Abstract
Background
Rapid point-of-care (POC) tests provide an economical alternative for rapid diagnosis and treatment of influenza, especially in public health emergency situations.
Objectives
To test the performance of a rapid influenza diagnostic test, QuickVue (Quidel) as a POC test against a real-time polymerase chain reaction (RT-PCR) assay for detection of influenza A and B in a developing country setting.
Study Design
In a prospective observational design, 600 patients with influenza-like illness (ILI) or with severe acute respiratory illness (SARI) who were referred to the Influenza Clinic of a tertiary care hospital in Srinagar, India from September 2012 to April 2013, were enrolled for diagnostic testing for influenza using QuickVue or RT-PCR. All influenza A-positive patients by RT-PCR were further subtyped using primers and probes for A/H1pdm09 and A/H3.
Results
Of the 600 patients, 186 tested positive for influenza A or B by RT-PCR (90 A/H1N1pdm09, 7 A/H3 and 89 influenza B), whereas only 43 tested positive for influenza (influenza A = 22 and influenza B = 21) by QuickVue. Thus, the sensitivity of the QuickVue was only 23% (95% confidence interval, CI: 17.3-29.8) and specificity was 100% (95% CI: 99.1-100) with a positive predictive value (PPV) of 100% (95% CI 91.8-100) and a negative predictive value (NPV) of 74.3% (95% CI: 70.5-77.9) as compared to RT-PCR.
Conclusions
The high specificity of QuickVue suggest that this POC test can be a useful tool for patient management or triaging during a public health crisis but a low sensitivity suggests that a negative test result need to be further tested using RT-PCR.
Keywords: Influenza, rapid tests, real-time PCR, sensitivity
Introduction
Influenza viruses cause outbreaks and epidemics that can spread rapidly, resulting in significant morbidity and mortality with an estimated 250,000 to 500,000 deaths annually.[1] In March 2009, an outbreak of novel H1N1 influenza A virus infection was detected in Mexico, with a rapid spread across many countries, resulting in pandemic.[2] This infection was associated with increased morbidity and mortality among children and younger adults compared with the usual seasonal influenza virus strains.[3,4] The emergence of the pandemic highlighted the challenges posed by rapid spread of viruses and fragility of health care infrastructure capacity.[5] Additionally, many countries could not handle the surge in virus testing because of the lack of laboratories with testing capacity, and the molecular tests being time-consuming and expensive. Hence simple bed-side point-of-care (POC) test systems are needed to handle testing capacity during public health emergency settings.
India witnessed its first case of pandemic A/H1N1pdm09 in May 2009 followed soon by a surge throughout the country, which overwhelmed the laboratory capacity for influenza testing. India has an extensive influenza surveillance network with many laboratories capable of testing for influenza.[6,7] While influenza circulation in most part of India reveals peaks during the monsoon periods in July–September, influenza circulation in the northern-most states is observed during the peak winter months of December–March.[6,8] Immediately following the pandemic in 2009, we reported that pandemic and seasonal influenza viruses contribute significantly to respiratory illness in the northern Indian state of Jammu and Kashmir[8,9] where uptake of influenza vaccination is poor, even in high-risk populations.[10]
The gold standard reference methods for the diagnosis of influenza include, virus isolation in past years and molecular detection using real-time polymerase chain reaction assays (RT-PCR) more recently. In comparison, commercially available rapid influenza diagnostic tests (RIDT) antigen detection ‘point of care’ immunoassay tests have the advantage of providing results much more quickly (within minutes).[11-13] These RIDT tests are simple to use and have the advantage of a fast turnaround, which can influence triage, diagnosis, initiation and duration of treatment and hospitalization. The rapid tests have a variable sensitivity ranging from 10-96%, even though the specificity consistently exceeds >90% for some tests.[12,13] While both molecular and POC assays have limitations for patient care, RIDTs might be useful tools during public health emergency response. During the initial phase of pandemic, a confirmatory test was required prior to initiation of antiviral. The QuickVue Influenza A and B test is a POC test for rapid diagnosis of influenza and differentiation of influenza A and B viruses.
