Abstract
Objective
Evaluate the feasibility, acceptability, and indicators of preliminary efficacy of the pilot of a parent-focused, phone-based intervention to improve glycemic control and parental and child well-being in young children newly diagnosed with type 1 diabetes (T1D).
Methods
Thirty mothers of young children ages 1–6 diagnosed with T1D for less than 6 months were randomized to either a phone-based intervention or physical activity education comparison program. Child HbA1c and parent report of depressive symptoms, stress, social support, and child quality of life were assessed at baseline, 1, 6, and 12 months post intervention.
Results
The program was feasible, as the majority of participants completed more than 80% of the intervention or comparison education sessions and reported high levels of satisfaction. Overall, there was a significant time by treatment intervention where the intervention group demonstrated improved social support and quality of life over time as compared to the comparison education group. The intervention demonstrated a trend towards moderating the association between baseline maternal depressive symptoms and prospective worsening of HbA1c.
Conclusions
Parents of young children newly diagnosed with T1D can be engaged in a phone-based program to provide support during this vulnerable period.
Keywords: Type 1 diabetes, young children, newly diagnosed, parent intervention
Type 1 diabetes (T1D) is a lifelong metabolic disorder that affects 1 out of every 400–600 American children each year. The incidence of T1D is on the rise (Dabelea et al., 2014) and although children are most often diagnosed between the ages of 10–14 years old, recent trends indicate that children are being diagnosed at significantly younger ages (Dahlquist, Nystrom, & Patterson, 2011; Patterson et al., 2009), with many new diagnoses now occurring in children under age 5 (Dabelea et al., 2007).
Adherence to a complex and time-consuming daily medical regimen is required to delay or prevent the onset of acute and chronic T1D-related complications (Bade-White & Obrzut, 2009; Silverstein et al., 2005). Until 2015, the American Diabetes Association (ADA) had outlined specific BG level and glycemic control goals for young children when the standards of care were made more universally applicable to all children (HbA1c <7.5%; American Diabetes Association, 2015). A recent examination of a large T1D registry found that 73% of youth ages 1–6 failed to meet glycemic targets with the new guidelines applied (Wood et al., 2013). Early intervention closer to diagnosis may positively impact the glycemic trajectory.
Parenting Challenges During the Newly Diagnosed Period
Following diagnosis, parents and young children must adapt to the diagnosis of a chronic disease with significant implications for their health and quality of life (Sundberg, Sand, & Forsander, 2015; Whittemore, Jaser, Chao, Myoungock, & Grey, 2012). Parents report being in a constant state of vigilance (Niedel, Traynor, McKee, & Grey, 2012; Sullivan-Bolyai, Deatrick, Gruppuso, Tamborlane, & Grey, 2003), and must rapidly become expert in their child’s T1D management while simultaneously teaching others about proper care (Sullivan-Bolyai & Lee, 2011).
The newly diagnosed period also increases the risk of parental psychological difficulties, including increased prevalence of post-traumatic stress-like symptoms (Landolt et al., 2002; Landolt, Vollrath, Ribi, Gnehm, & Sennhauser, 2003) and depression (Streisand et al., 2008). Maternal depressive symptoms may be particularly relevant as they have been associated with poorer diabetes self-care and glycemic control into adolescence (Mackey et al., 2014). Qualitative research has found that parents of children newly diagnosed with T1D value increased general support and education around diabetes management (Monaghan, Sanders, et al., 2011) and specific social support from parents who have experience managing T1D (Rearick, Sullivan-Bollyai, Bova, & Knafl, 2011). It is possible that provision of support during the newly diagnosed period may prevent future parental stress and poor glycemic control (Northam, Anderson, Adler, Werther, & Warne, 1996).
Management of T1D in Young Children
Young children exhibit increased insulin sensitivity and susceptibility to hypoglycemia as well as potential long-term neuropsychological effects, contributing to daily BG management challenges and parent stress (Desrocher & Rovet, 2004; Golden, Russell, Ingersoll, Gray, & Hummer, 1985; McNally, Raymond, Swift, Hearnshaw, & Burden, 1993; Silverstein et al., 2005; Svensson, Eriksson, & Dahlquist, 2004). Young children are often unable to reliably detect and/or report emerging symptoms of hypoglycemia (Sullivan-Bolyai et al., 2002). Parents may have difficulty discriminating between behavioral cues signifying a low or high BG level and normal developmental (mis)behavior (Wysocki, Huxtable, Linscheid, & Wayne, 1989), which can interfere with proper T1D management (Hilliard, Monaghan, Cogen, & Streisand, 2011).
T1D pervades nearly all of children’s activities, including play, meals, sleep, parent and sibling interactions, peer relationships, and school/daycare interactions, each of which affect and are affected by T1D (Estrada, Danielson, Drum, & Lipton, 2012; Hatton, Canam, Thorne, & Hughes, 1995; Linm, Mu, & Lee, 2008; Smaldone & Ritholz, 2011). Eating (Cathey & Gaylord, 2004), daytime naps and nighttime BG monitoring (Monaghan, Herbert, Cogen, & Streisand, 2012; Monaghan, Hilliard, Cogen, & Streisand, 2009), unpredictable activity patterns, and finding appropriate/safe childcare are all examples of the impact of T1D on children’s lives. This impact of a T1D diagnosis on daily life highlights the potential need for parents of young children with T1D for additional support and guidance in these areas of diabetes management and parenting. Therefore, these areas were all included in the current intervention in order to provide specific guidance and support to parents of young children newly diagnosed with T1D.
Current Study
Despite the need for additional support and intervention for parents of young children newly diagnosed with T1D, there are few existing behavioral interventions specifically for this population. Two published studies examining parent interventions provided soon after diagnosis demonstrated decreased maternal distress (Hoff et al., 2005) and increased perceived support and decreased family burden (Sullivan-Bolyai et al., 2004). Another published study investigating a behavior plus nutrition intervention for parents of young children with T1D demonstrated improved BG levels and decreased child and parent problematic mealtime behavior (Patton, Odar, Midyett, & Clements, 2014). However, most behavioral interventions, as well as cross-sectional descriptive studies in youth with T1D, typically limit study inclusion to children and adolescents diagnosed for a minimum of 6 to 12 months (Murphy, Rayman, & Skinner, 2006). Although this may assist research methodologies by enhancing internal validity, the findings of the majority of behavioral research in T1D are untested in the newly diagnosed period.
The current study examined the feasibility and acceptability of a phone-based pilot intervention among parents of young children immediately following diagnosis of T1D. A secondary focus examined preliminary efficacy of the intervention. It was hypothesized that: 1) recruiting families during the initial period after diagnosis would be feasible, 2) administering the intervention would be feasible and participants would evidence a high intervention completion rate (the majority completing at least 80% of sessions), 3) the intervention would be found acceptable and meaningful by participants and peer parents, and 4) there would be preliminary evidence of efficacy regarding decreased maternal depressive symptoms, decreased parenting stress, improved quality of life, and increased perceived social support. Given the difficulty assessing glycemic control during the honeymoon period (i.e., HbA1c may be artificially low and then appear to increase significantly due to the end of the honeymoon period rather than a change in diabetes management), we completed a secondary analysis hypothesizing that there would be decreased HbA1c among intervention group participants as compared to education condition participants. Literature has demonstrated consistent relations between depressive symptoms and child glycemic control, thus; associations among maternal depressive symptoms and change in HbA1c were also examined in the intervention and education comparison groups.
