Abstract
Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome secondary to germline fumarate hydratase (FH) mutation presents with cutaneous and uterine leiomyomas, and a distinctive aggressive renal carcinoma. Identification of HLRCC patients presenting first with uterine leiomyomas may allow early intervention for renal carcinoma. We reviewed the morphology and immunohistochemical (IHC) findings in patients with uterine leiomyomas and confirmed or presumed HLRCC. IHC was also performed on a tissue microarray of unselected uterine leiomyomas and leiomyosarcomas. FH-deficient leiomyomas underwent Sanger and massively parallel sequencing on formalin-fixed paraffin-embedded tissue. All 5 patients with HLRCC had at least 1 FH-deficient leiomyoma: defined as completely negative FH staining with positive internal controls. One percent (12/1152) of unselected uterine leiomyomas but 0 of 88 leiomyosarcomas were FH deficient. FH-deficient leiomyoma patients were younger (42.7 vs. 48.8 y, P=0.024) and commonly demonstrated a distinctive hemangiopericytomatous vasculature. Other features reported to be associated with FH-deficient leiomyomas (hypercellularity, nuclear atypia, inclusion-like nucleoli, stromal edema) were inconstantly present. Somatic FH mutations were identified in 6 of 10 informative unselected FH-deficient leiomyomas. None of these mutations were found in the germline. We conclude that, while the great majority of patients with HLRCC will have FH-deficient leiomyomas, 1% of all uterine leiomyomas are FH deficient usually due to somatic inactivation. Although IHC screening for FH may have a role in confirming patients at high risk for hereditary disease before genetic testing, prospective identification of FH-deficient leiomyomas is of limited clinical benefit in screening unselected patients because of the relatively high incidence of somatic mutations.
Key Words: leiomyoma, HLRCC, fumarate hydratase, fumarate hydratase–deficient leiomyoma
Hereditary leiomyomatosis and renal cell cancer (HLRCC) syndrome, also known as Reed syndrome,1 is a rare autosomal dominant hereditary tumor syndrome associated with inactivating germline mutations of the fumarate hydratase (FH) gene located at chromosome 1q42.3-q43.2–4 Patients with HLRCC are predisposed to the development of cutaneous and uterine leiomyomas and, more seriously, a unique type of aggressive renal cell carcinoma.3,5
The majority of female individuals with HLRCC will develop symptomatic uterine leiomyomas and require surgery at a young age, usually before the development of renal carcinoma.6–8 In 1 study, 98% (46/47) of female HLRCC patients with cutaneous leiomyomas also developed uterine leiomyoma, and these were sufficiently symptomatic to warrant surgery in 91% (42/46).6 Of particular note, 57% of these patients required a hysterectomy at or before the age of 30 years (mean, 30 y), which was significantly earlier than the development of renal carcinoma at a median age of 44 years.6 The presentation of patients with leiomyomas at a significantly younger age than renal carcinoma presents a clear opportunity for early diagnosis and intervention if these patients could be prospectively identified.
Unlike the syndrome of HLRCC, uterine leiomyomas are extremely common. Perhaps as many as 20% to 50% of women will develop uterine leiomyomas by 30 years, and up to 80% of females may have uterine leiomyomas by 50 years.9 Although the majority of those affected will not undergo surgery, uterine leiomyomas are still among the most common visceral tumors received in the diagnostic surgical pathology laboratory. Therefore, any screening test to identify patients with HLRCC presenting first with uterine leiomyomas must be highly specific.
It has previously been suggested that uterine leiomyomas arising in the setting of germline FH mutation may show distinctive morphologic features including prominent nucleoli with perinucleolar clearing, hypercellularity, symplastic-type nuclear atypia, a hemangiopericytomatous vascular pattern, cytoplasmic globules, and stromal edema.7,10,11 However, the significance and specificity of these features has recently been questioned,8 and it seems that morphology alone will be inadequate to identify HLRCC-associated uterine leiomyomas prospectively.10
Several groups have demonstrated that positive immunohistochemical (IHC) staining for S-(2-succino)-cysteine (2SC), a metabolite that accumulates when FH is inactivated, is highly sensitive for the identification of HLRCC-associated renal carcinomas12,13; however, it lacks specificity.14,15 This lack of specificity is a significant problem in a screening test for a rare entity. Although there is some evidence that 2SC IHC may be useful to identify HLRCC-associated uterine leiomyomas,10 others have found it less useful.8 Perhaps most importantly, to our knowledge, IHC for 2SC is not commercially available and, therefore, cannot be deployed in the routine surgical pathology laboratory.
IHC stain for FH is commercially available. In early investigations loss of staining for FH in conjunction with morphology has been suggested to be useful in the diagnosis of uterine leiomyomas,16 cutaneous leiomyomas,17 and renal carcinomas14,15 occurring in the setting of HLRCC. Our experience in renal carcinoma is that loss of staining for FH is less sensitive than positive staining for 2SC but highly specific for identifying loss of FH expression.14,15 Because of its commercial availability and high specificity, IHC screening for FH has the potential to identify HLRCC patients presenting with uterine leiomyomas.
We therefore sought to investigate the utility of FH IHC in the diagnosis of uterine leiomyomas associated with HLRCC, first by reporting the patterns of FH staining in uterine leiomyoma patients with known HLRCC and then assessing the results of FH IHC in unselected patients with uterine leiomyomas and leiomyosarcomas. We describe the pathologic features, FH mutation status, and clinical significance of the group of uterine leiomyomas, which show negative staining for FH, a class of tumor we term FH-deficient leiomyomas.
