Abstract
Numerous signaling molecules are altered following nerve injury, serving as a blueprint for drug delivery approaches that promote nerve repair. However, challenges with achieving the appropriate temporal duration of recombinant protein delivery have limited the therapeutic success of this approach. Genetic engineering of mesenchymal stem cells (MSCs) to enhance the secretion of proangiogenic molecules such as vascular endothelial growth factor (VEGF) may provide an alternative. We hypothesized that the administration of VEGF-expressing human MSCs would stimulate neurite outgrowth and proliferation of cell-types involved in neural repair. When cultured with dorsal root ganglion (DRG) explants in vitro, control and VEGF-expressing MSCs (VEGF-MSCs) increased neurite extension and proliferation of Schwann cells (SCs) and endothelial cells, while VEGF-MSCs stimulated significantly greater proliferation of endothelial cells. When embedded within a 3D fibrin matrix, VEGF-MSCs maintained overexpression and expressed detectable levels over 21 days. After transplantation into a murine sciatic nerve injury model, VEGF-MSCs maintained high VEGF levels for 2 weeks. This study provides new insight into the role of VEGF on peripheral nerve injury and the viability of transplanted genetically engineered MSCs. The study aims to provide a framework for future studies with the ultimate goal of developing an improved therapy for nerve repair.
Keywords: VEGF, Mesenchymal stem cells, Nerve regeneration, Peripheral nerve injury
INTRODUCTION
Treatments for nerve injuries have changed little in the past 30 years, despite our advanced understanding of developments during peripheral nerve regeneration. The two most common therapies remain direct end-to-end surgical reconnection of nerve stumps and implantation of autologous nerves18. However, direct suture is distance-limited and may result in stretching of the nerve, which can cause additional pain. Autografts are of limited availability, require sacrifice of donor nerves causing loss of sensation at the site of harvest, and rarely restore full motor function or sensation3,7.
After peripheral nerve injury, the injured nerve produces a host of growth factors to jumpstart the repair process including brain-derived neurotrophic factor (BDNF), leukemia inhibitory factor (LIF) 6, fibroblast growth factor (FGF) 33, nerve growth factor (NGF) 13, glial cell line-derived neurotrophic factor (GDNF) 11, and VEGF 32. To recapitulate the endogenous healing response, previous studies have sought to deliver recombinant growth factors to enhance nerve regeneration 40. However, the short half-lives of these molecules require the delivery of supraphysiologic dosages that can be cost-prohibitive or may cause unintended side effects. Furthermore, there are significant challenges in achieving the necessary local concentrations to achieve the desired therapeutic effect.
As an alternative to recombinant protein delivery, the implantation of supporting cells that naturally produce neurostimulatory factors is under investigation. Glial cells secrete neurotrophic factors after nerve injury and play an important role in remyelinating the regenerating axons. The presence of Schwann cells (SCs) and olfactory ensheathing cells improves regeneration in rodent models by forming support structures or secreting factors for regenerating axons 20,31. As with all primary cultures, the time required for expanding cells to clinically relevant numbers may prevent timely surgical intervention, and it is difficult to obtain a sufficient number of autologous cells without nerve sacrifice. After injury throughout the body, mesenchymal stem/stromal cells (MSCs) migrate to hypoxic, apoptotic, or inflamed areas and release trophic factors to suppress the local immune system, stimulate reparative activities of endogenous stem cells, enhance local angiogenesis, and inhibit fibrosis and apoptosis 27. In light of their native response to injury, MSCs are a promising alternative cell source for use in neural repair; they can be readily harvested and proliferate rapidly in culture 5,30. The MSC secretome includes numerous cues necessary in the regeneration process, yet native expression levels may not be therapeutically sufficient 9.
We hypothesized that the genetic manipulation of MSCs to overexpress VEGF would provide a sustained delivery strategy of the proangiogenic molecule for therapeutic benefit. This study utilizes human MSCs engineered to overexpress VEGF (VEGF-MSCs) to determine their effect on various cell types in peripheral nerves, while also examining their potential for implantation. The goal of the study was to determine which cell populations would benefit from the increased local presentation of VEGF and demonstrate that genetically engineered MSCs remain viable and maintain functionality after implantation.
MATERIALS AND METHODS
Sciatic nerve transection
C57BL/6 mice (Jackson West) underwent sciatic nerve transection to assess changes in endogenous growth factor secretion by host cells following injury. Treatment of all experimental animals was in accordance with UC Davis animal care guidelines and all National Institutes of Health animal-handling procedures. Animals were anesthetized using isoflurane (3% induction, 1.5% maintenance) before making an incision in the skin and upper region of the right gluteal muscle to expose the sciatic nerve. The nerve was transected 1–2 mm distal to the sciatic notch. The muscle and skin were closed with 6-0 silk sutures. Sham surgeries where the sciatic nerve was exposed but not transected served as controls. Animals were allowed access to food and water ad libitum.
Mice were euthanized with CO2 at 4, 6, 14 and 21 days post-transection and the sciatic nerves removed. The excised nerve extended from the sciatic notch to before the split into the common peroneal, sural and tibial nerves. Tissue was processed for analysis as previously described 37. The supernatant was collected and stored at −80°C until analysis.
Determination of VEGF concentrations in tissues or secreted by MSCs
Endogenous VEGF concentrations in explanted tissue were determined using a mouse-specific VEGF Quantikine ELISA kit (R&D systems) per manufacturer’s instructions. For human VEGF secretion from MSCs, the human-specific VEGF ELISA kit (Thermo Scientific) was used as described in the manufacturer’s instructions.
Cell isolation and culture
Bone marrow aspirates from healthy human donors were purchased (Lonza), and MSCs were isolated and expanded as previously described 9. Cells were maintained in MEM/EBSS (HyClone) supplemented with 10% FBS and 1% penicillin/streptomycin (P/S; Invitrogen) in 5% CO2/95% O2 at 37°C. MSCs from passages 4 to 10 were used for experimentation.
