Abstract
Several tissue engineering strategies in the form of protein therapy, gene therapy, cell therapy and its combinations are currently being explored for oral and cranio-facial regeneration and repair. Though each of these approaches has advantages, they all have common inherent drawbacks of being expensive and raising safety concerns. Using RNA (encoding therapeutic protein) has several advantages that have the potential to overcome these limitations. Chemically modifying the RNA improves its stability and mitigates immunogenicity allowing for the potential of RNA to become an alternative to protein and gene based therapies. This brief review article focuses on the potential of RNA therapeutics in the treatment of disorders in the oral and craniofacial regions.
Introduction
In dentistry, protein therapy utilizing growth factors or other proteins are approved for select clinical indications and are currently in clinical use. A commonly employed recombinant protein for craniofacial indications is recombinant human bone morphogenetic protein - 2 (rhBMP-2), which was cleared by the Food and Drug Administration in the United States for select clinical indications in dentistry (Pilipchuk et al., 2015). Growth factors such as recombinant human platelet derived growth factor–BB (rhPDGF-BB) and proteins such as enamel matrix derivative are also available in clinical dentistry for select indications and are used with varying degrees of clinical success (Pilipchuk et al., 2015).
Recombinant human BMP-2 is efficacious in augmenting maxillary sinus in humans (in order to place dental implants) but less effective than the use of autogenous bone (Freitas et al., 2015). Common side effects associated with rhBMP-2 use include significant facial swelling, erythema, edema or sensory loss. In order to compensate for the limited bioavailability of proteins due to short half-lives, growth factors are employed at supraphysiological doses, which can lead to local or systemic complications (Tannoury and An, 2014). Another major drawback of protein therapy is their high manufacturing cost. These drawbacks have led to the exploration of alternative molecular approaches that can overcome these pitfalls. One potential approach is gene therapy but gene therapy strategies using viral and non-viral vectors have their own set of challenges, most importantly safety concerns and lower transfection efficacy, respectively (Kimelman Bleich et al., 2012). Apart from tissue regeneration, there are several other areas such as cancer therapeutics, stem cell biology/cellular reprogramming, salivary gland therapeutics and pain management, where gene therapy is actively explored and they all have oral and craniofacial relevance. In this brief review, we describe one strategy that has the potential to overcome the above said limitations of both viral and non-viral gene therapy and hence has the potential to replace gene therapy in dentistry.
Messenger RNA Therapeutics
The idea of delivering mRNA has gained significant interest over recent years. The concept is very similar to plasmid DNA (pDNA) based therapies but instead of DNA, it’s the RNA that encodes the target protein that is delivered. RNA, upon entry into cells (with or without the aid of vectors) via lipid rafts and scavenger receptors, can get transcribed into the target proteins directly in the cytoplasm, circumventing the need for nuclear entry (Figure 1). Delivering mRNA has other significant advantages over DNA delivery that includes the following (Sahin et al., 2014):
Figure 1.
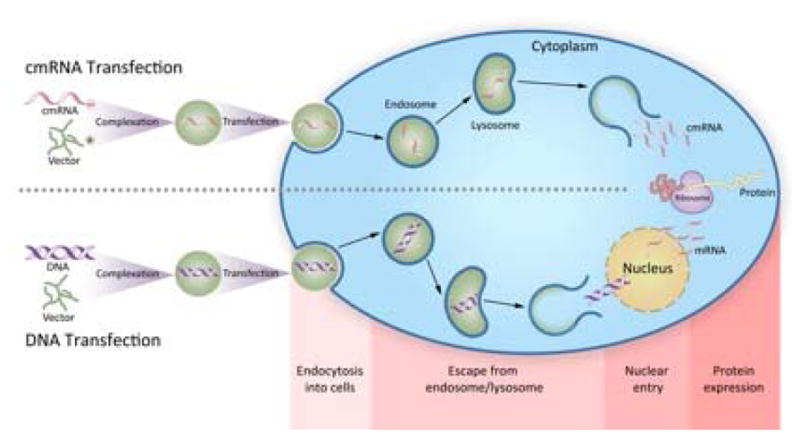
Schematic depicting the molecular mechanism including uptake and the mostly likely release mechanism of cmRNA in RNA based therapeutics in comparison with plasmid DNA based therapeutics.