Method
Participants
The current study was approved by the appropriate Institutional Review Boards. Primary caregivers (hereafter referred to as parents) of a young child (ages 1–6 years) newly diagnosed with T1D (within the last 6 months) were recruited from 2 tertiary diabetes care sites and enrolled in a pilot randomized controlled trial (RCT) to promote parental management of T1D. Parents who were fluent in written and spoken English and did not have a developmental disability, and whose young child with T1D did not have another major chronic illness or developmental disability were eligible.
Procedure
Following diagnosis, the study team was informed of upcoming clinic visits and parents were contacted by letter and telephone to assess eligibility. Interested participants provided verbal consent and completed baseline questionnaires by telephone with a trained study team member. Parents then met with one of the study counselors (either BA or PhD level in psychology) for an ‘orientation’ session, during which parents provided written informed consent and the study procedures were discussed. Parents were also asked to relate how their family was adjusting to the child’s diagnosis and indicate current T1D-related challenges as part of a structured interview to establish rapport and obtain information relevant to the intervention. Medical chart reviews were conducted at this time. Parents were then randomized to either the intervention group (up to 4 phone calls with a trained peer parent plus 5 telephone sessions with a trained PhD-level phone counselor focused on parental support of diabetes management) or the comparison group (5 telephone sessions focused on physical activity in young children with a trained BA-level phone counselor). Participants in both groups completed telephone-based follow up assessments at 1, 6, and 12 months post session completion, led by a research team member who was not the participant’s telephone counselor; medical chart reviews from the T1D clinic appointment closest to the telephone assessment were also completed. Parents received a modest gift card of incrementally higher amounts for data collection completion. Children received a diabetes ‘goodie bag’ at the orientation session and a set of 5 children’s books following completion of the parents’ telephone sessions.
Intervention
The Young Child-Newly Diagnosed (YC-ND) intervention was telephone-based and provided 5 sessions with a telephone counselor and 4 calls from a trained peer parent for support. For the 5 sessions with the phone counselor, content was adapted from our initial Young Child Project, which is described in depth in previously-published articles (Monaghan, Hilliard, Cogen, & Streisand, 2011; Monaghan et al., 2011) and provided developmentally tailored education, cognitive behavioral strategies to support parents in their daily management of their child’s diabetes, and parenting strategies related to young children with T1D. Given the range of development in children of participants (from preschoolers to kindergartners), time during Session 1 was spent discussing the child’s age-appropriate level of development and related expectations regarding diabetes management tasks; developmental considerations were incorporated into future sessions as well. Session 1 also included discussion of positive thinking, and the use of breathing as a stress reduction practice. Session 2 focused on glycemic goals and the strategy of problem solving. Session 3 incorporated various behavioral parenting strategies targeting eating, sleep, and behavior. Session 4 was a group phone call with other participants facilitated by a phone counselor to provide further social support for parenting a young child with T1D. Session 5 included a review of prior calls and discussion of the importance of parental self-care and well-being. The comparison group received 5 phone calls regarding physical activity and general child safety while being active. One of the 5 phone calls discussed diabetes and exercise safety, the other 4 calls focused on general topics such as injury prevention, bicycle helmet use, and supervision on playgrounds.
Given that parent mentors have been used successfully in prior interventions with mothers of young children with T1D (Sullivan-Bolyai et al., 2004), the model was also used in the current intervention. However, previous research has not found that simply the provision of parent mentors demonstrates efficacy for improving diabetes outcomes (Sullivan-Bolyai et al., 2010), so this portion was an adjunct to the primary pilot phone-based intervention delivered by a trained interventionist. Trained peer parents (PPs) were assigned to each participant and were instructed to call the participant 4 times, with one call following each of the first 4 intervention phone sessions with the phone counselor. Participants and PPs were informed that contact by the PP was optional, based on the participant’s preference. PPs were four mothers who had participated in the initial Young Child Project, with children who were now slightly older than children of study participants. Three were Caucasian; one was African-American. The average age at which PPs’ children were diagnosed with T1D was 3.72 years, and at the time of PPs’ participation, children had been diagnosed with T1D for a mean of 4.87 years. PPs received a group training, during which they provided informed consent, and received a training manual. PPs followed a general script and were trained to refer all medical questions back to the family’s diabetes team. PPs also completed training in ethical research and received regular phone contact with the project coordinator. PPs completed a follow up survey via REDCap (Harris et al., 2009) after each contact with an intervention participant, and a debriefing phone interview at the completion of the project. PPs received modest gift card compensation.
Measures
Demographic and medical questionnaire
Parents completed a general and medical information questionnaire including insulin regimen and date of diagnosis which were used for the current study; the medical information included on this form was also corroborated via medical record review.
Center for Epidemiological Studies- Depression
The frequency of parents’ depressive symptoms was measured using the Center for Epidemiological Studies- Depression (CES-D) scale, a 20-item self-report measure to assess the frequency of depressive symptoms during the previous week (Radloff, 1977). Items are rated on a 4-point Likert scale (possible range of 0 to 60). Higher scores indicate greater frequency of depressive symptoms. The CES-D has acceptable reliability and convergent validity with clinician ratings of depression (Radloff, 1977; Weissman, Sholomskas, Pottenger, Prusoff, & Locke, 1977). Internal consistency for the current sample was good (α = .91).
Pediatric Inventory for Parents
Parenting stress was assessed via the Pediatric Inventory for Parents (PIP), a 42-item parent self-report rating of stress associated with caring for a child with a medical illness (Streisand, Braniecki, Tercyak, & Kazak, 2001). Items are rated according to both the item’s frequency over the last week and level of difficulty associated with it. A total frequency and difficulty score are generated: higher scores indicate greater pediatric parenting stress. The PIP has been shown to be reliable in diabetes samples (α = .80–.96) (Lewin et al., 2005; Logan, Radcliffe, & Smith-Whitley, 2002; Preston et al., 2005; Streisand & Mednick, 2006). The difficulty subscale was used in the current analysis (α = .93 for current sample).
Pediatric Quality of Life Inventory
The Pediatric Quality of Life Inventory (PedsQL), Generic Core was used to obtain parent’s report of their child’s health-related quality of life (Varni, Burwinkle, Katz, Meeske, & Dickinson, 2002; Varni, Seid, & Rode, 1999). Subscales for the Generic Core scale include physical, emotional, social, and academic functioning and are reliable and valid (α = .86–.90) (Varni et al., 2003; Varni, Seid, & Kurtin, 2001). The total score for the Generic Core was used for the current study for both the younger and older child versions of the form. Internal consistency for the current sample was acceptable (α = .87 for older children, α = .70 for younger children).
