Abstract
Background:
This subgroup analysis of the European Cubicin Outcomes Registry Experience evaluated the safety and effectiveness of daptomycin in children and adolescent patients (<18 years).
Methods:
Clinical outcomes at the end of therapy were assessed as success (cured or improved), failure or nonevaluable. Safety was assessed for up to 30 days post treatment.
Results:
Eighty-one children and adolescent patients were included in this study. The most common primary infections were bacteremia (19.8%), complicated skin and soft-tissue infection (18.5%), osteomyelitis (13.6%), endocarditis (12.3%), foreign body/prosthetic infection (12.3%), uncomplicated skin and soft-tissue infection (9.9%) and other (13.6%). Daptomycin doses ranged from 4 to >10 mg/kg/day. Median duration of therapy was 12.5 (interquartile range, 7–25; mean, 16.7; standard deviation, 12.8) days. Staphylococcus aureus (46.7%) was the most commonly isolated pathogen (23.8% methicillin-resistant S. aureus). Forty-nine (60.5%) patients completed daptomycin therapy without further antibiotics, 27 (33.3%) switched to another antibiotic, 4 (4.9%) discontinued because of adverse events (AEs) and 1 (1.2%) discontinued because of other reason. Overall, 75 (92.6%; 95% confidence interval: 95.2–100.0%) patients achieved clinical success; 39 of 41 (95.1%) patients receiving daptomycin monotherapy and 36 of 40 (90.0%) patients receiving concomitant antibiotics. Six (7.4%) patients reported AEs, including 1 patient with increased blood creatine phosphokinase. Three (3.7%) patients had serious AEs; 1 (1.2%) had a serious AE possibly related to daptomycin.
Conclusion:
Daptomycin, alone or combined with other antibiotics and/or surgery, demonstrated high clinical success rates against a wide variety of infections and was well tolerated in children and adolescents.
Keywords: adolescents, children, daptomycin, EU-CORE, Gram-positive infections, staphylococci, effectiveness
Gram-positive bacteria, especially methicillin-resistant Staphylococcus aureus (MRSA), are known to be common pathogens in children and adolescents in both healthcare and community-associated infections.1–3 Treatment of resistant pathogens including MRSA and vancomycin-resistant enterococci remains challenging even with standard antimicrobial protocols.4–6 Higher mortality has been reported because of MRSA infections than infections caused by methicillin-susceptible S. aureus.7,8 Several treatment options are available for the management of MRSA infections in adults; however, these options are limited for pediatric patients owing to a lack of sufficient safety and efficacy data.1 Clindamycin, vancomycin and linezolid are currently the only antibiotics approved by the US Food and Drug Administration for the management of MRSA infections in pediatric patients.1
Daptomycin is a cyclic lipopeptide that is active against a wide range of Gram-positive bacteria. Its mechanism of action involves calcium-dependent binding to the bacterial cell membrane, resulting in rapid depolarization of the membrane potential and bacterial cell death, without cell lysis and release of inflammatory mediators.9,10 Daptomycin is a concentration-dependent bactericidal agent indicated for the treatment of adult patients with complicated skin and soft-tissue infection (cSSTI), right-sided endocarditis caused by S. aureus, and bacteremia associated with cSSTI or right-sided endocarditis.11
Daptomycin is not approved for the treatment of pediatric patients,12 and limited data are currently available regarding its use in the treatment of Gram-positive infections in this population.1 A few case reports and retrospective studies have described that use of daptomycin is beneficial in treating Gram-positive infections in children.2,12
The European Cubicin Outcomes Registry and Experience (EU-CORESM) study was a retrospective, noninterventional registry developed to collect real-world data on the use of daptomycin in the treatment of patients with Gram-positive infections. This subgroup analysis evaluated the safety and effectiveness of daptomycin in children and adolescent patients from the EU-CORE study.
METHODS
Patients and Data Collection
The EU-CORE study included patients who had received at least 1 dose of daptomycin between January 2006 and April 2012 for the treatment of a serious Gram-positive bacterial infection. Patients who might have received daptomycin as part of a controlled clinical trial were excluded. Local investigators collected the data using standardized case report forms. Safety was assessed up to 30 days after the end of daptomycin therapy to permit capture of adverse events (AEs) and serious AEs (SAEs). Interim data from the EU-CORE registry have been reported previously.13–15
The study was conducted according to the ethical principles of the Declaration of Helsinki. The protocol was approved by the health authority and the Institutional Review Board or Ethics Committee in each country and written informed consent was obtained from patients/legally acceptable representatives of patients according to the requirements of the Institutional Review Board or Ethics Committee and/or the local data privacy regulations.
