Abstract
Vertebrate MOXD2 encodes a monooxygenase DBH-like 2 protein that could be involved in neurotransmitter metabolism, potentially during olfactory transduction. Loss of MOXD2 in apes and whales has been proposed to be associated with evolution of olfaction in these clades. We analyzed 57 bird genomes to identify MOXD2 sequences and found frequent loss of MOXD2 in 38 birds. Among the 57 birds, 19 species appeared to have an intact MOXD2 that encoded a full-length protein; 32 birds had a gene with open reading frame-disrupting point mutations and/or exon deletions; and the remaining 6 species did not show any MOXD2 sequence, suggesting a whole-gene deletion. Notably, among 10 passerine birds examined, 9 species shared a common genomic deletion that spanned several exons, implying the gene loss occurred in a common ancestor of these birds. However, 2 closely related penguin species, each of which had an inactive MOXD2, did not share any mutation, suggesting an independent loss after their divergence. Distribution of the 38 birds without an intact MOXD2 in the bird phylogenetic tree clearly indicates that MOXD2 loss is widespread and independent in bird lineages. We propose that widespread MOXD2 loss in some bird lineages may be implicated in the evolution of olfactory perception in these birds.
Introduction
MOXD2 encodes a monooxygenase dopamine β-hydroxylase (DBH)-like 2 protein, and highly orthologous proteins are found in vertebrates [1, 2]. MOXD2 and its paralogs, MOXD1 and DBH, are members of a copper type II, ascorbate-dependent monooxygenase family, which was formed by sequential duplication during bilaterian evolution [1]. DBH is involved in the conversion of dopamine to norepinephrine (noradrenaline) in postganglionic sympathetic neurons, and its malfunction is implicated in a wide range of neuropsychiatric disorders [3–5]. It is likely that vertebrate MOXD2 is also involved in neurotransmitter metabolism, potentially during olfactory transduction, because mouse ortholog Moxd2 is highly expressed in the medial olfactory epithelium [6].
Human MOXD2 has a genomic deletion that spans 2 exons, which occurred after humans and chimpanzees diverged [1]. Orangutan MOXD2 has multiple nonsense mutations, and the gene has been completely deleted in gibbons [2]. Primates, especially Old World monkeys and apes, have enhanced visual perception, and they are less dependent on olfactory perception, which might have resulted in diminished olfaction and inactivation of olfaction-related genes [7, 8].
Interestingly, MOXD2 inactivation has also occurred in whales, as evidenced by many disruptive mutations such as small frameshift insertions/deletions, nonsense mutations, and whole-gene deletions [2]. The aquatic lifestyle of whales with vocal communication and sophisticate echolocation may have reduced the dependence on olfaction and led to the reduction in olfactory apparatus and inactivation of olfaction-related genes [9–12]. Therefore, convergent inactivation of MOXD2 in apes and whales could be an outstanding molecular signature of adaptive evolution for ecological and/or behavioral adaptation.
In this study, we examined 57 bird genomes and found widespread and independent loss of MOXD2 in 38 birds. Loss of functional MOXD2 may be associated with the evolution of olfaction in birds.
Materials and Methods
Identification of bird MOXD2 sequences
Bird MOXD2 sequences were identified by BLASTN searches (http://blast.ncbi.nlm.nih.gov/Blast.cgi) of the database for whole genome shotgun (WGS) contigs in the National Center for Biotechnology Information (NCBI). Initially, the chimpanzee MOXD2 cDNA sequence was used as a query to identify bird MOXD2 genomic sequences. Rifleman MOXD2 was chosen as the reference sequence for subsequent bird gene analyses simply because it was the first gene identified to have an intact coding sequence in this study. Pairwise sequence comparison was performed using FASTA (version 36.3.6f) (http://fasta.bioch.virginia.edu/fasta_www2/fasta_down.shtml) [13]. Exonic sequences that matched the corresponding rifleman MOXD2 exons from each bird genome were extracted and concatenated to generate virtual cDNA sequences. When a genomic contig contained only a partial region of an exon, raw WGS data, if available, were detected and retrieved from the NCBI Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra). CAP3 (version date 12/21/07) was used to align and assemble WGS data (http://seq.cs.iastate.edu) [14]. The resulting cDNA sequences were virtually translated into protein sequences. In October 2014, 57 bird genomes were available for analysis.
Comparative sequence analyses
Multiple sequence alignments of exon, cDNA, or protein sequences were performed using MUSCLE (v3.8.31) (http://www.drive5.com/muscle) [15]. Presence of a signal peptide at the N-terminal end of proteins was predicted using the SignalP 4.1 web server (http://www.cbs.dtu.dk/services/SignalP) [16]. Presence of a glycosylphosphatidylinositol (GPI) anchor at the C-terminal end of proteins was predicted using the PredGPI web server (http://gpcr.biocomp.unibo.it/predgpi) [17].
