Abstract
Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency is a common autosomal recessive disorder characterized by impaired cortisol biosynthesis, with or without aldosterone deficiency, and androgen excess. Patients with the classic (severe) form also have epinephrine deficiency. Patients with CAH have an increased prevalence of risk factors for cardiovascular disease including obesity, hypertension and insulin resistance. Androgen excess in women appears to be an additional risk factor for cardiovascular disease. Carotid intima media thickness, a measure of subclinical atherosclerosis also has been found to be increased in adults with CAH. The multiple hormonal imbalances present in the adult woman with CAH, in combination with chronic glucocorticoid therapy, contribute to cardiovascular disease risk. Further investigation of the predisposition to cardiovascular disease in women with CAH is warranted. Longitudinal studies are needed and interventions targeting obesity, insulin resistance, hypertension and hyperandrogenism may offer improved outcome.
Keywords: cardiovascular risk, congenital adrenal hyperplasia, metabolic syndrome, hyperandrogenism
INTRODUCTION
Congenital adrenal hyperplasia (CAH) is an autosomal recessive androgen excess disorder of adrenal origin. Ninety-five percent of cases are due to deficiency of the 21-hydroxylase enzyme1. Classic CAH, the most severe form, is characterized by impaired cortisol and aldosterone secretion, and impaired adrenomedullary function (Figure 1). Females with classic CAH are born with ambiguous genitalia due to exposure to excess androgens in utero. They have adrenal insufficiency, and 75 percent have severe aldosterone deficiency resulting in salt-wasting. These women receive life-time glucocorticoid, and usually, mineralocorticoid therapy. Adult women may present with nonclassic CAH (NCAH), the mild form of the disease, with manifestations of hyperandrogenism such as hirsutism or infertility. The mild impairment in 21-hydroxylase enzyme allows for sufficient cortisol production, but at the expense of androgen excess. Women with NCAH are born with normal genitalia and do not have adrenal insufficiency. They may have no apparent clinical symptoms and their presentation may overlap with polycystic ovarian syndrome (PCOS). Many, but not all, women with NCAH require glucocorticoid therapy.
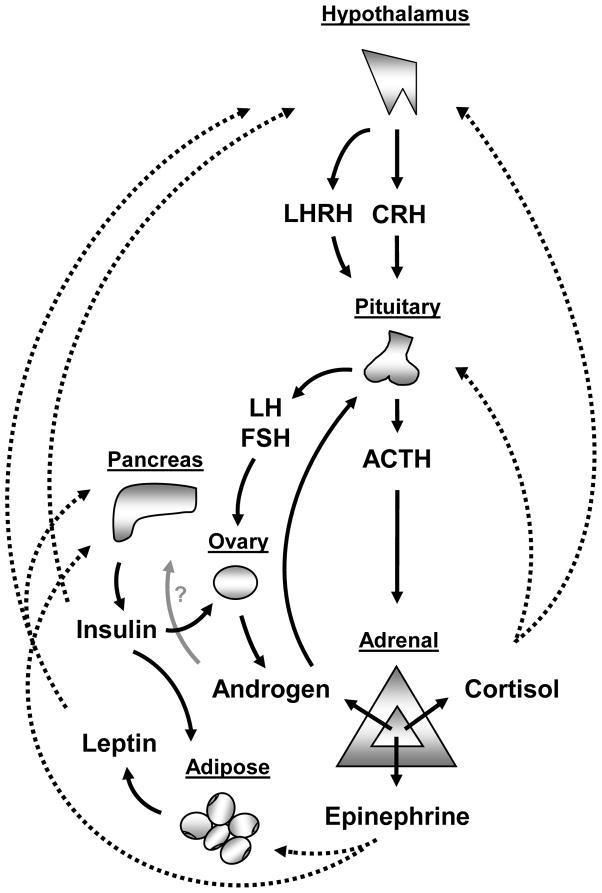
Figure 1.
Schematic representation of the complex interplay between: the hypothalamic-pituitary-adrenal axis, the hypothalamic-pituitary-ovarian axis, the adrenal production of cortisol, epinephrine and androgen, and the regulation of leptin and insulin. These pathways are altered in congenital adrenal hyperplasia.
Little is known about the metabolic long-term consequences in adult women with CAH. However, several cardiovascular disease (CVD) risk factors have been found to be increased in patients with CAH including obesity, hypertension and insulin resistance. PCOS, a more common endocrinopathy characterized by androgen excess in women, has been associated with an unfavorable cardiovascular risk profile. Studies of patients with PCOS suggest that androgen excess in women may be an independent risk factor for CVD2,3. Long-term glucocorticoid therapy and the hormonal imbalances characteristic of CAH play a role in the development of adverse CVD risk in women with CAH.