METHODS
The consultation files of one of the authors (A.J.G.) were searched for all patients with confirmed or presumed HLRCC who had also undergone surgical resection for uterine leiomyomas and had material available in formalin-fixed paraffin-embedded (FFPE) blocks. The database of the Department of Anatomical Pathology, Royal North Shore Hospital, Sydney, Australia was searched for all patients with uterine leiomyoma who underwent surgical resection during calendar years 2009 to 2013. A search was also made for all patients with leiomyosarcomas (including both uterine and extrauterine) who underwent surgery or biopsy from June 1998 to 2013 at the same institution. Material from patients with neoplastic tissue remaining in FFPE blocks was then used to construct tissue microarrays (TMAs) containing two 1 mm cores. In patients with multiple tumors the largest tumor was selected for annotation and TMA construction.
IHC for FH was performed on FFPE sections using previously described methods.8 Briefly, a commercially available anti-FH mouse monoclonal antibody was used at a dilution of 1 in 2000 (cloneJ-13, cat no sc-100743; Santa Cruz Biotechnology), using an automated staining platform—the Leica Bond III Autostainer (Leica Biosystems, Mount Waverley, Vic., Australia) with heat-induced epitope retrieval for 30 minutes at 97°C in the manufacturer’s alkaline retrieval solution ER2 (VBS part no: AR9640).
FH IHC was scored by a single observer (A.J.G.). Absent staining in all neoplastic cells in the presence of a positive internal control in non-neoplastic cells such as endothelial cells was interpreted as true negative staining. If tumor cells were negative but there was no internal positive control, staining was considered indeterminate and repeated on whole sections or different blocks. All other patterns of staining including focally positive staining were considered positive, provided the staining was cytoplasmic and granular (ie, mitochondrial).
For patients with known HLRCC, IHC was performed on whole sections, and the observer was not blinded to the underlying diagnosis. For all other patients with leiomyoma and/or leiomyosarcomas, the observer was blinded to all clinical and pathologic features at the time of interpreting IHC. For these unselected cases IHC was initially performed on TMA sections and then repeated on whole sections if staining was negative or indeterminate.
All patients with confirmed HLRCC were offered FH germline testing as part of their clinical care. Genetic testing was performed using massively parallel sequencing (MPS) for small nucleotide variants with Sanger confirmation and, multiplex ligation-dependent probe amplification (MLPA) for detection of large-scale deletions.
All patients without a clinically confirmed diagnosis of HLRCC but with a uterine leiomyoma that demonstrated negative staining for FH underwent mutation testing on DNA extracted from macrodissected neoplastic and non-neoplastic FFPE tissue. Mutation testing was performed by both Sanger sequencing and MPS. For Sanger sequencing, previously described custom primer sets were used.8 For MPS a MiSeq Platform and TruSeq Custom Amplicon Assay (Illumina, CA) was used. If a mutation was identified by MPS but not found on Sanger sequencing, repeat targeted Sanger sequencing of the exon of interest was performed before the mutation was considered confirmed. Loss of heterozygosity (LOH) studies were performed using a previously described set of 6 polymorphic short tandem repeat markers (D1S517, D1S2785, D1S180, AFM214xe11, D1S547, and D1S2842), surrounding the FH gene.8 This study was approved by the North Sydney Local Health District medical ethics review board.
RESULTS
The details of 5 patients with a clinical diagnosis of HLRCC who previously or subsequently underwent resection of uterine leiomyomas are presented in Table 1. Briefly, although some had previously undergone myomectomies with tissue unavailable for review, the material available for testing was from surgery performed at a mean age of 35 years (range, 25 to 41 y). The mean tumor size was 65 mm (range, 30 to 115 mm). Ten of 11 uterine leiomyomas from these patients demonstrated negative IHC staining for FH. One uterine leiomyoma from a patient who had a point mutation (c.689A>C, p.Lys230Arg) demonstrated patchy staining, which, although weaker than usual, was interpreted as positive. Four patients demonstrated some of the morphologic features previously reported to be associated with HLRCC-related uterine leiomyomas (symplastic-type nuclear atypia, hemangiopericytomatous vascular pattern, hypercellularity); however, these features were absent in all the leiomyomas from 1 patient.
TABLE 1.
Clinical, Morphologic, IHC, and Molecular Features of Uterine Leiomyomas in Patients With HLRCC
The database search identified 1176 patients with uterine leiomyomas who underwent surgical resection during the period 2009 to 2013. Of these patients, 1152 had sufficient material in TMA sections for IHC to be interpreted. Tumors from 25 patients demonstrated either negative staining for FH in the presence of a positive internal control or indeterminate staining when interpreted on TMA sections. When IHC was repeated on whole sections from these 25 patients it was definitively interpretable in all cases. Tumors from 13 patients demonstrated positive staining, and 12 were confirmed to be genuinely negative in the presence of an internal positive control. That is, 12 of 1152 (1%) unselected uterine leiomyomas demonstrated genuine negative IHC staining for FH and were therefore classified as FH-deficient leiomyomas (Table 2).
TABLE 2.
Morphologic and Demographic Features of Patients With FH-deficient Leiomyomas Identified by Screening IHC in a Cohort of 1152 Consecutive Patients
Of the 12 patients from the unselected cohort with FH-deficient tumors, 4 had multiple leiomyomas resected at the time of index surgery. All of these additional tumors demonstrated positive staining for FH. One patient had previously undergone myomectomy 9 years earlier (patient 1 in Table 2). This previously resected leiomyoma demonstrated negative staining for FH. No other patients with FH-deficient leiomyomas recurred, and all patients were alive and disease free at the last known follow-up.