Dotplots were created to identify and visually inspect exon deletions. MOXD2 genomic sequences of the rifleman and other birds were aligned using blastz (version 2003-05-14) (http://www.bx.psu.edu/miller_lab) with default options [18]. The blastz outputs were parsed using an ad hoc perl script to extract matched coordinates that were plotted using gnuplot (version 4.6 patchlevel 4) (http://www.gnuplot.info).
Results
Identification of MOXD2 from 57 bird genomes
We analyzed 57 bird genomes to identify MOXD2. The list and phylogenetic tree for the 57 birds examined in this study are shown in Fig 1. The phylogenetic tree is based on recently published genome data [19]. Among the 57 bird species, 19 appeared to have intact MOXD2 that encoded a full-length protein; 32 had a gene with deleterious mutations and/or exon deletions (21, both point mutations and exon deletions; 10, only point mutations; and 1, only an exon deletion); and 6 species did not yield any MOXD2 sequence, suggesting a complete gene deletion. Mutations identified in bird MOXD2 genes are listed in Table 1. Detailed information on bird MOXD2 genes, including accession numbers of genomic sequences, coordinates of exons, and cDNA and protein sequences (if available), is provided in S1 Fig.
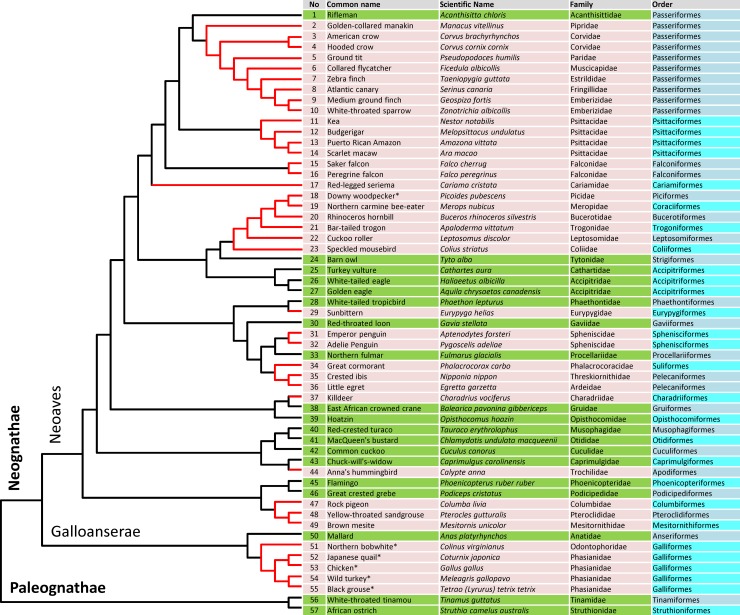
Fig 1. Phylogenetic tree for the birds.
A phylogenetic tree for the 57 birds analyzed in this study is presented. Species with intact MOXD2 are highlighted using a green background. Other species with MOXD2 with disruptive mutations are highlighted using a reddish background, and their branches are in red. Asterisks (*) indicate species that probably underwent complete-gene deletion. The orders of the birds are alternately colored (the last column). Major bird clades are mentioned above the corresponding branches. See S1 Fig for detailed sequence information on bird MOXD2 genes.
Table 1. Summary of mutations in MOXD2 in birds.
| Noa | Species | e1b | e2 | e3 | e4 | e5 | e6 | e7 | e8 | e9 | e10 | e11 | e12 | e13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | Golden-collared manakin | d17, sd | ed | ed | ed | ed | ed | ed | ed | ed | ed | ed | ed | |
| 3 | American crow | d1, ns | tl, d1, d2 | 5d69 | ed | ed | ed | ed | ed | ed | ed | ed | tl, 5d27, d10, sd | 5d54, d1 |
| 4 | Hooded crow | d1, ns | tl, d1, d2 | 5d69 | ed | ed | ed | ed | ed | ed | ed | ed | tl, 5d27, d10, sd | 5d54, d1 |
| 5 | Ground tit | d1, d2 | tl, 5d25, ns, d2 | 5d69 | ed | ed | ed | ed | ed | ed | ed | ed | ed | 5d55 |
| 6 | Collared flycatcher | ns, d1 | tl, ns, ns | 5d64, ns | ed | ed | ed | ed | ed | ed | ed | ed | ed | 5d117 |
| 7 | Zebra finch | d1, i1, ns | tl, ns, d1 | 5d64 | ed | ed | ed | ed | ed | ed | ed | ed | ed | 5d32, 3d20 |
| 8 | Atlantic canary | d1, ns | tl, d1, d1 | 5d64, ns | ed | ed | ed | ed | ed | ed | ed | ed | ed | 5d105, 3d35 |
| 9 | Medium ground finch | d1, ns | tl, ns, d1 | 5d64, ns | ed | ed | ed | ed | ed | ed | ed | ed | ed | 5d105 |
| 10 | White-throated sparrow | d11, d1, ns | tl, ns, d1 | 5d64, d2 | ed | ed | ed | ed | ed | ed | ed | ed | ed | 5d105 |
| 11 | Kea | d5, d5, d1 | ed | 5d37, d8 | sa, i4, i1 | ed | ed | ed | ed | ed | ed | ed | ed | |
| 12 | Budgerigar | d5 | ed | sa, i1 | sa, d7, i1 | ed | ed | ed | sd | ed | ed | ed | ed | ed |
| 13 | Puerto Rican Amazon | d5, ns, ns, d14 | ed | 5d19 | sa, d7, i1 | ed | ed | ed | ed | ed | ed | ed | ed | |
| 14 | Scarlet macaw | d5 | ed | sa, i1 | sa, d7, i1 | ed | ed | ed | ns | ed | ed | ed | ed | ed |