CVD is a leading cause of morbidity and mortality for women in the U.S. Traditional CVD risk factors include: hypertension, dyslipidemia, age >55 years, current smoker, diabetes, and family history of premature coronary artery disease in a 1st-degree relative (men <55 years, women <65 years)4. Newer CVD risk factors continue to emerge, including metabolic syndrome, insulin resistance, markers of subclinical atherosclerosis, impaired endothelial function, and inflammation. Some of these CVD risk factors have been studied in patients with CAH and others have yet to be studied.
HORMONAL IMBALANCES AND CVD RISK
Hypercortisolism
Patients with CAH are at risk for iatrogenic Cushing syndrome because supraphysiological glucocorticoid doses are often needed to adequately suppress adrenal androgen production1. Cushing syndrome has been found to be associated with adverse risk for CVD5,6. In one study, 74% of patients with Cushing syndrome were overweight or obese, and >60% of patients had at least three CVD risk factors6.
There is no standard glucocorticoid regimen for treating adults with CAH. Adults are treated with a variety of glucocorticoid regimens including short-acting hydrocortisone given twice or thrice daily, or longer-acting forms of glucocorticoid, such as dexamethasone or prednisone7. No form of glucocorticoid replacement is able to mimic the normal circadian rhythm of cortisol secretion8. This nonphysiological replacement might contribute to the development of signs and symptoms of hypercortisolism and the subsequent development of CVD risk factors.
Hyperandrogenism
Androgen excess appears to be a risk factor for CVD in women based on the unfavorable CVD risk profile found in women with hyperandrogenism. Obese women are characterized by functional hyperandrogenism, with elevated free testosterone likely playing a role in the development of the metabolic syndrome. In PCOS, elevated free testosterone has been associated with increased systolic blood pressure (SBP) and diastolic blood pressure (DBP), independent of other factors such as age, insulin resistance and dyslipidemia9. In 280 young healthy women, elevated DHEAS levels were found to have an independent association with blood pressure (BP) levels10.
Androgen excess may be an independent contributor to the development of dyslipidemia, with one study of 21 women with hyperandrogenism showing independent effects of hyperandrogenism and hyperinsulinism on dyslipidemia11. Women with PCOS are predisposed to developing abnormal, atherogenic lipid profiles, with increased total cholesterol, LDL-C and triglyceride levels, and decreased HDL-C levels closely associated with insulin resistance 12.
Hyperandrogenism may itself lead to hyperinsulinism, as studied in adolescent girls and women13,14. Interestingly, antiandrogen (flutamide) treatment results in partial reversal of insulin resistance in hyperandrogenic women15. In PCOS, a primarily ovarian androgen excess disorder, patients have insulin resistance, irregular menses and infertility, often combined with obesity. High insulin levels act on the ovary, with luteinizing hormone (LH), to increase thecal cell production of testosterone16 (Figure 1). Many of the findings in PCOS may be applicable to the adult female with CAH. However, much is unknown regarding the clinical implications of hyperandrogenism in relation to CVD risk in the adult female with CAH.
Insulin Resistance
Insulin resistance is a main component of the metabolic syndrome and a risk factor for coronary artery disease. In patients with CAH, insulin resistance has been noted in several studies, by different indices including fasting insulin, homeostasis model assessment-insulin resistance (HOMA) and frequently sampled IV glucose tolerance test (FSIVGTT). Higher insulin levels and HOMA have been noted in mixed cohorts of male and female patients with CAH, compared to body mass index (BMI) matched controls17,18, and in adult women >30 years old with classic CAH or NCAH19. In women with untreated NCAH, insulin resistance has been noted by FSIVGTT or HOMA method20,21. In a long-term follow-up study of adults with CAH, HOMA was found to increase with increasing BMI22.
Patients with classic CAH have impaired adrenomedullary function, and epinephrine deficiency in classic CAH has been associated with elevated insulin and leptin levels17,23–25. Leptin, secreted by adipose tissue, modulates energy homeostasis by acting centrally, and is in turn regulated by multiple factors including the adrenal medulla and insulin (Figure 1).