We report our findings of the comparison of the QuickVue Influenza A and B test with that of the RT-PCR in the setting of a developing country where the volumes of patients are high and it would be greatly advantageous to have a RIDT available for quick initiation of therapy, specifically during pandemic periods. The study, to the best of our knowledge, is the first from this part of world.
Material and Methods
All patients with influenza-like illness (ILI) or with severe acute respiratory illness (SARI), who were referred to the Influenza Clinic at Sher-i-Kashmir Institute of Medical Sciences, Srinagar, Kashmir for testing for influenza between September 2012 and April 2013, were enrolled for the prospective observational study for comparative diagnostic testing for influenza using QuickVue or RT-PCR. We defined ILI as fever of 100°F (>37.2° C) accompanied by cough and/or sore throat, whereas SARI was defined as those patients with ILI who also require hospitalisation. The timing of study was selected based on the peak circulation of influenza in Srinagar.[8] The specimens were either collected in an area designated for collection or from hospital wards. Combined throat and nasal swabs were collected simultaneously in viral transport medium for RT-PCR testing for influenza viruses.[6,7] The lab personnel performing the RT-PCR assay were blinded to the results of the QuickVue test. QuickVue Influenza A and B (Quidel Corporation, San Diego, USA) is a lateral-flow immunoassay using highly sensitive monoclonal antibodies that are specific for antigens of both Influenza A and B virus, with no known cross reactivity to other normal flora or other respiratory pathogens.[14] The test involves the extraction of influenza A and B viral antigens. The patient specimen is placed in the ‘Extraction Reagent Tube’, during which time the virus particles get disrupted exposing internal viral nucleoproteins. After extraction the test strip is placed in the ‘Extraction Reagent Tube’ where nucleoproteins in the specimen react with the reagents in the test strip. If the extracted specimen contains influenza A or B antigens, a pink to red ‘Test Line’ along with a blue procedural ‘Control Line’ appears indicating a positive result. The Test Line for Influenza A or B develop at separate locations on the strip. In case of a negative sample, only the blue procedural Control Line will appear.[14] The QuickVue Influenza A and B test was carried out strictly according to the manufacturer’s instructions. RT-PCR was performed within 1-3 hours of sample collection. All specimens that were influenza A-positive by RT-PCR were further subtyped using primers and probes for A/H1pdm09 and A/H3.
Specimens positive for influenza A or B virus in the RT-PCR were regarded as true positives. The sensitivity, specificity, positive (PPV) and negative (NPV) predictive values of the QuickVue Influenza A and B test results compared with those of the RT-PCR assay were calculated using two-by-two contingency tables. Continuous variables were tested by student’s t-test, whereas categorical variables were tested for statistical significance using Fisher’s exact/chi-square test using Medcalc Version 12.7 software. Values have been expressed as mean + SD and a P < 0.05 was considered significant.
The study was approved by the Institute Ethics Committee of SKIMS and informed consent for participation was obtained for all patients.
Results
A total of 600 patients presenting with ILI (n = 469) or SARI (n = 131) were enrolled from September 2012 to April 2013 at the tertiary care hospital at SKIMS. Among enrolled patients, almost half were males (n = 330) and 353 patients were 18 years or older. The date of enrolment ranged from 1-6 days after onset of symptoms (median 4 days).
Of the 600 recruited patients, 186 (31%) tested positive for influenza A or B by RT-PCR (97 for influenza A and 89 for influenza B), whereas only 43 (7.17%) tested positive for influenza A (n = 22, 21 were H1N1pdm09, 1 was H3N2) and influenza B (n = 21) by QuickVue testing. All 43 specimens positive by QuickVue were also positive by RT-PCR, thus giving a sensitivity of only 23.1% (95% confidence interval, CI 17.3-29.8) and a specificity of 100% (95% CI 99.1–100), with a PPV of 100% (95%CI: 91.8-100) and a NPV of 74.3% (95% CI 70.5-77.9) [Table 1]. There was no difference in sensitivity of RIDT in patients with ILI (22.8; 95%CI: 16.5-30.1) or SARI (25% 95% CI: 10.7-44.9) (P = 0.67), whereas the specificity was 100% in both the groups [Table 1].