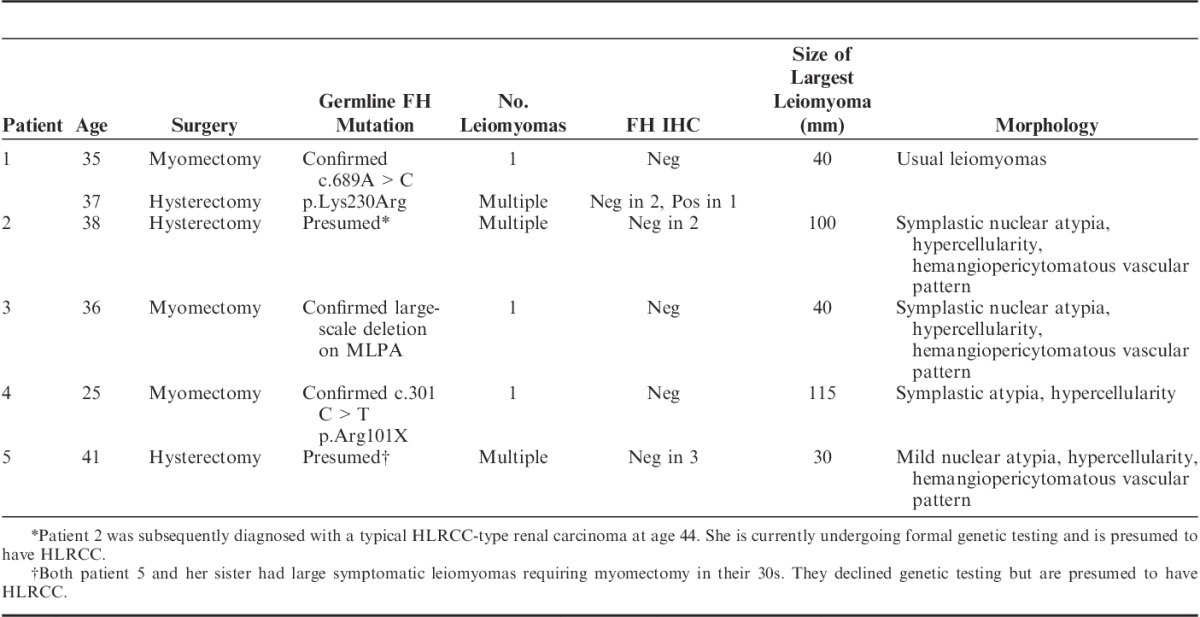
The pattern of FH IHC staining in FH-deficient leiomyomas is illustrated in Figure 1. Briefly, all non-neoplastic tissues (including adjacent non-neoplastic smooth muscle and endothelial cells within the leiomyomas) demonstrated positive staining in a mitochondrial pattern (ie, granular and cytoplasmic) (Fig. 1B), whereas the neoplastic cells were either negative or exhibited only a weak cytoplasmic blush of nonspecific staining (Fig. 1D).
FIGURE 1.
Serial hematoxylin and eosin–stained (A, C) and FH IHC-stained (B, D) sections. The non-neoplastic uterine smooth muscle (arrows) and endothelial cells within the main tumor mass (arrowheads) demonstrate positive staining for FH. This staining, which is distinctly mitochondrial (granular and cytoplasmic), serves as an internal positive control and contrasts with the leiomyoma, which is completely negative. The positive staining of the endothelial cells also serves to highlight the hemangiopericytomatous and slit-like vascular pattern.
The clinical and demographic features of the FH-deficient leiomyomas from the unselected cohort are presented in Table 2. Compared with other unselected uterine leiomyoma patients, patients with FH-deficient leiomyomas underwent surgery at a significantly younger mean age of 42.7 years versus 48.8 years (odds ratio, 0.919 [0.854-0.989]; P=0.024). Although the average tumor size was slightly larger in FH-deficient leiomyomas, the difference was not significant (54.4 vs. 51.6 mm, P=0.82).
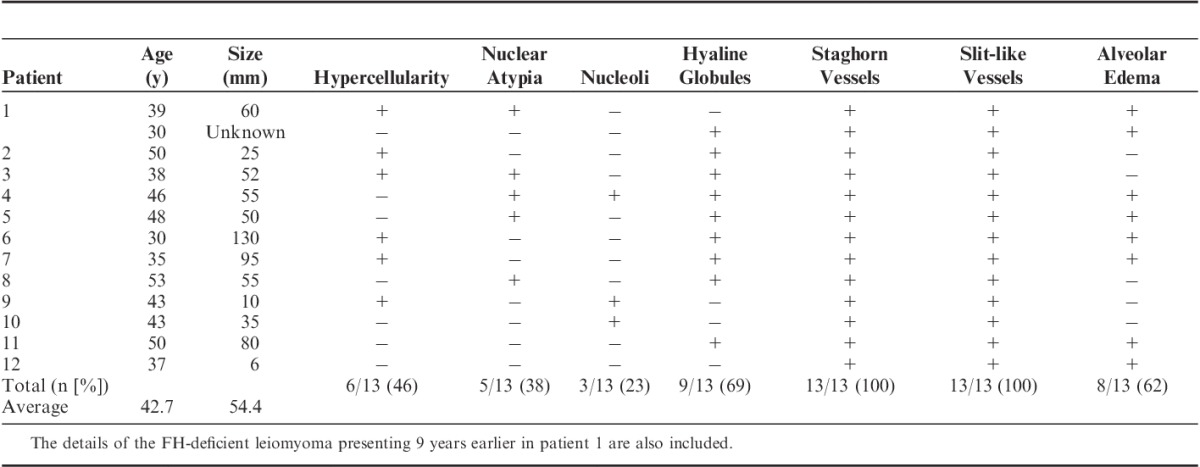
The morphology of the 13 FH-deficient leiomyomas from 12 patients thus identified (comprising the 12 tumors from the truly unselected cohort plus the tumor resected 9 years earlier from patient 1) were reviewed in a specific search for the features previously associated with HLRCC-related uterine leiomyomas—that is, hypercellularity, symplastic nuclear atypia, prominent inclusion-like nucleoli, hyaline globules, a hemangiopericytomatous vasculature, a slit-like vasculature, and alveolar edema. The findings are presented in Table 2 and illustrated in Figures 2 and 3. Briefly, all cases demonstrated a hemangiopericytomatous/staghorn vasculature at least focally. In some cases this was a focal finding, evident only after a careful search, whereas in other cases this was widespread. Only 3 cases (23%) demonstrated prominent eosinophilic inclusion-like nucleoli. Five cases (38%) demonstrated symplastic-type nuclear atypia, which in 1 case was diffuse and in 4 cases was focal. Six cases (46%) demonstrated notable hypercellularity. Nine cases (69%) contained eosinophilic cytoplasmic globules, although in some cases this finding was very subtle and evident only after a dedicated search. Alveolar edema, defined as prominent stromal edema, which, when mixed with spindled smooth muscle cells, imparted an alveolar architecture, was evident in 8 cases (62%) but was often very focal. There was no evidence of coagulative or hyaline necrosis in any of the 13 FH-deficient leiomyomas, and all cases had a mitotic count of <5 per 10 high-power fields.