Primary SCs were isolated from sciatic nerves of rats, as described previously 2. Cells were expanded in DMEM:F12 (ThermoFisher) supplemented with 10% FBS, 10 ng/mL glial growth factor-2 (GGF-2, Cambridge Neuroscience), and 2 μM forskolin (Tocris Bioscience). Basal media consisted of DMEM:F12 with 10% FBS.
Human aortic endothelial cells (HAECs, Lonza) were expanded in EBM-2 basal media (Lonza) supplemented with the EBM-2 BulletKit (Lonza) which consists of human epidermal growth factor (hEGF), hydrocortisone, gentamicin amphotericin-B (GA-1000), 2% FBS, VEGF, human bFGF- human insulin-like growth factor-1 (R3-IGF-1), ascorbic acid, and heparin. Basal media consisted of growth media without growth factors.
Transduction of MSCs using lentiviral vector
MSCs were transduced with third-generation lentiviral vectors using a multiplicity of infection (MOI) = 10. The construct’s general form is pCCLc-MNDU3-X-IRES-EGFP, where X is the insertion site for the full length cDNA of VEGF-A(165) or without a transgene as control, as we previously described 9. All cells were transduced with an EGFP transgene for in vitro and in vivo tracking.
Characterization of neurite outgrowth of DRG explants when co-cultured with MSCs
DRGs were collected from neonatal (postnatal day 0–4) C57BL/6 mice and cultured using previously described techniques 12,25. After 1 day, media was exchanged to basal media without NGF. Transwells (3.0 μm pore diameter, Corning) seeded with 20,000 control or VEGF-MSCs 24 hours prior were placed over the DRGs in a 24-well plate. As a positive control, DRGs were cultured in basal media supplemented with 5 or 100 ng/mL human recombinant VEGF (R&D systems). DRGs in basal media without NGF served as controls. To examine the mechanism by which MSCs acted upon DRGs, SU5416 (0.5 μM; Sigma), a synthetic inhibitor of the FLk-1/KDR VEGF receptor, was added to the media. The same volume of DMSO vehicle was added as a control. Digital images were captured after 2 days using an inverted phase-contrast microscope (Olympus IX70) equipped with a 10x objective (Olympus). Neurite extension was measured from the tip of the growing neurite to the edge of the DRG explant with NeuronJ 26.
Characterization of proliferation of SCs and HAECs when co-cultured with MSCs
SCs and HAECs were seeded in 24-well plates at 9,500 cells/well. Transwells were seeded with 20,000 control or VEGF-MSCs. After 1 day, media was exchanged to basal media of the respective cell type, and the transwells were placed over the SCs or HAECs. As a positive control, cells were cultured in their respective basal media supplemented with 5, 50 or 100 ng/mL human recombinant VEGF. SCs and HAECs in their respective basal media served as controls. SU5416 (0.5 μM) was added twice, first when both cell types were combined (Day 1) and 2 days after (Day 3). DMSO vehicle was added as a control. Digital images were captured after 4 days using an inverted phase-contrast microscope (Olympus IX70) equipped with a 4x objective (Olympus). Cells were counted using ImageJ (NIH).
Fabrication of poly-L-lactide acid (PLLA) conduits
We fabricated polymer conduits to retain fibrin gels when used to bridge nerve defects in vivo. PLLA (MW = 85,000–160,000, Sigma Aldrich) in chloroform (1.56% w/v) was cast onto a glass surface and evaporated overnight. Membranes were wrapped around a 22-gauge needle and sealed with chloroform, yielding tubes with an approximate diameter of 0.72 mm. Nerve conduits were approximately 6 mm in length 1.
Measurement of VEGF secretion by MSCs in 2D and 3D culture
MSCs were cultured on tissue culture plastic (TCP) in a 24-well plate at 9,500 cells/well in MEM/EBSS supplemented with 10% FBS and 1% P/S. After 2 days, media was exchanged. Supernatant was collected 2 days later and stored at −80°C until analysis.
MSCs were grown in a fibrin matrix to measure VEGF secretion in 3D culture. Fibrinogen solutions were prepared by dissolving human fibrinogen (Calbiochem) in distilled water containing 0.8% NaCl and sterilized by filtration through a 0.22 μm centrifuge tube filter (Spin-X, Corning). The fibrinogen solution was mixed with aprotinin (0.5 mg/mL, Santa Cruz Biotechnology) and heparin (167 μg/mL, Sigma Aldrich). 9,500 MSCs were resuspended in the fibrinogen solution. Fibrin gels were formed by mixing the fibrinogen solution with 20 mM CaCl2 and 136 U/mL thrombin (both from Calbiochem) in a 1.5 mL centrifuge tube, yielding fibrin gels with a final composition of 9.5 mg/mL fibrinogen, 40 μg/mL aprotinin, 1 μg/mL heparin, 2.5 mM CaCl2, and 2.5 U/mL thrombin. MSC-loaded gels were incubated at 37°C for 15 minutes to allow for complete polymerization and media was added. After 2 days, media was exchanged. Supernatant was collected 2 days later and stored at −80°C until analysis. VEGF concentrations in the conditioned medium from 2D and 3D culture were normalized to DNA content quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen) as described 28.
To examine long-term VEGF secretion by VEGF-MSCs in vitro, 100,000 cells were mixed in a 2 μL fibrin gel solution and pipetted into a 6 mm PLLA nerve conduit. Filled conduits were incubated at 37°C for 15 minutes to allow for complete polymerization. Conduits were cultured in MEM/EBSS supplemented with 10% FBS and 1% P/S. Supernatant was collected every 3 days for 21 days, and conduits were placed in a new well after each collection. Supernatant was stored at −80°C until measurement by ELISA. Fluorescent images of GFP-positive cells were captured every 3 days using an inverted microscope (Olympus IX70) equipped with a 4x objective (Olympus).