Clinical Outcome Assessment
The investigators assessed the clinical outcome at the end of daptomycin therapy as cured, improved, failed, or nonevaluable according to the following protocol-defined criteria—cured: clinical signs and symptoms resolved, no additional antibiotic therapy was necessary or infection cleared with a negative culture reported; improved: partial resolution of clinical signs and symptoms and/or additional antibiotic therapy was warranted; failed: inadequate response to daptomycin therapy, worsening or new/recurrent signs and symptoms, need for a change in antibiotic therapy or a positive culture reported at the end of therapy and nonevaluable: unable to determine response because of insufficient information. Clinical success was defined as an outcome of cured or improved. Time to improvement was recorded. The reasons for stopping daptomycin therapy and other antibiotics prescribed after daptomycin were also collected.13
Safety Assessment
AEs and SAEs during daptomycin treatment and the 30-day follow-up period were assessed by the investigators. All reported AEs were recorded, regardless of their relationship to daptomycin.
Statistical Analysis
Statistical analysis was performed using SAS version 9.3 (SAS Institute Inc., Cary, NC). Because of the nature of the trial, inferential analyses were not conducted, and no formal statistical methodology other than simple descriptive statistics was used. All analyses were considered to be explanatory. Numerical variables are summarized as arithmetic mean, standard deviation (SD), median, minimum, first quartile, third quartile and maximum for continuous variables. Categorical variables were summarized by absolute and relative frequencies. Exact confidence intervals (CIs, Pearson Clopper) were used around selected relative frequencies.
RESULTS
Patient Demographics and Clinical Characteristics
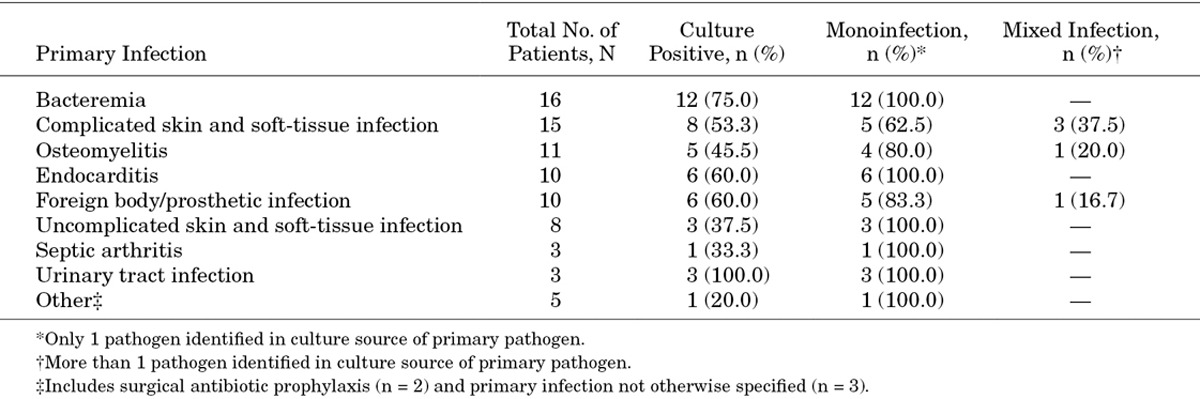
A total of 6075 patients were enrolled in EU-CORE, of whom 81 were children and adolescents. The patients in the pediatric subgroup were predominantly Caucasian (n = 74; 91.4%), with a median age of 13 [interquartile range (IQR): 8–16; mean: 11.4; SD: 4.8; 95% CI: 10.3–12.5%] years and a median body weight of 46.5 (IQR: 27–60; mean: 45.4; SD: 23.2; 95% CI: 40.2–50.6%) kg. Baseline demographics and underlying diseases are summarized in Table 1. Types of primary infection, including monoinfection and mixed infections, are presented in Table 2. The most common primary infection was bacteremia (n = 16; 19.8%). This was followed by cSSTI (n = 15; 18.5%); osteomyelitis, implant related and nonimplant related (n = 11; 13.6%); endocarditis (n = 10; 12.3%); foreign body/prosthetic infection (n = 10; 12.3%); uncomplicated skin and soft-tissue infection (uSSTI; n = 8; 9.9%); septic arthritis (n = 3; 3.7%); urinary tract infection (n = 3; 3.7%) and other (n = 5; 6.2%). A total of 49 (60.5%) patients completed daptomycin therapy and 27 (33.3%) patients switched to another antibiotic after the end of daptomycin therapy (eg, step-down to oral antibiotic therapy). Daptomycin therapy was discontinued in 4 (4.9%) patients because of AEs and in 1 (1.2%) patient because of other reason. All 81 patients were assessed for safety and efficacy.