FIGURE 2.
A, “Alveolar edema” (arrow) . B, Hemangiopericytomatous vascular pattern (arrowheads).
FIGURE 3.
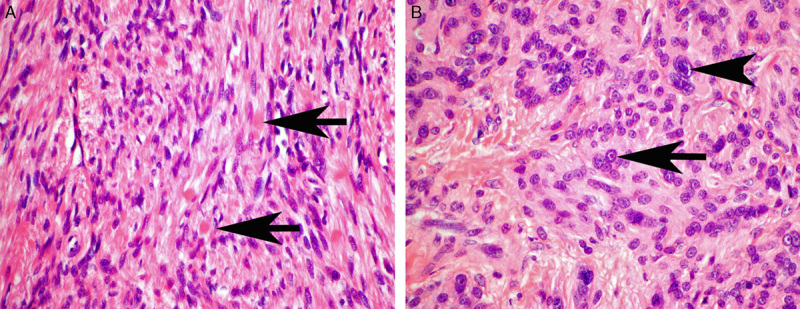
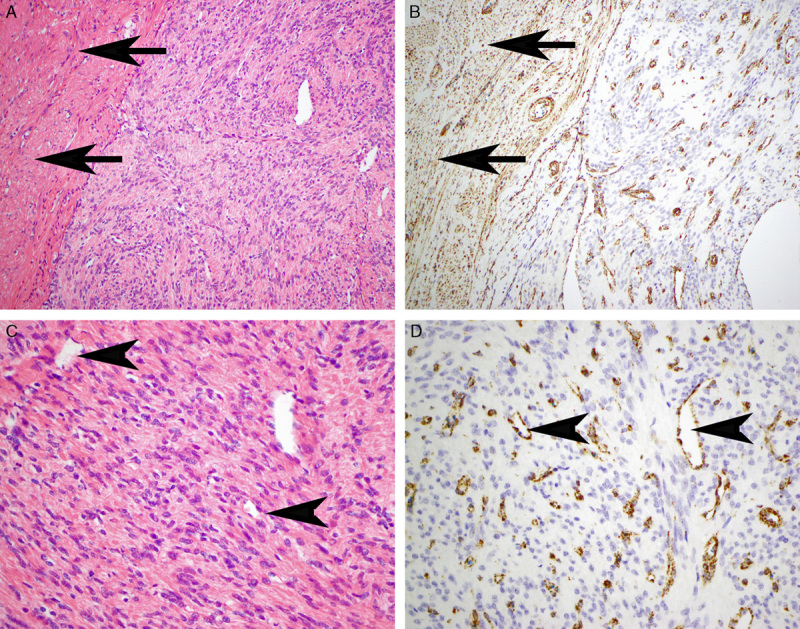
A, Eosinophilic cytoplasmic globules (arrows) were a subtle feature but commonly identified when sought. B, A few cases demonstrated either prominent inclusion-like nucleoli (arrow) or symplastic-type nuclear atypia (arrowhead).
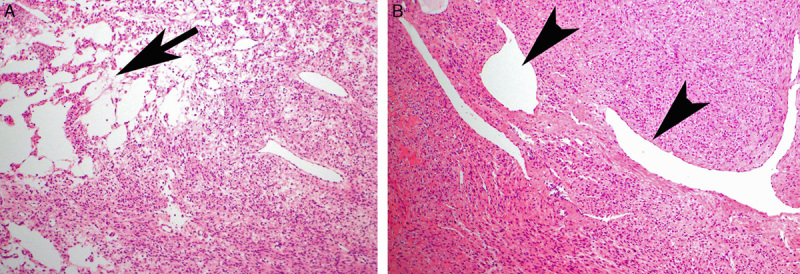
A total of 116 tumors from 88 patients who had received a diagnosis of leiomyosarcoma between 1998 and 2013 were available for IHC. Forty-four tumors from 35 patients were uterine leiomyosarcomas, 27 tumors from 22 patients were retroperitoneal, and the remainder arose in the soft tissue, subcutaneous connective tissue, or the gastrointestinal tract. All these leiomyosarcomas demonstrated positive staining for FH. However, we noted that 1 smooth muscle tumor of uncertain malignant potential (STUMP) was inadvertently included in the TMA of leiomyosarcomas because the initial reporting pathologist had favored a diagnosis of leiomyosarcomas, before retracting this diagnosis in favor of STUMP after consultation with a subspecialist gynecologic pathologist. This tumor, arising in a 31-year-old woman, demonstrated hypercellularity and areas of symplastic-type nuclear atypia but lacked coagulative necrosis or mitotic activity and is illustrated in Figure 4. IHC for FH was negative in both TMA and whole sections from this tumor. The patient was alive and disease free 8 years after surgery, confirming that the tumor was biologically benign but also illustrating that FH-deficient leiomyomas may mimic leiomyosarcomas.