| 15 | Saker falcon | d5, ns, sd | sa | sd | d1 | ns, d1 | ns | sa | ||||||
| 16 | Peregrine falcon | d5, ns, sd | sa | sd | d1 | ns, d1 | ns | sa | ||||||
| 17 | Red-legged seriema | ns | ed | ed | sa, sd | |||||||||
| 18 | Downy woodpecker | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd |
| 19 | Northern carmine bee-eater | d181 | ed | ed | ed | ed | ed | ed | d7 | ns, sd | sa, ns | |||
| 20 | Rhinoceros hornbill | d1 | ||||||||||||
| 21 | Bar-tailed trogon | sa, sd | 5d58, ns, d2 | i7 | 5d4, ns, ns | d10 | sa | i1, ns, 3d21 | ||||||
| 22 | Cuckoo roller | sa, ns | d1 | sd | sa, ns | d2, sd | sa | d2 | d76 | |||||
| 23 | Speckled mousebird | 5d12, d1, sd | sd | d8 | sa, ns, d2 | sa, sd | sa, ns, ns | ed | ed | ns, ns | sa, sd | |||
| 29 | Sunbittern | ed | ed | ns, d7 | i1 | sa, i1 | sa, ns, ns | ed | ed | ed | ed | ed | ed | |
| 31 | Emperor penguin | sa | i7 | sd | ed | ed | ||||||||
| 32 | Adelie Penguin | ns | i2 | |||||||||||
| 34 | Great cormorant | d5, d1, ns | d1 | 5d7, d7 | ns | ns | ns | sd | ns, sd | ns, 3d4 | i1, d11 | |||
| 35 | Crested ibis | d5 | ns, d1 | i1 | d4 | |||||||||
| 36 | Little egret | d5, ns | ns | sa, ns, sd | sa | ns | d7 | i13, d1, ns, sd | ns | d7, d1 | ||||
| 37 | Killdeer | ed | ||||||||||||
| 44 | Anna's hummingbird | ed | ed | d8 | 3d59 | ed | ||||||||
| 47 | Rock pigeon | ns | d1, sd | sd | sd | d20 | sa | |||||||
| 48 | Yellow-throated sandgrouse | d1, ns | ed | i1 | ed | ed | ed | d2 | ||||||
| 49 | Brown mesite | d5 | ed | ed | ns | d1, d8 | ns | ed | ns | sa | sd | ns | ||
| 51 | Northern bobwhite | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd |
| 52 | Japanese quail | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd |
| 53 | Chicken | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd |
| 54 | Wild turkey | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd |
| 55 | Black grouse | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd | gd |
a Species number used in this study. Species without mutations include: 1, rifleman; 24, barn owl; 25, turkey vulture; 26, white-tailed eagle; 27, golden eagle; 28, white-tailed tropicbird; 30, red-throated loon; 33, Northern fulmar; 38, East African crowned crane; 39, hoatzin; 40, red-crested turaco; 41, MacQueen’s bustard; 42, common cuckoo; 43, Chuck-will’s-widow; 45, flamingo; 46, great crested grebe; 50, mallard; 56, white-throated tinamou; and 57, African ostrich.
b Blank indicates no mutation in the given exon; sa, splice acceptor mutation; 5d#, #-nt deletion at the 5′-end; d#, internal deletion of # nt; i#, insertion of # nt; ns, nonsense codon; 3d#, #-nt deletion at the 3′-end; sd, splice donor mutation; ed, exon deletion; tl, translocation; gd, gene deletion.
MOXD2 genes that encoded a full-length protein were identified in 19 bird genomes: rifleman, barn owl, turkey vulture, white-tailed eagle, golden eagle, white-tailed tropicbird, red-throated loon, northern fulmar, East African crowned crane, hoatzin, red-crested turaco, MacQueen’s bustard, common cuckoo, Chuck-will’s-widow, flamingo, great crested grebe, mallard, white-throated tinamou, and African ostrich. Multiple sequence alignment of full-length MOXD2 proteins showed sequence conservation (S2 Fig). These bird MOXD2 proteins were predicted to have a signal peptide and a GPI anchor signal as other previously reported MOXD2 proteins do, suggesting that they are functional [1, 2]. Rifleman (order Passeriformes; species No. 1) MOXD2 was used as the reference sequence in subsequent analyses.