TABLE 1.
Baseline Demographics and Clinical Characteristics
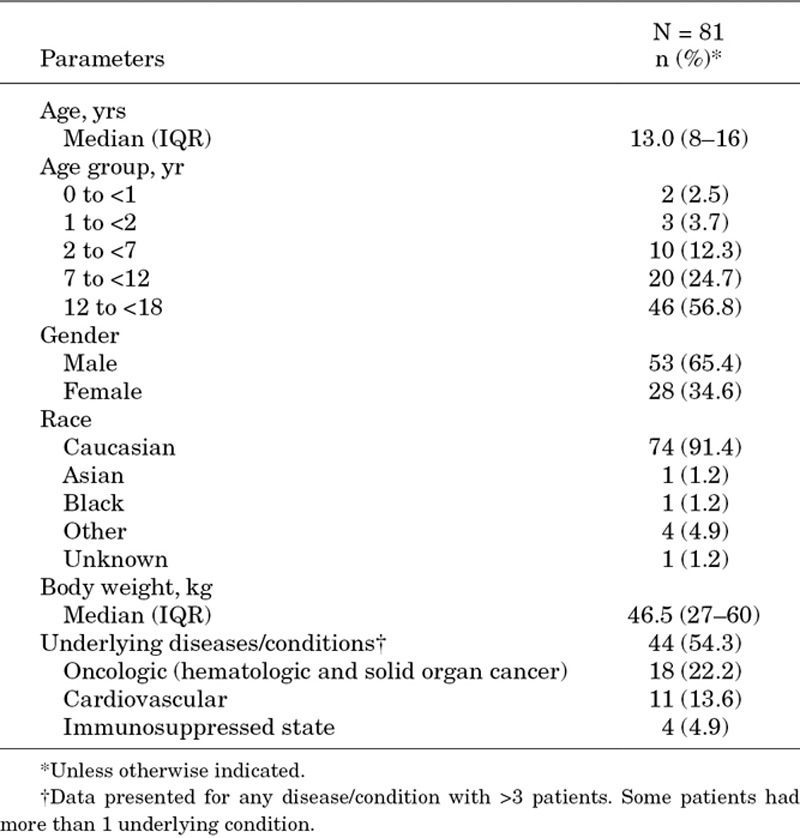
TABLE 2.
Types of Monoinfections and Mixed Infections by Type of Primary Infection
Microbiology
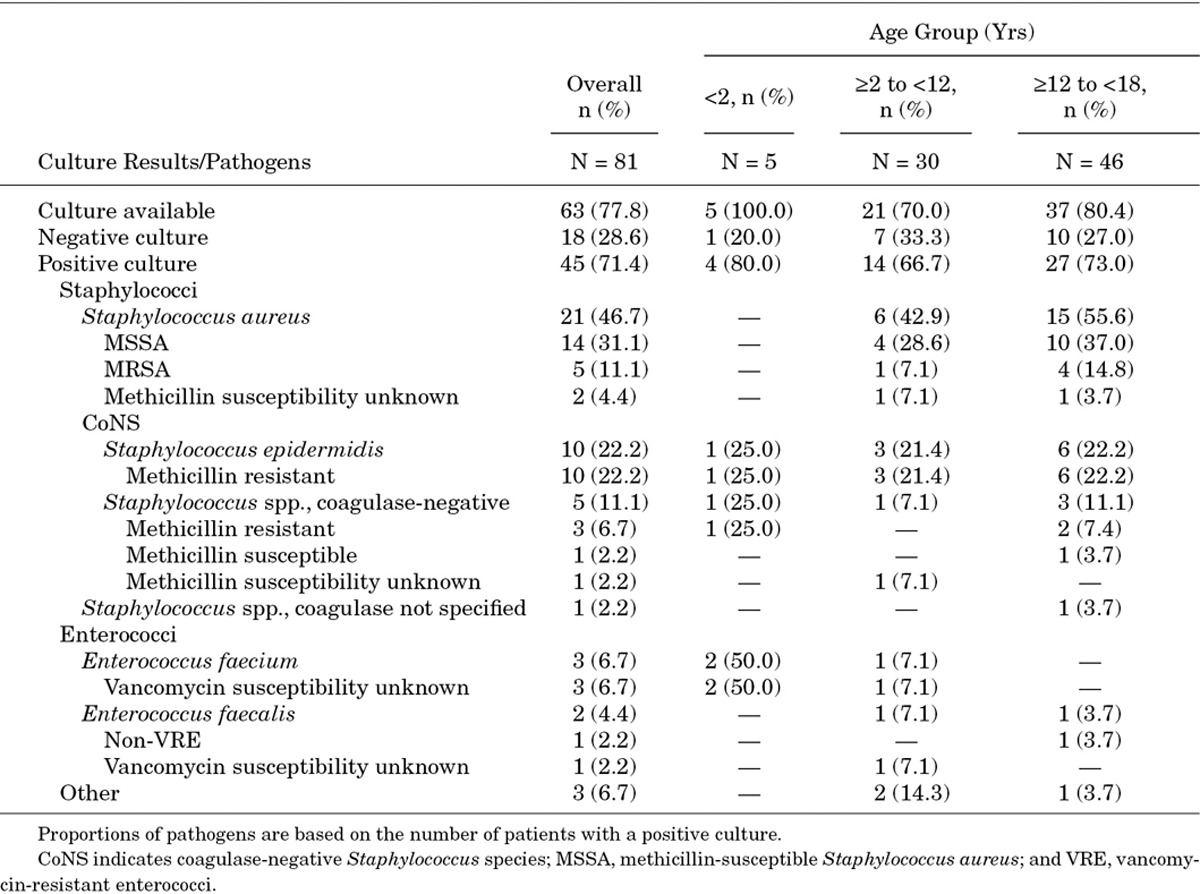
Culture results were available for 63 (77.8%) patients; of those, 45 (71.4%) patients had positive cultures. The most commonly reported pathogens in primary culture were S. aureus (n = 21; 46.7%; 23.8% MRSA), Staphylococcus epidermidis (n = 10; 22.2%) and coagulase-negative Staphylococcus species (n = 5; 11.1%; Table 3). In patients <2 years, coagulase-negative Staphylococcus and Enterococcus faecium were the only reported pathogens in primary culture. S. aureus and coagulase-negative Staphylococcus were the most common pathogens in patients ≥2 to <12 and ≥12 to <18 years (Table 3). The main culture sources of the primary pathogens were blood (n = 34), deep tissue (n = 10), skin swab (n = 8), needle aspirate (n = 3) and urine (n = 2). Other culture sources included venous catheter, intraoperative tissue biopsy and pleural fluid.
TABLE 3.
Culture Results—Overall and By Age Groups
Previous and Concomitant Antibiotic Therapies
A total of 50 (61.7%) patients received antibiotic therapy before daptomycin. Glycopeptides (n = 20; 24.7%) were the most frequently used prior antibiotics, followed by penicillins (n = 18; 22.2%) and aminoglycosides (n = 17; 21.0%). The main reason for switching to daptomycin was failure of the previous antibiotic therapy. A total of 37 (45.7%) inpatients and 4 (5.9%) outpatients received concomitant antibiotics.
Surgical Interventions
Several surgical interventions were performed as part of the treatment of primary infection during therapy with daptomycin. Thirteen (16.0%) patients underwent tissue debridement, and 9 (11.1%) patients underwent incision and drainage. Eight (9.9%) patients each underwent bone debridement, foreign device removal and other procedures. Heart valve replacement was performed in 3 (3.7%) patients. Forty (49.4%) patients did not undergo any surgical procedure.