FIGURE 4.
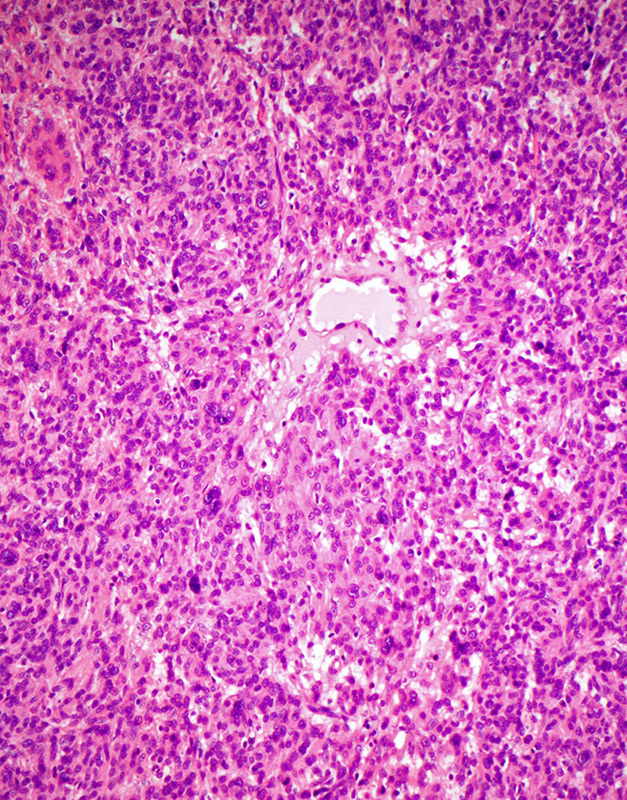
The diagnosis of leiomyosarcoma was originally considered in this FH-deficient leiomyoma. However, the tumor was reclassified as STUMP upon review. It is hypercellular and shows nuclear atypia but lacks significant mitotic activity or coagulative necrosis.
The results of molecular testing performed on DNA extracted from FFPE tissue from the 14 apparently sporadic FH-deficient leiomyomas, comprising 12 patients from the unselected cohort (1 with 2 FH-deficient leiomyomas presenting 9 y apart) and this additional STUMP patient, are presented in Table 3. Briefly, complete coverage by Sanger sequencing was achieved in 10 of 14 FH-deficient leiomyomas. Of these, somatic FH mutations were identified in 6 tumors from 6 separate patients (43% of all FH-deficient leiomyomas in the cohort, and 60% of those with complete coverage). Five of these mutations were also identified by MPS interpreted blinded to the results of Sanger sequencing, whereas 1 mutation was not identified by MPS due to incomplete coverage at the exon of interest but was confirmed on repeat Sanger sequencing. No confirmed somatic mutations were found in 4 FH-deficient leiomyomas with complete coverage by Sanger sequencing and partial coverage by MPS. LOH was identified in all 5 informative tumors with FH mutation. LOH at the FH locus was also identified in 1 FH-deficient leiomyoma in which no mutation was identified. LOH studies in the remaining tumors were not informative. Four FH-deficient leiomyomas had inadequate coverage by both Sanger sequencing and MPS to exclude mutation.
TABLE 3.
Summary of FH Status in Leiomyomas
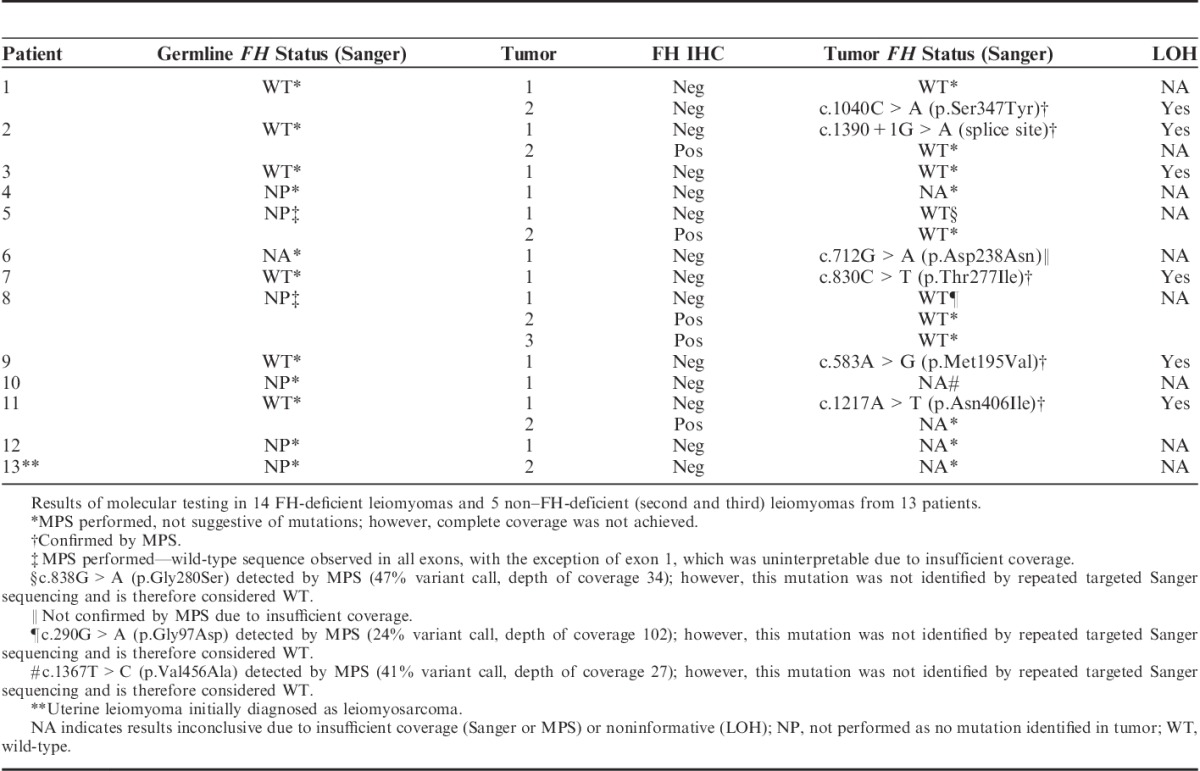
Sanger sequencing was successful in non-neoplastic tissue from 5 of 6 patients with FH-deficient leiomyomas harboring FH mutation. All were wild type for FH. No other mutations were identified by MPS on non-neoplastic tissue from any of the 13 patients; however, coverage was poor and insufficient to definitively exclude germline mutations in the 7 patients with no mutation identified in their tumors.
No FH mutations were identified in all 4 FH IHC-positive leiomyomas comprising second or third leiomyomas from patients with FH-deficient leiomyomas (patients 2, 5, and 8), which were successfully tested.
DISCUSSION
FH is the enzyme immediately following succinate dehydrogenase in the Krebs cycle.18 IHC for succinate dehydrogenase B, which is performed and interpreted in an identical way to FH, is used diagnostically as a marker of integrity of the succinate dehydrogenase complex. The standard nomenclature for tumors that show negative staining for succinate dehydrogenase B is succinate dehydrogenase deficient.18 Succinate dehydrogenase–deficient tumors include distinct subtypes of pheochromocytoma/paraganglioma, gastrointestinal stromal tumor, renal carcinoma, and pituitary adenoma—summarized in Gill.18 Using similar nomenclature, we therefore believe that FH-deficient leiomyoma is the most appropriate nomenclature for a leiomyoma, which shows IHC-negative staining for FH in the presence of an internal positive control. We prefer this terminology to HLRCC-associated leiomyoma, not only because of the obvious parallels with succinate dehydrogenase deficiency in terms of interpretation of the stain but also because it is clear from this study that, although most patients with HLRCC (ie, germline FH mutation) will develop FH-deficient leiomyoma, the great majority of FH-deficient leiomyomas seem to occur sporadically and are not associated with HLRCC.