Multidimensional Scale of Perceived Social Support
Parental perceptions of social support were measured with the Multidimensional Scale of Perceived Social Support (MSPSS) (Zimet, Dahlem, Zimet, & Farley, 1988), a 12-item self-report scale regarding respondents’ support from family, friends, and a significant other. Higher scores indicate better perceived social support. The MSPSS has good internal and test-retest reliability and adequate construct validity. Internal consistency for the total score has been high (α = .88), as has test-retest reliability (r = .85). Perceived support from family and friends has been significantly negatively related to symptoms of depression (Zimet et al., 1988). Internal consistency for the current sample was also excellent (α = .97).
Project Acceptability
Parental satisfaction with the intervention and perception of success of the intervention was assessed as part of the 1-month post telephone session completion follow up interview. A team member who was not the participant’s telephone counselor asked parents about their level of satisfaction with the program, relevance to their experiences, how informative they found it, how much it impacted their parenting, and how much it impacted their diabetes management. Parents in the intervention group responded to questions regarding their satisfaction with the PP component as well. The majority of questions were on a 5-point Likert scale (0 = Not at all to 4 = Extremely So); other questions were rated as Yes/No or open-ended.
Peer Parent Post-Session Questionnaire
PPs completed a follow up questionnaire that was developed by the research team after each phone call with an intervention participant via REDCap. Questions assessed the length of the call, the topics that were discussed, and whether or not participants discussed the topics targeted by their phone counselors. This was used to evaluate the content of the conversation and evaluate fidelity.
Peer Parent Project Acceptability
Three PPs completed phone interviews with a research team member upon completion of the project. Interview topics included their experiences as a PP, including the training process, what it was like working with the parents, if/how they benefited from participating as PPs, and how to improve the experience for them. Interviews lasted approximately 30 minutes.
Glycemic control
Hemoglobin A1c (HbA1c) is the most widely accepted measure of glycemic control and provides an average glucose level from the past 2–3 months (American Diabetes Association, 2013). HbA1c values were obtained during routine clinical care at each recruitment site. All assays were conducted with the DCA 2000 Analyzer and used high performance liquid chromatography to assure comparability between subjects (Tamborlane et al., 2005). HbA1c values were obtained by medical chart review from the clinic visit closest to the date of each assessment point from baseline to twelve months post-telephone session completion.
Data Analytic Plan
To examine feasibility and acceptability, descriptive and open-ended data were reviewed and summarized. In the secondary analyses examining preliminary efficacy, a repeated measures MANOVA using time and treatment group, as well as the time × treatment interaction, was used to predict outcome variables using baseline, 1, 6, and 12 month data. Multiple imputation (MI) was applied to handle missing values. MI has been shown to produce adequate results in the presence of high rates of missing data, and its performance is robust to small sample size and departure from normality assumptions (Rubin, 1987; Graham et al., 1997; Graham & Schafer, 1999; Schafer & Graham, 2002; Wayman, 2003). Missing values were imputed from Markov Chain Monte Carlo (MCMC) simulations. MI assumes missing at random (MAR), which is a much weaker assumption compared to the often assumed missing completely at random (MCAR). Importantly, MAR allows missingness to be dependent on observed variables (e.g., intervention assignment) (Arbuckle, 1996; Little & Rubin, 2002). To capture the uncertainty in missing value imputations, MI estimates the values multiple times (10 time in this study). The repeated MANOVA was performed separately on each data set, and parameter estimates were averaged over the set of analyses, and standard errors were computed using the average of the standard errors over the set of analyses and the between analysis parameter estimate variation (Rubin, 1987; Schafer, 1997).
To evaluate the hypothesis that the intervention moderated the association between maternal depressive symptoms and worsening of HbA1c over the first year following diagnoses, chi-square analyses were conducted using intervention/education comparison status, meeting criteria for clinically significant depressive symptoms, and change in HbA1c over time (each recoded into a dichotomous variable by computing the change in HbA1c from 3 months post-baseline to 12 month follow up and then coding decrease in HbA1c up to .1% increase as maintaining/improving and increase in ≥ .1% as worsening).
Results
Demographics
Participants were 30 mothers (M age = 33.64, 70% Caucasian) of young children ages 1–6 diagnosed with T1D for less than 6 months (child M age = 4.49, 50% female, M HbA1c = 8.28%). See Table 1.
Table 1.
Demographic/Medical Information (n = 30)
| Percentage | M | SD | Range | |
|---|---|---|---|---|
| Child age (years) | 4.49 | 1.71 | 1.33–6.92 | |
| Child sex (% female) | 50.00 | |||
| Parent age (years) | 33.64 | 6.66 | 20.14–48.57 | |
| Parent sex (% female) | 100.00 | |||
| Parent ethnicity (% Caucasian) | 70.00 | |||
| Secondary caregiver in the home (% yes) | 70.00 | |||
| Household income (% ≥$50,000) | 60.70 | |||
| Illness duration (years) | 0.23 | 0.10 | 0.05–0.48 | |
| Baseline HbA1c | 8.28% | 0.96 | 6.20–10.90 | |
| Insulin regimen (% intensive) | 50.00 |
Feasibility/Engagement
Letters detailing the study were mailed to 67 parents, with 16 unable to be reached and 6 determined to be ineligible. Of 45 parents deemed to be eligible and reached to query participation, 36 agreed to participate (80%) and 30 parents (67%) completed baseline data collection and the orientation session, and were randomized in the pilot RCT. Twenty-five parents (83%) completed at least 4 of the 5 phone sessions, 23 parents (77%) completed the 1 month follow-up data point, 21 parents (70%) completed the 6 month follow-up data point, and 21 parents (70%) completed follow-up at 12 months post phone sessions. These data are across both the intervention and education comparison groups as both had 5 telephone sessions and 3 follow up data points. See Figure 1. Phone calls for the intervention group ranged from 25–80 minutes (M = 52.87 min, SD = 8.71 min). Phone calls for the education comparison group ranged from 11–34 minutes (M = 18.47 minutes; SD = 3.94 minutes).
Figure 1.