ORF-disrupting point mutations in MOXD2 in 31 bird genomes
Among the 57 bird species, 31 species were identified to have MOXD2 with ORF-disrupting point mutations (Table 1). These species include golden-collared manakin, American crow, hooded crow, ground tit, collared flycatcher, zebra finch, Atlantic canary, medium ground finch, white-throated sparrow, kea, budgerigar, Puerto Rican Amazon, scarlet macaw, saker falcon, peregrine falcon, red-legged seriema, northern carmine bee-eater, rhinoceros hornbill, bar-tailed trogon, cuckoo roller, speckled mousebird, sunbittern, emperor penguin, Adelie penguin, great cormorant, crested ibis, little egret, killdeer, Anna’s hummingbird, rock pigeon, yellow-throated sandgrouse, and brown mesite.
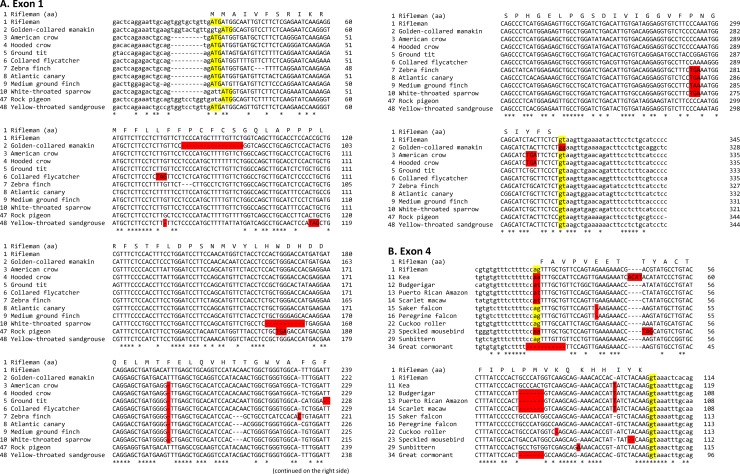
The ORF-disrupting point mutations included splice site mutations, frameshifting small insertions/deletions, and nonsense mutations. Two representative exons (exons 1 and 4) of selected species with such point mutations are shown in Fig 2. Other selected exons with point mutations are presented in S3 Fig. These point mutations are not attributable to sequencing errors; alignments and assemblies of WGS sequences derived from these exons confirmed that the exon sequences were assembled from a large amount of raw sequence data and therefore the mutations were genuine. Partial genomic assemblies that span the selected exons in S3 Fig are presented in S4 Fig.
Fig 2. Disruptive mutations in bird MOXD2.
ORF-disrupting mutations in exon 1 (A) and exon 4 (B) of representative birds are presented. Deleterious mutations, including nonsense codons, insertions, deletions, and splice-site mutations, are highlighted using a red background. Start codons and splice donor and acceptor sequences are highlighted using a yellow background. Amino acid sequences of the rifleman are shown above the DNA sequence alignments. Exonic and intronic sequences are in uppercase and lowercase letters, respectively. See S3 Fig for more disruptive mutations in other exons.
As a representative case, the exon 1 sequences of 12 species (11 selected species with point mutations and the rifleman) are shown in Fig 2A. The rifleman (order Passeriformes) MOXD2 gene, which may encode an intact full-length protein, was used as the reference sequence. Exon 1 of golden-collared manakin (order Passeriformes; species No. 2) MOXD2 showed a 17-nt deletion and a splice donor mutation (gt to gg). The other 8 passerine birds (American crow, hooded crow, ground tit, collared flycatcher, zebra finch, Atlantic canary, medium ground finch, and white-throated sparrow; species Nos. 3 to 10), rock pigeon, and yellow-throated sandgrouse had diverse ORF-disrupting point mutations, including small insertions/deletions, nonsense mutations, and a splice site mutation. Some mutations were shared by closely related species, for example, a 1-nt deletion was common in 8 passerine birds (see species Nos. 3 to 10 in Fig 2A), indicating this mutation occurred in a common ancestor of these birds.
As another representative case, exon 4 sequences of 11 species (10 selected species with point mutations and the rifleman) are shown in Fig 2B. These include 4 parrots (order Psittaciformes; kea, budgerigar, Puerto Rican Amazon, and scarlet macaw), 2 falcons (order Falconiformes; saker falcon and peregrine falcon), cuckoo roller, speckled mousebird, sunbittern, and great cormorant. As in exon 1, a variety of point mutations, including splice site mutations, small insertions/deletions, and nonsense mutations, were observed. Some mutations were shared by closely related species, for example, a 1-nt insertion was common in parrots (species Nos. 11 to 14 in Fig 2B) and a 1-nt deletion was common in falcons (Nos. 15 and 16).