Measurement of MSC proliferation within fibrin gels
MSCs were cultured on glass coverslips or within fibrin gels as described above. After 2 days, proliferation was assessed using the 594-Click iT® EdU Imaging Kit (Invitrogen). Coverslips were mounted and imaged by confocal microscopy. DAPI-positive and EdU-positive cells were counted from 4 random images from each sample. Data are represented as percentage of EdU-positive cells within all visualized cells
Characterization of MSC function in vivo
Sciatic nerves of nonobese diabetic/severe combined immune deficient (NOD/SCID)/β-2-microglobulin-deficient (NSG) mice (Jackson West) were transected as described above. After transection, 1 mm of the proximal and distal stumps was inserted into each end of 6 mm PLLA nerve conduits, creating a 4 mm nerve gap. The mice were randomly divided into 3 groups (n=3 per group). Conduits were loaded with 2 μL of fibrin gel containing the following: (1) 100,000 VEGF-MSCs, (2) 100,000 control MSCs, and (3) blank fibrin gel without cells. To retain the conduit in place, a 94.3 mg/mL fibrinogen concentration fibrin gel was pipetted over the conduit and allowed to polymerize for 3 minutes 25. The muscle and skin were then sutured.
To determine the duration of VEGF expression, conduits were harvested at 2 and 8 weeks after transection, tissue was prepared as described above, and VEGF protein levels were measured by ELISA.
To determine cell survival in vivo, mice were anesthetized with ketamine/xylazine after 2 weeks, perfused with PBS and 4% paraformaldehyde (PFA), and the contralateral and injured nerves were harvested. Nerves were fixed in 2% PFA for 30 minutes and washed 3 times with PBS. For cryoprotection, nerves were immersed in 30% (w/v) sucrose for 48 hours at 4°C and then embedded in OCT compound. Slides were air dried at room temperature and post-fixed in −20°C methanol for 8 minutes. The sections were overlaid with a blocking solution consisting of minimum essential medium containing 15 mM HEPES buffer, 10% (v/v) donkey serum, and 0.02% (w/v) sodium azide for 30 minutes. Sections were stained with DAPI (1:1000, Sigma Aldrich) for 10 minutes and mounted. Images were captured with a Nikon laser scanning confocal microscope equipped with a 40x objective (Nikon).
Statistical analysis
Data are presented as mean ± standard error of the mean. Statistical significance was assessed by two-way analysis of variance (ANOVA) followed by a Student-Newman-Keuls test for post hoc comparisons. p-values <0.05 were considered statistically significant.
RESULTS
Endogenous VEGF protein expression is acutely upregulated post-injury
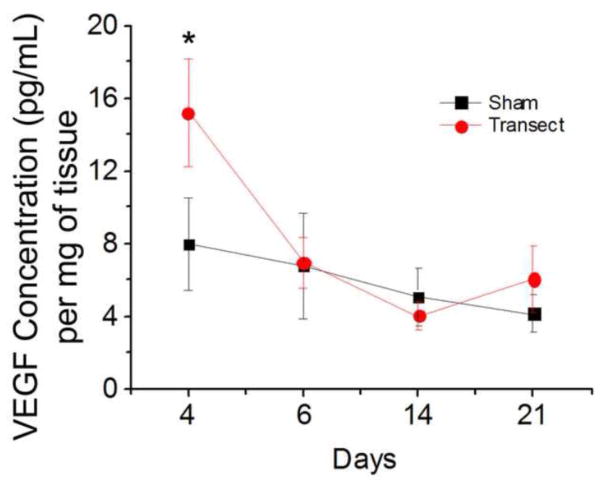
Sciatic nerves were surgically transected and harvested at various time points to measure endogenous VEGF expression levels after injury. Compared to sham controls, VEGF protein expression was strongly upregulated 4 days post-injury (Fig. 1). This injury-induced increase in VEGF was transient, as VEGF concentrations were comparable to control levels 6, 14 and 21 days after transection, suggesting the early importance of VEGF during nerve regeneration.
Figure 1.
Endogenous VEGF protein expression was upregulated 4 days post-sciatic nerve transection. Squares, sham surgery. Circles, nerve transection. Data are mean ± SEM, n=4, *p<0.01.
MSCs promote neurite outgrowth and cell proliferation
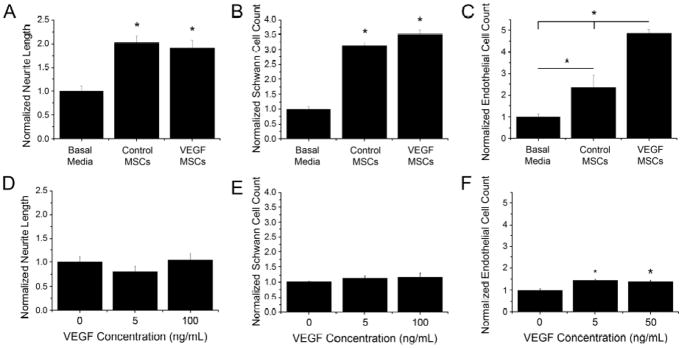
The effect of trophic factors secreted by control and VEGF-MSCs on DRG explants, SCs, and HAECs was examined with a co-culture assay. Both control and VEGF-MSCs significantly increased neurite extension compared to DRG explants grown in basal media (Fig. 2A), yet the increased VEGF presentation from VEGF-MSCs did not further enhance neurite outgrowth. In agreement with this finding, the presentation of recombinant VEGF to DRG explants did not increase neurite extension compared to basal media, even at the highest dosage studied (100 ng/mL) (Fig. 2D).
Figure 2.
Co-culture of DRG explants, SCs and HAECs with control or VEGF-MSCs increased neurite outgrowth and cell proliferation. (A) Neurite length was increased in DRG explants in co-cultures with control and VEGF-MSCs (*p<0.01 versus basal media). (B) SC proliferation was increased in co-cultures with control and VEGF-MSCs (*p<0.01 versus basal media). (C) HAEC proliferation was increased in co-cultures with control and VEGF-MSCs compared to basal media, and co-cultures with VEGF-MSCs increased proliferation compared to control MSCs (*p<0.01, n≥6). (D–F) Supplementation of cultures with human recombinant VEGF increased cell proliferation in (F) HAEC cultures (*p<0.01) but not (D) DRG explants or (E) SC cultures (n≥7). Data are normalized to their respective basal media controls. Data are mean ± SEM.