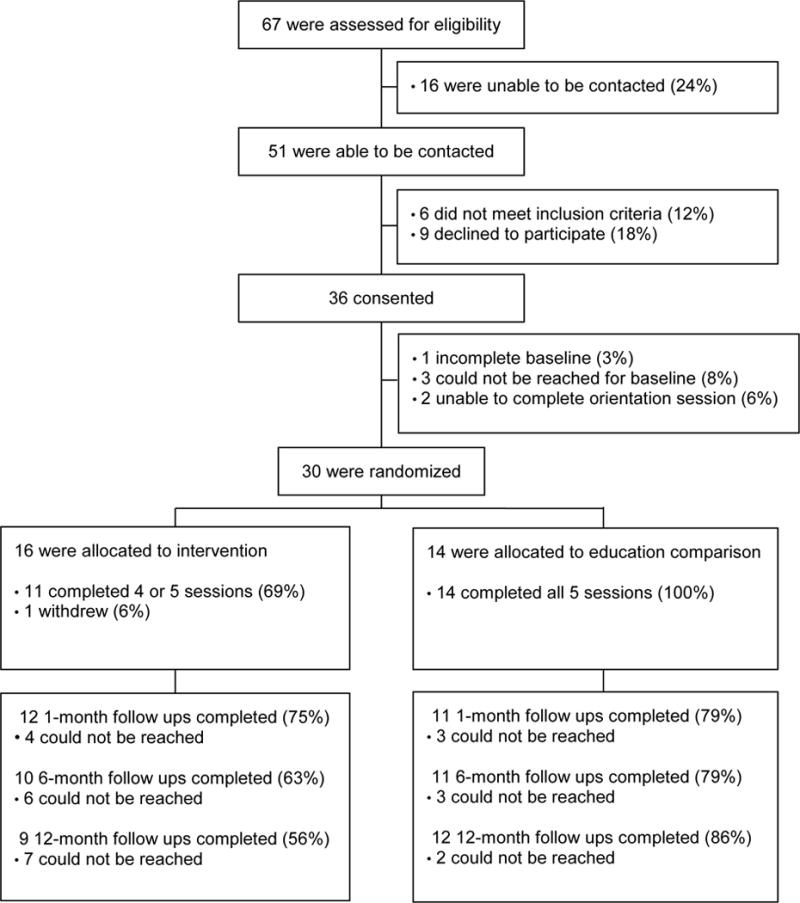
CONSORT Table
Acceptability
There was a high level of acceptability among both intervention and education comparison parents (see Table 3). The majority in both groups reported being “very” to “extremely” satisfied with the program (91% of both intervention and education comparison participants). With regard to parental perception of relevance of the program to their own experiences, 92% of intervention parents and 82% of education comparison parents reported that the program was either “very” or “extremely” relevant to their experience. Regarding how informative the program was found to be, 75% of intervention parents and 64% of education comparison parents reporting that it was either “very” or “extremely” informative.
Table 3.
Acceptability Ratings (percentage of participants in Intervention/Education Comparison (EC) endorsing each satisfaction rating)
| 0 Not at All |
1 A Little |
2 Somewhat |
3 Very |
4 Extremely |
|
|---|---|---|---|---|---|
| Program Satisfaction
|
|||||
| Intervention (N=11) | 0 | 0 | 9.1 | 45.5 | 45.5 |
| EC (N=11) | 0 | 0 | 8.3 | 25.0 | 66.7 |
|
| |||||
| Program Relevance
|
|||||
| Intervention (N=12) | 0 | 8.3 | 0 | 58.3 | 33.3 |
| EC (N=11) | 0 | 0 | 18.2 | 36.4 | 45.5 |
|
| |||||
| How Informative
|
|||||
| Intervention (N=12) | 0 | 8.3 | 16.7 | 41.7 | 33.3 |
| EC (N=11) | 0 | 0 | 36.4 | 27.3 | 36.4 |
|
| |||||
| Impact on Parenting
|
|||||
| Intervention (N=12) | 16.7 | 16.7 | 33.3 | 16.7 | 16.7 |
| EC (N=11) | 0 | 45.5 | 9.1 | 18.2 | 27.3 |
|
| |||||
| Impact on Diabetes Management
|
|||||
| Intervention (N=12) | 25.0 | 8.3 | 33.3 | 25.0 | 8.3 |
| EC (N=11) | 36.4 | 18.2 | 18.2 | 18.2 | 9.1 |
Note: No significant differences between groups on independent samples t-tests (p>.05).
The program was perceived to have an impact on parenting and diabetes management, as many in both groups of parents reported that the program impacted their parenting or diabetes management. Specifically, 33% of intervention parents reported that the program impacted their parenting and diabetes management “somewhat” and 33% reported the program impacted their parenting and diabetes management “very much” or “extremely.” For control parents, 9% reported that the program impacted their parenting “somewhat,” and 46% reported that the program impacted their parenting “very much,” or “extremely.” For control parents, 18% reported that the program impacted their diabetes management “somewhat,” and 27% reported that the program impacted their diabetes management “very much,” or “extremely.” Independent samples t-tests were conducted to determine if the groups differed in their ratings of the program. No significant differences (p > .05) were found on any of the ratings. Regarding PPs, many intervention parents (56%) reported that their PP was “very much so” or “extremely” helpful, and 44% reported that they wished they had even more contact with their PP.
When examining the open-ended question responses, one intervention parent reported that she believed everything in the program was helpful and that she learned new things. Another intervention parent noted that she felt that the program was “comprehensive and broken down into manageable chunks. It’s very applicable.” However, another intervention parent responded that she felt her existing family support and knowledge base decreased the relevance of the program.
PP Results
PP Post-Session Questionnaires
All but 1 intervention participant agreed to be contacted by a PP, and PPs made up to 3 attempts to contact their participant after each of the 4 telephone sessions. Eight intervention participants completed a total of 15 phone calls with a PP. The majority (70%) of PP-participant phone calls lasted for at least 20 minutes. The content of PP calls typically included eating (80%), general adjustment (80%), school (73%), and daily management (66%); sleep, childcare, child behavior, resources, special events, peers, and parent support were also discussed, but less frequently. The content of the phone counselor-led intervention sessions were discussed “somewhat” or “a lot” during over half of the PP calls.
PP Follow-Up Interviews
Three PPs completed follow up interviews about their experience with a research team member. Overwhelmingly they reported that they would like to serve as PPs in future. They reported that their training was sufficient, they appreciated the frequent contact with the project coordinator to discuss participant assignments, and thought the use of an online post-session questionnaire was an appropriate way to provide feedback about each call. PPs also expressed interest in meeting with other PPs in order to debrief and problem solve.
Regarding participant contact, PPs said that some participants were hard to reach, and suggested the use of “office hours” or times when the PP would always be available for calls. Another PP said that it was sometimes awkward to establish rapport without knowing more about families prior to calling, so she suggested having the phone counselor provide introductions for the first call. Finally, PPs said that they had positive experiences. One PP noted, “I always find comfort in helping people believe that it will get better,” and another PP indicated that her experience prompted her to launch her own community-based support group.
Secondary Analyses Regarding Preliminary Efficacy
To examine preliminary efficacy, one repeated measure MANOVA was conducted using time (baseline, 1, 6, and 12 months), treatment group, and the interaction between the two to predict outcomes. Time, treatment group, the interaction of time and treatment group, race, and gender were used to predict HbA1c, CES-D, MSPSS, PedsQL, and PIP-D scores. There were no significant time by treatment interaction findings and therefore only main effects were evaluated. There were no significant effects of time, treatment group, or gender on HbA1c (p>.05). However, there was a significant effect of race, such that non-Caucasian children had higher HbA1c (B= −.18, p<.01). For CES-D, MSPSS, PedsQL, and PIP-D scores, there were no significant effects for time, treatment group, race, or gender (p>.05).