Our study demonstrates that the identification of FH-deficient uterine leiomyomas may be an important clue to the diagnosis of HLRCC, but its significance should not be overinterpreted as it is not completely sensitive and it is far from specific. For example, in 11 leiomyomas from 5 confirmed HLRCC patients, only 10 tumors (91%) demonstrated negative IHC staining for FH. One tumor from a patient with a clearly pathogenic germline FH point mutation c.689A>C (the patient subsequently went on to develop typical HLRCC-related renal cell carcinoma) demonstrated positive staining for FH. Therefore, negative staining for FH will clearly not identify all patients with HLRCC presenting with leiomyoma.
Although FH-deficient leiomyomas account for only 12 of 1152 (1%) of all unselected uterine leiomyomas, uterine leiomyomas are among the most common visceral tumors submitted for pathologic examination. That is, because of the sheer volume of uterine leiomyomas resected, FH-deficient leiomyomas will be encountered frequently in most diagnostic surgical pathology practices. In this respect is it noteworthy that no germline FH mutations were identified in 12 patients with FH-deficient leiomyomas from an unselected cohort encountered over a 5-year period. We caution that Sanger sequencing and MPS were performed on DNA extracted from archived FFPE tissue, and this approach, being subject to uneven coverage, could not be expected to identify all FH mutations. Furthermore, we did not perform MLPA to look for large-scale deletions, which are not uncommon in HLRCC and in fact account for 1 of our patients from the confirmed germline mutation cohort (patient 3, Table 1). Therefore, it is possible that not all germline mutations would be identified by our approach. Nevertheless, the fact that FH-deficient leiomyomas are relatively common (1% of all uterine leiomyomas) but HLRCC is rare, and no germline mutations or subsequent clinical evidence of HLRCC were identified in our unselected patients, suggests that germline mutation testing for FH mutation is a low-yield test in the absence of other features to suggest syndromic disease even in patients presenting with FH-deficient leiomyomas.
Our findings are in keeping with previous studies, which primarily used molecular testing to screen for FH mutations in leiomyomas. For example, Barker et al19,20 detected no FH mutations in 129 unselected uterine leiomyomas but did identify LOH at 1q43 in 7 tumors, and Lehtonen et al21 found 2 somatic mutations in 153 uterine leiomyomas from 46 patients and also confirmed that no patients had germline mutation. Taken together, 2 of 282 (0.7%) unselected uterine leiomyomas from these molecular studies harbored somatic FH mutation, and neither of these were associated with germline mutation. Although our study was not intended or designed to assess the sensitivity of FH IHC in identifying FH mutations in uterine leiomyomas, given that we found an FH-deficient leiomyoma incidence of 1% it is likely that FH IHC identifies most but not all FH mutations.