Table 1. Performance of QuickVue versus RT-PCR for detection of influenza virus among patients with influenza-like illness or severe acute respiratory illness at Sher-i-Kashmir Institute of Medical Sciences, Srinagar, India.
| Overall | SARI | ILI | |
|---|---|---|---|
| Number tested | 600 | 131 | 469 |
| RT-PCR positive N (%) | 186 (31) | 28 (21.4) | 158 (33.7) |
| QuickVue positive N (%) | 43 (7.2) | 7 (5.3) | 36 (7.7) |
| Sensitivity (%) (95% CI) | 23.1 (17.3-29.9) | 25 (10.7-44.9) | 22.8 (16.5-30.1) |
| Specificity (%) (95% CI) | 100 (99.1-100) | 100 (96.5-100) | 100 (98.8-100) |
| PPV (%) (95% CI) | 100 (91.8-100) | 100 (59.0-100) | 100 (90.3-100) |
| NPV (%) (95% CI) | 74.3 (70.5-77.9) | 83.1 (75.3-89.2) | 71.8 (67.3-76) |
RT-PCR: Real-time polymerase chain reaction, CI: Confidence interval, PPV: Positive predictive value, NPV: Negative predictive value, ILI: Influenza-like illness, SARI: Severe acute respiratory illness
Any potential bias due to sampling or storage of kits was ruled out. All nasopharyngeal samples were collected for QuickVue and RT-PCR testing by well-trained staff members. The QuickVue kits were stored properly, not expired, and the tests were performed immediately as per the manufacturer’s instructions.
Analysis of QuickVue sensitivity by influenza types showed that there was no difference in the sensitivity for the influenza A and B and specificity was 100% for both influenza types [Table 2]. Further, both subtypes of influenza A/H1N1 pdm09 and A/H3 were detected by RITD assays (data not shown). We next assessed the performance of QuickVue among patients in different age groups. Of the 186 RT-PCR influenza positives, 51 were in those <18 years and 135 among those ≥18 years. While specificity and PPV was 100% for both age groups, the sensitivity was higher for children <18 years (37.3%, 95% CI 24.1-51.9), compared to those in ≥18 years (17.8%; 95% CI 11.7-25.3; P < 0.009) [Table 2].
Table 2. Sensitivity, specificity, positive and negative predictive values of QuickVue assay, by influenza types and age group, Sher-i-Kashmir Institute of Medical Sciences Srinagar, India.
| Influenza A | Influenza B | Children (≤18 years) | Adults (≥18 years) | |
|---|---|---|---|---|
| Number tested | 97 | 89 | 247 | 353 |
| RT-PCR positive N (%) | 97 (100) | 89 (100) | 51 (20.6) | 135 (38.2) |
| QuickVue positive N (%) | 22 (22.7) | 21 (23.6) | 19 (7.7) | 24 (6.8) |
| Sensitivity (%) (95% CI) | 22.7 (14.8-32.3) | 23.6 (15.2-33.8) | 37.3 (24.1-51.9)* | 17.8 (11.7-25.3)* |
| Specificity (%) (95% CI) | 100 (99.1-100) | 100 (99.1-100) | 100 (98.1-100) | 100 (98.3-100) |
| PPV (%) (95% CI) | 100 (84.6-100) | 100 (83.9-100) | 100 (82.4-100) | 100 (85.8-100) |
| NPV (%) (95% CI) | 84.7 (81.2-87.8) | 85.9 (82.5-88.9) | 86 (80.8-90.2) | 66.3 (60.9-71.4) |
Sensitivity of QuickVue higher for children≤18 years vs adults. RT-PCR: Real-time polymerase chain reaction, PPV: Positive predictive value, NPV: Negative predictive value, CI: Confidence interval
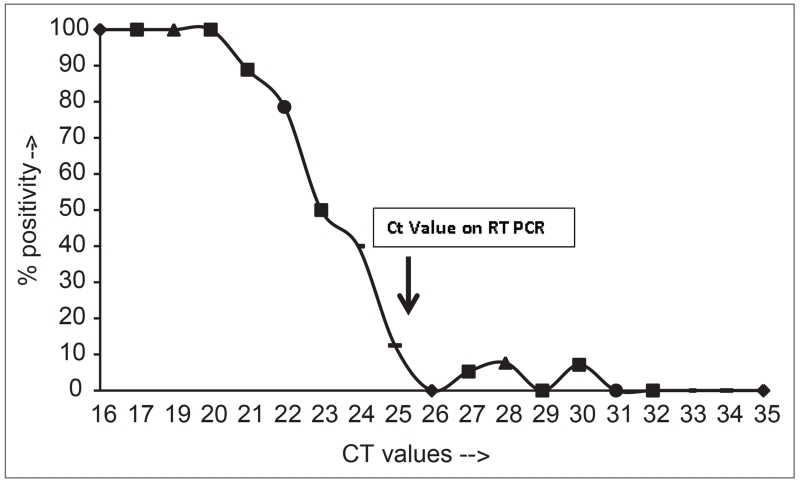
When the percentage positivity of the QuickVue tests were correlated against the cycle threshold threshold (Ct), a Ct value of < 21 was associated with a near 100% positivity of the rapid test, whereas those with higher values of CT had progressively lower positivity rates [Figure 1]. Thus the RT-PCR Ct values were lower in those with QuickVue test results that were positive (mean ± SD 22.02 + 3.04, range 16-31, median 22) vs negative (mean ± SD 28.35 + 3.1, range 21-35, median 28; P < 0.0001). This suggests that specimens with a higher viral load (as shown by low Ct values in the RT-PCR assay) are more likely to be positive in the QuickVue assay.
Figure 1.
Relationship between Ct values by RT-PCR and influenza positivity by Quickvue. The Ct values obtained for RT-PCR for influenza specific PCR (X-axis) are plotted against percent positivity by Quickvue test (Y-axis). Lower Ct values (proxy for high viral loads) correlated with higher positivity by Quickvue assay.