Exon deletions and translocations in MOXD2 in 22 bird genomes
Among the 32 birds with a disrupted MOXD2 gene, 22 were identified to have an exon deletion. When an exon was not present in any genomic contig and its 5′- and 3′-flanking regions were found in a single genomic contig, the missing exon was regarded to be deleted in the bird genome. For example, exons 1 to 10 and 13 of the emperor penguin MOXD2 gene were found in the contig “JMFQ01072246.1” and exons 11 and 12 were not present in this contig or any other contigs, suggesting that a genomic deletion that spanned exons 11 and 12 occurred in this species (S1 Fig, species No. 31). A genomic deletion that removed at least 1 exon was observed in 22 birds (marked as “ed” in Table 1): 21 of them also had at least 1 point mutation in other exons; the killdeer (No. 37) was the only species that had an exon deletion with no point mutation in other exons.
Some exons were identified to be translocated: an exon was considered to be translocated when its 5′- and the 3′-flanking regions were present in a genomic contig with other exons and the given exon itself was found in a different genomic contig. Exon translocation events were identified in MOXD2 in 8 bird genomes (marked as “tl” in Table 1). For example, exons 1, 3, and 13 of the American crow MOXD2 gene were present in the contig “JMFN01085921.1,” while exons 2 and 12 were found in different contigs, “JMFN01029801.1” and “JMFN01085927.1,” respectively (S1 Fig, species No. 3).
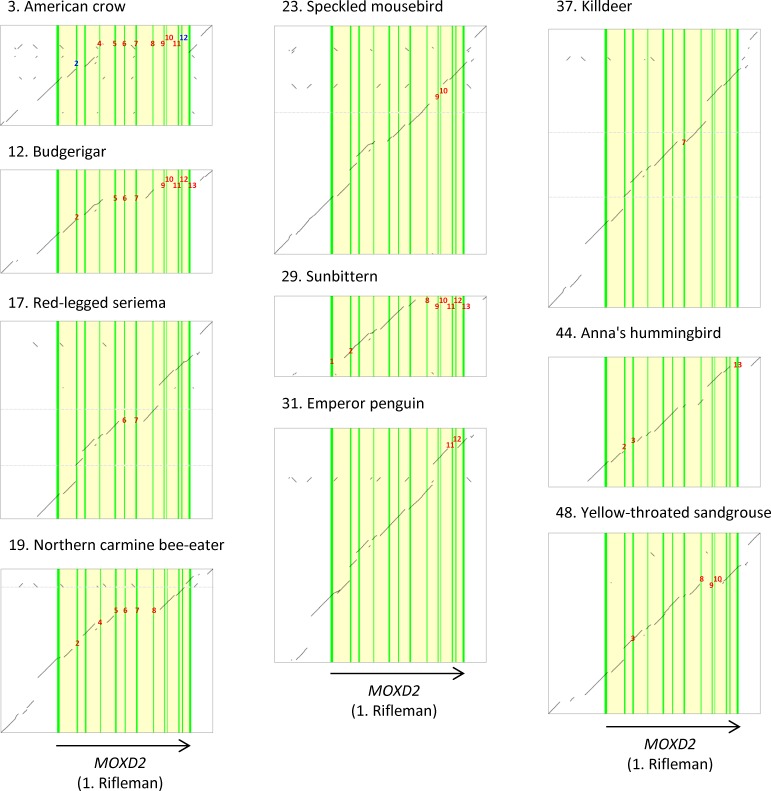
Dotplots between the rifleman MOXD2 genomic sequence and those of each of the 57 birds were produced to confirm and visualize genomic rearrangements that resulted in exon deletions and/or translocations (S5 Fig). Representative dotplots of 10 birds are shown in Fig 3. For example, American crow MOXD2 exhibited a genomic deletion that spanned exons 4 to 11 and two translocations involving exons 2 and 12 (Fig 3, species No. 3). The deletion spanning exons 4 to 11 was common in the 9 passerine birds (species Nos. 2 to 10), suggesting that this deletion occurred in a common ancestor of these birds after the rifleman diverged. The translocation event involving exon 2 was shared with the other 7 passerine birds (species Nos. 4 to 10). Exon 12 translocation was also found in the hooded crow (species No. 4), the closest relative of the American crow.
Fig 3. Examples of exon deletions and translocations in bird MOXD2.
Dotplots between MOXD2 genomic sequences of selected birds (vertical) and that of the rifleman MOXD2 gene (horizontal) are shown. Exonic and intronic segments of rifleman MOXD2 are marked in green and yellow, respectively. Diagonal lines indicate an aligned segment and hence the presence of corresponding genomic segments. Note that some exons are missing in these birds, as evidenced by the lack of a segment aligned with the rifleman MOXD2 exons. Red and blue numbers indicate deleted and translocated exons, respectively. See S5 Fig for dotplots of MOXD2 in other birds.
Possible complete deletion of MOXD2 in 6 bird genomes
In 6 bird genomes, no MOXD2 sequence was detected, raising the possibility of whole-gene deletion (marked as “gd” in Table 1 and with an asterisk in Fig 1). These birds include downy woodpecker, northern bobwhite, Japanese quail, chicken, wild turkey, and black grouse.