From a practical point of view we would recommend that if an FH-deficient uterine leiomyoma is diagnosed, the possibility of HLRCC should be considered clinically. However, genetic counseling and formal mutation testing may not be indicated in the absence of suspicious features identified after a detailed family history and physical examination—such as a personal or family history of cutaneous leiomyomas, renal carcinoma, or uterine leiomyomas with onset at a young age.
Compared with other uterine leiomyomas, FH-deficient leiomyomas were resected at a significantly younger age (mean, 42.7 vs. 48.8 y, P=0.024). Although a hemangiopericytomatous vascular pattern was a relatively constant feature, other morphologic clues to the diagnosis of FH-deficient leiomyomas (such as hypercellularity, prominent nucleoli, symplastic nuclear atypia, and hyaline globules) were subtle, inconstant, or infrequent. Therefore, although we emphasize that FH-deficient leiomyomas are overrepresented among symplastic, atypical, or hypercellular leiomyomas, and these features can certainly be a clue to the diagnosis, we are in agreement with Alsolami et al10 and Martinek et al8 who suggested that these morphologic features lack sufficient objectivity or robustness to be useful to definitively confirm or exclude a diagnosis of FH-deficient leiomyoma in routine clinical practice.
Our experience and that of others, in renal carcinoma is that not all HLRCC-associated tumors will be FH IHC negative and that some definite HLRCC-associated renal carcinomas will show positive staining for FH and could potentially be identified by positive staining for 2SC.14,15 The potential to identify HLRCC-associated uterine leiomyomas by positive staining for 2SC has been demonstrated by others,8,10,11 and we know that 1 of our uterine leiomyomas arising in a patient with a confirmed germline FH mutation but with positive IHC staining for FH was demonstrated to show positive staining for 2SC (data not shown). Perhaps in the future both positive staining for 2SC and negative staining for FH may be used to diagnose FH-deficient leiomyomas, and our experience has been that the combination shows potential in renal carcinoma albeit limited by the lack of sensitivity of FH and lack of specificity of 2SC.14 However, at the time of writing, IHC for 2SC is not commercially available and therefore not practical for routine clinical use. Because of this lack of availability we were unable to test the sensitivity and specificity of 2SC on the entire cohort for this study.
Although it is clear that FH-deficient leiomyomas commonly show cytologic atypia, the relationship between FH deficiency and true leiomyosarcomas (ie, biological evidence of malignancy) is unclear and muddied by previous reports of cases of uterine leiomyosarcoma diagnosed on the basis of histology, often without expert pathologic review, which do not provide evidence of the biological behavior of these tumors.3,22,23 In fact we are only aware of 1 case of uterine leiomyosarcoma arising in the setting of FH mutation in which biological evidence of malignant behavior is reported, and this patient was still alive 12 years after presentation.23 For this reason we believe that overinterpretation of the cytologic atypia and hypercellularity, which may occur in FH-deficient leiomyoma, may lead to “overdiagnosis” of leiomyosarcomas. This is well illustrated in our case of STUMP, which was initially considered a leiomyosarcoma but reclassified as symplastic leiomyoma on review. We note that none of the FH-deficient leiomyomas in our cohort behaved in a malignant manner, and we found no cases with negative FH IHC staining among 116 genuine leiomyosarcomas from 88 patients. This is similar to Reyes et al11 who demonstrated that none of 29 leiomyosarcomas showed positive staining for 2SC. Furthermore, it has previously been demonstrated that FH mutations are not a common driver of uterine leiomyosarcoma.24 Therefore, in the absence of more definitive evidence to the contrary, we believe FH mutations occur rarely (if at all) in association with biologically malignant smooth muscle tumors—that is, true leiomyosarcomas. However, we emphasize that FH-deficient leiomyomas may morphologically mimic leiomyosarcomas because of their hypercellularity and tendency to symplastic nuclear atypia. In this respect, loss of staining for FH may be considered a reassuring finding in a mildly atypical uterine smooth muscle tumor, and we would be hesitant to make a diagnosis of uterine leiomyosarcoma in the setting of FH deficiency unless there is unequivocal evidence of malignancy.
In conclusion, the great majority of uterine leiomyomas arising in the setting of HLRCC will show negative staining for FH, and therefore in the appropriate clinical context FH-deficient leiomyomas can be an important clue to the diagnosis of HLRCC. There are some morphologic clues to assist in the diagnosis of FH-deficient leiomyomas including hypercellularity, prominent nucleoli, symplastic-type nuclear atypia, cytoplasmic eosinophilic globules, and a staghorn vasculature. However, these features are inconstant and may be subtle. Morphology therefore cannot be used to replace IHC or molecular analysis in the routine clinical setting. Not all uterine leiomyomas arising in the setting of HLRCC will show negative staining for FH and positive staining for FH does not completely exclude the diagnosis of HLRCC. Loss of staining for FH occurs rarely, if at all, in true uterine leiomyosarcomas.
Most importantly, at least 1% of all unselected uterine leiomyomas show negative staining for FH. Although these leiomyomas are commonly associated with somatic FH mutation and show similar morphology to cases associated with germline FH mutation, these patients very rarely have demonstrable germline FH mutation. We therefore would not recommend genetic testing for HLRCC in these patients in the absence of other clinical risk factors such as a personal or family history of cutaneous leiomyomas, renal carcinoma, or symptomatic leiomyomas at a very young age. We do, however, believe there is merit in prospectively identifying these patients so that the possibility of syndromic disease can be considered clinically.