The cost of a QuickVue was approximately USD $10 per test, compared to an approximate cost of USD 50 per test for RT-PCR. The results of QuickVue are available in 15 minutes, whereas that of the RT-PCR are usually available the next day (unless during public health emergency when results are available in about 4-6 hours).
Discussion
India faced a huge public health crisis during the recent 2009 pandemic, with tremendous pressure and resource constraint on the health care system. We undertook a prospective observational analysis to compare the performance of a POC test (QuickVue) with a gold-standard RT-PCR assay to measure the utility of the POC test during public health emergencies. While we observed a very high specificity and PPV of the QuickVue Influenza A and B test for detection of influenza A or B, the sensitivity and NPV were very low, when compared to RT-PCR in both age groups that we studied. Thus a positive QuickVue test provides a rapid confirmation of influenza positivity which can help facilitate patient management and quick initiation of antiviral therapy; however, a negative test will require further assessment using more standard tests for confirmation of influenza. While in India a confirmatory test was required prior to initiation of antiviral, Centers for Disease Control (CDC) recommends that decisions about starting antiviral treatment should not await laboratory confirmation of influenza.[15]
Our observation of low sensitivity (23%) with high specificity is in agreement with some other published studies.[12,13,16-23] Overall, while the specificity of various RITD tests is high, sensitivity varies from study to study. A recent review of 159 studies with 26 RITD assays revealed a pooled sensitivity 62.3%.[12] Some investigators have reported a higher sensitivity of RIDT when compared to viral culture. Cheng et al.,[24] reported an overall sensitivity of 68% and specificity of 96% compared with viral culture, whereas Tai et al.,[25] reported a overall sensitivity of 82% and specificity of 99%. Gordon reported an agreement of 86% between the two tests.[26] In a meta-analysis of rapid tests for influenza, QuickVue RIDT had the highest sensitivity (51%) of all RITDs;[13] note that the figure of 51% is much higher than the sensitivity in current study and may be due to characteristics of the study population. In the current study, no difference in sensitivity of detection of influenza A (including pandemic H1N1pdm09) and B were observed. This is in contrast to previous studies, where RITDs were shown to be more sensitive for influenza A than B.[18] Similar to recently reported studies, we were also able to detect pandemic A/H1N1pdm09.[27,28]
We identified few factors that might account for the low sensitivity of RITDs in current study. No deviations from the manufacturer’s recommended specimen type, sample storage, handling and processing were practiced and all specimens were tested immediately (within 3 hours) of collection. However, a possible mechanism for the low sensitivity could be the use of twin swabs in RT-PCR, which could lead to a higher viral load, and only a single nasopharyngeal swab in the RIDT test. The choice of a reference standard has also been reported to affect the sensitivity of the RIDT tests. For example, sensitivities have generally been higher when RIDT influenza tests were compared with viral culture, than when they were compared with RT-PCR.[12,13] In the current study, we only used RT-PCR which could account for the lower measured sensitivity of detection by RIDT. The median duration of symptoms in our patients at the time of collection of swabs was 4 days (range 1–6 days). Performance of the rapid test has been reported to vary by the day of presentation, with a sensitivity of 41.7% for samples from children presenting on the day of symptom onset and a sensitivity of 72.1% for samples from children presenting one or more days post-symptom onset.[26] Thus a second swab collected after the day of the onset of the symptoms may help increase the sensitivity of the assay.
