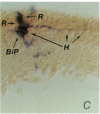
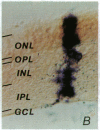
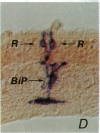
Abstract
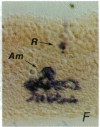
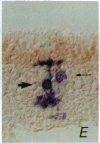
Recombinant retroviruses encoding the histochemically detectable enzyme beta-galactosidase have been used to investigate lineage in the vertebrate nervous system. Identification of the descendants of individual progenitors is straightforward when progeny cells are arranged in a reproducible, clustered pattern, but difficulties in interpretation arise when progeny migrate extensively and/or in an irregular pattern. To better resolve clonal boundaries, additional histochemical marker viruses that engender distinctive reaction products can be used in combination with lacZ-bearing viruses. To this end, we have created a retrovirus vector, DAP, encoding an easily assayable enzyme, human placental alkaline phosphatase. DAP was found to be at least as useful as a lacZ-encoding retrovirus (e.g., BAG) with respect to high viral titer, stability of expression, and in identification of infected cells in vivo. Moreover, it was found to be neutral with respect to postnatal rodent retinal development and offered superior staining characteristics relative to lacZ. Coinfection of rodent retina with DAP and BAG allowed an examination of the clonal nature of radial arrays of labeled retinal cells that previously had been described as products of a single infected progenitor. Of 1100 radial arrays examined for the presence of both DAP- and BAG-infected cells, only 1.2% were the result of infection with more than one virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Hasan N. S., Sutcliffe R. G. Placental alkaline phosphatase integrates via its carboxy-terminus into the microvillous membrane: its allotypes differ in conformation. Placenta. 1985 Sep-Oct;6(5):391–404. doi: 10.1016/s0143-4004(85)80016-3. [DOI] [PubMed] [Google Scholar]
- Austin C. P., Cepko C. L. Cellular migration patterns in the developing mouse cerebral cortex. Development. 1990 Nov;110(3):713–732. doi: 10.1242/dev.110.3.713. [DOI] [PubMed] [Google Scholar]
- BOURNE G. H. Histochemical demonstration of phosphatases in the central nervous system of the rat. Exp Cell Res. 1958;14(Suppl 5):101–117. [PubMed] [Google Scholar]
- Berger J., Hauber J., Hauber R., Geiger R., Cullen B. R. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988 Jun 15;66(1):1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- Berger J., Howard A. D., Gerber L., Cullen B. R., Udenfriend S. Expression of active, membrane-bound human placental alkaline phosphatase by transfected simian cells. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4885–4889. doi: 10.1073/pnas.84.14.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnerot C., Rocancourt D., Briand P., Grimber G., Nicolas J. F. A beta-galactosidase hybrid protein targeted to nuclei as a marker for developmental studies. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6795–6799. doi: 10.1073/pnas.84.19.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Austin C. P., Walsh C., Ryder E. F., Halliday A., Fields-Berry S. Studies of cortical development using retrovirus vectors. Cold Spring Harb Symp Quant Biol. 1990;55:265–278. doi: 10.1101/sqb.1990.055.01.029. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Cepko C. Retrovirus vectors and their applications in neurobiology. Neuron. 1988 Jul;1(5):345–353. doi: 10.1016/0896-6273(88)90184-5. [DOI] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galileo D. S., Gray G. E., Owens G. C., Majors J., Sanes J. R. Neurons and glia arise from a common progenitor in chicken optic tectum: demonstration with two retroviruses and cell type-specific antibodies. Proc Natl Acad Sci U S A. 1990 Jan;87(1):458–462. doi: 10.1073/pnas.87.1.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Blasco L., Harris H. Placental alkaline phosphatase in nonmalignant human cervix. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4226–4228. doi: 10.1073/pnas.77.7.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Rogers C. E., Harris H. Expression of alkaline phosphatase loci in mammalian tissues. Proc Natl Acad Sci U S A. 1980 May;77(5):2857–2860. doi: 10.1073/pnas.77.5.