The current sample reported means consistent with previously published samples of mothers of young children with chronic illness with regards to the CES-D (Jaser, Whittemore, Ambrosino, Lindemann, & Grey, 2009), PedsQL (Polloni et al., 2015), PIP-D (Patton, Dolan, Smith, Thomas, & Powers, 2011), and MSPSS (Zimet et al., 1988). As noted earlier, new ADA glycemic guidelines for youth under the age of 19 are HbA1c <7.5%. At baseline, 79% of participants did not meet these guidelines; this was not surprising at baseline given the recent diagnosis and, therefore, only short period of insulin use prior to HbA1c collection. However, there was a similar percentage (75%) of children not meeting the HbA1c target at 12 months post-diagnosis. A chi-square analysis revealed that this finding did not differ by treatment group (χ2(1, N = 28) = 0.43, p = .67). See Table 2 for descriptive information, by group, at each assessment time point.
Table 2.
Descriptive data for variables of interest at each study time point
| Baseline | One Month Follow Up | Six Month Follow Up | Twelve Month Follow Up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| M | SD | Range | M | SD | Range | M | SD | Range | M | SD | Range | |
| HbA1c (%)
|
||||||||||||
| Intervention | 8.31 | 0.90 | 7.00–10.90 | 8.63 | 1.66 | 7.00–13.70 | 8.42 | 0.71 | 7.10–9.60 | 8.61 | 1.11 | 6.90–11.00 |
| Educ Comparison | 8.11 | 0.92 | 6.20–9.70 | 8.33 | 1.24 | 6.40–11.30 | 8.96 | 1.53 | 7.50–13.10 | 8.53 | 1.06 | 7.10–10.20 |
|
| ||||||||||||
| CES-D
|
||||||||||||
| Intervention | 12.00 | 10.27 | 0.00–40.00 | 17.00 | 11.62 | 0.00–41.00 | 9.13 | 6.66 | 1.00–23.00 | 9.38 | 7.32 | 0.00–29.00 |
| Educ Comparison | 14.71 | 8.61 | 3.00–32.00 | 12.54 | 5.94 | 3.00–27.00 | 9.90 | 4.23 | 2.00–17.00 | 11.91 | 10.77 | 2.00–44.00 |
|
| ||||||||||||
| PedsQL
|
||||||||||||
| Intervention | 77.58 | 14.26 | 50.00–96.43 | 78.92 | 13.20 | 55.43–97.50 | 87.80 | 3.71 | 78.62–97.62 | 86.77 | 2.53 | 81.52–91.67 |
| Educ Comparison | 80.11 | 11.15 | 57.61–95.83 | 79.20 | 13.70 | 57.61–97.22 | 80.94 | 8.11 | 61.96–95.65 | 77.91 | 9.44 | 48.91–92.39 |
|
| ||||||||||||
| PIP- Difficulty
|
||||||||||||
| Intervention | 94.66 | 24.36 | 45.00–152.00 | 92.88 | 16.49 | 44.00–116.00 | 78.29 | 15.65 | 43.00–106.00 | 86.80 | 17.63 | 46.00–121.00 |
| Educ Comparison | 85.93 | 24.36 | 47.00–142.00 | 82.11 | 23.34 | 45.00–123.00 | 86.39 | 32.49 | 47.12–186.05 | 84.60 | 29.83 | 43.00–151.00 |
|
| ||||||||||||
| MSPSS
|
||||||||||||
| Intervention | 5.96 | 0.76 | 4.50–7.00 | 5.54 | 1.21 | 1.92–7.00 | 6.30 | 0.53 | 5.17–7.00 | 6.43 | 0.31 | 5.83–7.00 |
| Educ Comparison | 5.62 | 1.38 | 2.83–7.00 | 5.93 | 0.60 | 4.75–7.00 | 5.56 | 1.28 | 1.75–7.00 | 5.63 | 1.44 | 1.00–7.00 |
Note. CES-D = Center for Epidemiological Studies- Depression; PedsQL = Pediatric Quality of Life Inventory; PIP-Difficulty = Pediatric Inventory for Parents- Difficulty Scale; MSPSS = Multidimensional Scale of Perceived Social Support.
To test the hypothesis that participation in the intervention may moderate the association of maternal depression and glycemic control, chi-square analyses were used grouping participants by elevated maternal depression scores at baseline (CES-D ≥ 16) and relative change in HbA1c from 1 month to 12 months following baseline (improving/maintaining or worsening). Analyses were layered by treatment group (intervention/comparison). Eleven mothers across both groups reported depressive symptoms (CES-D ≥ 16) at baseline. This effect varied by treatment group, such that the effect between depressive symptoms and change in HbA1c was not significant for parents in the intervention (χ2(1, N = 5) = 1.25, p = .29). For comparison participants, a trend towards a significant effect was found (χ2(1, N = 6) = 3.90, p = .08); 83% (n = 5) of children with mothers reporting depressive symptoms at baseline demonstrated a worsening in HbA1c over time, as compared to only 40% of children with mothers not reporting depressive symptoms.
Discussion
The current study illustrates that a pilot intervention to support parents of young children newly diagnosed with T1D using parents as peers and a phone-based approach to intervention is feasible, with the majority of participants approached agreeing to participate and completing the majority of study sessions, and appears to be viewed favorably by parents. Recruitment for the current study was highly feasible, as a number of parents reported interest in receiving additional support beyond their standard of care. This is particularly important, as parents of young children newly diagnosed with T1D have many supports as part of standard of care, in terms of group and individual education about diabetes and frequent contact with their medical team. However, despite this relative intensity of contact in standard care during the newly diagnosed period, families still have a need for additional support. The current intervention was completely delivered via telephone with one in-person contact at a regularly scheduled medical clinic appointment. The phone calls were all scheduled at the convenience of the parent. This made both delivery and receipt of the intervention highly feasible and translatable, as it reduces barriers to delivery, such as travel, childcare, and work schedules.
Program satisfaction was high in both intervention and comparison groups, although opinion of the program was higher for those receiving the intervention. A large majority of parents reported satisfaction with the program, relevance to their current struggles, and changes to parenting or diabetes management as a result of their participation. These findings demonstrate that additional support in general, and especially support specific to parenting, emotional adjustment to diabetes, and diabetes management in young children, was well-received among parents. However, it is notable that despite these reported changes, there were no differences in change in HbA1c, demonstrating that although parental perception is important, changes to glycemic control may be significantly harder to impact. Regardless, the use of these strategies in future larger trials is warranted. Similarly, having someone associated with the child’s diabetes team contact parents on a regular basis following diagnosis may have proven helpful-even for parents receiving the educational/physical activity-focused information that was meant to serve as a no-treatment comparison group. This support received by both groups may in part explain our inability to detect differences between the two groups.