Our results emphasize that clinicians should understand that negative results of influenza testing do not exclude influenza virus infection, as a variety of factors can influence test results. Some of these factors include the time from the onset of illness to the collection of the specimen, prevalence of the circulating influenza viruses in the studied population, improper sample collection, use of clinical specimen or swab other than those recommended, prolonged time between the onset of illness and sample collection, improper storage or handling and testing of upper respiratory specimens in a patient with predominant lower respiratory disease.[5]
The current study was conducted in a period of high influenza activity. The rate of false-negative (and true-positive) results are more likely to occur when disease prevalence is high in the community.[6] Likewise, the PPV is the highest if the activity of influenza is high. The high PPV of the QuickVue assay observed in this study indicates that positive results do not need to be confirmed by RT-PCR and can be reported to the treating physician immediately in times of both low and high influenza rates. However, negative QuickVue Influenza A + B results, as well as negative results of other RIDT antigen assays, should be confirmed by RT-PCR. This is even more important during the influenza season, because of decreasing NPV with increasing influenza virus incidence.
We observed a higher sensitivity of RITD for influenza detection among children as compared to adults, even as the specificity in both age groups was similar. These observations are in agreement with previous studies where sensitivity was reported to be lower in adults when compared with children, even when adjusted for brand of RIDT, specimen type or reference standard.[18] Higher viral loads and prolonged shedding in children than in adults could contribute to the higher sensitivity seen in children.[6]
Lower Ct values on RT-PCR in our patients was associated with a significantly higher positivity of RIDT tests, suggesting that a higher viral load was associated with a higher positivity of the RIDT antigen test; this finding is similar to a recently published report.[28] The twin swabs used in the RT-PCR samples might also have yielded a higher positivity because of a higher viral load due to a greater volume of the specimen collected by two swabs as compared to one swab in the RIDT test. Recent reports suggest that there is an inverse relationship between Ct values and viral load and those therefore qualitative results from RT-PCR assays can be converted into quantitative viral load values in clinical samples without running standard curves in parallel.[29]
Based on this information, we propose an alternate algorithm for influenza testing during a public health emergency: RIDTs can be used as a first screening assay for patient management, expected to identify almost one-fourth of influenza-positive cases. The remaining RITD negative cases should be tested by RT-PCR for influenza confirmation. In addition, as RIDT-positive cases likely were detected among those with high viral loads, positive RIDT likely represent cases severe disease,[30] which can then receive immediate care and intervention. The use of RITD as first line of testing can further be assessed based on the cost; the RITD cost is US$10 and the RT-PCR cost five times as high. The lower cost along with a fast turnaround time argues for the use of RIDTs as the first screening procedure, particularly during times of high influenza activity.
Our results also reinforce CDC guidelines,[15] that decisions about starting antiviral treatment should not wait for laboratory confirmation of influenza. Instead, antiviral agents should be started as early as possible in patients with confirmed or suspected influenza who are hospitalised; have severe, complicated, or progressive illness; or are at higher risk for influenza complications.[15] Antiviral agents can also be considered for other patients if treatment can be started within 48-hours of illness onset. However, clinical practice may vary among countries, and some studies show that physicians base antiviral therapy decisions on the results of influenza testing. In India, government response included initiation of antiviral therapy only after positive influenza test results. Since RIDT tests are used as rapid tests for decisions about patient management, the test provides a tool for quick confirmation of influenza during public health emergency situations. Additionally, a positive RITD test would quickly indicate presence of influenza in population, which can help policy makers develop appropriate intervention strategies. The development of more sensitive POC rapid diagnostic tests with higher sensitivity is urgently needed.