To determine if MSCs could affect the proliferation of SCs, MSCs were co-cultured with SCs for 4 days, and the number of SCs was counted. Compared to basal media, SC proliferation was significantly increased in co-culture with control and VEGF-MSCs (Fig. 2B), yet SC proliferation was statistically similar using control or VEGF-MSCs. Similar to neurite extension from DRG explants, the addition of recombinant human VEGF did not increase SC proliferation (Fig. 2E).
Compared to basal media, HAEC number was increased when co-cultured with control and VEGF-MSCs. HAECs cultured with VEGF-MSCs exhibited significantly increased proliferation compared to control MSCs (Fig. 2C). Recombinant human VEGF positive controls significantly increased cell proliferation when added to HAEC cultures (Fig. 2F), yet control and VEGF-MSCs induced a significantly greater increase in HAEC proliferation.
SU5416 decreases HAEC proliferation in co-culture with VEGF-MSCs
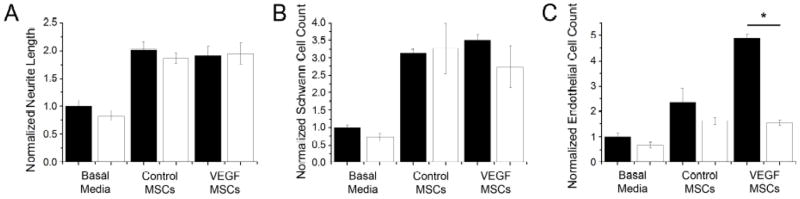
SU5416, a synthetic inhibitor of the VEGF receptor, was added to co-cultures with MSCs to determine whether the observed effects on neurite outgrowth and cell proliferation were caused by VEGF secretion by MSCs. The addition of SU5416 did not abrogate neurite extension of DRG explants or SC proliferation compared to cultures without the VEGF receptor inhibitor (Fig. 3A, B). The addition of SU5416 did not significantly impair HAEC proliferation in basal media or when co-cultured with control MSCs. However, SU5416 caused a significant decrease in HAEC proliferation when co-cultured with VEGF-MSCs compared to co-cultures without the inhibitor (Fig. 3C).
Figure 3.
Addition of the VEGF receptor inhibitor, SU5416, reduced proliferation of HAECs but did not effect neurite extension or SC proliferation. (A) Neurite length of DRG explant cultures. (B) Quantification of SC proliferation. (C) Quantification of HAEC proliferation. Filled columns represent data in the absence of SU5416; open columns represent data in the presence of 0.5 μM SU5416. Data are normalized to their respective basal media controls. Data are mean ± SEM, n≥6, *p<0.01.
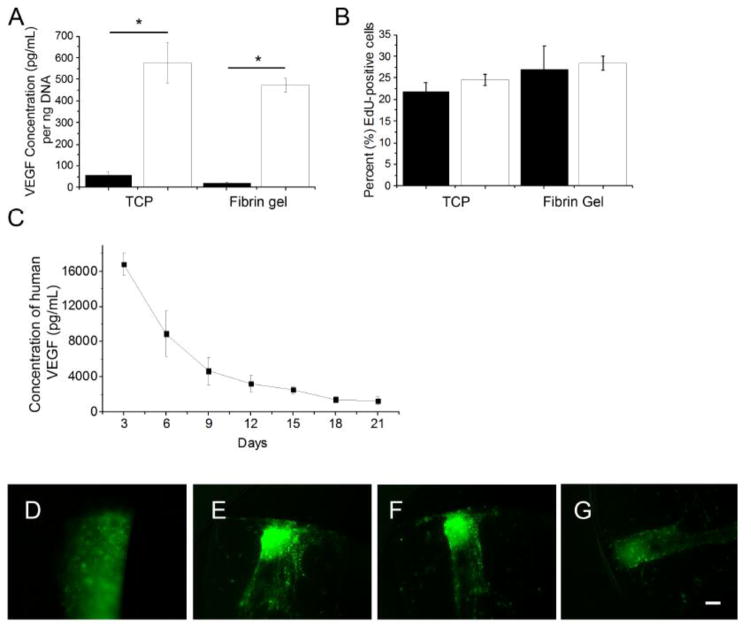
VEGF overexpression and proliferation rate is maintained by VEGF-MSCs in 3D culture
Control and VEGF-MSCs were embedded in fibrin gels to determine whether 3D culture affects VEGF secretion. VEGF concentration in the supernatant of VEGF-MSC cultures was significantly increased compared to control MSCs grown on tissue culture plastic (TCP) or in 3D fibrin gels. VEGF-MSCs exhibited VEGF concentrations statistically similar when grown on TCP or in fibrin gels (Fig. 4A). The percentage of proliferating (Edu-positive) cells was not significantly different among groups (Fig. 4B). PLLA conduits were then filled with VEGF-MSCs in a fibrin matrix to examine whether VEGF secretion was maintained in vitro over an extended period. VEGF protein gradually decreased over time but was still detectable in the culture medium after 21 days and displayed a peak 3-day concentration of approximately 16 ng/mL (Fig. 4C). GFP-positive VEGF-MSCs were present throughout the 21 day study period (Fig. 4D–G).
Figure 4.
VEGF-MSCs maintained their overexpression of growth factor and proliferation rate in 3D fibrin gels. (A) VEGF-MSCs maintain VEGF overexpression on tissue culture plastic (TCP) and in fibrin gels. Filled columns: control MSCs; open columns: VEGF-MSCs (n=6, *p<0.01 versus control MSCs). (B) Proliferation of control and VEGF-MSCs was not different when cultured on TCP or in fibrin gels. Filled columns, control MSCs. Open columns, VEGF-MSCs. (n=4). (C) Conduits filled with VEGF-MSCs embedded within fibrin gels secrete detectable levels of VEGF for up to 21 days (n=4). Data are mean ± SEM. Fluorescent images of conduits filled with green fluorescent protein (GFP)-positive VEGF-MSCs embedded within fibrin gels at Day (D) 0, (E) 6, (F) 12, and (G) 21. Scale bar=100 μm.