The downy woodpecker (species No. 18), which belongs to the order Piciformes, did not show any MOXD2 sequence. It is possible that the lack of the MOXD2 sequence is because of incomplete coverage of the WGS data. However, a sequence similarity search of the downy woodpecker WGS sequences using the MOXD2 genomic contigs of Northern carmine bee-eater, which is the closest species of downy woodpecker in our dataset, as queries, yielded 5 WGS contigs. Dotplot comparisons of downy woodpecker WGS contigs with Northern carmine bee-eater (S6 Fig) or rifleman genomic sequences (S5 Fig, species No. 18) suggested that the whole MOXD2 genomic segment is missing in the downy woodpecker. It is also noteworthy that an almost complete sequence of downy woodpecker MOXD1, a paralog of MOXD2, can be recovered from current genomic sequence data. Therefore, it is likely that the MOXD2 deletion is genuine.
All the other 5 species belong to the order Galliformes, suggesting that the MOXD2 deletion may be the ancestral state. It is also possible that the genomic segment containing the MOXD2 fragment needs to be sequenced. However, the gene is absent even in the chicken, the genome of which has been extensively studied. All these 5 Galliformes bird genomes yielded complete or partial sequences of MOXD1, a paralog of MOXD2, suggesting that the lack of MOXD2 sequences in these genomes may not be because of incomplete sequencing. A region from the chicken chromosome 1 was identified to be orthologous to the mallard genomic contig NW_004676532.1. A dotplot comparison of a 6,000,000-bp-long segment from the chicken chromosome 1 and the 1,916,416-bp-long mallard genomic contig confirmed the complete deletion of MOXD2 gene in the chicken (S7 Fig). Interestingly, the deleted segment was in an inversion boundary, suggesting that MOXD2 gene deletion might have been accompanied by a genomic rearrangement. Therefore, it is highly probable that the MOXD2 deletion is genuine in these 5 Galliformes birds, and it might have occurred in a common ancestor of these birds.
Discussion
Analysis of 57 bird genomes revealed that MOXD2 has been inactivated in 38 birds, as evidenced by ORF-disrupting point mutations, genomic rearrangements that cause exon deletions and/or translocations, or whole-gene deletions. Although in some cases ORF-disrupting mutations might lead to functional modifications which may be neutral or even result in evolution of advantageous phenotypes [20, 21], it is not likely that mutant bird MOXD2 genes produce functional proteins because they have multiple and/or highly disruptive mutations. As shown in Fig 1, 19 birdsn with an intact MOXD2 gene and 38 birds with a disrupted gene were distributed throughout the bird phylogenetic tree, indicating that MOXD2 inactivation is widespread and independent in bird lineages.
In some lineages, mutations were shared by closely related species, implying that the gene-disrupting mutation occurred in a common ancestor of those birds. For example, a genomic deletion that spanned exons 4 to 11 was commonly found in 9 passerine birds (see Table 1, species Nos. 2 to 10), suggesting that the deletion occurred in a common ancestor of these birds after the rifleman diverged. The 4 parrots (see Table 1, Nos. 11 to 14) shared many mutations in MOXD2, including a 5-nt deletion in exon 1, a 1-nt insertion in exon 4 (see Fig 2B), and 3 genomic deletions that spanned exons 2, 5 to 7, and 9 to 13, respectively.
The 6 birds, downy woodpecker, northern carmine bee-eater, rhinoceros hornbill, bar-tailed trogon, cuckoo roller, and speckled mousebird, in which MOXD2 was identified to be inactive, form a single clade, although they belong to different orders (Fig 1, species Nos. 18 to 23). Mutations in MOXD2 in these birds were not common, suggesting that the gene inactivation was of independent origin. The rhinoceros hornbill had only 1 mutation, a 1-nt deletion in exon 7 (S3E Fig), implying that the gene inactivation might have occurred quite recently in this bird. Even in the 2 closely related penguins, the emperor penguin and Adelie penguin, there was no common mutation (Table 1, species Nos. 31 and 32). This suggests that the gene might have become inactivated independently in each penguin lineage. Another possibility is that the gene might become of less or no use in an ancestral penguin species and accumulated different mutations after they diverged around 23 million years ago [22].
Widespread and independent inactivation of MOXD2 in bird lineages implies that this gene might have become generally dispensable during bird evolution. The phenotype associated with the inactivation of MOXD2 in birds has not been identified. MOXD2 was suggested to be involved in olfactory perception in mammals based on its strong expression in the mouse olfactory epithelium, although its molecular function has not yet been determined [6]. Inactivation of MOXD2 was proposed to be associated with diminished olfaction in apes and whales [1, 2]. As in birds, MOXD2 inactivation in apes and whales seemed to have occurred independently in lineages of each clade [1, 2]. The human MOXD2 gene has a genomic deletion that spanned exons 12 and 13, while chimpanzees, bonobos, and gorillas have a gene with intact ORF. Orangutans have a couple of nonsense mutations, while gibbons lost the whole gene by a genomic deletion. Both toothed and baleen whales have MOXD2 with disruptive mutations. However, no common mutation was found between these 2 whale clades [2].