Adrenomedullary function has not been evaluated in NCAH, but is expected to be normal or near-normal. Interestingly, in a study of 18 untreated NCAH women, hyperinsulinism, but not hyperleptinemia was found21. The relationship between insulin and leptin is complex, as they indirectly influence each other through their action on the central nervous system, with evidence of a possible direct effect of leptin on the β cell26 (Figure 1).
Insulin has also been shown to increase adrenal steroidogenesis, by increasing 17,20 lyase and 17 α-hydroxylase activity, key catalytic enzymes in androgen production 15,27. Insulin resistance may contribute to irregular menses in women with CAH19, similar to patients with PCOS. There is an increased incidence of polycystic ovaries on ultrasonography in females with CAH28, with an overall prevalence of 76% in postmenarchal women with CAH found in one study29.
Obesity
Obesity is reaching epidemic proportions in the U.S.30,31 with a concomitant increase in the prevalence of cardiovascular disease, type 2 diabetes and the metabolic syndrome32. Several measurements of obesity exist including BMI, waist circumference, and measurements of percent total body fat such as skin-fold thickness, whole body dual-energy x-ray absorption (DXA) scan, or bioelectrical impedance analysis33. Using these measures, obesity in U.S. women is defined as a BMI ≥30 kg/m2, waist circumference ≥88 cm, or percent total body fat ≥35%33. Abdominal obesity has been associated with an increased CVD risk and the development of type 2 diabetes34, with a higher visceral-to-subcutaneous fat ratio leading to worsened insulin sensitivity35,36.
In a long-term follow-up study of 45 adult females and males with classic and nonclassic forms of CAH, 47% were overweight based on BMI22. Falhammar et al. found a higher BMI and higher waist-to-hip ratio, compared to controls, in women 30 years or older with classic CAH and NCAH19. Higher body fat mass has also been reported in studies of young female and male adults with CAH, compared to controls37,38. In 13 women with classic CAH, increased body fat percentage was attributed to over-treatment with glucocorticoid39, although a positive correlation between glucocorticoid dose and measurements of obesity has not always been found38. In young adult patients with CAH treated with relatively low glucocorticoid doses, increased fat mass was found in CAH patients, compared to controls37. In children with CAH, a correlation between BMI and age, as well as a progressive increase in fat mass during childhood, has been reported40,41. In female children with CAH, bioelectrical impedance analysis of body fat mass showed an increased body fat ratio in CAH patients, compared to controls42.
Although functional hyperandrogenism is associated with increased abdominal adiposity in women43, the relationship between hyperandrogenism and abdominal adiposity in CAH has not been studied.
Hypertension
There is minimal data on hypertension in adult patients with CAH. In adult females with CAH, women ≥30 years old had a higher standing DBP compared to younger patients19.
Children with classic 21-hydroxylase deficiency have a higher prevalence of hypertension in several studies. On 24-hour ambulatory monitoring, children with salt-wasting CAH exhibited elevated blood pressures during the daytime, with an absent physiologic nocturnal nadir in SBP, which could have implications for future CVD risk44. The alterations in BP in these children were associated with BMI, particularly in female patients, and were not affected by mineralocorticoid or glucocorticoid replacement dose. In another study of children and adolescents with classic CAH, elevated SBP correlated with BMI and skin-fold thickness measures, along with leptin and insulin levels45. There was no correlation between elevated BP and hormone replacement dosages. Essential hypertension (hypertension of unknown etiology) was noted in 5 of 91 children with classic 21-hydroxylase deficiency46 possibly due to dysregulation inherent to CAH.
Dyslipidemia
Patients with CAH might be at risk for dyslipidemia due to chronic glucocorticoid therapy. A case-control study examining the effect of glucocorticoid treatment on prepubertal Hispanic children with CAH, versus controls, showed abnormalities primarily in triglyceride levels of CAH patients on prednisone treatment47. Patients on glucocorticoid therapy for other conditions have been noted to have dyslipidemia. Patients with Cushing syndrome can have abnormal lipids, and may also exhibit insulin resistance and hyperandrogenism6. Risk for dyslipidemia in CAH is an area that requires further study.
Subclinical Atherosclerosis
Increased intima media thickness (IMT) is a leading modality to measure subclinical atherosclerosis. Increased carotid IMT has been noted in adult female and male patients with different forms of CAH due to 21-hydroxylase deficiency18. In these patients, IMT was independent of glucocorticoid doses and androgen concentrations. Long-term follow-up study, including the relationship between IMT and hormonal imbalances, is needed to understand the significance of these findings.