Nuclear entry is a rate limiting step in DNA therapy but not for RNA therapy.
There is no risk of insertional mutagenesis.
RNA therapy works in non-dividing mammalian cells.
The protein is produced by the cells and therefore undergoes the normal modifications and folding prior to secretion, making it native and non-immunogenic.
mRNA production does not include complex steps and thus represents a powerful molecular means to synthesize intra-cellular proteins.
Major barriers in using mRNA over DNA, include its inherent instability and immunogenicity. RNA undergoes several modifications within the cells that allow them to remain stable and therefore, these modifications are required in vitro before it can be used for clinical applications. In addition, mRNA is highly immunogenic. In the intracellular space, mRNA binds to specific, endosomal Toll-like receptors (7 and 8) as well as certain cytoplasmic receptors and induce a strong inflammatory response. Therefore, modifications are also required in mRNA to mitigate their immunogenic properties. Recent work has shown that the binding affinity of mRNA to innate immune receptors can be reduced (Karikó et al., 2008). Partial substitution of combinations of various nucleotides to more closely mimick those observed in endogenous transcripts, can yield mRNA transcripts with further increased stability (Kormann et al., 2011). For example, a twice weekly local application of cmRNA (surfactant protein-B) in the form of aerosol restored 71% of the wild-type SP-B expression in the mouse model of lethal congenital lung disease, and treated mice survived until the end of the study period (after 28 days) (Kormann et al., 2011). It should be pointed out that the design of chemically modified mRNA (cmRNA) may have substantially different effects in specific organs in vivo.
In recent years, a number of studies have demonstrated the safety and efficacy of mRNA therapeutics for a variety of applications including gene editing (utilizing ZFN mRNA, TALEN mRNA or CRISPR-Cas9 mRNA), cellular reprogramming, allergy toleration, protective RNA vaccination, cancer immunotherapy and protein replacement therapy (Sahin et al., 2014). In a recent study, nuclease-encoding chemically modified mRNA (nec-mRNA, a special form of cmRNA) was described as a novel vehicle for delivering genome-editing components directly to the lung (Mahiny et al., 2015). Using a murine model of SP-B deficiency, nec-mRNA-encoded ZFNs were able to demonstrate the first report of life-prolonging gene correction specifically in the lung tissue (Mahiny et al., 2015).
Almost all of the above studies involve systemic or topical delivery of cmRNA. Though it can be applicable in some domains of dentistry, due to the accessible nature of oral and craniofacial structures, a direct local delivery of cmRNA is far more relevant in this field than a systemic delivery approach.
Potential Applications
Bone Tissue Engineering
Bone tissue engineering strategies typically involve the use of recombinant proteins such as rhBMP-2 (protein therapy), cells (cell therapy), gene encoding growth factors and morphogens, biomimetic scaffolds, and their combinations. Our group successfully demonstrated the application of non-viral gene based delivery system to enhance bone regeneration (Elangovan et al., 2014). We employed a gene activated matrix (GAM) consisting of collagen containing nanoplexes of polyethylenimine (PEI) complexed with plasmid DNA encoding platelet derived growth factor-BB. We demonstrated the superior bone regeneration capacity of GAM in calvarial defects in rats. For example, In vivo studies showed significantly higher new bone volume/total volume (BV/TV) % in calvarial defects treated with the complex-activated scaffolds following 4 weeks of implantation (14- and 44-fold higher) when compared to empty defects or empty scaffolds, respectively. (Elangovan et al., 2014). This study clearly demonstrated the potential of nucleic acid based therapies loaded in collagen matrices for bone regeneration. Due to the inherent advantages with using cmRNA over DNA, our group recently tested the in vivo efficacy of cmRNA encoding BMP-2 for bone regeneration application in rats using the same animal model (Elangovan et al., 2015). This is the first study to explore the use of cmRNA therapeutics for any tissue regeneration applications. In bone marrow stromal cells, we investigated the transfection efficiency, cytotoxicity, osteogenicity and in vivo bone regenerative capacity