The findings of the current study indicate that there were no significant effects of the intervention on parenting stress, depression, social support, quality of life, or HbA1c. It is possible that the study was underpowered to detect these effects and it is also possible that HbA1c levels were affected by the honeymoon period, which can result in more stable glycemic control for up to one year post-diagnosis. Regardless of power, it is possible that the intervention was not effective for depression, parenting stress, or glycemic control in this newly diagnosed sample, whether it is because contact even with a BA-level interventionist during this time period was as helpful as an intervention, or that the intervention itself was not potent enough to affect the outcomes of interest. Regardless of group, maternal depressive symptoms decreased over time. Therefore, it is possible that not all families in the newly diagnosed period required this level of intervention and future research may want to examine different levels of intervention depending on family functioning and glycemic control.
The moderating effect of maternal depressive symptoms was more closely examined because these symptoms may be prominent in the newly diagnosed period, may sustain over time (Mackey et al., 2014), and therefore may have a negative impact on diabetes management across development. The newly diagnosed period therefore represents a unique period to provide additional support in order to buffer the negative effects of maternal depressive symptoms on child glycemic control. Indeed, the current intervention shows promise as children of mothers with elevated depressive symptoms in the intervention group showed no significant deterioration in HbA1c, whereas children of mothers with elevated depressive symptoms in the education comparison group showed worsening of HbA1c. This suggests that the intervention may buffer children of mothers with elevated depressive symptoms from worsening glycemic control. However, these results must be interpreted with caution given the very small sample size and represent an area for future research with larger samples.
A particularly novel feature of the current study was the use of parent peer consultants, which has significant potential for future clinical work and research. Current global guidelines indicate the need to rethink the delivery of mental health services through “task-shifting,” or training lay community members to deliver services (World Health Organization, 2008). This shift enables widespread, low-cost dissemination and has demonstrated efficacy across a number of patient populations, cultures, and presenting problems. Given that the current study found this approach to be both feasible and acceptable from participant and PP perspectives, this may be an important intervention component in future research. However, the targeted 4 contacts per participant were not met, highlighting the difficulty of contacting busy parents by phone. Future studies may want to incorporate other strategies, such as more use of mobile technology or an in-person meeting to facilitate rapport building.
Limitations
A primary limitation of the current study was the small sample size. In order to generalize the findings and to determine efficacy, a larger trial of the program is warranted. Moreover, the newly diagnosed period presents a challenge to examining effects of an intervention on glycemic control, as many children are still experiencing the honeymoon period, and transition out of the honeymoon period occurs at variable rates (Abdul-Rasoul, Habib, & Al-Khouly, 2006; Bowden, Duck, & Hoffman, 2008). Therefore, it is impossible to know whether the intervention affected glycemic control in the short term and future research needs to examine effects on glycemic control across a longer time period after which no participants are in their honeymoon period, as well as other indicators of diabetes management and adherence, such as blood glucose monitoring.
Other study limitations include the fact that the interventionists for the intervention and education comparison groups had different levels of training and credentials; PhD level interventionists conducted intervention sessions whereas BA-level interventionists conducted the comparison group sessions. Future research should control for interventionist qualifications or examine the utility of BA-level interventionists only as a means of improving translatability and cost effectiveness of the intervention. An additional difference between the two groups was the length of the phone calls, with the amount of contact for intervention participants significantly more than for control participants. This difference, rather than the content of the intervention group might have contributed to study findings. Moreover, given that following the newly diagnosed period a number of factors might improve, such as parent stress or maternal depressive symptoms, it may be important to include an additional “usual care” comparison group in order to account for natural change in outcome variables in order to better evaluate the difference between the intervention and comparison groups.
Clinical Applications
The findings of the current study suggest potential areas of clinical application. First and foremost, the parental report of the desire to obtain more support during the newly diagnosed period, the positive response of participants to a phone-based intervention providing more regular contact with a professional, as well as important information regarding diabetes management and parenting in the context of caring for a child with T1D suggests that parents may appreciate additional support from their clinical team during the newly diagnosed period. Although these sessions could not be billed for as is, they could be bundled as part of a larger endocrine team effort to improve outcomes for newly diagnosed or targeted to those with identified need. Additionally, given new directions in billing for telehealth services, this program could be conducted in a telehealth setting, allowing for billing and possibly increasing the potency of the intervention itself, given the face to face contact. Perhaps a universal prevention program that may be administered by peer parents or BA-level trained interventionists may be helpful to all families. Such a program could also offer a more intensive level of intervention indicated for those families at higher risk for difficulties with psychosocial or diabetes outcomes, including presence of maternal depressive symptoms or early difficulty with adherence.
Conclusions
In summary, a pilot intervention delivered via telephone aimed at parents of young children newly diagnosed with T1D was feasible and highly acceptable, despite no evidence of preliminary efficacy. However, the current study found that the intervention may have the potential of serving as a buffer against the impact of maternal depression on poor glycemic control. A larger trial is warranted in order to evaluate broader efficacy. Additionally, future research examining other outcomes, such as health care utilization and cost effectiveness may be warranted in order to evaluate the effect of such interventions on reducing health care usage and cost.
Acknowledgments
This research was supported by NIDDK R01DK080102 awarded to Randi Streisand, PhD.