Similar inactivation patterns for sensory perception genes occurred along with ecological habitat shift and/or changes in feeding or communication behaviors. For examples, a large portion of olfactory receptor (OR) genes are inactive in catarrhine primates, possibly because of reduced reliance on olfaction [7, 11]. On the basis of the same reason, TRPC2, which encodes the transient receptor potential cation channel, subfamily C, member 2 protein, a crucial component of pheromone transduction, is inactive in catarrhine primates and whales, and it is frequently inactivated in bats and other aquatic mammals [23–26]. Tas1r2, which encodes a component of the sweet receptor, is inactive in many carnivorous mammals such as cats, spotted hyenas, and seals [27]. It is probable that the sweet receptor became dispensable in these exclusive meat-eaters and accumulated disruptive mutations under absence of selection.
Interestingly, Tas1r2 was found to be deleted in 16 bird genomes [28]. In addition, penguins do not have genes for the umami and bitter taste receptors, probably because they swallow food whole and have no dependence on the taste perception, which might have allowed the loss of these taste receptor genes. Bird genome analysis also revealed that two diet-related genes, AGT and GULO, that encode alanine/glyoxylate aminotransferase and l-gulonolactone oxidase, respectively, had been inactivated independently in some bird lineages: AGT is inactive in the cuckoo roller, American crow, zebra finch, medium ground-finch, and Anna’s hummingbird, while GULO is a pseudogene in the golden-collared manakin, zebra finch, and medium ground finch [29]. MOXD2 seems to be another example of a gene that was independently inactivated during bird evolution.
Recent studies have shown that the olfactory bulb (OB) size and OR gene repertoires in birds are correlated with their ecological adaptations and behavioral characteristics [30, 31]. For example, semi-aquatic birds have relatively larger OBs than terrestrial birds, suggesting that the former rely on olfaction more than the latter. Interestingly, the mallard, an Anseriformes, which inhabits a semi-aquatic environment, has an intact MOXD2 gene, while its close relatives, Galliformes including chicken and turkey that are terrestrial, lost the gene by complete gene deletion. Songbirds (Passeriformes), or vocal-learning species, which more rely on cognitive ability than olfaction, have the smallest OBs and least number of OR genes [30, 31]. As expected, the MOXD2 gene is inactive in all passerine birds. This observation strengthens our notion that loss of MOXD2 gene is associated with evolution of olfactory function in birds although detailed further study is required for a conclusive answer.
In summary, 57 bird genomes were analyzed and widespread and independent losses of MOXD2 were found in 38 birds. Frequent MOXD2 inactivation in some birds may be associated with the evolution of olfaction in these birds depending on their ecological and/or behavioral adaptations.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1B3001513), Republic of Korea. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hahn Y, Jeong S, Lee B. Inactivation of MOXD2 and S100A15A by exon deletion during human evolution. Mol Biol Evol. 2007;24(10):2203–12. 10.1093/molbev/msm146 . [DOI] [PubMed] [Google Scholar]
- 2.Kim DS, Wang Y, Oh HJ, Lee K, Hahn Y. Frequent loss and alteration of the MOXD2 gene in catarrhines and whales: a possible connection with the evolution of olfaction. PLoS One. 2014;9(8):e104085 10.1371/journal.pone.0104085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Combarros O, Warden DR, Hammond N, Cortina-Borja M, Belbin O, Lehmann MG, et al. The dopamine beta-hydroxylase -1021C/T polymorphism is associated with the risk of Alzheimer's disease in the Epistasis Project. BMC Med Genet. 2010;11:162 10.1186/1471-2350-11-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cubells JF, Sun X, Li W, Bonsall RW, McGrath JA, Avramopoulos D, et al. Linkage analysis of plasma dopamine beta-hydroxylase activity in families of patients with schizophrenia. Hum Genet. 2011;130(5):635–43. 10.1007/s00439-011-0989-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timmers HJ, Deinum J, Wevers RA, Lenders JW. Congenital dopamine-beta-hydroxylase deficiency in humans. Ann N Y Acad Sci. 2004;1018:520–3. 10.1196/annals.1296.064 . [DOI] [PubMed] [Google Scholar]
- 6.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101(16):6062–7. 10.1073/pnas.0400782101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton RA. Olfactory evolution and behavioral ecology in primates. Am J Primatol. 2006;68(6):545–58. 10.1002/ajp.20251 . [DOI] [PubMed] [Google Scholar]
