Abstract
Registering and combining anatomical components from different image modalities, like MRI and CT that have different tissue contrast, could result in patient-specific models that more closely represent underlying anatomical structures.
In this study, we combined a pair of CT and MRI scans of a pig thorax to make a tetrahedral mesh and compared different registration techniques including rigid, affine, thin plate spline morphing (TPSM), and iterative closest point (ICP), to superimpose the segmented bones from the CT scan on the soft tissues segmented from the MRI. The TPSM and affine-registered bones remained close to, but not overlapping, important soft tissue.
Simulation models, including an ECG forward model and a defibrillation model, were computed on generated multi-modality meshes after TPSM and affine registration and compared to those based on the original torso mesh.
1. Introduction
Generating image based models for simulation can be difficult due to limitations of different image modalities. One reason is the differing tissue contrast between image modalities that can affect anatomical representation in models. For example, magnetic resonance imaging (MRI) has high soft tissue contrast but lacks bone contrast to robustly segment bones for registration. X-ray computed tomography (CT), however, has high bone contrast, but the heart, lungs, and blood volume can be hard to distinguish.
Combined-modality models, using both MRI and CT, could more closely represent anatomical features for use in simulation. Specifically, it could advance applications of cardiac modeling like the electrocardiography (ECG) inverse and forward problems [1] or the placement of Implantable Cardiac Defibrillators (ICDs) [2] given the close proximity of the sternum, ribcage and bones to the heart.
This study therefore aimed to generate mutli-modality, tetrahedral meshes for cardiac simulations by superimposing CT bones of an adult pig into an MRI of a different adult pig specimen. Registration techniques including thin plate spline morphing (TPSM), affine, rigid, and iterative closest point (ICP) [3] were quantitively and qualitatively compared for this purpose using error metrics like the Dice coefficient [4, 5], Hausdorff distance [6], root mean square (RMS) error, and amount of tissue overlap.
The registered CT bones of the highest quality were then used to create combined-modality meshes for ECG and ICD simulation. Given same simulation parameters and electrode placement, simulation results were compared between the generated multi-modality meshes and a mesh generated solely from the MRI.
2. Methods
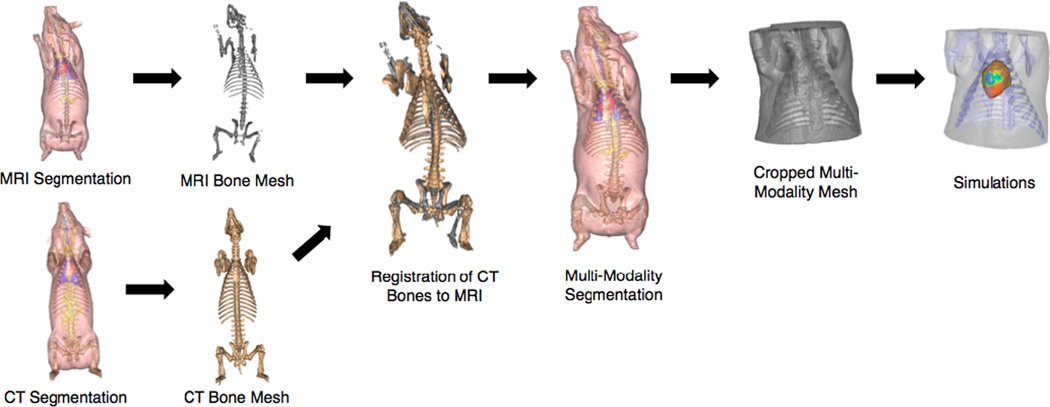
A generation pipeline for multi-modality, tetrahedral meshes was developed (Figure 1) using software for the Center for Integrate Biomedical Computing software.
Figure 1.
A general pipeline for generating combined image-based tetrahedral meshes for simulation.
2.1. Preliminary Mesh Generation
Two adult pigs were scanned, one with MRI and the other with CT. The image data were segmented separately into 11 different tissue types using Seg3D. Tetrahedral meshes were then created using Cleaver2 of the primary tissues layers of torso, heart, lungs, blood, and bones.
2.2. Image Registration
A total of 70 correspondence points were selected from the entire porcine torso for image registration using affine, TPSM, and rigid techniques. A subset of 37 points was subsampled from the original set with use of a Ransac algorithm [7–9] implemented within MatLab. Both affine and TPSM techniques were then performed within SCIRun, and rigid registration was performed within Mat- Lab. ICP [3] registration was also implemented within MatLab on the torso surface meshes.
Each registration technique was evaluated quantitively within MatLab. Error metrics include the DICE coefficient [4, 5], Hausdorff distance [6] and RMS error. The amount of both tissue and bone overlap was also calculated. Additionally the superimposed CT Bones were qualitatively compared against the original MRI bone mesh.