Daptomycin Prescribing Patterns
The most frequently prescribed dose of daptomycin was 6 mg/kg/day in 37 (45.7%) patients, followed by 4 mg/kg/day in 15 (18.5%) patients. Other prescribed doses were ≥8 to ≤10 mg/kg/day in 11 (13.6%) patients, >6 to <8 mg/kg/day in 9 (11.1%) patients and >10 mg/kg/day in 5 (6.2%) patients. Four (4.9%) patients received a dose of >4 to <6 mg/kg/day. Children and adolescent patients received daptomycin for a median duration of 12.5 (IQR, 7–25; mean 16.7; SD, 12.8) days. The median duration of therapy on the basis of patient disposition was 15.5 (IQR, 7–34; mean, 19.8; SD, 15.0) days for outpatients, 11.0 (IQR, 7–24; mean 15.8; SD, 12.6) days for inpatients and 7.0 (IQR, 3–11; mean, 8.8; SD, 7.1) days for intensive care patients. The median duration of daptomycin therapy varied widely depending on the type of primary infection. Patients received daptomycin either as first-line (n = 30) or second-line (n = 50) treatment; 1 patient had such information missing.
Clinical Outcome
Overall clinical success was achieved in 75 (92.6%; 95% CI: 95.2–100.0%) out of 81 patients; 42 (51.9%) patients were cured and 33 (40.7%) patients were improved. Clinical success with daptomycin monotherapy (n = 41) was achieved in 39 [95.1%; cured, 20 (48.8%); improved, 19 (46.3%)] patients and 2 (4.9%) patients were nonevaluable. Of the patients who received concomitant antibiotics (n = 40), 36 (90.0%) patients demonstrated clinical success [cured, 22 (55.0%); improved, 14 (35.0%)], whereas 4 (10.0%) patients were nonevaluable. Also, of the 27 patients who switched to another antibiotic, 25 patients were either cured [10 (37.0%)] or improved [15 (55.6%)] at the time of switching. Clinical outcomes by type of primary infection are summarized in Figure 1. The overall clinical success rate in children and adolescent patients was 100% for bacteremia (95% CI: 79.4–100.0%), endocarditis (95% CI: 69.2–100.0%), foreign body/prosthetic infection (95% CI: 69.2–100.0%), osteomyelitis including implant-related infection (95% CI: 71.5–100.0%), uSSTI (95% CI: 63.1–100.0%) and urinary tract infection (95% CI: 29.2–100.0%). Clinical outcomes by duration of daptomycin treatment are summarized in Figure 2. Children and adolescent patients receiving ≥5 days of daptomycin had an overall clinical success rate ranging from 92.3% to 100%. The median time for improvement of all patients was 3.0 (IQR, 2–5) days, with 80.2% of patients improving between 1 and 4 days of treatment. Although no treatment failures were reported, 6 (7.4%) patients were nonevaluable at the end of daptomycin therapy. Clinical success rates were high regardless of whether daptomycin was used as first-line or second-line treatment (n = 28; 93.3% and n = 46; 92.0%, respectively).
FIGURE 1.
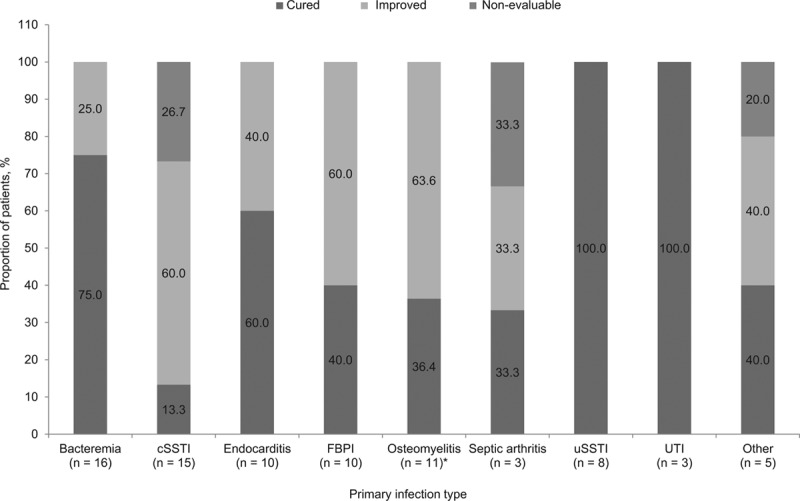
Clinical outcomes by primary infection. *Nonimplant related, n = 8; implant related, n = 3. c/uSSTI indicates complicated/uncomplicated skin and soft-tissue infection; FBPI, foreign body/psrosthetic (without orthopedic device) infection; and UTI, urinary tract infection.
FIGURE 2.