Markers of chronic inflammation
Atherosclerosis is a process that is in part due to local inflammation. Markers of inflammation that have been shown to predict CVD risk include: high sensitivity C-reactive protein, plasminogen activator inhibitor-1, and increased lymphocytes and monocytes. Both obesity and insulin resistance have been linked to inflammation48. Obesity is associated with chronic low-grade inflammation, with a higher prevalence of macrophages in adipose tissue of obese individuals49, and release of cytokines (IL-6, TNF-alpha) and adipokines from the adipose tissue which can lead to inflammation and stimulate the hypothalamic-pituitary-adrenal (HPA) axis50. This area has yet to be studied in patients with CAH.
CONCLUSIONS
Several CVD risk factors have been identified in patients with CAH, including obesity, hypertension and insulin resistance. These risk factors are inter-related, and androgen excess in women may play an important role. Alterations in the HPA axis, chronic glucocorticoid therapy, and adrenomedullary dysfunction also likely contribute to the development of CVD risk factors. The interplay between metabolic abnormalities and hormonal imbalances, especially excess androgen in women, in both classic and nonclassic forms of CAH requires further study.
A multifactorial intervention approach, including behavior modification and targeted pharmacologic therapy, should be considered in women with CAH who have major CVD risk factors. Future prospective, longitudinal studies of women with CAH to assess cardiovascular events, morbidity and mortality, and long-term outcomes in relation to hormonal imbalances are needed. Greater knowledge in the pathophysiology of CAH and the relationship to CVD risk will lead to therapeutic strategies that aim to treat and prevent adverse CVD outcomes.
Acknowledgments
This research was supported (in part) by the Intramural Research Program of the National Institutes of Health and (in part) by the Congenital Adrenal Hyperplasia Research, Education and Support (CARES) Foundation.
Abbreviations Key
- CAH
congenital adrenal hyperplasia
- NCAH
nonclassic congenital adrenal hyperplasia
- PCOS
polycystic ovarian syndrome
- CVD
cardiovascular disease
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- BP
blood pressure
- LH
luteinizing hormone
- HOMA
homeostasis model assessment-insulin resistance
- FSIVGTT
frequently sampled IV glucose tolerance test
- BMI
body mass index
- DXA
dual-energy x-ray absorption
- IMT
intima media thickness
- HPA
hypothalamic-pituitary-adrenal
References
- 1.Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365(9477):2125–2136. doi: 10.1016/S0140-6736(05)66736-0. [DOI] [PubMed] [Google Scholar]
- 2.Orio F, Palomba S, Colao A. Cardiovascular risk in women with polycystic ovary syndrome. Fertil Steril. 2006;86(Suppl 1):S20–21. doi: 10.1016/j.fertnstert.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Orio F, Jr, Palomba S, Spinelli L, et al. The cardiovascular risk of young women with polycystic ovary syndrome: an observational, analytical, prospective case-control study. J Clin Endocrinol Metab. 2004;89(8):3696–3701. doi: 10.1210/jc.2003-032049. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health. Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Bethesda, MD: National Institutes of Health; 2001. NIH Publication 01-3670. [Google Scholar]
- 5.Kristo C, Ueland T, Godang K, Aukrust P, Bollerslev J. Biochemical markers for cardiovascular risk following treatment in endogenous Cushing’s syndrome. J Endocrinol Invest. 2008;31(5):400–405. doi: 10.1007/BF03346383. [DOI] [PubMed] [Google Scholar]
- 6.Arnaldi G, Mancini T, Polenta B, Boscaro M. Cardiovascular risk in Cushing’s syndrome. Pituitary. 2004;7(4):253–256. doi: 10.1007/s11102-005-1172-7. [DOI] [PubMed] [Google Scholar]
- 7.Merke DP. Approach to the adult with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2008;93(3):653–660. doi: 10.1210/jc.2007-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debono M, Ghobadi C, Rostami-Hodjegan A, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. 2009 Feb 17; doi: 10.1210/jc.2008-2380. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen MJ, Yang WS, Yang JH, et al. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension. 2007;49(6):1442–1447. doi: 10.1161/HYPERTENSIONAHA.106.083972. [DOI] [PubMed] [Google Scholar]