2.3. Multi-Modality Mesh Generation
The highest quality registered CT bones were superimposed into the original MRI thorax within Seg3D. It was ensured that the CT bones did not intersect directly through important tissue layers including the heart and lungs. In Seg3D, the combined segmentations were cropped within the transverse plane to just above the neck and below the rib cage. Tetrahedral meshes were then created from the cropped, combined-modality segmentations using Cleaver 2.
2.4. ICD and ECG Simulations
ECG and ICD Simulations were performed on the affine multi-modality, TPSM multi-modality, and original MRI meshes. For the ECG model [1], recorded, epicardial potentials from a canine heart were mapped onto the heart surface of the MRI by means of registration. Using the Forward/Inverse Toolbox in SCIRun, the torso surface potentials were solved for and error metrics of percent error, correlation coefficient, and RMS error were calculated within MatLab. The ICD simulations [2] were performed by first placing the can and coil electrodes within each model using modular software within SCIRun. Orientation and placement remained constant. Defibrillation thresholds and the torso potential distribution were then calculated using SCIRun.
3. Results
3.1. Registration
Quantitative error metrics for registration are displayed in Table 1. Comparatively, the TPSM-registered bones have the smallest tissue overlap, highest bone overlap, highest Dice coefficient, and a lower RMS error. Though rigid registration exhibited the lowest Hausdorff distance, the method also had the highest tissue overlap and lowest bone overlap. Both ICP and affine registration quantitatively preformed similarly, though affine has a higher Dice coefficient and higher bone overlap while ICP has a lower RMS error and tissue overlap.
Table 1.
Error metrics for different registration techniques.
| Error Metric | TPSM | Affine | Rigid | ICP |
|---|---|---|---|---|
| Dice Coefficient | 0.23 | 0.11 | 0.07 | 0.10 |
| Hausdorff Distance (mm) | 165 | 172 | 118 | 150 |
| RMS Error (mm) | 25.3 | 32.8 | 31.8 | 23.8 |
| Bone Overlap (%) | 39.0 | 18.6 | 13.2 | 16.4 |
| Tissue Overlap (%) | 3.5 | 6.1 | 7.9 | 4.9 |
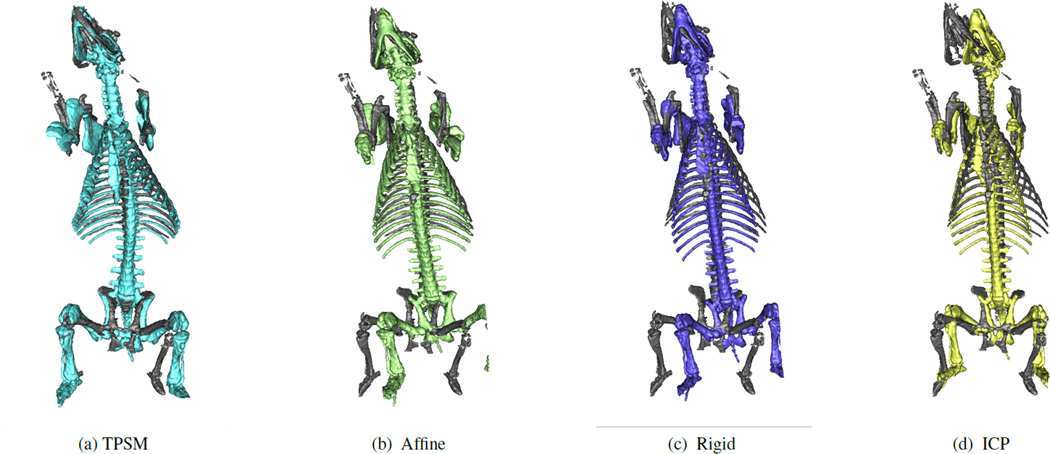
Qualititive comparison indicates that both TPSM (Figure 2a) and affine (Figure 2b) follow the general spinal, sternum and ribcage curvature and remain close to, but not overlapping important soft tissue. Both the rigid (Figure 2c) and ICP (Figure 2d) registration show noticeable deviation from the MRI bones, especially along the spine, leading to overlap with the heart and lungs.
Figure 2.
CT Bones registered using TPSM, rigid, affine, and ICP techniques compared to the original MRI Bones.