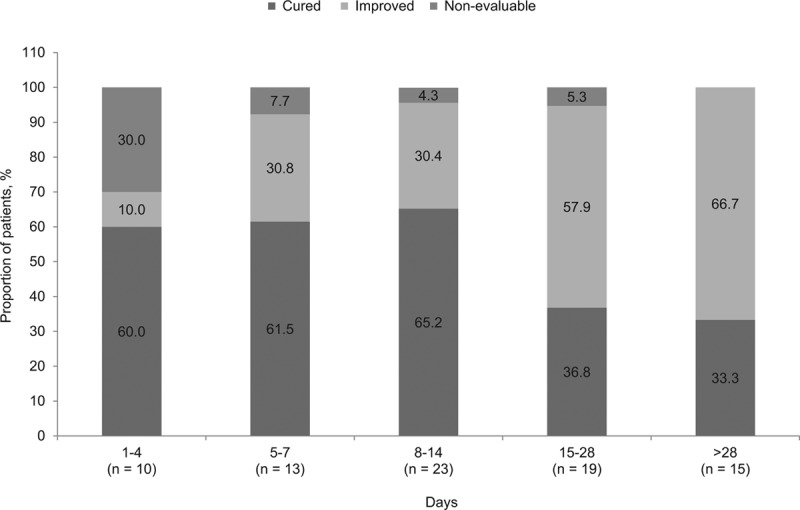
Clinical outcomes by duration of daptomycin treatment.
Safety
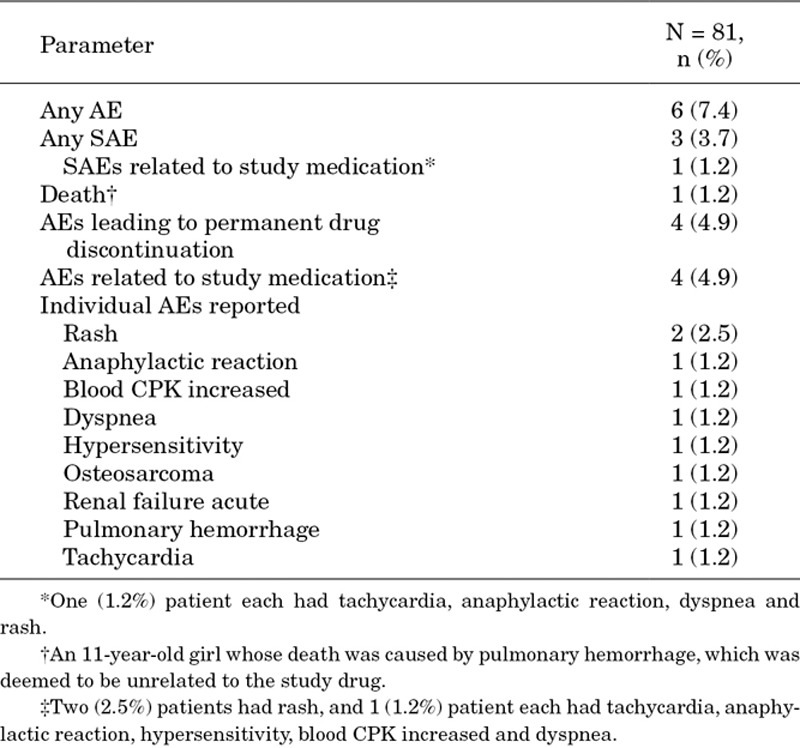
AEs, regardless of relationship to daptomycin, were reported in 6 (7.4%) patients. Three (3.7%) patients experienced SAEs. A total of 4 (4.9%) patients discontinued daptomycin because of AEs, of whom 3 (3.7%) patients discontinued because of SAEs. One death was reported in an 11-year-old girl with cSSTI because of pulmonary hemorrhage 3 days after starting daptomycin therapy, and this was deemed to be unrelated to the treatment. Immune system disorders; respiratory, thoracic and mediastinal disorders; and skin and subcutaneous disorders were the most commonly involved system organ classes [2 (2.5%) patients each]. Details of individual AEs and SAEs are presented in Table 4. Three patients had AEs after 1–4 days of exposure to daptomycin, and 1 patient each had an AE after 5–7, 15–28 and >28 days of exposure to daptomycin.
TABLE 4.