MSCs remain localized upon implantation in vivo and secrete VEGF
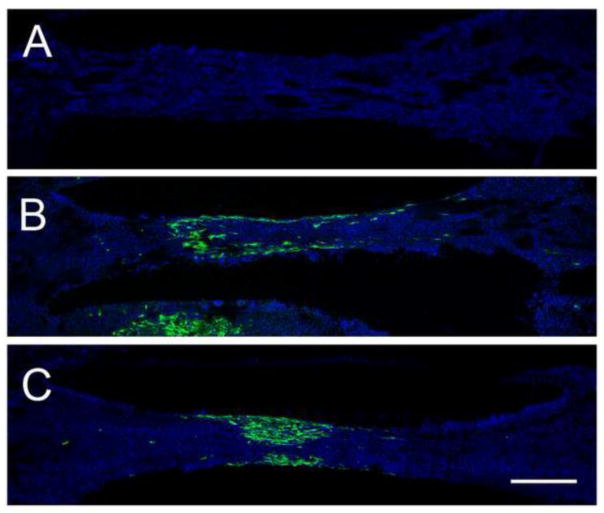
Two weeks after implantation, conduits were harvested to determine whether GFP-positive MSCs were still present. No cells were visible in the sham controls (Fig. 5A). In both control and VEGF-MSC conditions, GFP-positive cells remained localized within the implanted conduit (Fig. 5B–C). Axon regeneration was complete in all groups as early as 2 weeks post-transection (Supplementary Fig. 1–3).
Figure 5.
GFP-positive MSCs remained localized at the site of implantation at week 2. Confocal images of transected sciatic treated with a PLLA conduit containing (A) fibrin alone, (B) a fibrin matrix with control MSCs, or (C) a fibrin matrix with VEGF-MSCs. Conduits are oriented with the proximal end on the left and the distal end on the right. Green, GFP for endogenous green fluorescent protein positive-MSCs. Blue, DAPI for cell nuclei. Scale bar = 500 μm.
Conduits were harvested 2 and 8 weeks post-transplantation, and VEGF levels were measured to determine whether VEGF overexpression was maintained after transplantation. Conduits containing VEGF-MSCs had significantly higher levels of VEGF compared to conduits filled with control MSCs at 2 weeks (Fig. 6). By week 8, VEGF levels were not significantly different between MSC-containing conduits.
Figure 6.
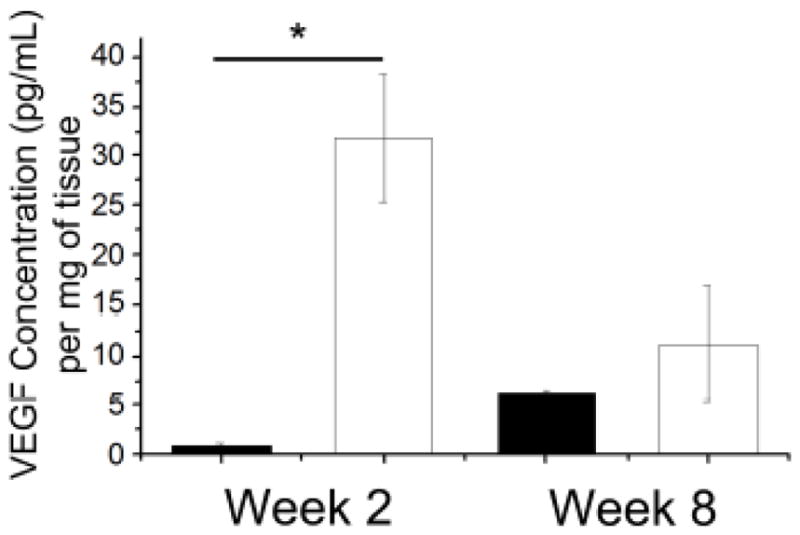
Conduits filled with VEGF-MSCs embedded within fibrin gels maintain VEGF overexpression 2 weeks post-transplantation. Filled columns: control MSCs; open columns: VEGF-MSCs. Data are mean ± SEM, n≥3, *p<0.01.
DISCUSSION
Endogenous VEGF levels are strongly upregulated after nerve transection. Thus, we aimed to utilize MSCs overexpressing VEGF to deliver the proangiogenic factor at the site of nerve injury with an eye toward nerve regeneration therapies. When co-cultured with DRG explants, SCs or HAECs, MSCs increased DRG neurite extension and proliferation of both SCs and HAECs. The bioavailability of greater concentrations of VEGF from VEGF-MSCs increased endothelial cell proliferation in a dose-dependent manner, yet changes in cell response as a function of VEGF presentation were not apparent for DRG explants or SCs. After transplantation to treat a sciatic nerve injury model, human MSCs remained localized at the implantation site and secreted VEGF for at least 2 weeks post-transplantation. The study suggests the potential of MSCs secreting VEGF as a possible therapy for nerve injury.
Despite more robust regeneration compared to the CNS, peripheral nerve regeneration is still limited. The most common strategy for accelerating recovery is the delivery of recombinant growth factors to the site of injury. When nerve grafts are used to bridge injury gaps, adequate vascularization is the most important factor in determining success to avoid graft necrosis 36. VEGF, a potent endothelial cell mitogen, has been effectively used to increase angiogenesis in many tissues including skin, bone, and muscle 4,24,39. In the PNS, delivery of VEGF after nerve injury increased SC invasion and neovascularization, which was associated with nerve regeneration 15,35. Delivery of a plasmid vector transiently expressing the human VEGF165 gene was associated with an increase in myelinated fibers and blood vessels 29, but this strategy has poor transfection efficiency and requires the uptake of the gene by neighboring host cells, thereby delaying the local presentation of therapeutic VEGF. Similar studies using a more efficient, stably incorporating lentiviral vector exhibited hypervascularization that inhibited axonal regrowth 17, suggesting that both the dose and duration of VEGF presentation is a critical aspect for consideration. The results of these studies suggest that transduction with this particular lentiviral vector is an effective means to achieve high transfection efficiency with transient VEGF presentation, which has broad appeal to many applications in tissue repair and regeneration, even beyond nerve repair.