Acknowledgement
Authors acknowledge Drs. J. Tokars, M-A Widdowson and S. Saha for excellent comments on the draft.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.World Health Organization [Last accessed on 2013 Jun 14];Influenza (seasonal) Available from: http://www.who.int/mediacentre/factsheets/fs211/en/
- 2.Centers for Disease Control and Prevention (CDC) [Last accessed on 2013 Feb 25];The 2009 H1N1 Pandemic: Summary Highlights, April 2009-April 2010. Available from: http://www.cdc.gov/h1n1flu/cdcresponse.htm.
- 3.Wijngaard CC, Asten LV, Koopmans MP, Pelt WV, Nagelkerke NJ, Wielders CC, et al. Comparing pandemic to seasonal influenza mortality: Moderate impact overall but high mortality in young children. PLoS One. 2012;7:e31197. doi: 10.1371/journal.pone.0031197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: A modelling study. Lancet Infect Dis. 2012;12:687–95. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 5.Leung GM, Nicoll A. Reflections on pandemic (H1N1) 2009 and the international response. PLoS Med. 2010;7:e1000346. doi: 10.1371/journal.pmed.1000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chadha MS, Broor S, Gunassekaran P, Potdar VA, Krishnan A, Chawla-Sarkar M, et al. Multisite virological influenza surveillance in India: 2004-2008. Influenza Other Respir Viruses. 2012;6:196–203. doi: 10.1111/j.1750-2659.2011.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potdar VA, Chadha MS, Jadhav SM, Mullick J, Cherian SS, Mishra AC. Genetic characterization of the influenza A pandemic (H1N1) 2009 virus isolates from India. PLoS One. 2010;5:e9693. doi: 10.1371/journal.pone.0009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koul PA, Mir MA, Bali NK, Chawla-Sarkar M, Sarkar M, Kaushik S, et al. Pandemic and seasonal influenza viruses among patients with acute respiratory illness in Kashmir (India) Influenza Other Respir Viruses. 2011;5:e521–7. doi: 10.1111/j.1750-2659.2011.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan UH, Mir MA, Ahmad F, Mir MH, Bali NK, Lal RB, et al. An outbreak of influenza B in an isolated nomadic community in Jammu and Kashmir, India. Indian J Med Res. 2013;138:1012–5. [PMC free article] [PubMed] [Google Scholar]
- 10.Bali N, Ashraf M, Ahmad F, Khan UH, Widdowson MA, Lal RB, et al. Knowledge, attitude and practices about the seasonal influenza vaccination among healthcare workers in Srinagar, India. Influenza Other Respir Viruses. 2013;7:540–5. doi: 10.1111/j.1750-2659.2012.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . WHO recommendations on the use of rapid testing for influenza diagnosis. World Health Organization; Geneva: [Last accessed on 2010 Jul 22]. Jul, 2005. Available from: http://www.who.int/influenza/resources/documents/rapid_testing/en/index.html. [Google Scholar]
- 12.Chartrand C, Leeflang MM, Minion J, Brewer T, Pai M. Accuracy of rapid influenza diagnostic tests: A meta-analysis. Ann Intern Med. 2012;156:500–11. doi: 10.7326/0003-4819-156-7-201204030-00403. [DOI] [PubMed] [Google Scholar]
- 13.Chu H, Lofgren ET, Halloran ME, Kuan PF, Hudgens M, Cole SR. Performance of rapid influenza H1N1 diagnostic tests: A meta-analysis. Influenza Other Respir Viruses. 2012;6:80–6. doi: 10.1111/j.1750-2659.2011.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [Last accessed on 2014 Jun 10];Quick Vue A+B Test: Quidel. Available from: http://www.quidel.com/sites/quidel.com/files/product/documents/pi_qvinfluenzaab_modcomplex.pdf. [Google Scholar]
- 15.CDC [Last accessed on 2013 Jul 25];Seasonal Influenza: Use of antivirals-Guidance of use of Influenza antiviral agents. Available from: http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm.