Safety of Daptomycin in Children and Adolescents
Serum creatine phosphokinase (CPK) was measured in 32 (39.5%) patients at baseline and 31 (38.3%) patients during daptomycin treatment. At baseline, 28 (87.5%) patients had CPK at ≤1 × upper limit of normal (ULN), with 1 patient in each of the following categories: >1 to 2 × ULN, >2 to 5 × ULN, >5 to 10 × ULN and >10 × ULN. The majority of patients showed no increase in maximum CPK level during daptomycin therapy. Three patients had an increase to >1 to 2 × ULN, and 1 patient had an increase to >5 to 10 × ULN from normal values at baseline (≤1 × ULN). One patient showed a decrease from >5 to 10 × ULN at baseline to >2 to 5 × ULN during treatment.
DISCUSSION
The emergence of resistant Gram-positive bacteria such as MRSA and vancomycin-resistant enterococci as major pathogens of serious pediatric infections has highlighted the current limitations of existing therapies.1–5 Daptomycin exhibits rapid, concentration-dependent bactericidal activity against both susceptible and resistant Gram-positive bacteria. This and its once-daily administration make it an attractive therapeutic option for infections caused by these pathogens.16 EU-CORE was initiated to capture real-world data regarding the use of daptomycin in the treatment of Gram-positive infections. The results from this analysis showed that children and adolescent patients with a variety of Gram-positive infections treated with daptomycin had a high clinical success rate, whether daptomycin was used as first- or second-line therapy.
Although this study showed that outpatients received daptomycin for a longer mean duration than inpatients, it should be noted that there were only 6 patients treated as outpatients compared with 78 as inpatients.
Daptomycin was well tolerated, and no unexpected or infusion-related reactions were reported. A limited number of AEs were reported, with no new safety signals. CPK levels remained at <1 × ULN in the majority of patients. No indication of CPK-related AEs was observed in these real-world data.
Results from pharmacokinetic studies suggest that higher doses may be required in pediatric patients due to increased plasma clearance and hence lower area under the curve when compared with adults.1 However, in the present registry, the most commonly used dose was 6 mg/kg/day. In a recently completed study of daptomycin in patients aged 1–17 years with cSSTI, age-adjusted daptomycin doses were administered once daily to achieve exposures demonstrated to be successful in adult studies of cSSTI: 12–17 years, 5 mg/kg; 7–11 years, 7 mg/kg; 2–6 years, 9 mg/kg; and 1 to <2 years, 10 mg/kg (NCT00711802). Treatment guidelines published in 2011 (after the start of this study in January 2006) suggest that an intravenous dosage of daptomycin 6–10 mg/kg/day may be used as an alternative agent in the management of MRSA bacteremia, infective endocarditis, acute hematogenous osteomyelitis and septic arthritis in pediatric patients.17 There are currently 2 trials registered in Clinical Trials.gov that focus on the treatment of Gram-positive infections with daptomycin in pediatric patients. One of these studies will examine the safety and efficacy of daptomycin versus standard of care in pediatric patients with bacteremia caused by S. aureus (NCT01728376). The second trial will examine the safety and efficacy of daptomycin, compared with vancomycin or nafcillin, in the treatment of pediatric patients with acute hematogenous osteomyelitis (NCT01922011). These randomized studies aim to recruit a relatively large number of patients and should help to increase the overall knowledge surrounding the efficacy and safety of daptomycin in children and adolescent patients.
Limitations
This was a retrospective, noncomparative registry that included selected patients and data. The number of patients treated in this study is an obvious limitation to drawing any conclusions about the effectiveness or safety of daptomycin in this population. An additional limitation was the prior/concomitant use of other antibiotic agents. Furthermore, patients were followed for only up to 30 days post treatment. Finally, there was no blinding or independent evaluation, and the outcome was determined by the treating physician. However, despite these limitations, the results showed interesting trends in a population for which there is scarcity of data.
ACKNOWLEDGMENTS
Editorial assistance was provided by Anupam Ghose (Novartis Healthcare Private Limited, Hyderabad, India) and Farid Khalfi (Novartis Ireland Ltd., Dublin, Ireland).
Footnotes
Funding for the study and editorial assistance was provided by Novartis Pharma AG. V. Syriopoulou had received grant from Novartis. N. Dmitriy has no funding or conflicts of interest to disclose. Z. Dailiana had received honoraria for consultancy services and support for travel to meetings from Novartis. R. Utili had received grant support and travel grants to attend meetings from Novartis. He also provided consultancy services and lectures for Novartis and was a member of the board of Novartis. R. Pathan is an employee of Novartis Healthcare Pvt. Ltd. K. Hamed is an employee of Novartis Pharmaceuticals Corporation.