A variety of cell populations are under investigation for cell-based therapies of nerve repair. Transplanted SCs improved axonal regeneration in small nerve gaps 14. Transplantation of MSCs promotes angiogenesis 21 and resulted in greater organization of the regenerating nerve, more myelinated fibers, greater sensory neuron survival, and improved functional recovery by week 6 10. However, native MSCs do not secrete therapeutic relevant levels of trophic factors needed for recovery from large nerve defects 9. To address this significant limitation and enhance the secretion of desired trophic factors, we genetically engineered MSCs to overexpress VEGF, a potent proangiogenic factor, and examined its capacity to stimulate neurogenic activity in vitro and in vivo.
To understand how VEGF secretion from MSCs may modulate the activities of cells that play a critical role in nerve repair, we co-cultured MSCs overexpressing VEGF with 3 cell types found in peripheral nerves: sensory axons, SCs, and endothelial cells. Others have shown that MSCs secrete trophic factors that promote neurite growth and proliferation in these cell types 19,23,38. In agreement with these data, we observed increases in DRG explant neurite outgrowth and SC and endothelial cell proliferation with control MSCs. VEGF-MSCs induced even greater increases in endothelial cell proliferation, but other cells were unaffected by the increased VEGF. Sondell et al. reported that VEGF increased neurite outgrowth and SC proliferation in superior cervical ganglia (SCG) and DRG explants 34. While the potential contribution of VEGF is consistent with these studies, the age of DRG explants used in these studies remains an important difference. Embryonic and postnatal DRG explants do not require VEGF for neuronal survival and growth, and the tyrosine kinase VEGF receptor VEGFR2 is not found on sensory neurons or other non-endothelial cells of the DRG 22. This study harvested DRG explants from postnatal day 0–4 mice while Sondell et al. used 5-week-old mice. To further confirm the endothelial specific activity of VEGF, we supplemented co-cultures with recombinant human VEGF, as well as a VEGF receptor inhibitor. The addition of soluble VEGF increased endothelial cell proliferation but not neurite outgrowth or SC proliferation. Upon addition of the VEGF receptor antagonist, only co-cultures of VEGF-MSCs and endothelial cells displayed an inhibition of proliferation, suggesting that the complex MSC secretome has many components that may stimulate endothelial cell proliferation 16. The results from this study suggest that the primary role of VEGF in this system is to increase endothelial proliferation. However, the interplay between VEGF and surrounding cells (e.g., axons, SCs, and endothelial cells) merits further study to understand how physical and indirect interactions during regeneration drive cell function. For example, VEGF may act indirectly on nerve regeneration by inducing angiogenesis, which leads to SC proliferation and axonal regeneration through increased secretion of NGF by recruited host endothelial cells. Recently, Egervari et al. found that VEGF secreted by astrocytes integrate into the extracellular matrix (ECM) to stabilize β1 integrin in cell-matrix adhesions. VEGF accumulated at the ECM-integrin complexes turned over rapidly, suggesting the necessity of a continuous delivery of VEGF to maintain stability. The authors believe the immobilization of VEGF enhances regeneration after injury by regulating glial and endothelial functions in vivo 8. The presentation of VEGF by MSCs may provide the necessary signals to maintain activation of these complexes during the healing process.
To deliver MSCs to the site of injury in vivo, we fabricated tubes from a biodegradable polymer to serve as a nerve conduit and localize cell delivery using an engineered fibrin matrix. VEGF-MSCs in 3D culture maintained overexpression of VEGF, and MSCs exhibited similar proliferation within fibrin gels as on TCP. Additionally, VEGF-MSCs entrapped in fibrin suspended within PLLA conduits secreted gradually decreasing but detectable VEGF levels for up to 21 days. The maintenance of VEGF expression suggests that VEGF-MSCs may be a suitable source for prolonged delivery of angiogenic factors throughout the healing process.
Conduits were harvested up to 8 weeks post-transplantation to determine whether MSCs remained localized at the site of harvest and continued to secrete VEGF at detectable levels. MSCs, both control and VEGF-overexpressing, were transduced to express GFP to aid in their visualization in vivo. GFP-positive MSCs were observed 2 weeks post-implantation via immunohistochemistry of explants with few after 4 weeks and none after 8 weeks (data not shown), suggesting that the regenerative boost by MSCs would occur during the first few weeks after injury. Compared to control MSCs, we detected significant increases in local VEGF availability in defects treated with VEGF-MSCs at 2 weeks, but VEGF levels were not significantly different by week 8. MSCs may have migrated out of the conduit or apoptosed by this time point. Future studies merit an alternative animal model that better mimics poor peripheral nerve regeneration in humans. Sciatic nerve defects of 4 mm were created in this study. This subcritical-sized defect, which heals spontaneously in nerve guide conduits 41, likely masked the therapeutic benefit of sustained presentation of VEGF from engineered MSCs. The results of this study reveal that axon regeneration was complete as early as 2 weeks post-transection, despite no functional recovery (data not shown). In addition to the animal’s robust recovery from peripheral nerve injury, the size of the animal presents technical difficulties in surgical manipulations. As an alternative, rat models allow for nerve gaps of up to 13 mm and the implantation of conduits with inner diameters of 1.5 mm 40. While the transplanted VEGF-MSCs secreted higher levels of the bioactive factor than control MSCs, the levels may not be sufficient for increasing angiogenesis. The peak 3-day concentration of VEGF secreted by VEGF-MSCs in vitro was approximately 16 ng/mL. Other studies reported success in accelerating axonal regeneration with a VEGF concentration of 500–700 ng/mL 15. A rat model would allow for the implantation of a higher number of cells, translating to higher local concentrations of VEGF to discern potential differences between the MSC populations.