References
- Abdul-Rasoul M, Habib H, Al-Khouly M. ‘The honeymoon phase’ in children with type 1 diabetes mellitus: Frequency, duration, and influential factors. Pediatric Diabetes. 2006;7(2):101–107. doi: 10.1111/j.1399-543X.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care. 2013;36(S1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes – 2015. Diabetes Care. 2015;38(S1):S70–S76. [Google Scholar]
- Arbuckle JL. Full information estimation in the presence of incomplete data. In: Marcoulides GA, Schumacker RE, editors. Advanced Structural Equation Modeling: Issues and Techniques. Mahwak, NJ: Lawrence Erlbaum Associates; 1996. pp. 243–277. [Google Scholar]
- Bade-White PA, Obrzut JE. The neurocognitive effects of type 1 diabetes mellitus in children and young adults with and without hypoglycemia. Journal of Developmental and Physical Disabilities. 2009;21:425–440. [Google Scholar]
- Bowden S, Duck M, Hoffman R. Young children (<5 yr) and adolescents (>12 yr) with type 1 diabetes mellitus have low rate of partial remission: Diabetic ketoacidosis is an important risk factor. Pediatric Diabetes. 2008;9(3 Pt 1):197–201. doi: 10.1111/j.1399-5448.2008.00376.x. [DOI] [PubMed] [Google Scholar]
- Cathey M, Gaylord N. Picky eating: A toddler’s approach to mealtime. Pediatric Nursing. 2004;30(2):101–106. [PubMed] [Google Scholar]
- Dabelea D, Bell R, D’Agostino RB, Imperatore G, Johansen JM, Linder B, Waitzfelder B. Incidence of diabetes in youth in the United States. Journal of the American Medical Association. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Mayer-Davis E, Saydah S, Imperatore G, Linder B, Divers J, Hamman RF. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. Journal of the American Medical Association. 2014;311(17):1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlquist G, Nystrom L, Patterson C, The Swedish Childhood Diabetes Study Group & The Diabetes Incidence in Sweden Study Group Incidence of type 1 diabetes in Sweden among individual aged 0–34 years, 1983–2007: An analysis of time trends. Diabetes Care. 2011;34(8):1754–1759. doi: 10.2337/dc11-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrocher M, Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychology. 2004;10(1):36–52. doi: 10.1076/chin.10.1.36.26241. [DOI] [PubMed] [Google Scholar]
- Estrada CL, Danielson KK, Drum ML, Lipton RB. Insufficient sleep in young patients with diabetes and their families. Biological Research for Nursing. 2012;14(1):48–54. doi: 10.1177/1099800410395569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden MP, Russell BP, Ingersoll GM, Gray DL, Hummer KM. Management of diabetes mellitus in children younger than 5 years of age. American Journal of Diseases of Children. 1985;139(5):448–452. doi: 10.1001/archpedi.1985.02140070022019. [DOI] [PubMed] [Google Scholar]
- Goonetilleke R, Pollitzer M, Mann N. Insulin for toddlers with difficult diabetes. Diabetes Care. 2004;27(6):1505. doi: 10.2337/diacare.27.6.1505. [DOI] [PubMed] [Google Scholar]
- Graham JW, Hofer SM, Donaldson SI, MacKinnon DP, Schafer JL. Analysis with missing data in prevention research. In: Bryant K, Windle M, West S, editors. The Science of Prevention: Methodological Advances From Alcohol and Substance Abuse Research. Washington, D.C.: American Psychological Association; 1997. pp. 325–366. [Google Scholar]
- Graham JW, Schafer JL. On the performance of multiple imputation for multivariate data with small sample size. In: Hoyle R, editor. Statistical Strategies for Small Sample Research. Thousand Oaks, CA: Sage; 1999. [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DL, Canam C, Thorne S, Hughes AM. Parents’ perceptions of caring for an infant or toddler with diabetes. Journal of Advanced Nursing. 1995;22(3):569–577. doi: 10.1046/j.1365-2648.1995.22030569.x. [DOI] [PubMed] [Google Scholar]
- Hentinen M, Kyngas H. Factors associated with the adaptation of parents with a chronically ill child. Journal of Clinical Nursing. 1998;7(4):316–324. doi: 10.1046/j.1365-2702.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- Herbert L, Clary L, Owen V, Monaghan M, Alvarez V, Streisand R. Relations among school/daycare functioning, fear of hypoglycemica, and quality of life in parents of young children with type 1 diabetes. Journal of Clinical Nursing. 2015;24(9–10):1199–1209. doi: 10.1111/jocn.12658. [DOI] [PubMed] [Google Scholar]
- Hilliard ME, Monaghan M, Cogen FR, Streisand R. Parent stress and child behaviour among young children with type 1 diabetes. Child: Care, Health, and Development. 2011;37(2):224–232. doi: 10.1111/j.1365-2214.2010.01162.x. [DOI] [PubMed] [Google Scholar]
- Jaser SS, Whittemore R, Ambrosino JM, Lindemann E, Grey M. Coping and psychosocial adjustment in mothers of young children with type 1 diabetes. Children’s Health Care. 2009;38(2):91–106. doi: 10.1080/02739610902813229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff AL, Mullins LL, Gillaspy SR, Page MC, Van Pelt JC, Chaney JM. An intervention to decrease uncertainty and distress among parents of children newly diagnosed with diabetes: A pilot study. Families, Systems, & Health. 2005;23(3):329–342. [Google Scholar]
- Landolt MA, Ribi K, Laimbacher J, Vollrath M, Gnehm HE, Sennhauser FH. Posttraumatic stress disorder in parents of children with newly diagnosed type 1 diabetes. Journal of Pediatric Psychology. 2002;27(7):647–652. doi: 10.1093/jpepsy/27.7.647. [DOI] [PubMed] [Google Scholar]
- Landolt MA, Vollrath M, Ribi K, Gnehm HE, Sennhauser FH. Incidence and associations of parental and child posttraumatic stress symptoms in pediatric patients. Journal of Child Psychology and Psychiatry. 2003;44(8):1199–1207. doi: 10.1111/1469-7610.00201. [DOI] [PubMed] [Google Scholar]
- Lewin AB, Geffken GR, Heidgerken AD, Duke DC, Novoa W, Williams LB, Storch EA. The Diabetes Family Behavior Checklist: A psychometric evaluation. Journal of Clinical Psychology in Medical Settings. 2005;12(4):315–322. [Google Scholar]
- Linm H, Mu P, Lee Y. Mothers’ experience supporting life adjustment in children with T1DM. Western Journal of Nursing Research. 2008;30(1):96–110. doi: 10.1177/0193945907302456. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2nd. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- Logan DE, Radcliffe J, Smith-Whitley K. Parent factors and adolescent sickle cell disease: Associations with patterns of health service use. Journal of Pediatric Psychology. 2002;27(5):475–484. doi: 10.1093/jpepsy/27.5.475. [DOI] [PubMed] [Google Scholar]
- Mackey ER, Struemph K, Powell P, Chen R, Streisand R, Holmes C. Maternal depressive symptoms and disease care status in youth with type 1 diabetes. Health Psychology. 2014;33(8):783–791. doi: 10.1037/hea0000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally P, Raymond N, Swift P, Hearnshaw J, Burden A. Does the prepubertal duration of diabetes influence the onset of microvascular complications? Diabetic Medicine. 1993;10:906–908. doi: 10.1111/j.1464-5491.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Monaghan M, Herbert LJ, Cogen FR, Streisand R. Sleep behaviors and parent functioning in young children with type 1 diabetes. Children’s Health Care. 2012;41:246–259. doi: 10.1080/02739615.2012.685385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan M, Hilliard M, Cogen F, Streisand R. Supporting parents of very young children with type 1 diabetes: Results from a pilot study. Patient Education and Counseling. 2011;82(2):271–274. doi: 10.1016/j.pec.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan M, Hilliard ME, Cogen FR, Streisand R. Nighttime caregiving behaviors among parents of young children with type 1 diabetes: Associations with illness characteristics and parent functioning. Families, Systems, & Health. 2009;27(1):28–38. doi: 10.1037/a0014770. [DOI] [PubMed] [Google Scholar]