- 8.Dong D, He G, Zhang S, Zhang Z. Evolution of olfactory receptor genes in primates dominated by birth-and-death process. Genome Biol Evol. 2009;1:258–64. 10.1093/gbe/evp026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishida T, Kubota S, Shirayama Y, Fukami H. The olfactory receptor gene repertoires in secondary-adapted marine vertebrates: evidence for reduction of the functional proportions in cetaceans. Biol Lett. 2007;3(4):428–30. 10.1098/rsbl.2007.0191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGowen MR, Clark C, Gatesy J. The vestigial olfactory receptor subgenome of odontocete whales: phylogenetic congruence between gene-tree reconciliation and supermatrix methods. Syst Biol. 2008;57(4):574–90. 10.1080/10635150802304787 . [DOI] [PubMed] [Google Scholar]
- 11.Hayden S, Bekaert M, Crider TA, Mariani S, Murphy WJ, Teeling EC. Ecological adaptation determines functional mammalian olfactory subgenomes. Genome Res. 2010;20(1):1–9. 10.1101/gr.099416.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yim HS, Cho YS, Guang X, Kang SG, Jeong JY, Cha SS, et al. Minke whale genome and aquatic adaptation in cetaceans. Nat Genet. 2014;46(1):88–92. 10.1038/ng.2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson WR, Lipman DJ. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988;85(8):2444–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X, Madan A. CAP3: A DNA sequence assembly program. Genome Res. 1999;9(9):868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113 10.1186/1471-2105-5-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–6. 10.1038/nmeth.1701 . [DOI] [PubMed] [Google Scholar]
- 17.Pierleoni A, Martelli PL, Casadio R. PredGPI: a GPI-anchor predictor. BMC Bioinformatics. 2008;9:392 10.1186/1471-2105-9-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz S, Kent WJ, Smit A, Zhang Z, Baertsch R, Hardison RC, et al. Human-mouse alignments with BLASTZ. Genome Res. 2003;13(1):103–7. 10.1101/gr.809403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarvis ED, Mirarab S, Aberer AJ, Li B, Houde P, Li C, et al. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science. 2014;346(6215):1320–31. 10.1126/science.1253451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahn Y, Lee B. Identification of nine human-specific frameshift mutations by comparative analysis of the human and the chimpanzee genome sequences. Bioinformatics. 2005;21 Suppl 1:i186–94. 10.1093/bioinformatics/bti1000 . [DOI] [PubMed] [Google Scholar]
- 21.Hahn Y, Lee B. Human-specific nonsense mutations identified by genome sequence comparisons. Hum Genet. 2006;119(1–2):169–78. 10.1007/s00439-005-0125-6 . [DOI] [PubMed] [Google Scholar]
- 22.Li C, Zhang Y, Li J, Kong L, Hu H, Pan H, et al. Two Antarctic penguin genomes reveal insights into their evolutionary history and molecular changes related to the Antarctic environment. Gigascience. 2014;3(1):27 10.1186/2047-217X-3-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liman ER, Innan H. Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proc Natl Acad Sci U S A. 2003;100(6):3328–32. 10.1073/pnas.0636123100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiselyov K, van Rossum DB, Patterson RL. TRPC channels in pheromone sensing. Vitam Horm. 2010;83:197–213. 10.1016/S0083-6729(10)83008-0 . [DOI] [PubMed] [Google Scholar]
- 25.Yu L, Jin W, Wang JX, Zhang X, Chen MM, Zhu ZH, et al. Characterization of TRPC2, an essential genetic component of VNS chemoreception, provides insights into the evolution of pheromonal olfaction in secondary-adapted marine mammals. Mol Biol Evol. 2010;27(7):1467–77. 10.1093/molbev/msq027 . [DOI] [PubMed] [Google Scholar]
- 26.Zhao H, Xu D, Zhang S, Zhang J. Widespread losses of vomeronasal signal transduction in bats. Mol Biol Evol. 2011;28(1):7–12. 10.1093/molbev/msq207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang P, Josue J, Li X, Glaser D, Li W, Brand JG, et al. Major taste loss in carnivorous mammals. Proc Natl Acad Sci U S A. 2012;109(13):4956–61. 10.1073/pnas.1118360109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao H, Li J, Zhang J. Molecular evidence for the loss of three basic tastes in penguins. Curr Biol. 2015;25(4):R141–2. 10.1016/j.cub.2015.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang G, Li C, Li Q, Li B, Larkin DM, Lee C, et al. Comparative genomics reveals insights into avian genome evolution and adaptation. Science. 2014;346(6215):1311–20. 10.1126/science.1251385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corfield JR, Price K, Iwaniuk AN, Gutierrez-Ibanez C, Birkhead T, Wylie DR. Diversity in olfactory bulb size in birds reflects allometry, ecology, and phylogeny. Front Neuroanat. 2015;9:102 10.3389/fnana.2015.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan I, Yang Z, Maldonado E, Li C, Zhang G, Gilbert MT, et al. Olfactory Receptor Subgenomes Linked with Broad Ecological Adaptations in Sauropsida. Mol Biol Evol. 2015;32(11):2832–43. 10.1093/molbev/msv155 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.