The results of this study demonstrate the potential of genetically engineered MSCs to contribute to treatment of peripheral nerve injuries. MSCs were transduced to secrete higher levels of VEGF to augment endogenous levels. In vitro studies demonstrated a compelling benefit for VEGF-overexpressing MSCs, and we observed sustained elevations in VEGF concentrations in vivo for more than 2 weeks. While the results demonstrate the potential of genetically engineered MSCs in treating peripheral nerve injury as demonstrated by increased VEGF presentation and prolonged transient survival, further studies should be performed in order to achieve the ultimate goal of functional nerve recovery. The nerve injury site represents a complex environment with a variety of permissive and non-permissive cues and different cell types. An effective therapy may require a combinatorial approach that provides an extracellular matrix substrate, stimulatory factors and supporting cells, as well as topographical and/or soluble guidance cues. This study represent an important building block in designing a tissue engineered nerve conduit by providing a cell-based approach for revascularizing nerve defects.
Supplementary Material
Acknowledgments
This work was partially supported by R03DE021704 (to JKL) and CIRM Disease Team Grant DR2A-05423 for Critical Limb Ischemia (to FAF). The authors acknowledge Dr. Tony Passerini for providing HAECs.
Footnotes
CONFLICTS OF INTEREST
Alan Man, Gregory Kujawski, Travis Burns, Elaine Miller, Fernando Fierro, Kent Leach, and Peter Bannerman declare that they have no conflicts of interest.
ETHICAL STANDARDS
Treatment of all experimental animals was in accordance with UC Davis animal care guidelines and all National Institutes of Health animal-handling procedures and was approved by the appropriate institutional committees. No human subject research was performed.
References
- 1.Aframian DJ, Redman RS, Yamano S, Nikolovski J, Cukierman E, Yamada KM, Kriete MF, Swaim WD, Mooney DJ, Baum BJ. Tissue compatibility of two biodegradable tubular scaffolds implanted adjacent to skin or buccal mucosa in mice. Tissue Eng. 2002;8:649–59. doi: 10.1089/107632702760240562. [DOI] [PubMed] [Google Scholar]
- 2.Ara J, Bannerman P, Shaheen F, Pleasure DE. Schwann cell-autonomous role of neuropilin-2. J Neurosci Res. 2005;79:468–75. doi: 10.1002/jnr.20370. [DOI] [PubMed] [Google Scholar]
- 3.Beazley WC, Milek MA, Reiss BH. Results of nerve grafting in severe soft tissue injuries. Clin Orthop Relat Res. 1984:208–12. [PubMed] [Google Scholar]
- 4.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A. 2010;107:3287–92. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–5. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis R, Scherer SS, Somogyi R, Adryan KM, Ip NY, Zhu Y, Lindsay RM, DiStefano PS. Retrograde axonal transport of LIF is increased by peripheral nerve injury: correlation with increased LIF expression in distal nerve. Neuron. 1994;12:191–204. doi: 10.1016/0896-6273(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 7.Dellon AL, Mackinnon SE. An alternative to the classical nerve graft for the management of the short nerve gap. Plast Reconstr Surg. 1988;82:849–56. doi: 10.1097/00006534-198811000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Egervari K, Potter G, Guzman-Hernandez ML, Salmon P, Soto-Ribeiro M, Kastberger B, Balla T, Wehrle-Haller B, Kiss JZ. Astrocytes spatially restrict VEGF signaling by polarized secretion and incorporation of VEGF into the actively assembling extracellular matrix. Glia. 2015 doi: 10.1002/glia.22939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fierro FA, Kalomoiris S, Sondergaard CS, Nolta JA. Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells. 2011;29:1727–37. doi: 10.1002/stem.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frattini F, Lopes FR, Almeida FM, Rodrigues RF, Boldrini LC, Tomaz MA, Baptista AF, Melo PA, Martinez AM. Mesenchymal stem cells in a polycaprolactone conduit promote sciatic nerve regeneration and sensory neuron survival after nerve injury. Tissue Eng Part A. 2012;18:2030–9. doi: 10.1089/ten.TEA.2011.0496. [DOI] [PubMed] [Google Scholar]
- 11.Hammarberg H, Piehl F, Cullheim S, Fjell J, Hokfelt T, Fried K. GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury. Neuroreport. 1996;7:857–60. doi: 10.1097/00001756-199603220-00004. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins RL, Seeds NW. Protease inhibitors influence the direction of neurite outgrowth. Brain Res Dev Brain Res. 1989;45:203–9. doi: 10.1016/0165-3806(89)90039-4. [DOI] [PubMed] [Google Scholar]
- 13.Heumann R, Lindholm D, Bandtlow C, Meyer M, Radeke MJ, Misko TP, Shooter E, Thoenen H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc Natl Acad Sci U S A. 1987;84:8735–9. doi: 10.1073/pnas.84.23.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoben G, Yan Y, Iyer N, Newton P, Hunter DA, Moore AM, Sakiyama-Elbert SE, Wood MD, Mackinnon SE. Comparison of accellular nerve allograft modification wih Schwann cells of VEGF. Hand. 2014 doi: 10.1007/s11552-014-9720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobson MI, Green CJ, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat. 2000;197(Pt 4):591–605. doi: 10.1046/j.1469-7580.2000.19740591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoch AI, Binder BY, Genetos DC, Leach JK. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One. 2012;7:e35579. doi: 10.1371/journal.pone.0035579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoyng SA, De Winter F, Gnavi S, de Boer R, Boon LI, Korvers LM, Tannemaat MR, Malessy MJ, Verhaagen J. A comparative morphological, electrophysiological and functional analysis of axon regeneration through peripheral nerve autografts genetically modified to overexpress BDNF, CNTF, GDNF, NGF, NT3 or VEGF. Exp Neurol. 2014;261:578–93. doi: 10.1016/j.expneurol.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Isaacs J. Treatment of acute peripheral nerve injuries: current concepts. J Hand Surg Am. 2010;35:491–7. doi: 10.1016/j.jhsa.2009.12.009. quiz 498. [DOI] [PubMed] [Google Scholar]