Footnotes
W.J.H. and J.A. contributed equally.
Conflicts of Interest and Sources of Funding: Supported by the Cancer Institute NSW through the Sydney Vital Translational Research Centre program (A.J.G.) and the Royal College of Pathologists of Australasia through the medical student pathology scholarship program (W.J.H.). The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Reed WB, Walker R, Horowitz R.Cutaneous leiomyomata with uterine leiomyomata. Acta Derm Venereol. 1973;53:409–416. [PubMed] [Google Scholar]
- 2.Tomlinson IP, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. [DOI] [PubMed] [Google Scholar]
- 3.Launonen V, Vierimaa O, Kiuru M, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98:3387–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alam NA, Rowan AJ, Wortham NC, et al. Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet. 2003;12:1241–1252. [DOI] [PubMed] [Google Scholar]
- 5.Merino MJ, Torres-Cabala C, Pinto P, et al. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31:1578–1585. [DOI] [PubMed] [Google Scholar]
- 6.Toro JR, Nickerson ML, Wei MH, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanz-Ortega J, Vocke C, Stratton P, et al. Morphologic and molecular characteristics of uterine leiomyomas in hereditary leiomyomatosis and renal cancer (HLRCC) syndrome. Am J Surg Pathol. 2013;37:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinek P, Grossmann P, Hes O, et al. Genetic testing of leiomyoma tissue in women younger than 30 years old might provide an effective screening approach for the hereditary leiomyomatosis and renal cell cancer syndrome (HLRCC). Virchows Arch. 2015;467:185–191. [DOI] [PubMed] [Google Scholar]
- 9.Payson M, Leppert P, Segars J. Epidemiology of myomas. Obstet Gynecol Clin North Am. 2006;33:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsolami S, El-Bahrawy M, Kalloger SE, et al. Current morphologic criteria perform poorly in identifying hereditary leiomyomatosis and renal cell carcinoma syndrome-associated uterine leiomyomas. Int J Gynecol Pathol. 2014;33:560–567. [DOI] [PubMed] [Google Scholar]
- 11.Reyes C, Karamurzin Y, Frizzell N, et al. Uterine smooth muscle tumors with features suggesting fumarate hydratase aberration: detailed morphologic analysis and correlation with S-(2-succino)-cysteine immunohistochemistry. Mod Pathol. 2014;27:1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YB, Brannon AR, Toubaji A, et al. Hereditary leiomyomatosis and renal cell carcinoma syndrome-associated renal cancer: recognition of the syndrome by pathologic features and the utility of detecting aberrant succination by immunohistochemistry. Am J Surg Pathol. 2014;38:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bardella C, El-Bahrawy M, Frizzell N, et al. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol. 2011;225:4–11. [DOI] [PubMed] [Google Scholar]
- 14.Trpkov K, Hes O, Agaimy A, et al. IHC screening of unclassified RCC detects tumors associated with HLRCC. Mod Pathol. 2015;28:263A. [Google Scholar]
- 15.Chen YB, Kong MX, Bialik A, et al. Hereditary leiomyomatosis and renal cell carcinoma (HLRCC)-associated renal cancer: a comparison of fumarate hydratase (FH) and S-(2-succino)-cysteine (2SC) immunohistochemistry as ancillary tools. Mod Pathol. 2015;28:271–318. [Google Scholar]
- 16.Bennett JA, Chiang S, Chen Y-B, et al. Leiomyoma with bizarre nuclei: correlation between morphology and fumarate/S-(2-succino)-cysteine expression. Mod Pathol. 2015;28:276A. [Google Scholar]
- 17.Buelow B, Cohen J, Joseph NM, et al. Immunohistochemistry for 2SC and FH in cutaneous leiomyomas may aid in identification of patients with HLRCC (hereditary leiomyomatosis and renal cell carcinoma syndrome). Mod Pathol. 2015;28:117A. [DOI] [PubMed] [Google Scholar]
- 18.Gill AJ. Succinate dehydrogenase (SDH) and mitochondrial driven neoplasia. Pathology. 2012;44:285–292. [DOI] [PubMed] [Google Scholar]
- 19.Barker KT, Spendlove HE, Banu NS, et al. No evidence for epigenetic inactivation of fumarate hydratase in leiomyomas and leiomyosarcomas. Cancer Lett. 2006;235:136–140. [DOI] [PubMed] [Google Scholar]
- 20.Barker KT, Bevan S, Wang R, et al. Low frequency of somatic mutations in the FH/multiple cutaneous leiomyomatosis gene in sporadic leiomyosarcomas and uterine leiomyomas. Br J Cancer. 2002;87:446–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehtonen R, Kiuru M, Vanharanta S, et al. Biallelic inactivation of fumarate hydratase (FH) occurs in nonsyndromic uterine leiomyomas but is rare in other tumors. Am J Pathol. 2004;164:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ylisaukko-oja SK, Kiuru M, Lehtonen HJ, et al. Analysis of fumarate hydratase mutations in a population-based series of early onset uterine leiomyosarcoma patients. Int J Cancer. 2006;119:283–287. [DOI] [PubMed] [Google Scholar]
- 23.Kiuru M, Lehtonen R, Arola J, et al. Few FH mutations in sporadic counterparts of tumor types observed in hereditary leiomyomatosis and renal cell cancer families. Cancer Res. 2002;62:4554–4557. [PubMed] [Google Scholar]
- 24.Alam NA, Olpin S, Leigh IM. Fumarate hydratase mutations and predisposition to cutaneous leiomyomas, uterine leiomyomas and renal cancer. Br J Dermatol. 2005;153:11–17. [DOI] [PubMed] [Google Scholar]