of cmRNA encoding BMP-2 and complexed with the cationic non-viral vector polyethylenimine (PEI). We also made comparisons with PEI complexed with pDNA encoding BMP-2. The PEI-cmRNA polyplexes were fabricated at an amine (N) to phosphate (P) ratio of 10 and characterized for transfection efficiency using human bone marrow stromal cells (BMSCs). In addition, the expression of bone-specific genes, osteocalcin and alkaline phosphatase was assessed in transfected BMSCs and bone matrix deposition was evaluated to validate the functionality of transfection. In all of the above assessments, we demonstrated the superiority of PEI-cmRNA (BMP-2), when compared to its pDNA counterpart. Using a calvarial bone defect model in rats, we demonstrated the superior bone regeneration capacity of PEI-cmRNA (encoding BMP-2)-activated matrices compared to PEI-pDNA (BMP-2)-activated matrices. Our proof of concept study clearly demonstrated that collagen scaffolds loaded with non-viral vectors complexed to cmRNA encoding BMP-2 is an effective strategy for local bone regeneration (Elangovan et al., 2015).
Periodontal Tissue Engineering
Periodontal tissue engineering involves the application of tissue engineering principles to regenerate the lost periodontium (that comprises of periodontal ligament, bone and cementum) on a previously diseased root surface. As of now, for periodontal tissue engineering, innovative biomimetic scaffolds, protein (growth factor) therapy, cell/gene based approaches and their combinations were explored (Cochran et al., 2015). Scaffolds containing cmRNA encoding a growth factor that is known to have a positive effect on periodontal regeneration such as platelet derived growth factor (PDGF) should be explored as this has the potential to overcome several limitations that exists with current periodontal regenerative strategies.
Cancer Therapeutics
Both pre-clinical as well as human clinical trials have clearly demonstrated the safety and efficacy of cmRNA based immune therapeutics for various forms of cancers. This strategy utilizes cmRNA encoding a specific tumor antigen that can be utilized to stimulate an antigen specific anti-tumor immune response including CD8+ T-cells. For example, mRNA encoding several different antigens including, oncofetal antigen, chimeric antigen, survivin, and melanoma-associated antigen gp 100 has shown promising results in animal studies (Sahin et al., 2014). Several human clinical trials were conducted that systemically delivered cmRNA encoding a cocktail of different tumor specific antigens and demonstrated safety and efficacy in inducing vaccine directed immune cells that led to improved clinical outcomes in patients with a variety of cancers including metastatic melanoma (Weide et al., 2009). Several highly aggressive forms of cancer occur in the head and neck region and oral squamous cell carcinoma is one of the most commonly occurring aggressive cancers. The utility of gene therapy targeting squamous cell carcinoma is currently being investigated in several trials and certainly cmRNA can substitute DNA and possibly enhance the outcomes.
Stem Cell Engineering and Genetic Reprogramming
In 2006, a Nobel prize winning breakthrough by Yamanaka and his team led to the creation of induced pluripotent stem cells (iPS). They showed that transfecting somatic cells such as fibroblasts with a cocktail of four transcription factors (Klf4, c-Myc, Oct3/4, Sox2) led to the conversion of differentiated somatic cells into pluripotent cells (Takahashi and Yamanaka, 2006). Initially viral vectors were utilized to transfect these factors into cells, which was followed by the use of non-viral vectors (Okita et al., 2008). The lower transfection efficiency and a slight possibility of insertional mutagenesis were major limitations that led to several recent in vitro investigations utilizing cmRNA (encoding the required transcription factors) to produce iPS cells from a variety of somatic cells (Sahin et al., 2014; Mandal and Rossi, 2013). CmRNA was shown to be as efficient as viral DNA based approaches in generating iPS with conversion efficiency of more than 2% and took only 17 days for the emergence of embryonic stem cell-like colonies (versus 4 weeks with viral gene delivery approaches (Warren et al., 2010). With several potential and proven applications of iPS in dental research ranging from bone regeneration to whole tooth regeneration, cmRNA has a definite role to play in the near future in these areas (Liu et al., 2014).