- 19.Johansson U, Rasmusson I, Niclou SP, Forslund N, Gustavsson L, Nilsson B, Korsgren O, Magnusson PU. Formation of composite endothelial cell-mesenchymal stem cell islets: a novel approach to promote islet revascularization. Diabetes. 2008;57:2393–401. doi: 10.2337/db07-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalbermatten DF, Kingham PJ, Mahay D, Mantovani C, Pettersson J, Raffoul W, Balcin H, Pierer G, Terenghi G. Fibrin matrix for suspension of regenerative cells in an artificial nerve conduit. J Plast Reconstr Aesthet Surg. 2008;61:669–75. doi: 10.1016/j.bjps.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Kniazeva E, Kachgal S, Putnam AJ. Effects of extracellular matrix density and mesenchymal stem cells on neovascularization in vivo. Tissue Eng Part A. 2011;17:905–14. doi: 10.1089/ten.tea.2010.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kutcher ME, Klagsbrun M, Mamluk R. VEGF is required for the maintenance of dorsal root ganglia blood vessels but not neurons during development. FASEB J. 2004;18:1952–4. doi: 10.1096/fj.04-2320fje. [DOI] [PubMed] [Google Scholar]
- 23.Ladak A, Olson J, Tredget EE, Gordon T. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp Neurol. 2011;228:242–52. doi: 10.1016/j.expneurol.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Leach JK, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27:3249–55. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 25.Man AJ, Davis HE, Itoh A, Leach JK, Bannerman P. Neurite outgrowth in fibrin gels is regulated by substrate stiffness. Tissue Eng Part A. 2011;17:2931–42. doi: 10.1089/ten.tea.2011.0030. [DOI] [PubMed] [Google Scholar]
- 26.Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 2004;58:167–76. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- 27.Meyerrose T, Olson S, Pontow S, Kalomoiris S, Jung Y, Annett G, Bauer G, Nolta JA. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv Drug Deliv Rev. 2010;62:1167–74. doi: 10.1016/j.addr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy KC, Hughbanks ML, Binder BY, Vissers CB, Leach JK. Engineered Fibrin Gels for Parallel Stimulation of Mesenchymal Stem Cell Proangiogenic and Osteogenic Potential. Ann Biomed Eng. 2015;43:2010–21. doi: 10.1007/s10439-014-1227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira Lopes FR, Lisboa BC, Frattini F, Almeida FM, Tomaz MA, Matsumoto PK, Langone F, Lora S, Melo PA, Borojevic R, Han SW, Martinez AM. Enhancement of sciatic nerve regeneration after vascular endothelial growth factor (VEGF) gene therapy. Neuropathol Appl Neurobiol. 2011;37:600–12. doi: 10.1111/j.1365-2990.2011.01159.x. [DOI] [PubMed] [Google Scholar]
- 30.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 31.Radtke C, Aizer AA, Agulian SK, Lankford KL, Vogt PM, Kocsis JD. Transplantation of olfactory ensheathing cells enhances peripheral nerve regeneration after microsurgical nerve repair. Brain Res. 2009;1254:10–7. doi: 10.1016/j.brainres.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 32.Scarlato M, Ara J, Bannerman P, Scherer S, Pleasure D. Induction of neuropilins-1 and -2 and their ligands, Sema3A, Sema3F, and VEGF, during Wallerian degeneration in the peripheral nervous system. Exp Neurol. 2003;183:489–98. doi: 10.1016/s0014-4886(03)00046-3. [DOI] [PubMed] [Google Scholar]
- 33.Scarlato M, Xu T, Bannerman P, Beesley J, Reddy UR, Rostami A, Scherer SS, Pleasure D. Axon-Schwann cell interactions regulate the expression of fibroblast growth factor-5 (FGF-5) J Neurosci Res. 2001;66:16–22. doi: 10.1002/jnr.1193. [DOI] [PubMed] [Google Scholar]
- 34.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–40. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor stimulates Schwann cell invasion and neovascularization of acellular nerve grafts. Brain Res. 1999;846:219–28. doi: 10.1016/s0006-8993(99)02056-9. [DOI] [PubMed] [Google Scholar]
- 36.Tarlov IM, Epstein JA. Nerve Grafts - the Importance of an Adequate Blood Supply. Journal of Neurosurgery. 1945;2:49–71. [Google Scholar]
- 37.Tokumine J, Kakinohana O, Cizkova D, Smith DW, Marsala M. Changes in spinal GDNF, BDNF, and NT-3 expression after transient spinal cord ischemia in the rat. J Neurosci Res. 2003;74:552–61. doi: 10.1002/jnr.10760. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Ding F, Gu Y, Liu J, Gu X. Bone marrow mesenchymal stem cells promote cell proliferation and neurotrophic function of Schwann cells in vitro and in vivo. Brain Res. 2009;1262:7–15. doi: 10.1016/j.brainres.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 39.Wilcke I, Lohmeyer JA, Liu S, Condurache A, Kruger S, Mailander P, Machens HG. VEGF(165) and bFGF protein-based therapy in a slow release system to improve angiogenesis in a bioartificial dermal substitute in vitro and in vivo. Langenbecks Archives of Surgery. 2007;392:305–314. doi: 10.1007/s00423-007-0194-1. [DOI] [PubMed] [Google Scholar]
- 40.Wood MD, MacEwan MR, French AR, Moore AM, Hunter DA, Mackinnon SE, Moran DW, Borschel GH, Sakiyama-Elbert SE. Fibrin matrices with affinity-based delivery systems and neurotrophic factors promote functional nerve regeneration. Biotechnol Bioeng. 2010;106:970–9. doi: 10.1002/bit.22766. [DOI] [PubMed] [Google Scholar]
- 41.Yannas IV, Hill BJ. Selection of biomaterials for peripheral nerve regeneration using data from the nerve chamber model. Biomaterials. 2004;25:1593–600. doi: 10.1016/s0142-9612(03)00505-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.