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Rogers C., Harris H. Evolution of alkaline phosphatases in primates. Proc Natl Acad Sci U S A. 1982 Feb;79(3):879–883. doi: 10.1073/pnas.79.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. E., Glover J. C., Majors J., Sanes J. R. Radial arrangement of clonally related cells in the chicken optic tectum: lineage analysis with a recombinant retrovirus. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7356–7360. doi: 10.1073/pnas.85.19.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn P., Zervos P., Raducha M., Harris H., Kadesch T. Expression of a human placental alkaline phosphatase gene in transfected cells: use as a reporter for studies of gene expression. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6342–6346. doi: 10.1073/pnas.85.17.6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. M., Blau H. M. Migration of myoblasts across basal lamina during skeletal muscle development. Nature. 1990 May 24;345(6273):350–353. doi: 10.1038/345350a0. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kam W., Clauser E., Kim Y. S., Kan Y. W., Rutter W. J. Cloning, sequencing, and chromosomal localization of human term placental alkaline phosphatase cDNA. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8715–8719. doi: 10.1073/pnas.82.24.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll B. J., Rothblum K. N., Longley M. Nucleotide sequence of the human placental alkaline phosphatase gene. Evolution of the 5' flanking region by deletion/substitution. J Biol Chem. 1988 Aug 25;263(24):12020–12027. [PubMed] [Google Scholar]
- Korman A. J., Frantz J. D., Strominger J. L., Mulligan R. C. Expression of human class II major histocompatibility complex antigens using retrovirus vectors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2150–2154. doi: 10.1073/pnas.84.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber S. M., Breedlove S. M., Sanes J. R. Lineage, arrangement, and death of clonally related motoneurons in chick spinal cord. J Neurosci. 1990 Jul;10(7):2451–2462. doi: 10.1523/JNEUROSCI.10-07-02451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin M. B., Pearlman A. L., Sanes J. R. Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus. Neuron. 1988 Oct;1(8):635–647. doi: 10.1016/0896-6273(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Millán J. L. Molecular cloning and sequence analysis of human placental alkaline phosphatase. J Biol Chem. 1986 Mar 5;261(7):3112–3115. [PubMed] [Google Scholar]
- NEALE F. C., CLUBB J. S., HOTCHKIS D., POSEN S. HEAT STABILITY OF HUMAN PLACENTAL ALKALINE PHOSPHATASE. J Clin Pathol. 1965 May;18:359–363. doi: 10.1136/jcp.18.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posen S., Cornish C. J., Horne M., Saini P. K. Placental alkaline phosphatase and pregnancy. Ann N Y Acad Sci. 1969 Oct 14;166(2):733–744. doi: 10.1111/j.1749-6632.1969.tb46431.x. [DOI] [PubMed] [Google Scholar]
- Price J. Retroviruses and the study of cell lineage. Development. 1987 Nov;101(3):409–419. doi: 10.1242/dev.101.3.409. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R. Analysing cell lineage with a recombinant retrovirus. Trends Neurosci. 1989 Jan;12(1):21–28. doi: 10.1016/0166-2236(89)90152-5. [DOI] [PubMed] [Google Scholar]
- Stoker A. W., Bissell M. J. Quantitative immunocytochemical assay for infectious avian retroviruses. J Gen Virol. 1987 Sep;68(Pt 9):2481–2485. doi: 10.1099/0022-1317-68-9-2481. [DOI] [PubMed] [Google Scholar]
- TEWARI H. B., BOURNE G. H. Histochemical studies on the distribution of alkaline and acid phosphatases and 5-nucleotidase in the cerebellum of rat. J Anat. 1963 Jan;97:65–72. [PMC free article] [PubMed] [Google Scholar]
- Turner D. L., Cepko C. L. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987 Jul 9;328(6126):131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Turner D. L., Snyder E. Y., Cepko C. L. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990 Jun;4(6):833–845. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Walsh C., Cepko C. L. Clonally related cortical cells show several migration patterns. Science. 1988 Sep 9;241(4871):1342–1345. doi: 10.1126/science.3137660. [DOI] [PubMed] [Google Scholar]
- Zoellner H. F., Hunter N. Histochemical identification of the vascular endothelial isoenzyme of alkaline phosphatase. J Histochem Cytochem. 1989 Dec;37(12):1893–1898. doi: 10.1177/37.12.2584695. [DOI] [PubMed] [Google Scholar]