Salivary Gland Applications
Several studies were performed in the past to explore direct local gene therapeutics in salivary glands for a range of applications from protein replacement to correction of salivary gland pathologies. Being exocrine in nature, it makes logical sense to transfect the cells in these glands to produce the target protein of interest that can be actively secreted into saliva which can ultimately reach the blood stream (Baum et al., 2002). Range of genes from those encoding growth hormones (such as insulin) to membrane proteins such as aquaporin-5 (Shan Z et al., 2005), has been explored delivered using viral vectors. In addition, gene therapy encoding anti-inflammatory cytokine such as interleukin-27 in mice was able to inhibit the immunological damage seen in auto-immune conditions such as Sjoägren’s syndrome, ultimately improving salivary gland function (Lee et al., 2002). Being easily accessible, direct local delivery of cmRNA encoding the required factors can be successfully employed in salivary glands for the above mentioned applications.
Other Potential Application - Pain Management
Gene therapy offers a potential strategy to eliminate pain, which is a major part of patient management in dentistry. In addition, several patients suffer from severe chronic pain in the oro-facial region. Past studies utilizing viral vectors demonstrated reduction of pain by delivering genes encoding endorphin peptides or opiate peptides (Guedon et al., 2014; Tzabazis et al., 2014; Nasirinezhad et al., 2015). This is another potential area for cmRNA therapeutics in dentistry.
We can safely predict that therapeutic strategies involving cmRNA will have a significant translational impact in oral, dental and craniofacial applications. Our study on the utilization of cmRNA for bone regeneration will hopefully be the starting point for several studies in the dental field to test its safety and efficacy in the important domain of regenerative oral therapeutics and other key areas such as stem cell biology and cancer therapy.
Acknowledgments
This study was supported by NIH R21 grant (1R21DE024206-01A1 to S.E. and A.K.S), the University of Iowa Start-up Grant to S.E., the ITI Foundation for the Promotion of Implantology, Switzerland (ITI Research Grant No. 855 2012 to S.E. and A.K.S.), the Osteology Foundation Grant (12-054 to S.E. and A.K.S.), the Sunstar — American Academy of Periodontology Foundation Research Fellowship to S.E., the Deutsche Forschungsgemeinschaft (DFG KO 4258/2-1, to M.S.D.K.), the European Research Council (ERC Starting Grant “BREATHE”, to M.S.D.K.) and the Lyle and Sharon Bighley Professorship to A.K.S.
References
- Baum BJ, Kok M, Tran SD, Yamano S. The impact of gene therapy on dentistry: a revisiting after six years. J Am Dent Assoc. 2002;133(1):35–44. doi: 10.14219/jada.archive.2002.0019. [DOI] [PubMed] [Google Scholar]
- Cochran DL, Cobb CM, Bashutski JD, Chun YH, Lin Z, Mandelaris GA, McAllister BS, Murakami S, Rios HF. Emerging regenerative approaches for periodontal reconstruction: a consensus report from the AAP Regeneration Workshop. J Periodontol. 2015;86(S2):S153–156. doi: 10.1902/jop.2015.140381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangovan S, D’Mello SR, Hong L, Ross RD, Allamargot C, Dawson DV, Stanford CM, Johnson GK, Sumner DR, Salem AK. The enhancement of bone regeneration by gene activated matrix encoding for platelet derived growth factor. Biomaterials. 2014;35(2):737–747. doi: 10.1016/j.biomaterials.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangovan S, Khorsand B, Do AV, Hong L, Dewerth A, Kormann M, Ross RD, Rick Sumner D, Allamargot C, Salem AK. Chemically modified RNA activated matrices enhance bone regeneration. J Control Release. 2015;218:22–28. doi: 10.1016/j.jconrel.2015.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas RM, Spin-Neto R, Marcantonio E, Pereira LA, Wikesjö UM, Susin C. Alveolar ridge and maxillary sinus augmentation using rhBMP-2: a systematic review. Clin Implant Dent Relat Res. 2015;17(S1):e192–201. doi: 10.1111/cid.12156. [DOI] [PubMed] [Google Scholar]