- 16.Harper SA, Bradley JS, Englund JA, File TM, Gravenstein S, Hayden FG, et al. Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children--diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: Clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1003–32. doi: 10.1086/604670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention . Guidance for clinicians on the use of rapid influenza diagnostic tests. Centers for Disease Control and Prevention; Atlanta: [Last accessed on 2011 Mar 8]. Available from: http://www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm. [Google Scholar]
- 18.Uyeki TM. Influenza diagnosis and treatment in children: A review of studies on clinically useful tests and antiviral treatment for influenza. Pediatr Infect Dis J. 2003;22:164–77. doi: 10.1097/01.inf.0000050458.35010.b6. [DOI] [PubMed] [Google Scholar]
- 19.Grijalva CG, Poehling KA, Edwards KM, Weinberg GA, Staat MA, Iwane MK, et al. Accuracy and interpretation of rapid influenza tests in children. Pediatrics. 2007;119:e6–11. doi: 10.1542/peds.2006-1694. [DOI] [PubMed] [Google Scholar]
- 20.Mehlmann M, Bonner AB, Williams JV, Dankbar DM, Moore CL, Kuchta RD, et al. Comparison of the MChip to viral culture, reverse transcription-PCR, and the QuickVue Influenza A+B test for rapid diagnosis of influenza. J Clin Microbiol. 2007;45:1234–7. doi: 10.1128/JCM.02202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurt AC, Alexander R, Hibbert J, Deed N, Barr IG. Performance of six influenza rapid tests in detecting human influenza in clinical specimens. J Clin Virol. 2007;39:132–5. doi: 10.1016/j.jcv.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Rashid H, Shafi S, Haworth E, El Bashir H, Ali KA, Memish ZA, et al. Value of rapid testing for influenza among Hajj pilgrims. Travel Med Infect Dis. 2007;5:310–3. doi: 10.1016/j.tmaid.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Uyeki TM, Prasad R, Vukotich C, Stebbins S, Rinaldo CR, Ferng YH, et al. Low sensitivity of rapid diagnostic test for Influenza. Clin Infect Dis. 2009;48:e89–92. doi: 10.1086/597828. [DOI] [PubMed] [Google Scholar]
- 24.Cheng CK, Cowling BJ, Chan KH, Fang VJ, Seto WH, Yung R, et al. Factors affecting QuickVue Influenza A+B rapid test performance in the community setting. Diagn Microbiol Infect Dis. 2009;65:35–41. doi: 10.1016/j.diagmicrobio.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Tai CF, Lu CY, Shao PL, Lee PI, Chang LY, Huang LM. Rapid-test sensitivity for novel swine-origin pandemic influenza A. J Formos Med Assoc. 2012;111:427–30. doi: 10.1016/j.jfma.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Gordon A, Videa E, Saborío S, López R, Kuan G, Balmaseda A, et al. Diagnostic accuracy of a rapid influenza test for pandemic influenza A H1N1. PLoS One. 2010;5:e10364. doi: 10.1371/journal.pone.0010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poeppl W, Herkner H, Burgmann H, Pustelnik T, Mooseder G, Popow-Kraupp T, et al. Performance of the QuickVue Influenza A+B rapid test for pandemic H1N1 (2009) virus infection in adults. PLoS One. 2011;6:e28089. doi: 10.1371/journal.pone.0028089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louie JK, Guevara H, Boston E, Dahlke M, Nevarez M, Kong T, et al. Rapid influenza antigen test for diagnosis of pandemic (H1N1) 2009. Emerg Infect Dis. 2010;16:824–6. doi: 10.3201/eid1605.091794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piralla A, Daleno C, Pariani E, Conaldi P, Esposito S, Zanetti A, et al. Virtual quantification of influenza A virus load by real-time RT-PCR. J Clin Virol. 2013;56:65–8. doi: 10.1016/j.jcv.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Li CC, Wang L, Eng HL, You HL, Chang LS, Tang KS, et al. Correlation of pandemic (H1N1) 2009 viral load with disease severity and prolonged viral shedding in children. Emerg Infect Dis. 2010;16:1265–72. doi: 10.3201/eid1608.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]