REFERENCES
- 1.Durand C, Brueckner A, Sampadian C, et al. Daptomycin use in pediatric patients. Am J Health Syst Pharm. 2014;71:1177–1182. doi: 10.2146/ajhp130601. [DOI] [PubMed] [Google Scholar]
- 2.Ardura MI, Mejías A, Katz KS, et al. Daptomycin therapy for invasive Gram-positive bacterial infections in children. Pediatr Infect Dis J. 2007;26:1128–1132. doi: 10.1097/INF.0b013e31814523f8. [DOI] [PubMed] [Google Scholar]
- 3.Dukic VM, Lauderdale DS, Wilder J, et al. Epidemics of community-associated methicillin-resistant Staphylococcus aureus in the United States: a meta-analysis. PLoS One. 2013;8:e52722. doi: 10.1371/journal.pone.0052722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akins RL, Haase KK. Gram-positive resistance: pathogens, implications, and treatment options: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2005;25:1001–1010. doi: 10.1592/phco.2005.25.7.1001. [DOI] [PubMed] [Google Scholar]
- 5.Wu G, Abraham T, Rapp J, et al. Daptomycin: evaluation of a high-dose treatment strategy. Int J Antimicrob Agents. 2011;38:192–196. doi: 10.1016/j.ijantimicag.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Kullar R, McKinnell JA, Sakoulas G. Avoiding the perfect storm: the biologic and clinical case for reevaluating the 7-day expectation for methicillin-resistant Staphylococcus aureus bacteremia before switching therapy. Clin Infect Dis. 2014;59:1455–1461. doi: 10.1093/cid/ciu583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blot SI, Vandewoude KH, Hoste EA, et al. Outcome and attributable mortality in critically Ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med. 2002;162:2229–2235. doi: 10.1001/archinte.162.19.2229. [DOI] [PubMed] [Google Scholar]
- 8.Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 9.Sader HS, Farrell DJ, Flamm RK, et al. Daptomycin activity tested against 164457 bacterial isolates from hospitalised patients: summary of 8 years of a Worldwide Surveillance Programme (2005–2012). Int J Antimicrob Agents. 2014;43:465–469. doi: 10.1016/j.ijantimicag.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Lee SY, Fan HW, Kuti JL, et al. Update on daptomycin: the first approved lipopeptide antibiotic. Expert Opin Pharmacother. 2006;7:1381–1397. doi: 10.1517/14656566.7.10.1381. [DOI] [PubMed] [Google Scholar]
- 11.Arbeit RD, Maki D, Tally FP, et al. Daptomycin 98-01 and 99-01 Investigators. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis. 2004;38:1673–1681. doi: 10.1086/420818. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Rahman SM, Chandorkar G, Akins RL, et al. Single-dose pharmacokinetics and tolerability of daptomycin 8 to 10 mg/kg in children aged 2 to 6 years with suspected or proved Gram-positive infections. Pediatr Infect Dis J. 2011;30:712–714. doi: 10.1097/INF.0b013e31820fc8e1. [DOI] [PubMed] [Google Scholar]
- 13.Dohmen PM, Guleri A, Capone A, et al. Daptomycin for the treatment of infective endocarditis: results from a European registry. J Antimicrob Chemother. 2013;68:936–942. doi: 10.1093/jac/dks467. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Ruiz A, Beiras-Fernandez A, Lehmkuhl H, et al. Effectiveness and safety of daptomycin in complicated skin and soft-tissue infections and bacteraemia in clinical practice: results of a large non-interventional study. Int J Antimicrob Agents. 2013;41:372–378. doi: 10.1016/j.ijantimicag.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Seaton RA, Gonzalez-Ramallo VJ, Prisco V, et al. Daptomycin for outpatient parenteral antibiotic therapy: a European registry experience. Int J Antimicrob Agents. 2013;41:468–472. doi: 10.1016/j.ijantimicag.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Principi N, Caironi M, Venturini F, et al. Daptomycin in paediatrics: current knowledge and the need for future research. J Antimicrob Chemother. 2015;70:643–648. doi: 10.1093/jac/dku453. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]


