–39. [PubMed] [Google Scholar]
- 12.World Health Organization. Pneumococcal vaccines WHO position paper – 2012 – recommendations. Vaccine. 2012;30:4717–8. doi: 10.1016/j.vaccine.2012.04.093. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Japanese encephalitis vaccines: WHO position paper. Wkly Epidemiol Rec. 2006;81:331–40. [Google Scholar]
- 14.World Health Organization. WHO position paper on Haemophilus influenzae type b conjugate vaccines. Wkly Epidemiol Rec. 2006;81:339–445. [PubMed] [Google Scholar]
- 15.Hossain MJ, Gurley ES, Montgomery S, Petersen L, Sejvar J, Fischer M, et al. Hospital-based surveillance for Japanese encephalitis at four sites in Bangladesh, 2003–2005. Am J Trop Med Hyg. 2010;82:344–9. doi: 10.4269/ajtmh.2010.09-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul RC, Rahman M, Gurley ES, Hossain MJ, Diorditsa S, Hasan ASMM, et al. A novel low-cost approach to estimate the incidence of Japanese encephalitis in the catchment area of three hospitals in Bangladesh. Am J Trop Med Hyg. 2011;85:379–85. doi: 10.4269/ajtmh.2011.10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ. 2011;89:766–74. doi: 10.2471/BLT.10.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parida M, Dash PK, Tripathi NK. Japanese encephalitis outbreak, India, 2005. Emer Infect Dis. 2006;12:1427. doi: 10.3201/eid1209.060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain P, Jain A, Kumar A, Prakash S, Khan DN, Singh KP, et al. Epidemiology and etiology of acute encephalitis syndrome in North India. Jpn J Infect Dis. 2014;67:197–203. doi: 10.7883/yoken.67.197. [DOI] [PubMed] [Google Scholar]
- 20.McIntyre PB, O’Brien KL, Greenwood B, van de Beek D. Effect of vaccines on bacterial meningitis worldwide. Lancet. 2012;380:1703–11. doi: 10.1016/S0140-6736(12)61187-8. [DOI] [PubMed] [Google Scholar]
- 21.Luby SP, Brooks WA, Saha SK, El-Arifeen S, Naheed A, Sack D, et al. Use of multiple surveillance modalities to assess the epidemiology of Streptococcus pneumoniae infection in Bangladesh. Clin Infect Dis. 2009;48:S97–102. doi: 10.1086/596487. [DOI] [PubMed] [Google Scholar]
- 22.Naheed A, Saha SK, Breiman RF, Khatun F, Brooks WA, El Arifeen S, et al. Multihospital surveillance of pneumonia burden among children aged <5 years hospitalized for pneumonia in Bangladesh. Clin Infect Dis. 2009;48:S82–9. doi: 10.1086/596485. [DOI] [PubMed] [Google Scholar]
- 23.Shao A, Li W, Ren J, Liang X, Xu L, Diao B, et al. Identification of a new Neisseria meningitidis serogroup C clone from Anhui province, China. Lancet. 2006;367:419–23. doi: 10.1016/S0140-6736(06)68141-5. [DOI] [PubMed] [Google Scholar]
- 24.Minz S, Balraj V, Lalitha M, Murali N, Cherian T, Manoharan G, et al. Incidence of Haemophilus influenzae type b meningitis in India. Indian J Med Res. 2008;128:57. [PubMed] [Google Scholar]
- 25.World Health Organization. Field guide for supplementary activities aimed at achieving polio eradication, 1996 revision. Geneva: WHO; 1997. [Google Scholar]
- 26.Centers for Disease Control Prevention. Expanding poliomyelitis and measles surveillance networks to establish surveillance for acute meningitis and encephalitis syndromes – Bangladesh, China, and India, 2006–2008. Morb Mortal Wkly Rep. 2012;61:1008. [PubMed] [Google Scholar]
- 27.Ravi V, Robinson JS, Russell BJ, Desai A, Ramamurty N, Featherstone D, et al. Evaluation of IgM antibody capture enzyme-linked immunosorbent assay kits for detection of IgM against Japanese encephalitis virus in cerebrospinal fluid samples. Am J Trop Med Hyg. 2009;81:1144–50. doi: 10.4269/ajtmh.2009.09-0144. [DOI] [PubMed] [Google Scholar]