- 10.Mantzoros CS, Georgiadis EI, Young R, et al. Relative androgenicity, blood pressure levels, and cardiovascular risk factors in young healthy women. Am J Hypertens. 1995;8(6):606–614. doi: 10.1016/0895-7061(95)00051-P. [DOI] [PubMed] [Google Scholar]
- 11.Wild RA, Applebaum-Bowden D, Demers LM, et al. Lipoprotein lipids in women with androgen excess: independent associations with increased insulin and androgen. Clin Chem. 1990;36(2):283–289. [PubMed] [Google Scholar]
- 12.Dokras A. Cardiovascular disease risk factors in polycystic ovary syndrome. Semin Reprod Med. 2008;26(1):39–44. doi: 10.1055/s-2007-992923. [DOI] [PubMed] [Google Scholar]
- 13.Huppert J, Chiodi M, Hillard PJ. Clinical and metabolic findings in adolescent females with hyperandrogenism. J Pediatr Adolesc Gynecol. 2004;17(2):103–108. doi: 10.1016/j.jpag.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Golden SH, Ding J, Szklo M, et al. Glucose and insulin components of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women: the atherosclerosis risk in communities study. Am J Epidemiol. 2004;160(6):540–548. doi: 10.1093/aje/kwh250. [DOI] [PubMed] [Google Scholar]
- 15.Moghetti P, Tosi F, Castello R, et al. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J Clin Endocrinol Metab. 1996;81(3):952–960. doi: 10.1210/jcem.81.3.8772557. [DOI] [PubMed] [Google Scholar]
- 16.Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med. 2008;358(1):47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]
- 17.Charmandari E, Weise M, Bornstein SR, et al. Children with classic congenital adrenal hyperplasia have elevated serum leptin concentrations and insulin resistance: potential clinical implications. J Clin Endocrinol Metab. 2002;87(5):2114–2120. doi: 10.1210/jcem.87.5.8456. [DOI] [PubMed] [Google Scholar]
- 18.Sartorato P, Zulian E, Benedini S, et al. Cardiovascular risk factors and ultrasound evaluation of intima-media thickness at common carotids, carotid bulbs, and femoral and abdominal aorta arteries in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92(3):1015–1018. doi: 10.1210/jc.2006-1711. [DOI] [PubMed] [Google Scholar]
- 19.Falhammar H, Filipsson H, Holmdahl G, et al. Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92(1):110–116. doi: 10.1210/jc.2006-1350. [DOI] [PubMed] [Google Scholar]
- 20.Speiser PW, Serrat J, New MI, Gertner JM. Insulin insensitivity in adrenal hyperplasia due to nonclassical steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1992;75(6):1421–1424. doi: 10.1210/jcem.75.6.1464643. [DOI] [PubMed] [Google Scholar]
- 21.Saygili F, Oge A, Yilmaz C. Hyperinsulinemia and insulin insensitivity in women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency: the relationship between serum leptin levels and chronic hyperinsulinemia. Horm Res. 2005;63(6):270–274. doi: 10.1159/000086363. [DOI] [PubMed] [Google Scholar]
- 22.Bachelot A, Plu-Bureau G, Thibaud E, et al. Long-term outcome of patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res. 2007;67(6):268–276. doi: 10.1159/000098017. [DOI] [PubMed] [Google Scholar]
- 23.Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43(5):533–549. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- 24.Riepe FG, Krone N, Kruger SN, et al. Absence of exercise-induced leptin suppression associated with insufficient epinephrine reserve in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Exp Clin Endocrinol Diabetes. 2006;114(3):105–110. doi: 10.1055/s-2005-865836. [DOI] [PubMed] [Google Scholar]
- 25.Weise M, Mehlinger SL, Drinkard B, et al. Patients with classic congenital adrenal hyperplasia have decreased epinephrine reserve and defective glucose elevation in response to high-intensity exercise. J Clin Endocrinol Metab. 2004;89(2):591–597. doi: 10.1210/jc.2003-030634. [DOI] [PubMed] [Google Scholar]
- 26.Niswender KD, Magnuson MA. Obesity and the beta cell: lessons from leptin. J Clin Invest. 2007;117(10):2753–2756. doi: 10.1172/JCI33528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martikainen H, Salmela P, Nuojua-Huttunen S, et al. Adrenal steroidogenesis is related to insulin in hyperandrogenic women. Fertil Steril. 1996;66(4):564–570. doi: 10.1016/s0015-0282(16)58568-9. [DOI] [PubMed] [Google Scholar]