- Guedon JM, Zhang M, Glorioso JC, Goins WF, Kinchington PR. Relief of pain induced by varicella-zoster virus in a rat model of post-herpetic neuralgia using a herpes simplex virus vector expressing enkephalin. Gene Ther. 2014;21(7):694–702. doi: 10.1038/gt.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16(11):1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman Bleich N, Kallai I, Lieberman JR, Schwarz EM, Pelled G, Gazit D. Gene therapy approaches to regenerating bone. Adv Drug Deliv Rev. 2012;64(12):1320–1330. doi: 10.1016/j.addr.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormann MS, Hasenpusch G, Aneja MK, Nica G, Flemmer AW, Herber-Jonat S, Huppmann M, Mays LE, Illenyi M, Schams A, Griese M, Bittmann I, Handgretinger R, Hartl D, Rosenecker J, Rudolph C. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat Biotechnol. 2011;29(2):154–157. doi: 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- Lee BH, Carcamo WC, Chiorini JA, Peck AB, Nguyen CQ. Gene therapy using IL-27 ameliorates Sjögren’s syndrome-like autoimmune exocrinopathy. Arthritis Res Ther. 2012;14(4):R172. doi: 10.1186/ar3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Zhang Y, Chen S, Cai J, Pei D. Application of iPS cells in dental bioengineering and beyond. Stem Cell Rev. 2014;10(5):663–670. doi: 10.1007/s12015-014-9531-2. [DOI] [PubMed] [Google Scholar]
- Mahiny AJ, Dewerth A, Mays LE, Alkhaled M, Mothes B, Malaeksefat E, Loretz B, Rottenberger J, Brosch DM, Reautschnig P, Surapolchai P, Zeyer F, Schams A, Carevic M, Bakele M, Griese M, Schwab M, Nürnberg B, Beer-Hammer S, Handgretinger R, et al. In vivo genome editing using nuclease-encoding mRNA corrects SP-B deficiency. Nat Biotechnol. 2015;33(6):584–586. doi: 10.1038/nbt.3241. [DOI] [PubMed] [Google Scholar]
- Mandal PK, Rossi DJ. Reprogramming human fibroblasts to pluripotency using modified mRNA. Nat Protoc. 2013;8(3):568–82. doi: 10.1038/nprot.2013.019. [DOI] [PubMed] [Google Scholar]
- Nasirinezhad F, Gajavelli S, Priddy B, Jergova S, Zadina J, Sagen J. Viral vectors encoding endomorphins and serine histogranin attenuate neuropathic pain symptoms after spinal cord injury in rats. Mol Pain. 2015;11:2. doi: 10.1186/1744-8069-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Pilipchuk SP, Plonka AB, Monje A, Taut AD, Lanis A, Kang B, Giannobile WV. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent Mater. 2015;31(4):317–338. doi: 10.1016/j.dental.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- Shan Z, Li J, Zheng C, Liu X, Fan Z, Zhang C, Goldsmith CM, Wellner RB, Baum BJ, Wang S. Increased fluid secretion after adenoviral-mediated transfer of the humanaquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther. 2005;11(3):444–451. doi: 10.1016/j.ymthe.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tannoury CA, An HS. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014;14(3):552–559. doi: 10.1016/j.spinee.2013.08.060. [DOI] [PubMed] [Google Scholar]
- Tzabazis AZ, Klukinov M, Feliciano DP, Wilson SP, Yeomans DC. Gene therapy for trigeminal pain in mice. Gene Ther. 2014;21(4):422–426. doi: 10.1038/gt.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, Pawelec G, Hoerr I, Rammensee HG, Garbe C. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32(5):498–507. doi: 10.1097/CJI.0b013e3181a00068. [DOI] [PubMed] [Google Scholar]