3.2. Simulations
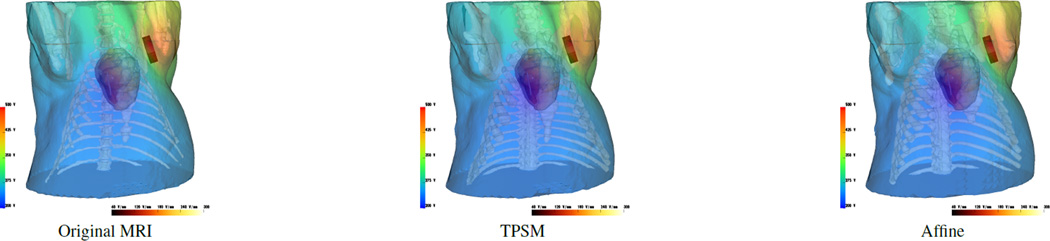
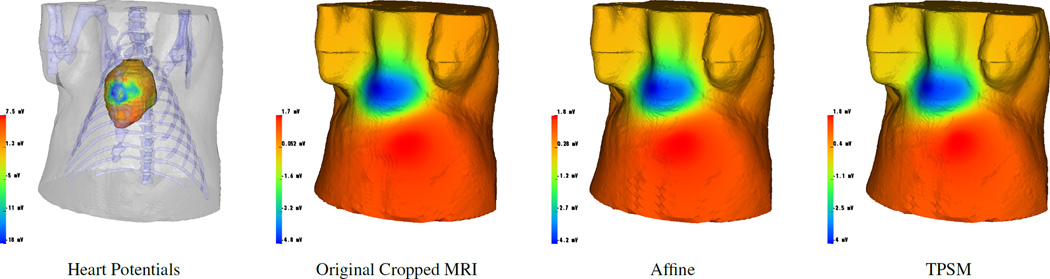
Both multi-modality meshes performed similarly to the original MRI mesh in the ICD and ECG simulations. The ICD simulations (Figure 3) yielded defibrillation thresholds of 8.1 J, 8.2 J, and 9.7 J for the original MRI, affine mutli-modality, and TPSM mutli-modality meshes, respectively. The correlation coefficient, RMS error, and percent error for the affine multi-modality mesh was 0.98, 53 V, and 3.9 %. The TPSM mutli-modality mesh had metric values of 0.98, 57 V, and 4.5%, respectively. The ECG simulations (Figure 4) resulted in a correlation coefficient, RMS error, and percent error of 0.98, 0.16 mV and 3.7% for the TPSM multi-modality mesh and 0.98, 0.17 mV, and 4.1% for the affine multi-modality mesh.
Figure 3.
ICD defibrillation discharge potentials of the original torso mesh (first) compared to the affine (second) and TPSM (third) composite meshes given the same ICD placement and an initial 500 V shock.
Figure 4.
ECG Forward simulations show heart surface potentials (first) and corresponding surface potential estimation on the original torso mesh (second), affine composite mesh (third), and TPSM composite mesh (fourth).
4. Discussion
The tetrahedral meshes generated, using both TPSM and affine registration techniques, were of high enough quality to use in simulated applications that compare in performance to the original MRI Mesh (Figures 3 and 4). Other registration techniques could also be effective for generating multi-modality meshes given correct spinal curvature in the region of the heart and lungs is attained with minimal soft tissue overlap (Figure 2). Additionally, registration techniques could be used sequentially. For example, preliminary data suggests that TPSM could be followed by ICP to get further refinement of the registered CT Bones. Furthermore, performing registration on cropped, localized meshes instead of the entire specimen could lead to better error metrics with less soft tissue overlap, especially around the domain of the heart.
In general, taking advantage of each modality's strengths by generating mutli-modality meshes is feasible. The pipeline we developed (Figure 1) can be used to generate torso geometries from different modalities, like MRI or CT, for simulations regardless of differing specimens. Patient specific models could subsequently be created using an arbitrary CT scan of an alternative patient, thus limiting radiation exposure and improving clinical use.
Acknowledgments
This project was supported by the National Institute of General Medical Sciences of the National Institutes of Health under grant number P41 GM103545-17.
References
- 1.Burton B, Tate J, Erem B, Swenson D, Wang D, Brooks D, van Dam P, MacLeod R. A toolkit for forward/inverse problems in electrocardiography within the scirun problem solving environment. IEEE Eng. in Med. and Biol. Soc. 2011 doi: 10.1109/IEMBS.2011.6090052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jolley M, Stinstra J, Pieper S, MacLeod R, Brooks DH, Cecchin F, Triedman JK. A computer modeling tool for comparing novel ICD electrode orientations in children and adults. Heart Rhythm. 2008;5(4):565–572. doi: 10.1016/j.hrthm.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besl P, McKay N. A method for registration of 3-D shapes. IEEE Trans Pat Anal Mach Intellig. 1992;14(2):239–256. [Google Scholar]
- 4.Sorenson T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on danish commons. Biol Skr. 1948;5:1–34. [Google Scholar]
- 5.Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;27:297–302. [Google Scholar]
- 6.Huttenlocher DP, Klanderman GA, Rucklidge WJ. Comparing images using the hausdorff distance. IEEE Trans Pat Anal Mach Intellig. 1993;15:850–863. [Google Scholar]
- 7.Fischler MA, Bolles RC. Random sample consensus: A paradigm for model fitting with applications to image analysis and automated cartography. Communications of the ACM. 1981;24:381–395. [Google Scholar]
- 8.Zuliani M. Ransac toolbox for matlab. 2008 Nov; [web page] http://www.mathworks.com/matlabcentral/fileexchange/18555. [Google Scholar]
- 9.Zuliani M. Ransac for dummies. Technical report. 2008 Nov [Google Scholar]