- Monaghan M, Sanders RE, Kelly KP, Cogen FR, Streisand R. Using qualitative methods to guide clinical trial design: Parent recommendations for intervention modification in Type 1 diabetes. Journal of Family Psychology. 2011;25(6):868–872. doi: 10.1037/a0024178. [DOI] [PubMed] [Google Scholar]
- Murphy H, Rayman G, Skinner T. Psycho-educational interventions for children and young people with Type 1 diabetes. Diabetic Medicine. 2006;23:935–943. doi: 10.1111/j.1464-5491.2006.01816.x. [DOI] [PubMed] [Google Scholar]
- Niedel A, Traynor M, McKee M, Grey M. Parallel vigilance: Parents’ dual focus following diagnosis of type 1 diabetes mellitus in their young child. Health. 2012;17(3):246–265. doi: 10.1177/1363459312451180. [DOI] [PubMed] [Google Scholar]
- Northam E, Anderson P, Adler R, Werther G, Warne G. Psychosocial and family functioning in children with insulin-dependent diabetes at diagnosis and one year later. Journal of Pediatric Psychology. 1996;21(5):699–717. doi: 10.1093/jpepsy/21.5.699. [DOI] [PubMed] [Google Scholar]
- Patterson C, Dahlquist G, Gyurus E, Green A, Soltesz G, EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: A multicentre prospective registration study. Lancet. 2009;373(9680):2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- Patton S, Odar C, Midyett L, Clements M. Pilot study results for a novel behavior plus nutrition intervention for caregivers of young children with type 1 diabetes. Journal of Nutrition Education and Behavior. 2014;45(5):429–433. doi: 10.1016/j.jneb.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton SR, Dolan LM, Smith LB, Thomas IH, Powers SW. Pediatric parenting stress and its relation to depressive symptoms and fear of hypoglycemia in parents of young children with type 1 diabetes mellitus. Journal of Clinical Psychology in Medical Settings. 2011;18(4):345–352. doi: 10.1007/s10880-011-9256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polloni L, Ferruzza E, Ronconi L, Lazzarotto F, Toniolo A, Bonaguro R, Muraro A. Perinatal stress and food allergy: a preliminary study on maternal reports. Psychology, Health, and Medicine. 2015;20(6):732–741. doi: 10.1080/13548506.2014.993406. [DOI] [PubMed] [Google Scholar]
- Preston A, Storch EA, Lewin A, Geffken GR, Baumeister AL, Strawser MS, Silverstein JH. Parental stress and maladjustment in children with short stature. Clinical Pediatrics. 2005;44(4):327–331. doi: 10.1177/000992280504400407. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rearick E, Sullivan-Bolyai S, Bova C, Knafl K. Parents of children newly diagnosed with type 1 diabetes: Experiences with social support and family management. The Diabetes Educator. 2011;37(4):508–518. doi: 10.1177/0145721711412979. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- Silverstein J, Klingensmith G, Copeland K, Plotnick LP, Kaufman F, Laffel L, Clark N. Care of children and adolescents with Type 1 Diabetes: A statement of the American Diabetes Association. Diabetes Care. 2005;28(1):186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- Smaldone A, Ritholz MD. Perceptions of parenting children with type 1 diabetes diagnosed in early childhood. Journal of Pediatric Health Care. 2011;25:87–95. doi: 10.1016/j.pedhc.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisand R, Mackey E, Elliot B, Mednick L, Slaughter I, Turek J, Austin A. Parental anxiety and depression associated with caring for a child newly diagnosed with type 1 diabetes: Opportunities for education and counseling. Patient Education & Counseling. 2008;73(2):333–338. doi: 10.1016/j.pec.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Streisand R, Braniecki S, Tercyak KP, Kazak A. Childhood illness-related parenting stress: The Pediatric Inventory for Parents. Journal of Pediatric Psychology. 2001;26(3):155–162. doi: 10.1093/jpepsy/26.3.155. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Bova C, Leung K, Trudeau A, Lee MM, Gruppuso P. Social Support to Empower Parents (STEP): An intervention for parents of young children newly diagnosed with type 1 diabetes. The Diabetes Educator. 2010;36(1):88–97. doi: 10.1177/0145721709352384. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Deatrick J, Gruppuso P, Tamborlane W, Grey M. Mothers’ experiences raising young children with type 1 diabetes. Journal for Specialists in Pediatric Nursing. 2002;7(3):93–103. doi: 10.1111/j.1744-6155.2002.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Deatrick J, Gruppuso P, Tamborlane W, Grey M. Constant vigilance: Mothers’ work parenting young children with type 1 diabetes. Journal of Pediatric Nursing. 2003;18(1):21–29. doi: 10.1053/jpdn.2003.4. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Grey M, Deatrick J, Gruppuso P, Giraitis P, Tamborlane W. Helping other mothers effectively work at raising young children with type 1 diabetes. Diabetes Educator. 2004;30(3):476–484. doi: 10.1177/014572170403000319. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Lee M. Parent mentor perspectives on providing social support to empower parents. The Diabetes Educator. 2011;37(1):34–43. doi: 10.1177/0145721710392248. [DOI] [PubMed] [Google Scholar]
- Sundberg F, Sand P, Forsander G. Health-related quality of life in preschool children with Type 1 diabetes. Diabetic Medicine. 2015;32(1):116–119. doi: 10.1111/dme.12557. [DOI] [PubMed] [Google Scholar]
- Svensson M, Eriksson J, Dahlquist G. Early glycemic control, age at onset, and development of microvascular complications in childhood-onset type 1 diabetes: A population-based study in northern Sweden. Diabetes Care. 2004;27(4):955–962. doi: 10.2337/diacare.27.4.955. [DOI] [PubMed] [Google Scholar]
- Tamborlane W, Kollman C, Steffes M, Ruedy K, Dongyuan X, Beck R, DirecNet Study Group Comparison of fingerstick hemoglobin A1c levels assayed by DCA 2000 with the DCCT/EDIC central laboratory assay: Results of a Diabetes Reseach in Children Network (DirecNet) study. Pediatric Diabetes. 2005;6(1):13–16. doi: 10.1111/j.1399-543X.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabetes Care. 2003;26(3):631–637. doi: 10.2337/diacare.26.3.631. [DOI] [PubMed] [Google Scholar]
- Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: Reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94(7):2090–2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Varni JW, Seid M, Rode CA. The PedsQL-super: Measurement model for the pediatric quality of life inventory. Medical Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- Wayman JC. Multiple Imputation For Missing Data: What Is It And How Can I Use It?. Paper presented at the 2003 Annual Meeting of the American Educational Research Association; Chicago, IL. 2003. [Google Scholar]
- Weissman M, Sholomskas D, Pottenger M, Prusoff B, Locke B. Assessing depressive symptoms in five psychiatric populations: A validation study. American Journal of Epidemiology. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- Whittemore R, Jaser S, Chao A, Myoungock J, Grey M. Psychological experience of parents of children with type 1 diabetes. The Diabetes Educator. 2012;38(4):562–579. doi: 10.1177/0145721712445216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JR, Miller KM, Maahs DM, Beck RW, DiMeglio LA, Libman IM, Woerner SE. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36(7):2035–2037. doi: 10.2337/dc12-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Task Shifting : Rational Redistribution of Tasks Among Health Workforce Teams : Global Recommendations and Guidelines. Geneva, Switzerland: WHO Document Production Services; 2008. [Google Scholar]
- Wysocki T, Huxtable K, Linscheid TR, Wayne W. Adjustment to diabetes mellitus in preschoolers and their mothers. Diabetes Care. 1989;12(8):524–529. doi: 10.2337/diacare.12.8.524. [DOI] [PubMed] [Google Scholar]
- Zimet G, Dahlem N, Zimet S, Farley G. The multidimensional scale of perceived social support. Journal of Personality Assessment. 1988;52:30–41. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]