- 28.New MI. Polycystic ovarian disease and congenital and late-onset adrenal hyperplasia. Endocrinol Metab Clin North Am. 1988;17(4):637–648. [PubMed] [Google Scholar]
- 29.Hague WM, Adams J, Rodda C, et al. The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin Endocrinol (Oxf) 1990;33(4):501–510. doi: 10.1111/j.1365-2265.1990.tb03887.x. [DOI] [PubMed] [Google Scholar]
- 30.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288(14):1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 31.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 32.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 33.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 34.Burke V, Beilin LJ, Simmer K, et al. Predictors of body mass index and associations with cardiovascular risk factors in Australian children: a prospective cohort study. Int J Obes (Lond) 2005;29(1):15–23. doi: 10.1038/sj.ijo.0802750. [DOI] [PubMed] [Google Scholar]
- 35.Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362(9388):951–957. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34(11–12):616–621. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- 37.Stikkelbroeck NM, Oyen WJ, van der Wilt GJ, Hermus AR, Otten BJ. Normal bone mineral density and lean body mass, but increased fat mass, in young adult patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2003;88(3):1036–1042. doi: 10.1210/jc.2002-021074. [DOI] [PubMed] [Google Scholar]
- 38.Christiansen P, Molgaard C, Muller J. Normal bone mineral content in young adults with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res. 2004;61(3):133–136. doi: 10.1159/000075588. [DOI] [PubMed] [Google Scholar]
- 39.Hagenfeldt K, Martin Ritzen E, Ringertz H, Helleday J, Carlstrom K. Bone mass and body composition of adult women with congenital virilizing 21-hydroxylase deficiency after glucocorticoid treatment since infancy. Eur J Endocrinol. 2000;143(5):667–671. doi: 10.1530/eje.0.1430667. [DOI] [PubMed] [Google Scholar]
- 40.Cornean RE, Hindmarsh PC, Brook CG. Obesity in 21-hydroxylase deficient patients. Arch Dis Child. 1998;78(3):261–263. doi: 10.1136/adc.78.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volkl TM, Simm D, Beier C, Dorr HG. Obesity among children and adolescents with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics. 2006;117(1):e98–105. doi: 10.1542/peds.2005-1005. [DOI] [PubMed] [Google Scholar]
- 42.Isguven P, Arslanoglu I, Mesutoglu N, Yildiz M, Erguven M. Bioelectrical impedance analysis of body fatness in childhood congenital adrenal hyperplasia and its metabolic correlates. Eur J Pediatr. 2008;167(11):1263–1268. doi: 10.1007/s00431-007-0665-y. [DOI] [PubMed] [Google Scholar]
- 43.Pasquali R, Vicennati V, Gambineri A, Pagotto U. Sex-dependent role of glucocorticoids and androgens in the pathophysiology of human obesity. Int J Obes (Lond) 2008;32(12):1764–1779. doi: 10.1038/ijo.2008.129. [DOI] [PubMed] [Google Scholar]
- 44.Roche EF, Charmandari E, Dattani MT, Hindmarsh PC. Blood pressure in children and adolescents with congenital adrenal hyperplasia (21-hydroxylase deficiency): a preliminary report. Clin Endocrinol (Oxf) 2003;58(5):589–596. doi: 10.1046/j.1365-2265.2003.01757.x. [DOI] [PubMed] [Google Scholar]
- 45.Volkl TM, Simm D, Dotsch J, Rascher W, Dorr HG. Altered 24-hour blood pressure profiles in children and adolescents with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2006;91(12):4888–4895. doi: 10.1210/jc.2006-1069. [DOI] [PubMed] [Google Scholar]
- 46.Nebesio TD, Eugster EA. Observation of hypertension in children with 21-hydroxylase deficiency: a preliminary report. Endocrine. 2006;30(3):279–282. doi: 10.1007/s12020-006-0005-4. [DOI] [PubMed] [Google Scholar]
- 47.Botero D, Arango A, Danon M, Lifshitz F. Lipid profile in congenital adrenal hyperplasia. Metabolism. 2000;49(6):790–793. doi: 10.1053/meta.2000.6261. [DOI] [PubMed] [Google Scholar]
- 48.Bloomgarden ZT. Inflammation and insulin resistance. Diabetes Care. 2003;26(6):1922–1926. doi: 10.2337/diacare.26.6.1922. [DOI] [PubMed] [Google Scholar]
- 49.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29(24):2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 50.Witchel SF, DeFranco DB. Mechanisms of disease: regulation of glucocorticoid and receptor levels--impact on the metabolic syndrome. Nat Clin Pract Endocrinol Metab. 2006;2(11):621–631. doi: 10.1038/ncpendmet0323. [DOI] [PubMed] [Google Scholar]