- 28.Robinson JS, Featherstone D, Vasanthapuram R, Biggerstaff BJ, Desai A, Ramamurty N, et al. Evaluation of three commercially available Japanese encephalitis virus IgM enzyme-linked immunosorbent assays. Am J Trop Med Hyg. 2010;83:1146–55. doi: 10.4269/ajtmh.2010.10-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin Z, Wang H, Yang J, Luo H, Hadler SC, Sandhu HS, et al. Japanese encephalitis disease burden and clinical features of Japanese encephalitis in four cities in the People’s Republic of China. Am J Trop Med Hyg. 2010;83:766–73. doi: 10.4269/ajtmh.2010.09-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Yin Z, Shao Z, Li M, Liang X, Sandhu HS, et al. Population-based surveillance for bacterial meningitis in China, September 2006–December 2009. Emerg Infect Dis. 2014;20:61–9. doi: 10.3201/eid2001.120375. www.cdc.gov/eid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Establishing surveillance for acute meningitis and encephalitis syndromes through expansion of poliomyelitis and measles surveillance networks in Bangladesh, China, and India 2006–2008. Wkly Epidemiol Rec. 2012;51 and 52:513–9. www.cdc.gov/eid. [PubMed] [Google Scholar]
- 32.World Health Organization, Department of Immunization Vaccines and Biologicals. WHO-recommended standards for surveillance of selected vaccine-preventable diseases. 2003 http://whqlibdoc.who.int/hq/2003/WHO_v&b_03.01.pdf [accessed 10.10.14].
- 33.Centers for Disease Control and Prevention. US Department of Health and Human Services updated guidelines for evaluating public health surveillance systems. Morb Mortal Wkly Rep. 2001;50:1–35. [Google Scholar]
- 34.Roush SW, McIntyre L, Baldy LM. Manual for the surveillance of vaccine-preventable diseases. 2008 [Google Scholar]
- 35.Centers for Disease Control and Prevention. US Department of Health and Human Services. Progress toward polio eradication – India, January 2007–May 2009. Morb Mortal Wkly Rep. 2009;58:719–23. [Google Scholar]
- 36.Mhlanga B, Katsande R, Toscano C, Cherian T, O’Loughlin R, Rainey J, et al. Pediatric bacterial meningitis surveillance – African region, 2002–2008. Morb Mortal Wkly Rep. 2009;58:493–7. [PubMed] [Google Scholar]
- 37.Thomas K. Prospective multicentre hospital surveillance of Streptococcus pneumoniae disease in India. Lancet. 1999;353:1216–21. [PubMed] [Google Scholar]
- 38.Gupta M, Kumar R, Deb AK, Bhattacharya SK, Bose A, John J, et al. Multi-center surveillance for pneumonia & meningitis among children (<2 yr) for Hib vaccine probe trial preparation in India. 2010 [PubMed] [Google Scholar]
- 39.Hills S, Dabbagh A, Jacobson J, Marfin A, Featherstone D, Hombach J, et al. Evidence and rationale for the World Health Organization recommended standards for Japanese encephalitis surveillance. BMC Infect Dis. 2009;9:214–23. doi: 10.1186/1471-2334-9-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Touch S, Grundy J, Rani M, Samnang C, Khalakdina A, Jacobson J. The rationale for integrated childhood meningoencephalitis surveillance: a case study from Cambodia. Bull World Health Organ. 2009;87:320–4. doi: 10.2471/BLT.08.052951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu H, Yang W, Varma JK. To save children’s lives, China should adopt an initiative to speed introduction of pneumonia vaccines. Health Aff. 2012;31:2545–53. doi: 10.1377/hlthaff.2011.1272. [DOI] [PubMed] [Google Scholar]


